Influences of the Introduced O-Containing Functional Groups on the Gaseous Pyrolysis Product of Superfine Pulverized Coal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Modified Methods
2.2. Characterization of the Carbon Skeleton
2.3. Characterization of the Pore Structure
2.4. Thermogravimetry and Pyrolysis
2.5. Naming Rules for Samples
3. Results and Discussion
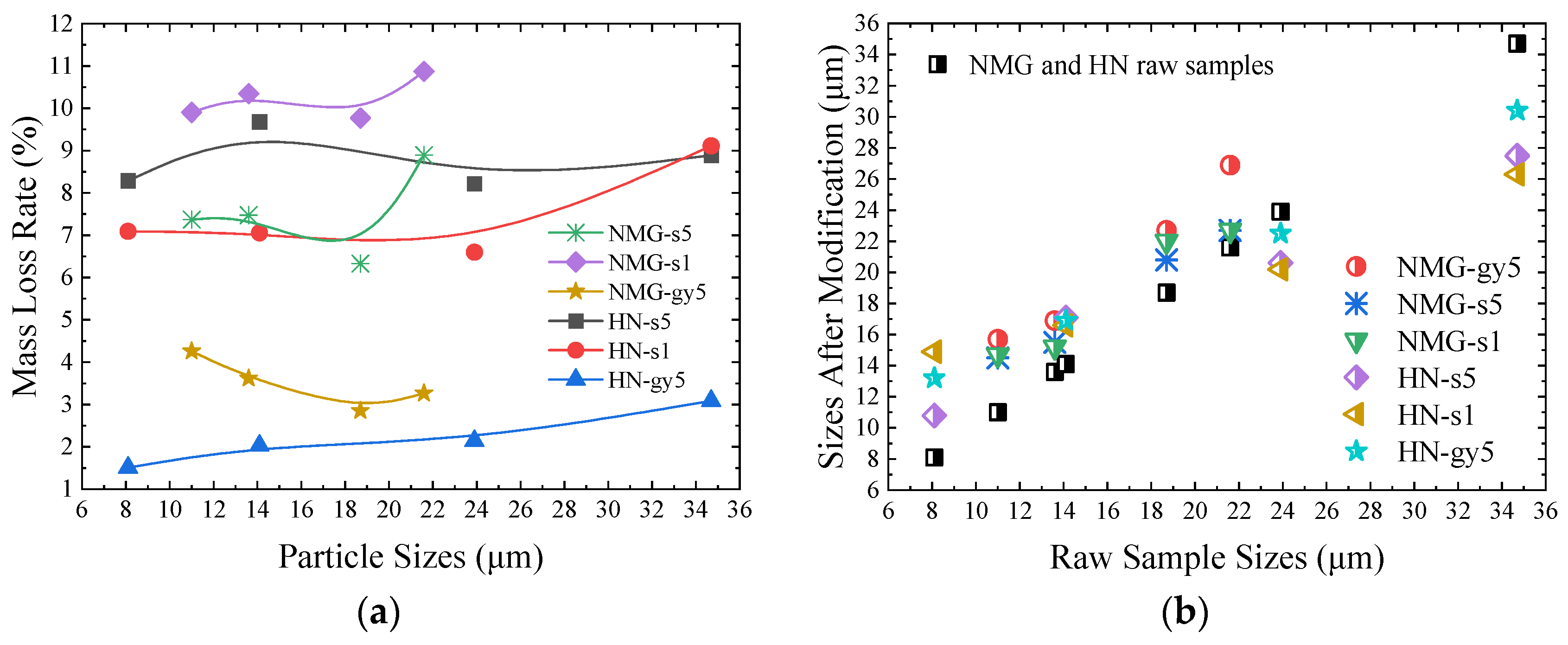
3.1. Mass and Particle Sizes Variation
3.2. Change in the Carbon Structure
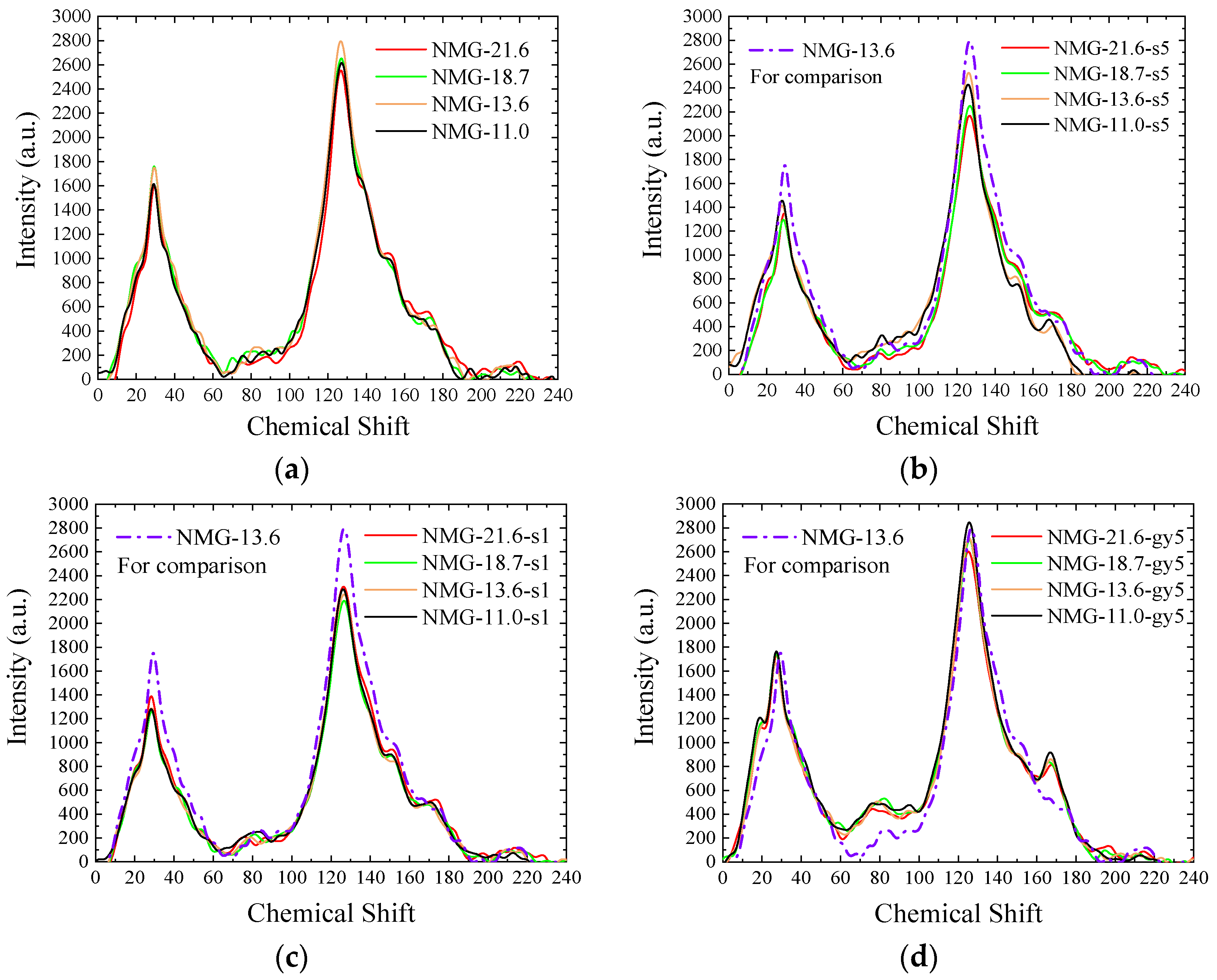
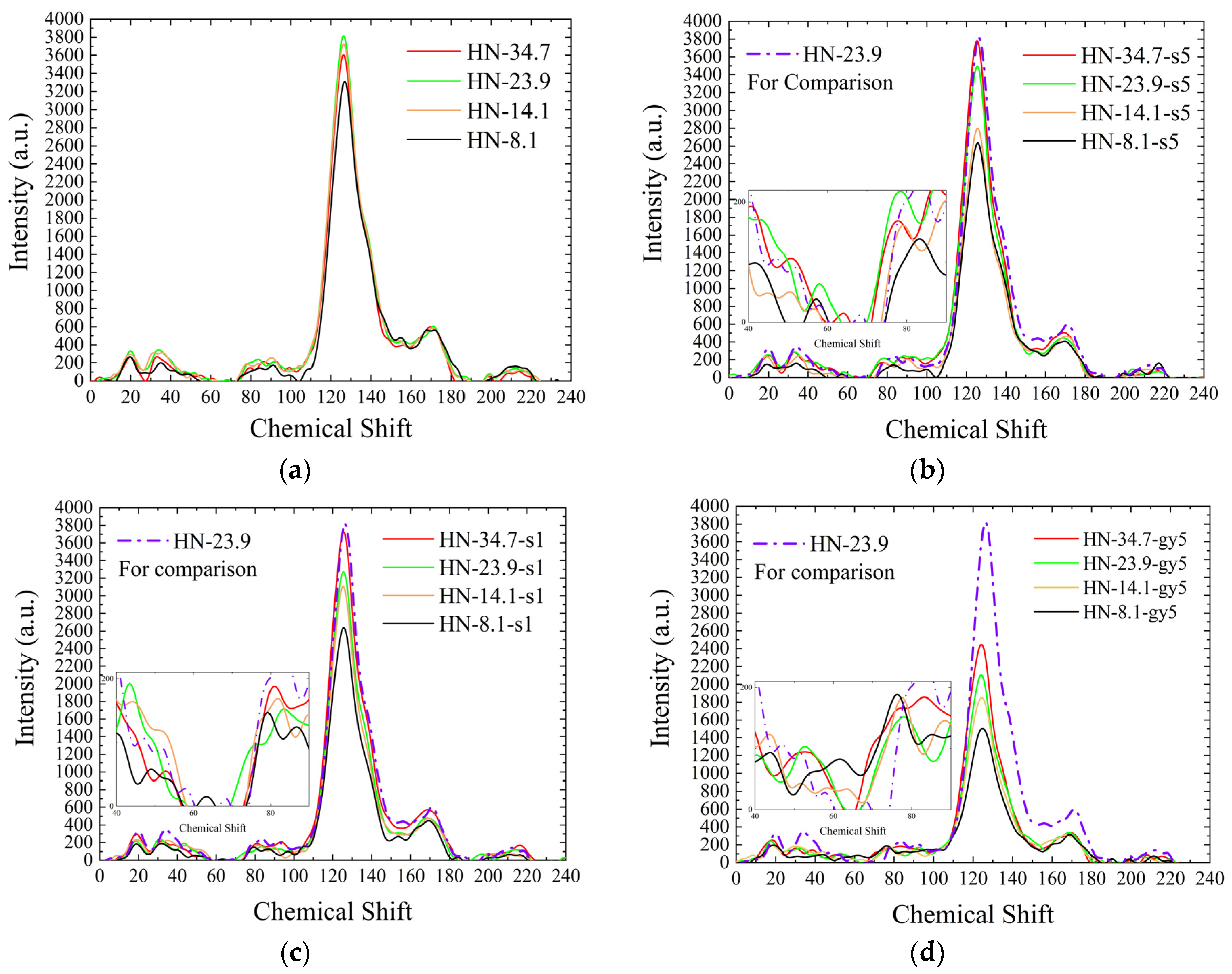
3.2.1. Analysis of 13C-NMR Spectra
NMG Samples
HN Samples
3.2.2. The Change in the Surface Structure
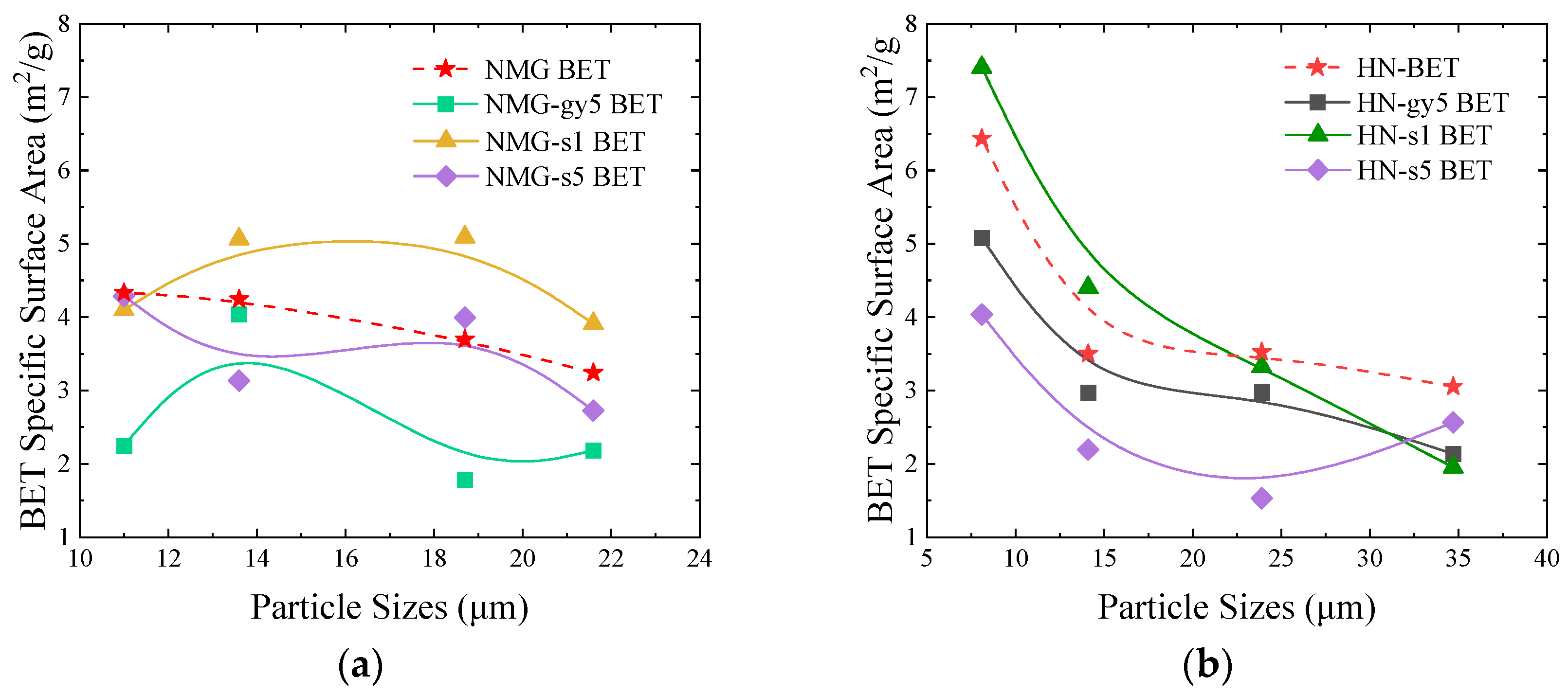
3.3. Pore structure Analysis
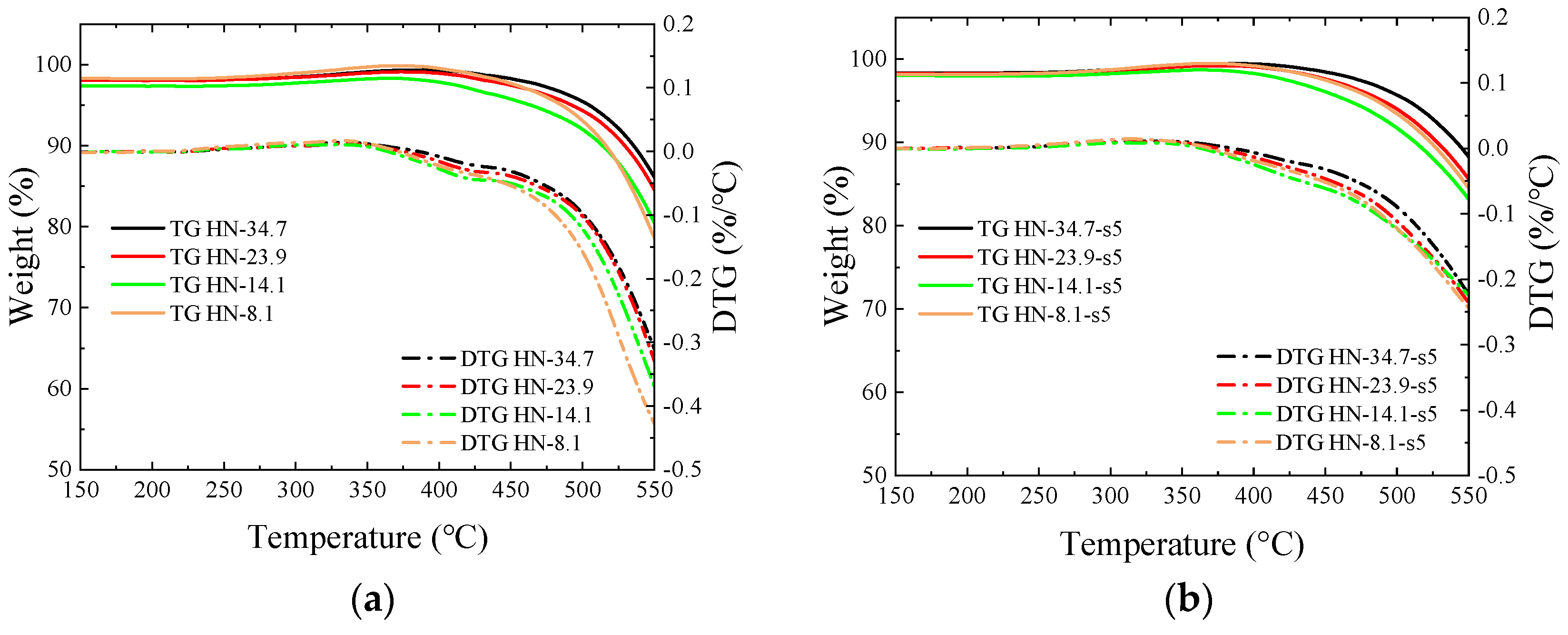
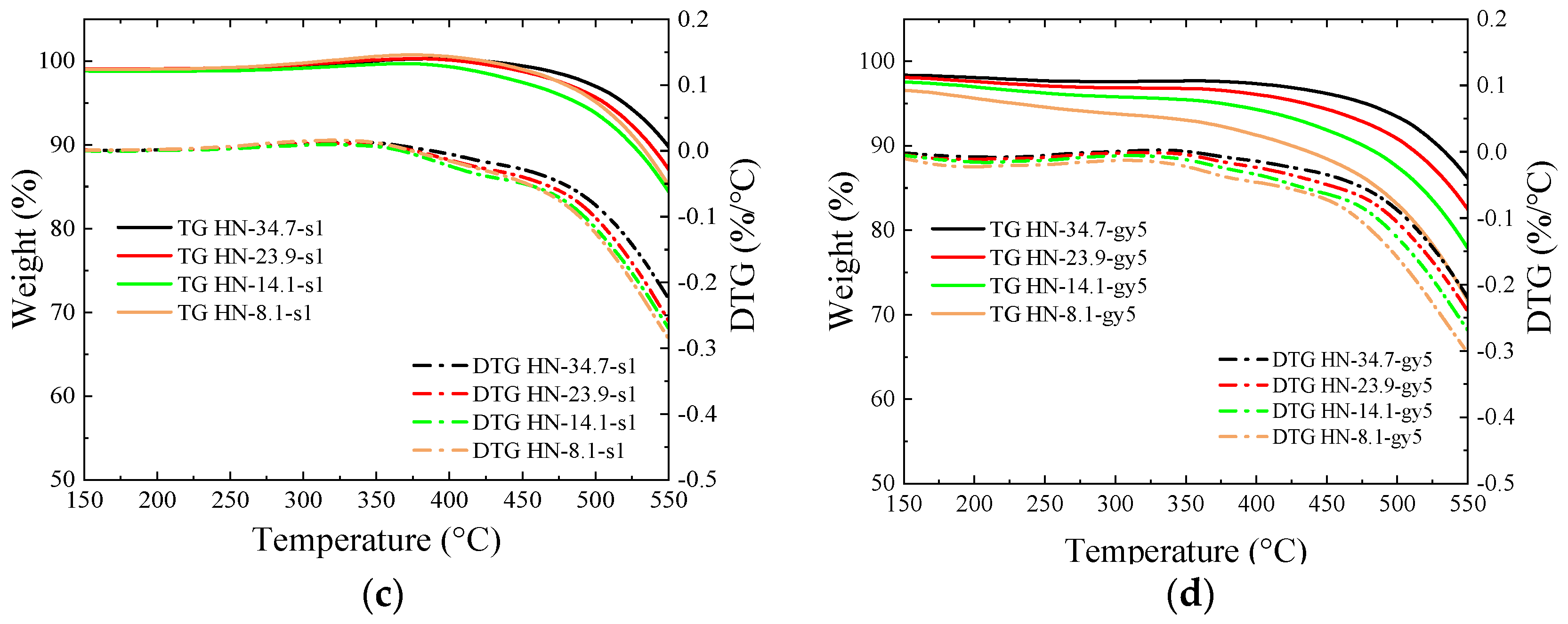
3.4. Thermogravimetric Analysis
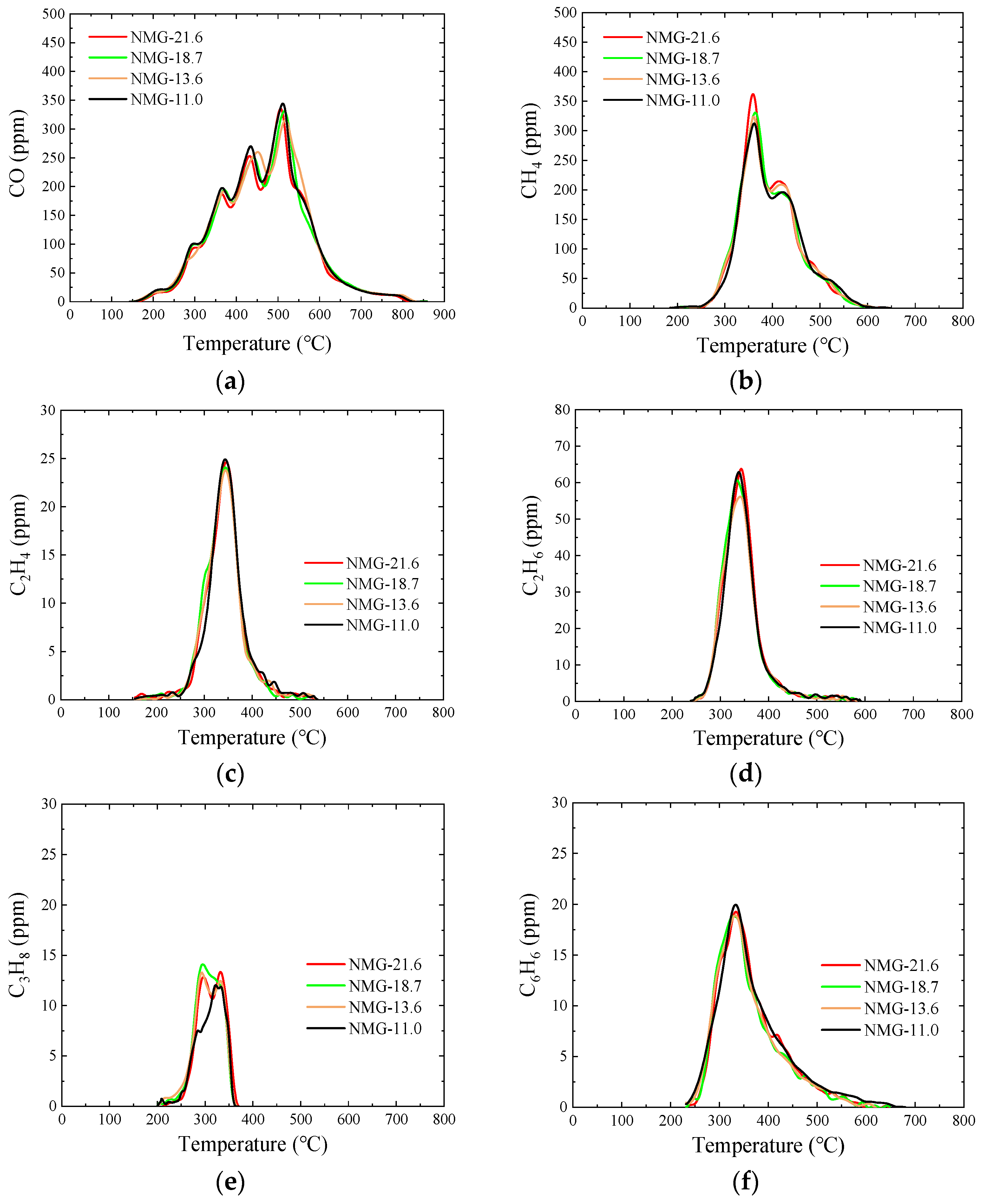
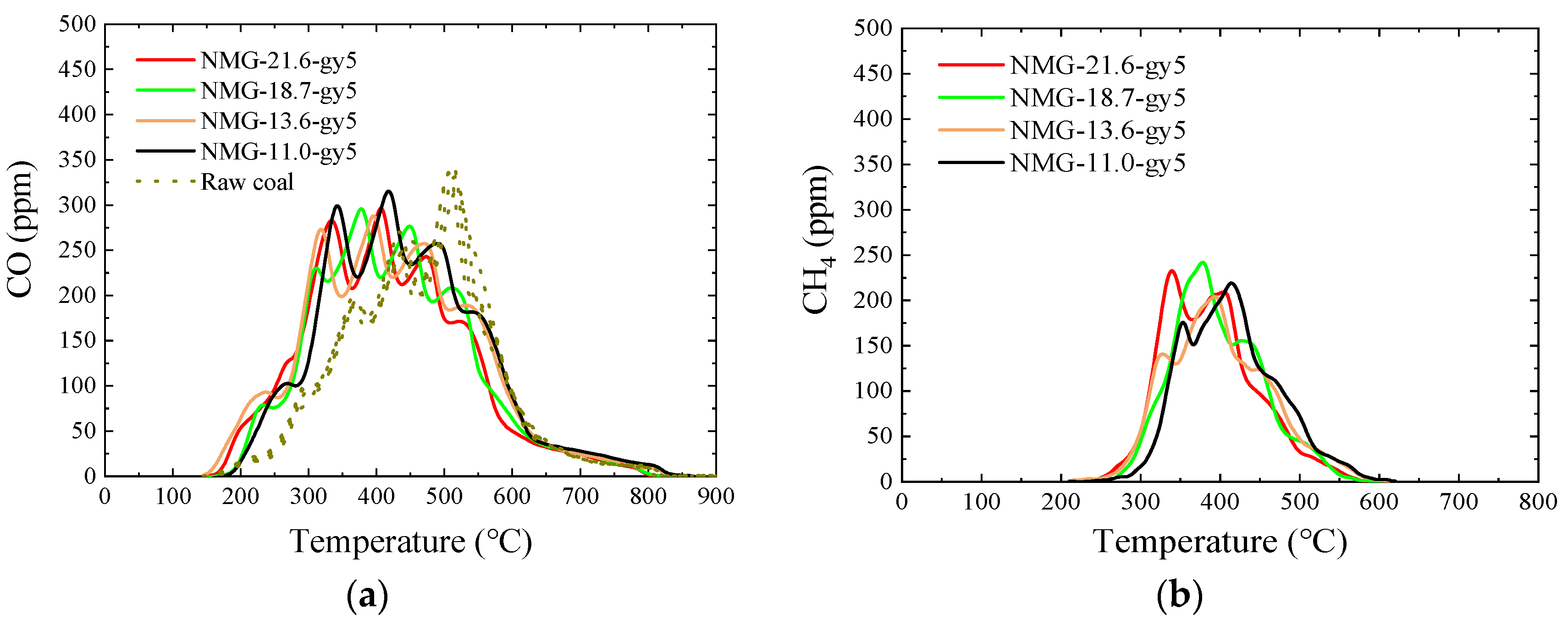
3.5. Pyrolysis Gas
3.5.1. NMG Raw Samples
3.5.2. NMG–gy5 Samples
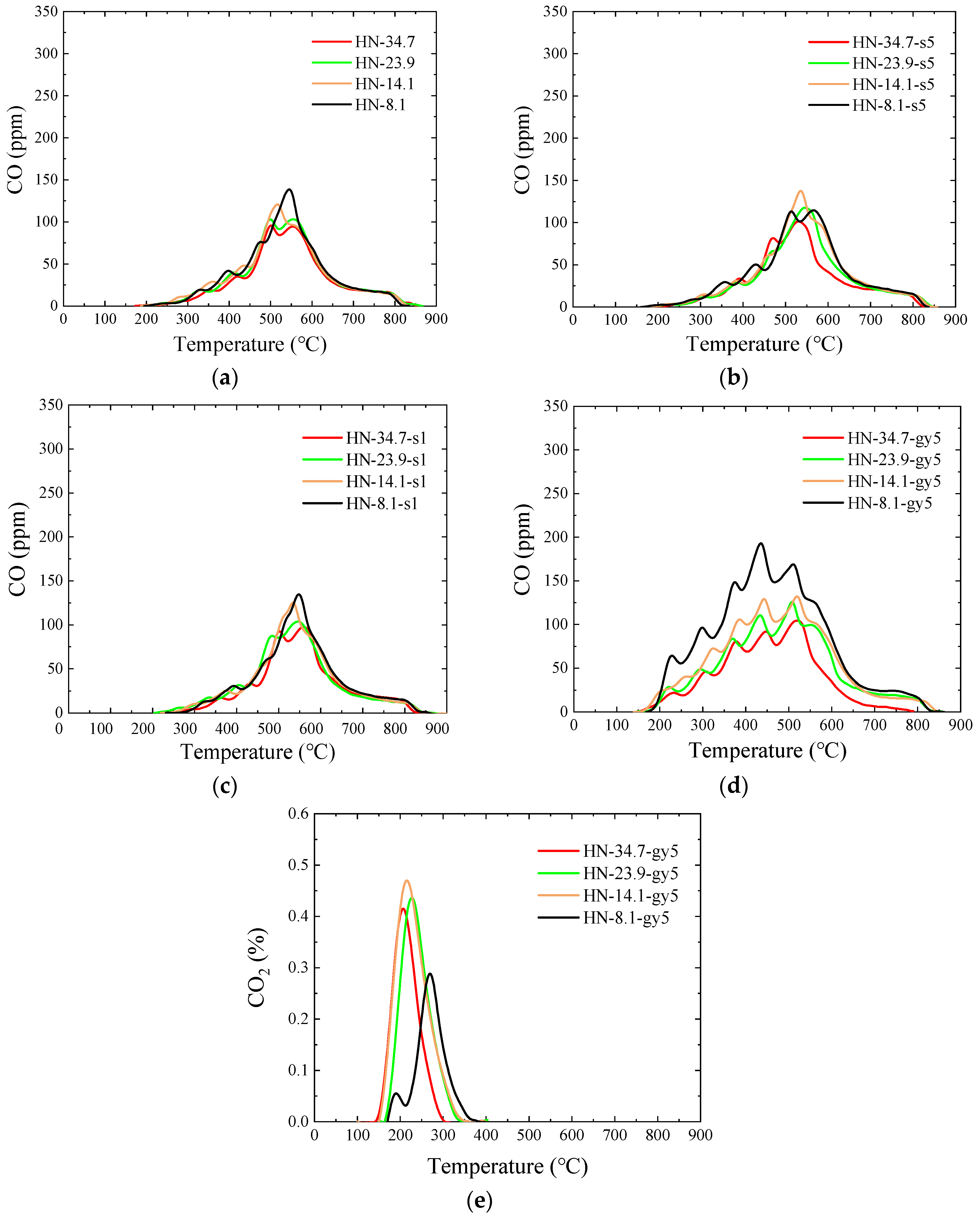
3.5.3. HN Raw and Modified Samples
4. Conclusions
- Peracetic acid of 5 wt% can introduce O-containing functional groups, such as a carboxyl groups and alcoholic hydroxyl groups, into the carbon molecular chain of coal, while 1 and 5 wt% H2O2 have no such effects.
- The introduced O-containing functional groups reduce the binding sites with oxygen in coal and accelerate combustion. During the thermogravimetric test in the air atmosphere, weight gain disappears at temperatures below 400 °C, and the ignition temperature decreases.
- After peracetic acid modification, the carboxyl functional groups introduced into the coal increase the amount of CO generated in the pyrolysis process. Additionally, the commencing temperatures of CO are lowered by about 50 °C. In addition, the formation pathways and amounts of C2H4 and C6H6 have changed. After modification, a new generation peak appears at a low temperature. Due to the introduction of the carboxyl group, a CO2 generation curve appears distinctly in the pyrolysis process of peracetic-acid-modified samples.
- A 1 wt% H2O2 solution can expand the pore structure and increase the specific surface area of the coal powder by a small margin; however, these changes essentially cannot alter the generation path of pyrolysis products. Additionally, the influence of a variation in the pore structure on the pyrolysis products is far less than the introduced O-containing functional groups.
- The superfine pulverized coal has a synergistic effect on the modification of peracetic acid. More O-containing functional groups are introduced as the particle sizes become smaller. For anthracite, this phenomenon is more obvious.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zheng, M.; Ren, C.; Guo, L. ReaxFF molecular dynamics simulations of thermal reactivity of various fuels in pyrolysis and combustion. Energy Fuels 2021, 35, 11707–11739. [Google Scholar] [CrossRef]
- Zou, C.; Li, X.; Ren, M.; Wang, W.; Wu, H. Thermal behavior and kinetics of Shenmu coal pyrolyzed under hydrogen-rich or methane-gas-rich atmosphere. Energies 2023, 16, 2339. [Google Scholar] [CrossRef]
- Feng, G.; Cui, J.; Niu, X.; Liao, J.; Li, W.; Bai, Z.; Han, Y. Effect of O-containing functional groups and meso- and micropores on content and re-adsorption behavior of water in upgraded brown coal. Fuel 2019, 257, 116100. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X.; Luukkanen, S.; Qu, J.; Zhang, C.; Xue, J.; Li, H.; Yang, W.; Yang, S.; Bu, X. Effect of the oxygen-containing functional group on the adsorption of hydrocarbon oily collectors on coal surfaces. Physicochem. Probl. Miner. Process. 2022, 58, 149937. [Google Scholar] [CrossRef]
- Wang, J.; Du, J.; Chang, L.; Xie, K. Study on the structure and pyrolysis characteristics of Chinese western coals. Fuel Process. Technol. 2010, 91, 430–433. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Jin, L.; Zhu, J.; Li, J.; Li, Y.; Fan, H.; Hu, H. Effect of functional groups on volatile evolution in coal pyrolysis process with in situ pyrolysis photoionization time-of-flight mass spectrometry. Fuel 2020, 260, 116322. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, Y.; Huang, S.; Wu, S.; Gao, J. Effect of decomposition of oxygen-containing structures on the conversion of lignite in the presence of hydrogen donor solvent. J. Anal. Appl. Pyrolysis 2020, 145, 104743. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, Y.; Teng, H.; Liu, J.; Tian, F.; Cui, L.; Li, W.; Liu, J.; Wang, C.; Li, D. Analysis of oxygen-containing species in coal tar by comprehensive two-dimensional GC×GC-TOF and ESI FT-ICR mass spectrometry through a new subfraction separation method. J. Energy Inst. 2022, 101, 209–220. [Google Scholar] [CrossRef]
- Gao, H.; Zong, Z.; Li, J.; Wei, X.; Guo, Q.; Zhao, T.; Bai, H. Characterization of oxygen-containing aromatics in a low-temperature coal tar. Energy Fuels 2021, 35, 283–289. [Google Scholar] [CrossRef]
- Qin, X.; Yang, T.; Liu, Z.; Liu, Q. Molecular structure, bond cleavage and their relation of four low rank coals. Fuel Process. Technol. 2022, 236, 107391. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Yang, Y.; Wang, C. Study on oxidation and gas release of active sites after low-temperature pyrolysis of coal. Fuel 2018, 233, 237–246. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Zhu, D.; Qin, M. Effect of O-containing functional groups produced by preoxidation on Zhundong coal gasification. Fuel Process. Technol. 2020, 206, 06480. [Google Scholar] [CrossRef]
- Xu, J.; Tang, H.; Su, S.; Liu, J.; Xu, K.; Qian, K.; Wang, Y.; Zhou, Y.; Hu, S.; Zhang, A.; et al. A study of the relationships between coal structures and combustion characteristics: The insights from micro-Raman spectroscopy based on 32 kinds of Chinese coals. Appl. Energy 2018, 212, 46–56. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Sun, R.; Li, Y.; Ren, X.; Xu, J. Effect of reaction conditions on the evolution of surface functional groups in O2/H2O combustion process of demineralized coal char. Fuel Process. Technol. 2019, 195, 106144. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Naganuma, R.; Tsubouchi, N. Influence of inherently present oxygen-functional groups on coal fluidity and coke strength. Energy Fuels 2018, 32, 1657–1664. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.; Xu, D.; Jiang, Q.; Xin, H.; Fu, C.; He, W. Study on the reaction path of -CH3 and -CHO functional groups during coal spontaneous combustion: Quantum chemistry and experimental research. Energies 2022, 15, 4891. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Xing, Y.; Zhang, H.; Luo, L.; Jiang, X. Application of TG-FTIR analysis to superfine pulverized coal. J. Anal. Appl. Pyrolysis 2018, 133, 154–161. [Google Scholar] [CrossRef]
- Luo, L.; Yao, W.; Liu, J.; Zhang, H.; Ma, J.; Jiang, X. The effect of the grinding process on pore structures, functional groups and release characteristic of flash pyrolysis of superfine pulverized coal. Fuel 2019, 235, 1337–1346. [Google Scholar] [CrossRef]
- Yao, Q.; Ma, M.; Liu, Y.; Ma, D.; Chen, H.; Hao, Q.; Sun, M.; Ma, X. The structural and pyrolysis characteristics of vitrinite and inertinite from Shendong coal and the gasification performance of chars. J. Anal. Appl. Pyrolysis 2022, 164, 105519. [Google Scholar] [CrossRef]
- Kim, D.; Yoshikawa, K.; Park, K. Characteristics of biochar obtained by hydrothermal carbonization of cellulose for renewable energy. Energies 2015, 8, 14040–14048. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; Chen, L.; Yang, Z.; Li, L. Effect of oxidation processing on the surface properties and floatability of Meizhiyou long-flame coal. Fuel 2017, 210, 177–186. [Google Scholar] [CrossRef]
- Sun, W.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K. Study on the evolution pattern of the aromatics of lignin during hydrothermal carbonization. Energies 2023, 16, 1089. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Y.; Tahmasebi, A.; Han, Y.; Li, X.; Lucas, J.; Wall, T. Coal oxidation under mild conditions: Current status and applications. Chem. Eng. Technol. 2014, 37, 1635–1644. [Google Scholar] [CrossRef]
- Mae, K.; Maki, T.; Araki, J.; Miura, K. Extraction of low-rank coals oxidized with hydrogen peroxide in conventionally used solvents at room temperature. Energy Fuels 1997, 11, 825–831. [Google Scholar] [CrossRef]
- Liu, F.; Wei, X.; Zhu, Y.; Wang, Y.; Li, P.; Fan, X.; Zhao, Y.; Zong, Z.; Zhao, W.; Wei, Y. Oxidation of Shengli lignite with aqueous sodium hypochlorite promoted by pretreatment with aqueous hydrogen peroxide. Fuel 2013, 11, 211–215. [Google Scholar] [CrossRef]
- Sun, S.; Xu, D.; Wei, Y.; Zhi, Y.; Jiang, G.; Guo, Y. Influence laws of operating parameters on coal pyrolysis characteristics. J. Anal. Appl. Pyrolysis 2022, 167, 105684. [Google Scholar] [CrossRef]
- Pechacek, N.; Osorio, M.; Caudill, J.; Peterson, B. Evaluation of the toxicity data for peracetic acid in deriving occupational exposure limits: A minireview. Toxicol. Lett. 2015, 233, 45–57. [Google Scholar] [CrossRef]
- Jiao, A.; Tian, S.; Lin, H. Analysis of outburst coal structure characteristics in Sanjia coal mine based on FTIR and XRD. Energies 2022, 15, 1956. [Google Scholar] [CrossRef]
- Xiong, G.; Li, Y.; Jin, L.; Hu, H. In situ FT-IR spectroscopic studies on thermal decomposition of the weak covalent bonds of brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 262–267. [Google Scholar] [CrossRef]
- Benfell, K.; Beamish, B.; Rodgers, K. Thermogravimetric analytical procedures for characterizing New Zealand and eastern Australian coals. Thermochim. Acta 1996, 286, 67–74. [Google Scholar] [CrossRef]
- Cui, X.; Li, X.; Li, Y.; Li, S. Evolution mechanism of oxygen functional groups during pyrolysis of Datong coal. J. Therm. Anal. Calorim. 2017, 129, 1169–1180. [Google Scholar] [CrossRef]
- Lin, X.; Luo, M.; Li, S.; Yang, Y.; Chen, X.; Tian, B.; Wang, Y. The evolutionary route of coal matrix during integrated cascade pyrolysis of a typical low-rank coal. Appl. Energy 2017, 199, 335–346. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, G.; Yin, H.; Zhou, C.; Wu, D.; Tan, F. A comparative study on thermal behavior of functional groups in coals with different ranks during low temperature pyrolysis. J. Anal. Appl. Pyrolysis 2021, 158, 105258. [Google Scholar] [CrossRef]
- Arenillas, A.; Rubiera, F.; Pis, J.J. Simultaneous thermogravimetric–mass spectrometric study on the pyrolysis behaviour of different rank coals. J. Anal. Appl. Pyrolysis 1999, 50, 31–46. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, L.; Zhou, Y.; Li, Y.; Hu, H. Online analysis of initial volatile products of Shenhua coal and its macerals with pyrolysis vacuum ultraviolet photoionization mass spectrometry. Fuel Process. Technol. 2017, 163, 67–74. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Jin, L.; Tang, Z.; Hu, H. In situ analysis of volatile products from lignite pyrolysis with pyrolysis-vacuum ultraviolet photoionization and electron impact mass spectrometry. Fuel Process. Technol. 2015, 133, 232–236. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, M.; Jiang, X. Nitrogenous gas formation mechanisms of chemically modified superfine pulverized coal during pyrolysis. Energy Sources Part A 2022, 44, 3544–3554. [Google Scholar] [CrossRef]



| Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Particle Sizes D50 (µm) | Mar | FCar | Var | Aar | Cdaf | Hdaf | Odaf | Ndaf | Sdaf |
| 11.0 | 6.5 | 56.2 | 31.0 | 6.3 | 72.6 | 4.7 | 21.4 | 0.9 | 0.4 |
| 13.6 | 8.3 | 56.4 | 30.2 | 5.1 | 73.3 | 4.7 | 20.7 | 0.9 | 0.4 |
| 18.7 | 8.1 | 56.6 | 29.5 | 5.8 | 72.4 | 4.7 | 21.6 | 0.9 | 0.4 |
| 21.6 | 8.9 | 56.2 | 29.67 | 5.3 | 72.8 | 4.6 | 21.3 | 0.9 | 0.4 |
| Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Particle Sizes D50 (µm) | Mar | FCar | Var | Aar | Cdaf | Hdaf | Odaf | Ndaf | Sdaf |
| 8.1 | 1.5 | 75.6 | 9.4 | 13.5 | 88.5 | 3.8 | 5.9 | 1.4 | 0.4 |
| 14.1 | 2.3 | 75.7 | 9.1 | 12.9 | 88.4 | 3.9 | 5.9 | 1.4 | 0.4 |
| 23.9 | 2.5 | 76.6 | 8.3 | 12.6 | 88.9 | 4.0 | 5.3 | 1.4 | 0.4 |
| 34.7 | 2.8 | 76.2 | 8.5 | 12.5 | 89.0 | 3.7 | 5.5 | 1.4 | 0.4 |
| Reagents | Specifications | Concentration | Tabs | |
|---|---|---|---|---|
| 1 | Hydrogen peroxide | AR solution (30 wt%) | 5 wt% | s5 |
| 2 | 1 wt% | s1 | ||
| 3 | Peracetic acid | AR solution (20 wt%) | 1 wt% | gy5 |
| Ignition Temperature (°C) | 21.6 (μm) | 18.7 (μm) | 13.6 (μm) | 11.0 (μm) |
|---|---|---|---|---|
| NMG–raw samples | 271.3 | 264.8 | 262.3 | 257.5 |
| NMG–gy5 | 264.9 | 258.5 | 254.5 | 249.1 |
| HN–raw samples | 345.5 | 342.4 | 339.1 | 338.8 |
| HN–gy5 | 322.0 | 317.1 | 302.5 | 297.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Gao, Y.; Jiang, X. Influences of the Introduced O-Containing Functional Groups on the Gaseous Pyrolysis Product of Superfine Pulverized Coal. Energies 2023, 16, 4418. https://doi.org/10.3390/en16114418
Ma Y, Gao Y, Jiang X. Influences of the Introduced O-Containing Functional Groups on the Gaseous Pyrolysis Product of Superfine Pulverized Coal. Energies. 2023; 16(11):4418. https://doi.org/10.3390/en16114418
Chicago/Turabian StyleMa, Yang, Yan Gao, and Xiumin Jiang. 2023. "Influences of the Introduced O-Containing Functional Groups on the Gaseous Pyrolysis Product of Superfine Pulverized Coal" Energies 16, no. 11: 4418. https://doi.org/10.3390/en16114418





