The Use of Methanol Vapour for Effective Drying of Cellulose Insulation
Abstract
1. Introduction
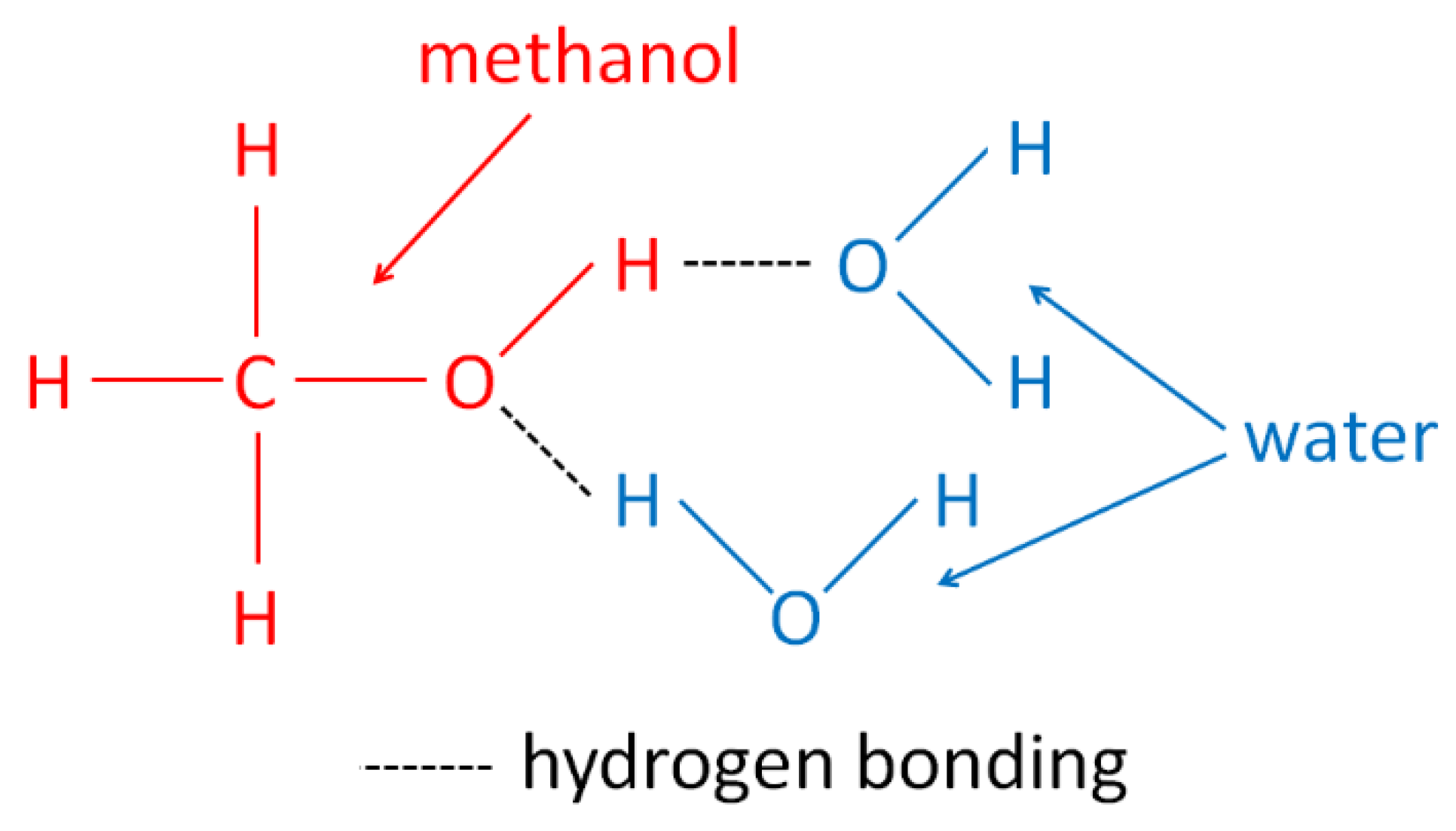
2. The Concept of Using Methanol for Drying Cellulose Materials—State of Art
3. Drying of Cellulose Materials Using Methanol Vapour
3.1. The Aim of the Research
- What is the difference in the effectiveness of drying cellulose materials using nitrogen and methanol vapour whose carrier gas is nitrogen?
- Is there delamination after drying the pressboard with methanol vapour? Such a problem has been found in the case of drying cellulose materials by directly immersing them in methanol.
- What is the influence of pressboard drying time using methanol vapour on the effectiveness of this process?
- Is it possible to dry cellulose materials to a satisfactory level of water content below 1%?
- What is the influence of the thickness of cellulose materials on the effectiveness of their drying using methanol vapour?
- What is the methanol residue in cellulose materials after their drying?
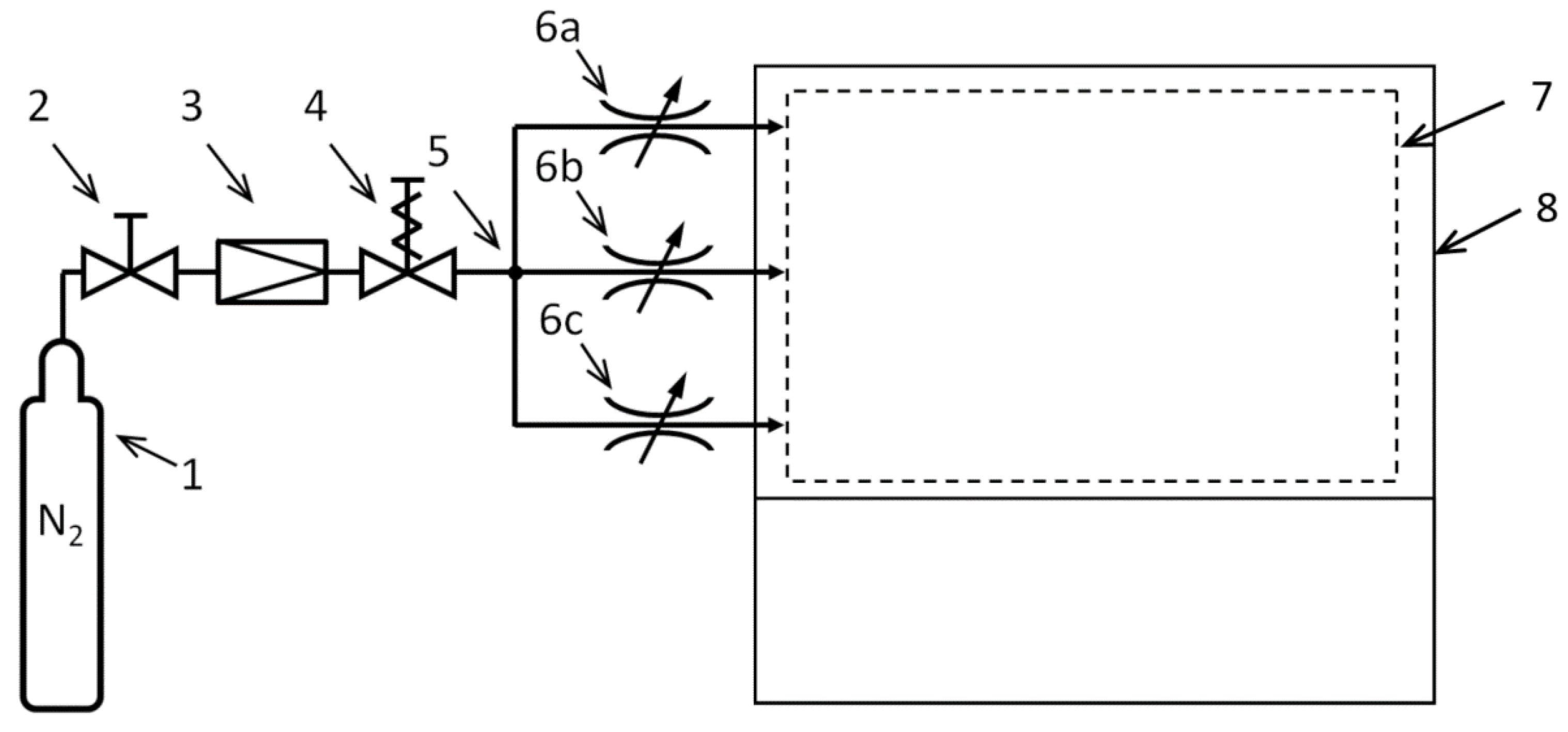
3.2. Carrier Gas Flow Control Laboratory System
3.3. Test Results and Their Discussion
3.3.1. Stage 1
Test Objects and Measurement Procedure
Research Results and Their Discussion
- In the case of sample no. 2 dried in the system with a lower nitrogen flow (1.8 L/h), a better drying efficiency was found than in the case of sample no. 3 dried in the system with a nitrogen flow of 6.0 L/h. The difference in water content in both samples after the drying process is 0.35 p.p. The explanation for this result is the higher concentration of methanol vapour in the nitrogen used for drying sample no. 2, which was obtained by using a lower nitrogen flow;
- After the drying process, a lower methanol content was found in the pressboard sample no. 3, which was dried in a system with a higher nitrogen flow, than in the case of sample no. 2;
- The applied drying procedures did not allow to reduce the water content in the 5 mm thick pressboard to a level below 1.0%. To achieve the assumed goal, it is necessary to extend the drying time and optimize the concentration of methanol vapour in nitrogen. In the case of currently used drying methods, the excessive residual moisture can also remain in some bulky insulating components. Typically, these are supports for leads, cylinders, core support insulation, etc. [28].
- After drying, there was no problem with pressboard delamination as was the case in the method requiring immersion of the material in methanol.
3.3.2. Stage 2
Test Objects and Measurement Procedure
Research Results and Their Discussion
- A significant influence of the drying time on the efficiency of this process was found.
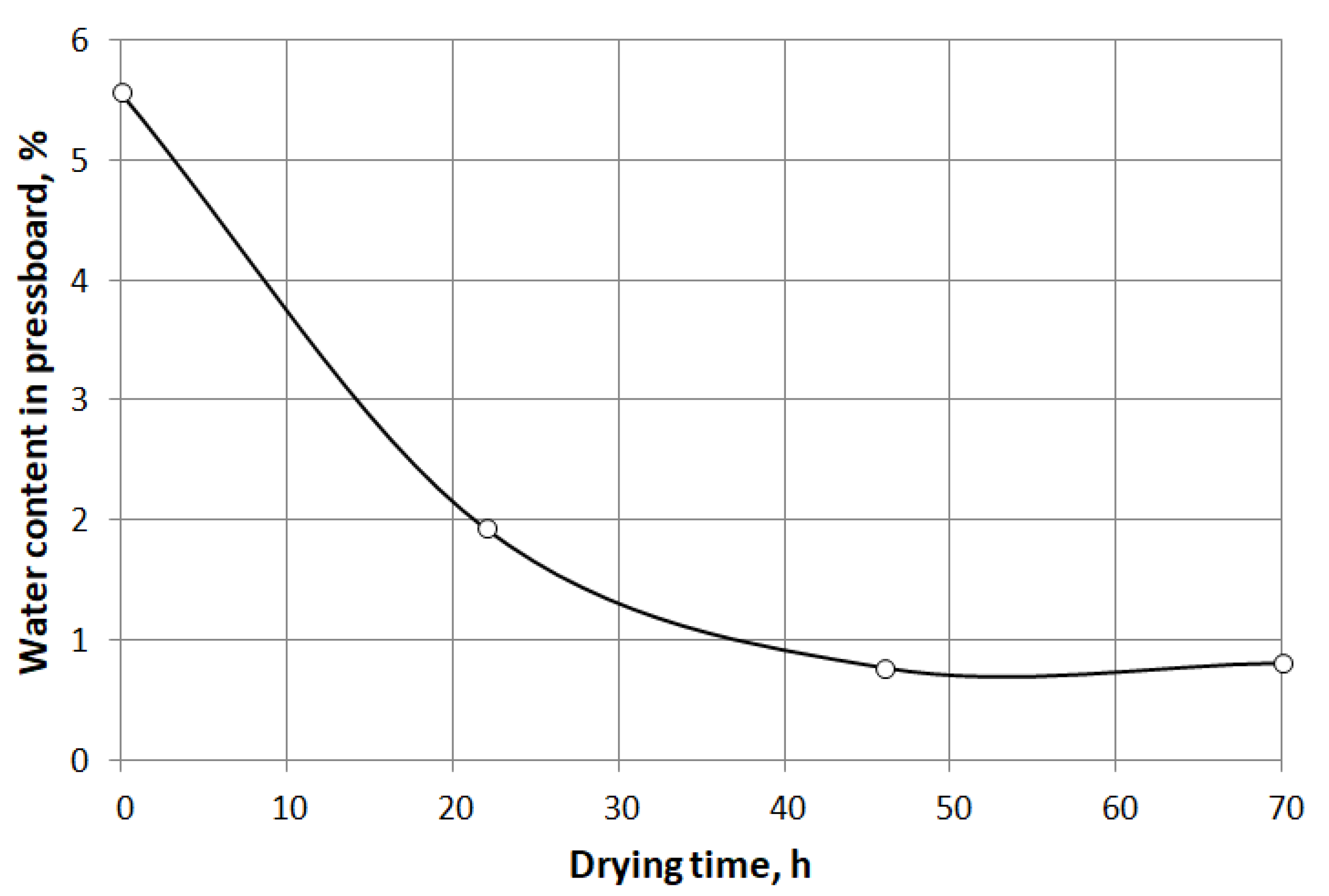
- The drying time of 46 h, used in the case of samples 3 and 4, allowed to reduce the water content to 0.77%. Extending the drying time to 70 h did not result in a further reduction of moisture content (Figure 7). The obtained moisture level is acceptable considering the water content levels usually obtained during the conventional drying method. The time it takes to get a moisture level below 1% is also satisfactory.
- After drying, there was no problem with pressboard delamination.
3.3.3. Stage 3
Test Objects and Measurement Procedure
Research Results and Their Discussion
- The use of methanol vapour for drying paper samples allowed to obtain a very low moisture level, not exceeding 0.5% (samples 2 and 3). This result was obtained after 70 h of drying;
- In the case of sample 2, after the drying process, nitrogen purging was used to reduce the methanol content. This allowed for a lower methanol content in the sample (1.56%) compared to the methanol content in sample 3 (4.03%), which was not purged with nitrogen;
- In the case of sample 2, a higher water content level was obtained (0.41%) than in the case of sample 3 (0.09%). This was caused by the secondary moisture of sample no. 2 during the process of its purging with nitrogen.
3.3.4. Stage 4
Test Objects and Measurement Procedure
4. Conclusions and Direction of Further Research
- Studies of dielectric properties of cellulose materials dried with methanol vapour;
- Analysis of the influence of methanol residues in cellulose materials on the dynamics of their aging;
- Analysis of the problem of methanol migration from cellulose material to mineral oil and assessment of the impact of methanol content in oil on its properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lundgaard, L.E.; Allan, D.; Atanasowa-Höhlein, I.; Clavreul, R.; Dahlund, M.O.; Gasser, H.P.; Heywood, R.; Krause, C.; Lessard, M.C.; Saha, T.K.; et al. Ageing of Cellulose in Mineral-Oil Insulated Transformers; Cigré Technical Brochure 323; International Council on Large Electric Systems (CIGRE): Paris, France, 2007. [Google Scholar]
- Muth, T.; Jenau, F.; Gasser, H.P. Ageing of thermally and electrically stressed mineral oil impregnated presspaper insulation systems. In Proceedings of the International Conference on High Voltage Engineering and Application (ICHVE), Poznan, Poland, 8–11 September 2014; pp. 1–4. [Google Scholar] [CrossRef]
- Fort, E.M.; Pietsch, H.E. Aging of Insulation by Thermal and Electrical Stresses, In Proceedings of the 12th Electrical and Electronics Insulation Conference. Boston, MA, USA, 11–14 November 1975; pp. 143–146. [Google Scholar] [CrossRef]
- Münster, T.; Werle, P.; Hämel, K.; Preusel, J. Thermally Accelerated Aging of Insulation Paper for Transformers with Different Insulating Liquids. Energies 2021, 14, 3036. [Google Scholar] [CrossRef]
- Höhlein, I.; Kachler, A.J. Aging of Cellulose at Transformer Service Temperatures. Part 2. Influence of Moisture and Temperature on Degree of Polymerization and Formation of Furanic Compounds in Free-breathing Systems. IEEE Electr. Insul. Mag. 2005, 21, 20–24. [Google Scholar] [CrossRef]
- N’cho, J.S.; Fofana, I.; Hadjadj, Y.; Beroual, A. Review of Physicochemical-based Diagnostic Techniques for Assessing Insulation Condition in Aged Transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Cybulski, M.; Przybylek, P. The application of a molecular sieve for drying the insulation system of a power transformer in terms of improving its perational reliability. Eksploat. I Niezawodn. Maint. Reliab. 2022, 24, 502–509. [Google Scholar] [CrossRef]
- Moser, H.P.; Dahinden, V. Transformerboard II; Printing Styria: Graz, Switzerland, 1987. [Google Scholar]
- Moser, H.P. Transformerboard; Special Print of Scientia Electrica; Weidmann AG: Rapperswil, Switzerland, 1979. [Google Scholar]
- Gielniak, J.; Graczkowski, A.; Moranda, H.; Przybylek, P.; Walczak, K.; Nadolny, Z.; Moscicka-Grzesiak, H.; Feser, K.; Gubanski, S.M. Moisture in Cellulose Insulation of Power Transformers-Statistics. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 982–987. [Google Scholar] [CrossRef]
- Pikus, I.F.; Gimpeleva, L.S.; Koshepavo, L.A. Basic Laws of the Vacuum Drying Process Applied to Cellulose Materials for Electrical Insulation. J. Eng. Phys. Thermophys. 1972, 22, 290–296. [Google Scholar] [CrossRef]
- Gasser, H.P.; Krause, C.; Prevost, T. Water Absorption of Cellulosic Insulating Materials Used in Power Transformer. In Proceedings of the IEEE International Conference on Solid Dielectrics (ICSD), Winchester, UK, 8–13 July 2007. [Google Scholar] [CrossRef]
- Betie, A.; Meghnefi, F.; Fofana, I.; Yeo, Z. Modeling the Insulation Paper Drying Process from Thermogravimetric Analyses. Energies 2018, 11, 517. [Google Scholar] [CrossRef]
- Hasterman, Z.; Mosinski, F.; Maliszewski, A. Electric Strength of Power Transformer; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1983. (In Polish) [Google Scholar]
- Rindlisbacher, G.; von Ah, M. Cut and Dry: Low Frequency Drying Process in Transformer Production Can Cut Energy Costs in Half. ABB Review 2005. Available online: https://library.e.abb.com/public/22f229958625da3ec125707b004c4466/66-69%203M557_ENG72dpi.pdf (accessed on 21 March 2023).
- Koestinger, P.; Aronsen, E.; Boss, P.; Rindlisbacher, G. Practical Experience with the Drying of Power Transformers in the Field, Applying the LFH Technology. CIGRE Session Papers & Proceedings, Reference: A2-205_2004, Paris, France. Available online: https://www.transform.ru/articles/pdf/sigre/a2-205.pdf (accessed on 3 May 2023).
- Muntener, W. LFH—Trocknung: Erfahrungen und Trends; Technical Paper; Symposium Stuttgart: Stuttgart, Germany, 1999; pp. 1–14. [Google Scholar]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Ageing of Oil-impregnated Paper in Power Transformers. IEEE Trans. Power Del. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Heywood, R.J.; Emsley, A.M.; Ali, M. Degradation of Cellulose Insulation in Power Transformers. Part 1: Factors Affecting the Measurement of the Average Viscometric Degree of Polymerization of New and Aged Electrical Papers. IEE Proc.–Sci. Meas. Technol. 2000, 147, 86–90. [Google Scholar] [CrossRef]
- Przybylek, P.; Gielniak, J. Opracowanie metody suszenia izolacji stałej transformatora energetycznego na etapie jego produkcji (Development of a method for drying the solid insulation of a power transformer at the stage of its production). 2019, unpublished work done within statutory activity; No. 04/41/SBAD/4413. (In Polish)
- Przybylek, P. A New Concept of Applying Methanol to Dry Cellulose Insulation at the Stage of Manufacturing a Transformer. Energies 2018, 11, 1658. [Google Scholar] [CrossRef]
- Przybylek, P. Sposób i Urządzenie do Suszenia Układów Izolacyjnych Urządzeń Elektroenergetycznych. Method and Device for Drying Insulation Systems of Power Equipment) Patent No. PL 226674, 2017. (In Polish).
- IEC 60814; Insulating Liquids—Oil-Impregnated Paper and Pressboard—Determination of Water by Automatic Coulometric Karl Fischer Titration. International Electrotechnical Commission (IEC): Geneva, Switzerland, 1997.
- Przybylek, P. A New Method for Indirect Measurement of Water Content in Fibrous Electro-insulating Materials Using Near-infrared Spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1798–1804. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, Z.; Chen, C.; He, G.; Hao, C. Effect of hydrogen bonding on self-diffusion in methanol/water liquid mixtures: A molecular dynamics simulation study. J. Mol. Liq. 2015, 203, 90–97. [Google Scholar] [CrossRef]
- Han, C.; Gao, J.; Sun, W.; Han, C.; Li, F.; Li, B. Structure Study of Water in Alcohol-Water Binary System Based on Raman Spectroscopy. J. Phys. Conf. Ser. 2022, 2282, 012021. [Google Scholar] [CrossRef]
- Yang, B.; Cao, X.; Lang, H.; Wang, S.; Sun, C. Study on Hydrogen Bonding Network in Aqueous Methanol Solution by Raman Spectroscopy. Spectrochem. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117488. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.; Aubin, J.; Davydov, V.; Gasser, H.P.; Griffin, P.; Koch, M.; Lundgaard, L.; Roizman, O.; Scala, M.; Tenbohlen, S.; et al. Moisture Equilibrium and Moisture Migration within Transformer Insulation Systems; Cigré Technical Brochure 349; International Council on Large Electric Systems (CIGRE): Paris, France, 2008. [Google Scholar]




| No. | WCPi, % | WCPd, % | ΔWCP, p.p. | Va, L/h | Cm, g/L | WCM, % | Sample Description |
|---|---|---|---|---|---|---|---|
| 1 | 4.49 | 3.07 | 1.42 | 1.8 | - | - | not delaminated |
| 2 | 4.58 | 1.26 | 3.32 | 1.8 | 0.44 | 7.9 | not delaminated |
| 3 | 4.48 | 1.61 | 2.87 | 6.0 | 0.20 | 6.2 | not delaminated |
| No. | WCPi, % | WCPd, % | ΔWCP, p.p. | Va, L/h | Cm, g/L | WCM, % | Sample Description |
|---|---|---|---|---|---|---|---|
| 1 | 5.57 | 1.93 | 3.64 | 2.4 | 0.25 | 7.34 | not delaminated |
| 2 | 5.57 | 0.77 | 4.80 | 2.4 | 0.25 | 2.04 | not delaminated |
| 3 | 5.57 | 0.81 | 4.76 | 2.4 | 0.25 | 1.90 | not delaminated |
| No. | WCPi, % | WCPd, % | ΔWCP, p.p. | Va, L/h | Cm, g/L | WCM, % | Sample Description |
|---|---|---|---|---|---|---|---|
| 1 | 4.47 | 1.65 | 2.82 | 2.4 | - | - | - |
| 2 | 4.37 | 0.41 | 3.96 | 2.4 | 0.25 | 1.56 | - |
| 3 | 4.13 | 0.09 | 4.04 | 2.4 | 0.25 | 4.03 | - |
| No. | WCPi, % | WCPd, % | ΔWCP, p.p. | Va, L/h | Cm, g/L | WCM, % | Sample Description |
|---|---|---|---|---|---|---|---|
| 1 | 4.59 | 0.75 | 3.84 | 1.2 | 0.25 | 1.40 | not delaminated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylek, P.; Gielniak, J. The Use of Methanol Vapour for Effective Drying of Cellulose Insulation. Energies 2023, 16, 4465. https://doi.org/10.3390/en16114465
Przybylek P, Gielniak J. The Use of Methanol Vapour for Effective Drying of Cellulose Insulation. Energies. 2023; 16(11):4465. https://doi.org/10.3390/en16114465
Chicago/Turabian StylePrzybylek, Piotr, and Jaroslaw Gielniak. 2023. "The Use of Methanol Vapour for Effective Drying of Cellulose Insulation" Energies 16, no. 11: 4465. https://doi.org/10.3390/en16114465
APA StylePrzybylek, P., & Gielniak, J. (2023). The Use of Methanol Vapour for Effective Drying of Cellulose Insulation. Energies, 16(11), 4465. https://doi.org/10.3390/en16114465






