Study of Ash Sintering Temperature and Ash Deposition Behavior during Co-Firing of Polish Bituminous Coal with Barley Straw Using Non-Standard Tests
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Samples Analsyis
3.2. Pressure Drop Test and Mechanical Test Compared to the FactSage Analyzes
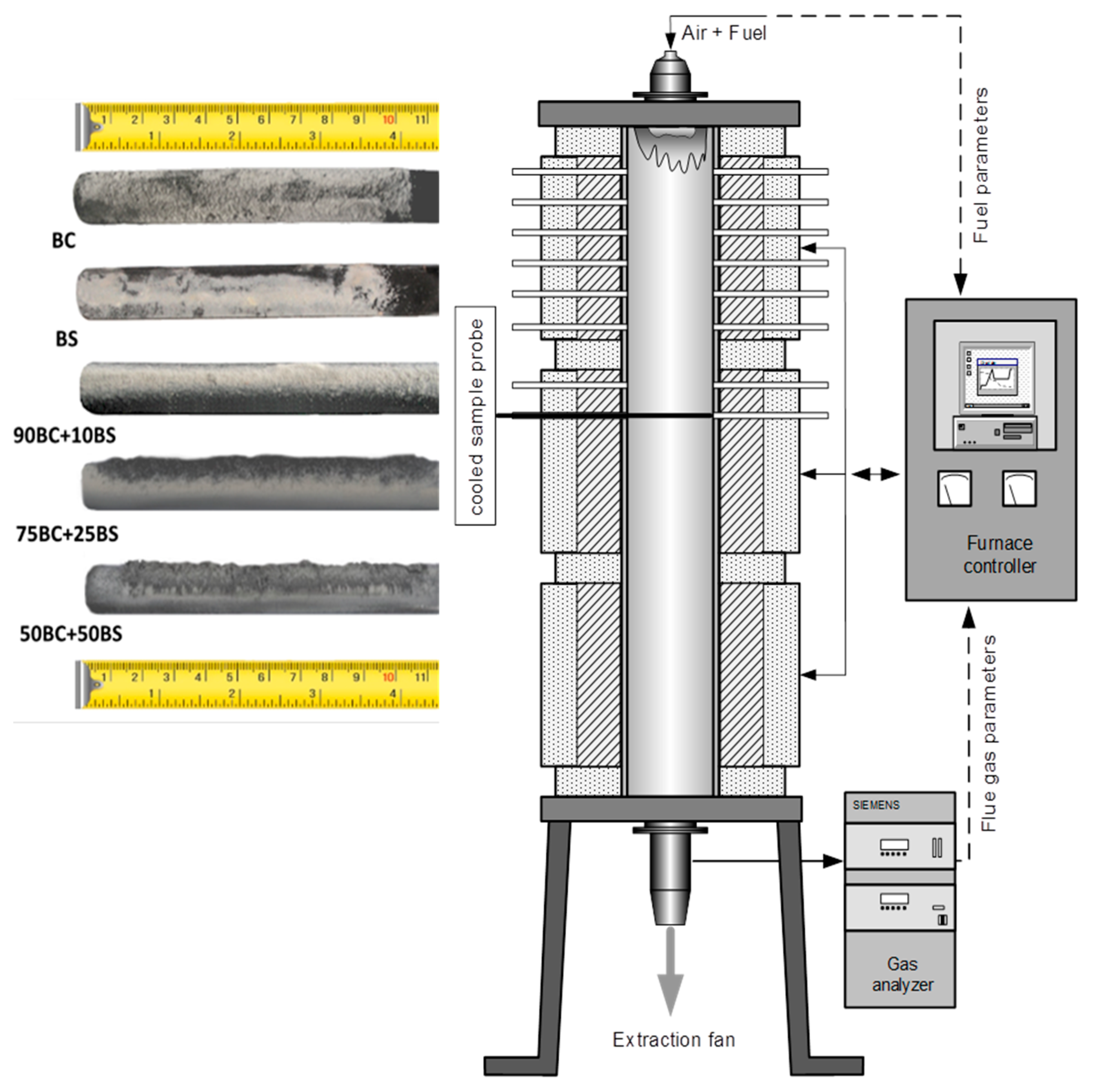
3.3. Deposition Rate Test
4. Conclusions
- Non-standard methods such as the pressure drop test and the mechanical test well reflect the nature of processes observed during the sintering of the tested ashes with bituminous coal from Polish mine Makoszowa, barley straw biomass and their blends.
- The characteristic temperature ranges for the discontinuities of the specific heat cp and density predicted by FactSage 8.0 are consistent with the temperature range of the pressure drop in the pressure drop test and the increase in the fracture stress in the mechanical method.
- Tested fuel ashes: bituminous coal from Polish Mine Makoszowa (BC), barley straw (BS) biomass and their blends: 90 wt.% BC/10 wt.% BS, 75 wt.% BC/25 wt.% BS and 50 wt.% BC/50 wt.% BS can be divided into three groups due to sintering temperatures.
- The first group includes BC and 90 wt.% BC/10 wt.% BS. Within this group, the sintering temperatures are the highest and the deposition rate the lowest. The difference of the sintering temperature and deposition rate for BC and 90 wt.% BC/10 wt.% BS are small (<5%).
- The second group—75 wt.% BC/25 wt.% BS and 50 wt.% BC/50 wt.% BS—is characterized by a much lower sintering temperature and visibly higher deposition rate. This effect is clear. For the BC/BS blends, the slagging/fouling tendency increases.
- The third group, which is biomass BS, is characterized by the lowest sintering temperature. The deposition rate for BS is lower than for the fuels from the second group, but higher than for the fuels from the first. This effect is a consequence of the low content of ash in BS compared to BC—the flow of mass in our experiment was the same for all fuels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission; Joint Research Centre; Camia, A.; Giuntoli, J.; Jonsson, R.; Robert, N.; Cazzaniga, N.E.; Jasinevičius, G.; Avitabile, V.; Grassi, G.; et al. The Use of Woody Biomass for Energy Production in the EU; Publications Office of the European Union: Luxembourg, 2021; Available online: https://data.europa.eu/doi/10.2760/831621 (accessed on 13 May 2023).
- Sami, M.; Annamalai, K.; Wooldridge, M. Co-firing of coal and biomass fuel blends. Prog. Energy Combust. Sci. 2001, 27, 171–214. [Google Scholar] [CrossRef]
- The European Bioeconomy Network (EuBioNet). Available online: https://eubionet.eu/ (accessed on 13 May 2023).
- Poland’s Energy Policy until 2040 (PEP2040). Available online: https://www.gov.pl/ (accessed on 13 May 2023).
- Hansson, J.; Berndes, G.; Johnsson, F.; Kjärstad, J. Co-firing biomass with coal for electricity generation—An assessment of the potential in EU27. Energy Policy 2009, 37, 1444–1455. [Google Scholar] [CrossRef]
- Cardoso, J.S.; Silva, V.; Chavando, J.A.M.; Eusébio, D.; Hall, M.J. Numerical modelling of the coal phase-out through ammonia and biomass co-firing in a pilot-scale fluidized bed reactor. Fuel Commun. 2022, 10, 100055. [Google Scholar] [CrossRef]
- Bentson, S.; Evitt, D.; Still, D.; Lieberman, D.; MacCarty, N. Retrofitting stoves with forced jets of primary air improves speed, emissions, and efficiency: Evidence from six types of biomass cookstoves. Energy Sustain. Dev. 2022, 71, 104–117. [Google Scholar] [CrossRef]
- Ndou, N.R.; Bada, S.O.; Falcon, R.M.S.; Weiersbye, I.M. Co-combustion of Searsia lancea and Tamarix usneoides with high ash coal. Fuel 2020, 267, 117282. [Google Scholar] [CrossRef]
- Marangwanda, G.T.; Madyira, D.M.; Chihobo, H.C.; Babarinde, T.O. Modelling co-combustion of bituminous coal and pine sawdust: Thermal behavior. Fuel Commun. 2021, 9, 100035. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 2. Potential utilisation, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.; Cao, Z.; Di, H. Investigation on minerals migration during co-firing of different straw/coal blending ratios. Energy Convers. Manag. 2013, 74, 279–285. [Google Scholar] [CrossRef]
- Yang, T.; Kai, X.; Sun, Y.; He, Y.; Li, R. The effect of coal sulfur on the behavior of alkali metals during co-firing biomass and coal. Fuel 2011, 90, 2454–2460. [Google Scholar] [CrossRef]
- Wang, G.; Pinto, T.; Costa, M. Investigation on ash deposit formation during the co-firing of coal with agricultural residues in a large-scale laboratory furnace. Fuel 2014, 117, 269–277. [Google Scholar] [CrossRef]
- Khodier, A.H.M.; Hussain, T.; Simms, N.J.; Oakey, J.E.; Kilgallon, P.J. Deposit formation and emissions from co-firing miscanthus with Daw Mill coal: Pilot plant experiments. Fuel 2012, 101, 53–61. [Google Scholar] [CrossRef]
- Li, R.; Kai, X.; Yang, T.; Sun, Y.; He, Y.; Shen, S. Release and transformation of alkali metals during co-combustion of coal and sulfur- rich wheat straw. Energy Convers. Manag. 2014, 83, 197–202. [Google Scholar] [CrossRef]
- Król, K.; Moroń, W.; Nowak-Woźny, D. Biomass and Coal Ash Sintering—Thermodynamic Equilibrium Modeling versus Pressure Drop Test and Mechanical Test. Energies 2023, 16, 362. [Google Scholar] [CrossRef]
- CEN/TS 14775:2004; Solid Biofuels-Method for the Determination of Ash Content. Slovenian Institute of Standardization: Ljubljana, Slovenia, 2004. Available online: https://standards.iteh.ai/catalog/standards/sist/7a423bc5-65a2-40ae-90f7-319c45968a59/sist-ts-cen-ts-14775-2004 (accessed on 13 May 2023).
- CEN/TS 15148:2006; Solid Biofuels—Method for the Determination of the Content of Volatile Matter. Slovenian Institute of Standardization: Ljubljana, Slovenia, 2006. Available online: https://standards.iteh.ai/catalog/standards/sist/ee9f431c-a3c0-4b7f-bfcd-9ed100a6dc08/sist-ts-cen-ts-15148-2006 (accessed on 19 May 2023).
- CEN/TS 14774-2:2004; Solid Biofuels—Methods for the Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method. Slovenian Institute of Standardization: Ljubljana, Slovenia, 2006. Available online: https://standards.iteh.ai/catalog/standards/cen/b288cb93-644a-49f9-9a5a-ef7236367eab/cen-ts-14774-2-2004 (accessed on 19 May 2023).
- PN-G-04502:2014-11; Hard and Brown Coals—Sampling and Preparation of Samples for the Laboratory Tests—Primary Methods. ISO: London, UK, 2014.
- Król, K.; Nowak-Woźny, D. Application of the Mechanical and Pressure Drop Tests to Determine the Sintering Temperature of Coal and Biomass Ash. Energies 2021, 14, 1126. [Google Scholar] [CrossRef]
- PN-EN ISO 18134-1:2015-11; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 1: Total Moisture—Reference Method. ISO: London, UK, 2015.
- PN-EN ISO 18123:2016-01; Solid Biofuels—Determination of the Content of Volatile Matter. ISO: London, UK, 2015.
- PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. ISO: London, UK, 2015.
- PN-ISO 1928:2020-05; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. ISO: London, UK, 2020.
- Magdziarz, A.; Wilk, M.; Gajek, M.; Nowak-Woźny, D.; Kopia, A.; Kalemba-Rec, I.; Koziński, J. Properties of ash genarated during sewage sludge combustion: A multifaceded analysis. Energy 2016, 113, 85–94. [Google Scholar] [CrossRef]
- Król, K.; Iskra, K.; Ferens, W.; Miodonski, J.M. Testing properties of sewage sludge for energy use. Environ. Prot. Eng. 2019, 45, 61–73. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Gądek, W.; Pronobis, M.; Kalisz, S. Investigation of ash deposition in PF boiler during combustion of torrefied biomass. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Krakow, Poland, 14–17 November 2017; Volume 214, p. 012080. [Google Scholar]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical review of predictive coefficients for biomass ash deposition tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Jeong, T.-Y.; Sh, L.; Kim, J.-H.; Lee, B.-H.; Jeon, C.-H. Experimental Investigation of Ash Deposit Behavior during Co-Combustion of Bituminous Coal with Wood Pellets and Empty Fruit Bunches. Energies 2019, 12, 2087. [Google Scholar] [CrossRef]










| Samples | Proximate Analysis | Ultimate Analysis | Heating Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | A | VM | FC | FR | C | H | N | S | O | Cl | HHV | |
| wt.% | wt.% | wt.% | wt.% | – | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | MJ/kg | |
| 100%BC | 2.0 | 17.3 | 26.9 | 53.8 | 2.00 | 65.4 | 3.7 | 1.3 | 1.2 | 8.6 | 0.51 | 27.8 |
| 93%BC + 7%BS | 2.5 | 16.7 | 29.4 | 51.5 | 1.88 | 64.2 | 3.8 | 1.2 | 1.1 | 10.0 | 0.51 | 27.0 |
| 86%BC + 14%BS | 2.9 | 16.0 | 31.9 | 49.2 | 1.77 | 62.9 | 4.0 | 1.2 | 1.0 | 11.4 | 0.50 | 26.2 |
| 67%BC + 33%BS | 4.1 | 14.3 | 38.7 | 42.8 | 1.45 | 59.6 | 4.4 | 1.0 | 0.8 | 15.3 | 0.49 | 23.9 |
| 100%BS | 8.5 | 8.2 | 62.7 | 20.6 | 0.33 | 47.8 | 5.7 | 0.4 | 0.1 | 28.9 | 0.45 | 16.1 |
| Samples | 100%BC | 90%BC + 10%BS | 75%BC + 25%BS | 50%BC + 50%BS | 100%BS |
|---|---|---|---|---|---|
| Oxides | wt.% | wt.% | wt.% | wt.% | wt.% |
| SiO2 | 51.4 | 53.0 | 55.4 | 59.4 | 67.5 |
| Al2O3 | 25.5 | 22.9 | 19.2 | 12.8 | 0.2 |
| Fe2O3 | 6.6 | 5.9 | 5.0 | 3.4 | 0.2 |
| CaO | 4.6 | 4.6 | 4.7 | 4.8 | 4.9 |
| MgO | 3.3 | 3.1 | 2.9 | 2.4 | 1.5 |
| Na2O | 1.0 | 0.9 | 0.8 | 0.7 | 0.4 |
| K2O | 2.6 | 4.5 | 7.2 | 11.8 | 21.0 |
| P2O5 | 0.5 | 0.7 | 1.0 | 1.6 | 2.7 |
| TiO2 | 1.1 | 1.0 | 0.8 | 0.5 | 0.0 |
| SO3 | 3.2 | 3.0 | 2.8 | 2.4 | 1.5 |
| Mn3O4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 |
| BaO | 0.2 | 0.2 | 0.1 | 0.1 | 0.0 |
| SrO | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Parameter/Symbol | Value | Fuel | Unit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Extremely High | 100%BC | 90%BC + 10%BS | 75%BC + 25%BS | 50%BC + 50%BS | BS | |||
| Silica content in ash | SiO2 | <20 | 20–25 | >25 | 51.35 | 52.97 | 55.38 | 59.41 | 67.46 | wt.% | |
| Chlorine content in fuel | Cl d | <0.2 | 0.2–0.3 | 0.3–0.5 | >0.5 | 0.52 | 0.52 | 0.51 | 0.51 | 0.49 | wt.% |
| Basic to acidic compounds ratio | B/A | <0.5 | 0.5–1.0 | 1.0–1.75 | 0.24 | 0.26 | 0.29 | 0.34 | 0.45 | – | |
| Bed agglomeration index | BAI | <0.15 | 1.83 | 1.11 | 0.62 | 0.27 | 0.01 | – | |||
| Babcock index | Rs | <0.6 | 0.6–2.0 | 2–2.6 | >2.6 | 0.29 | 0.29 | 0.27 | 0.23 | 0.04 | – |
| Fouling index | Fu | 0.6–2.0 | 2.0–40 | >40 | 0.86 | 1.38 | 2.31 | 4.24 | 9.75 | – | |
| Fouling factor | Rf | <0.2 | 0.2–0.5 | 0.5–1.0 | >1.0 | 0.64 | 0.99 | 1.61 | 2.88 | 6.49 | – |
| Slag viscosity index | Sr | >72 | 65–72 | <65 | 77.96 | 79.41 | 81.53 | 84.88 | 91.04 | – | |
| Mechanical test temperature | T(MT) | >1100 | 900–1100 | <900 | 975 | 975 | 925 | 825 | 725 | °C | |
| Pressure drop test temperature | T(PDT) | >1100 | 900–1100 | <900 | 942 | 942 | 878 | 790 | 649 | °C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, K.; Nowak-Woźny, D.; Moroń, W. Study of Ash Sintering Temperature and Ash Deposition Behavior during Co-Firing of Polish Bituminous Coal with Barley Straw Using Non-Standard Tests. Energies 2023, 16, 4424. https://doi.org/10.3390/en16114424
Król K, Nowak-Woźny D, Moroń W. Study of Ash Sintering Temperature and Ash Deposition Behavior during Co-Firing of Polish Bituminous Coal with Barley Straw Using Non-Standard Tests. Energies. 2023; 16(11):4424. https://doi.org/10.3390/en16114424
Chicago/Turabian StyleKról, Karol, Dorota Nowak-Woźny, and Wojciech Moroń. 2023. "Study of Ash Sintering Temperature and Ash Deposition Behavior during Co-Firing of Polish Bituminous Coal with Barley Straw Using Non-Standard Tests" Energies 16, no. 11: 4424. https://doi.org/10.3390/en16114424
APA StyleKról, K., Nowak-Woźny, D., & Moroń, W. (2023). Study of Ash Sintering Temperature and Ash Deposition Behavior during Co-Firing of Polish Bituminous Coal with Barley Straw Using Non-Standard Tests. Energies, 16(11), 4424. https://doi.org/10.3390/en16114424








