The Effect of Explosions on the Protective Wall of a Containerized Hydrogen Fuel Cell System
Abstract
:1. Introduction
2. Simulation Methods
2.1. Governing Equations
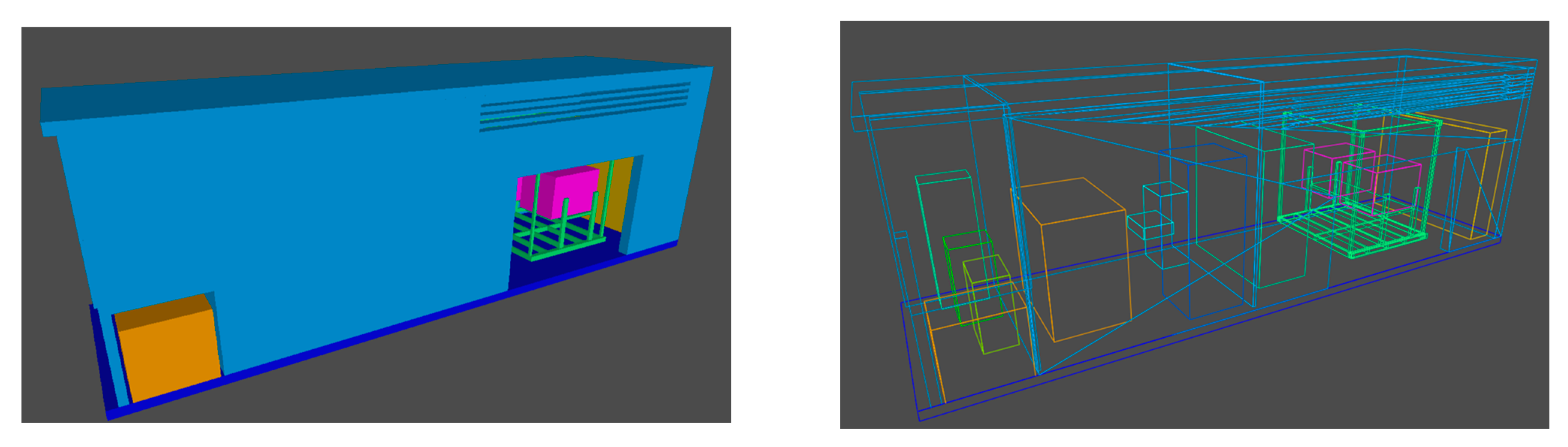
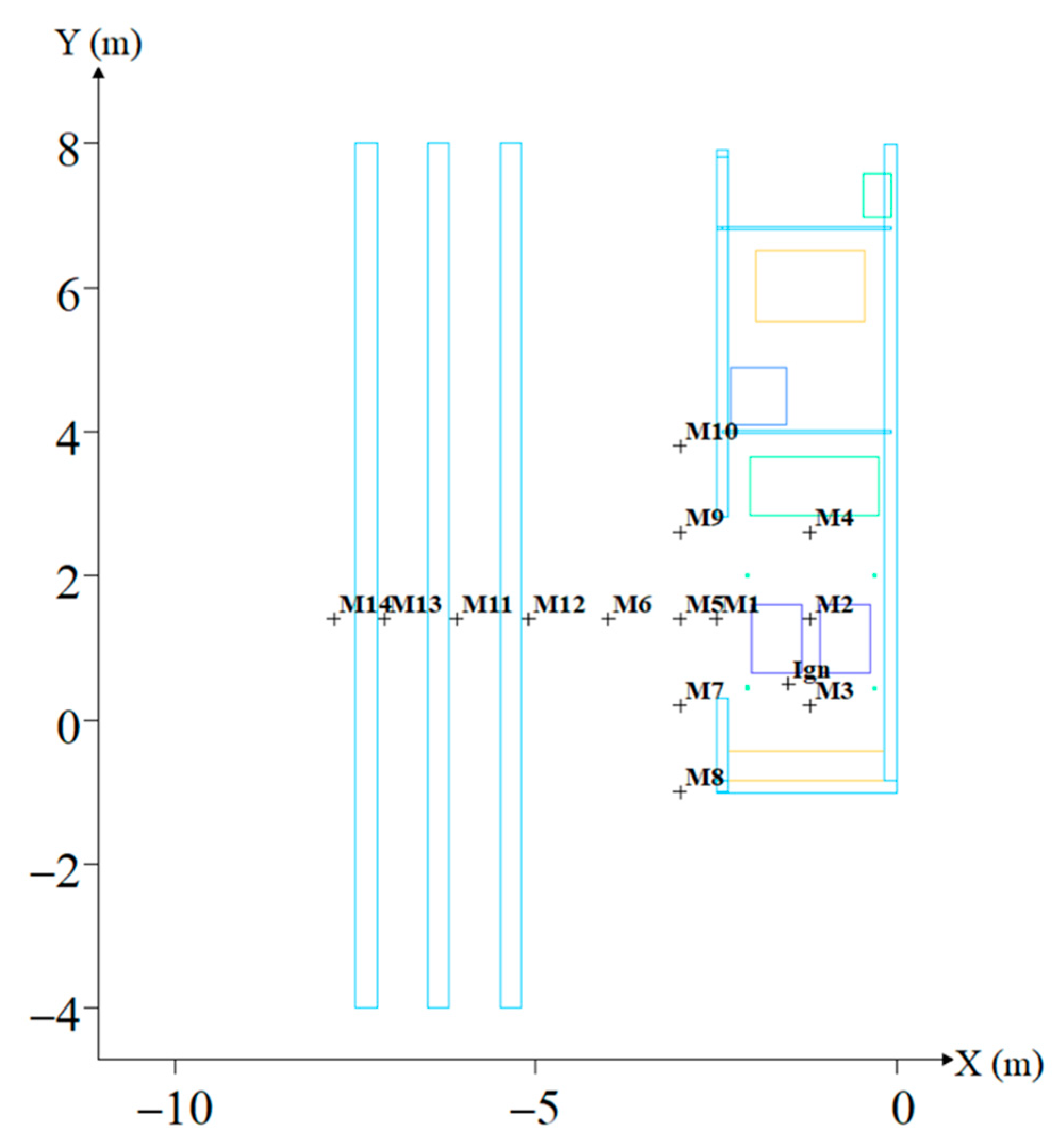
2.2. Geometry and Model Settings
2.3. Grid Sensitivity Analysis
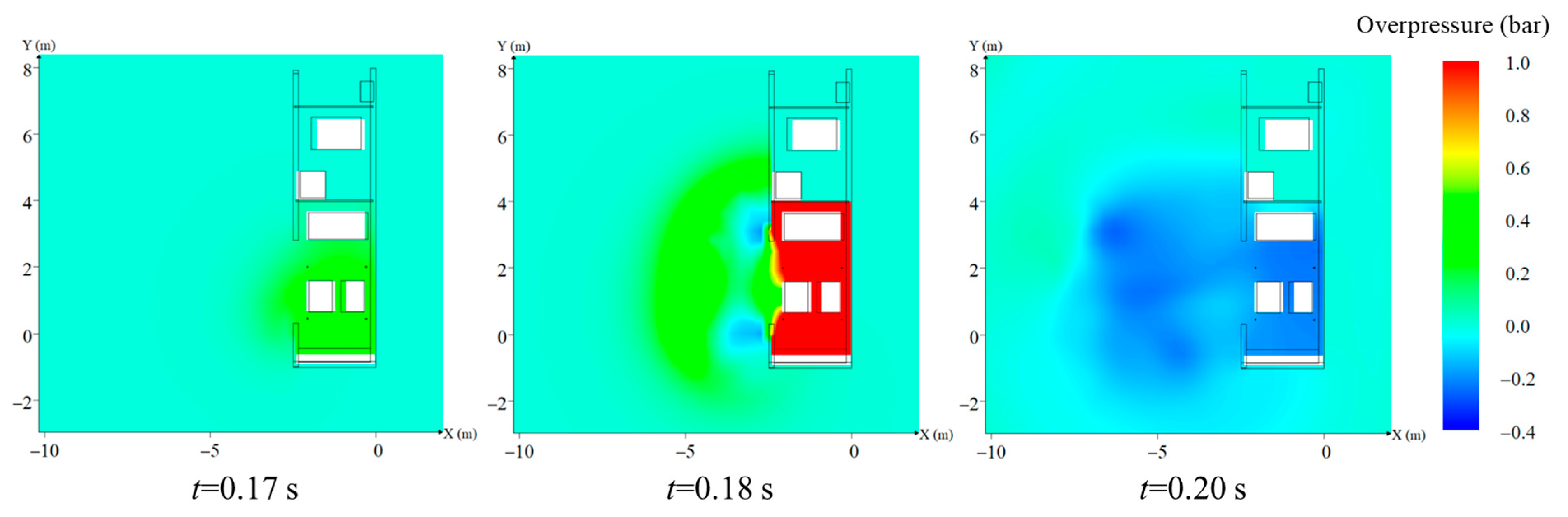
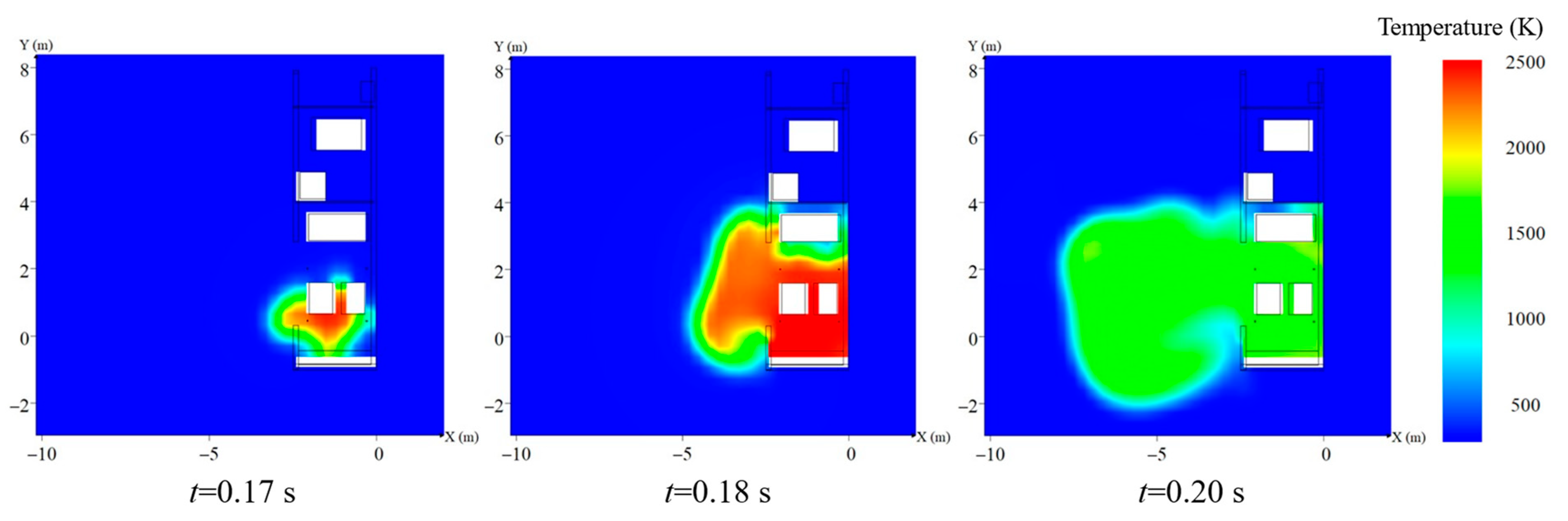
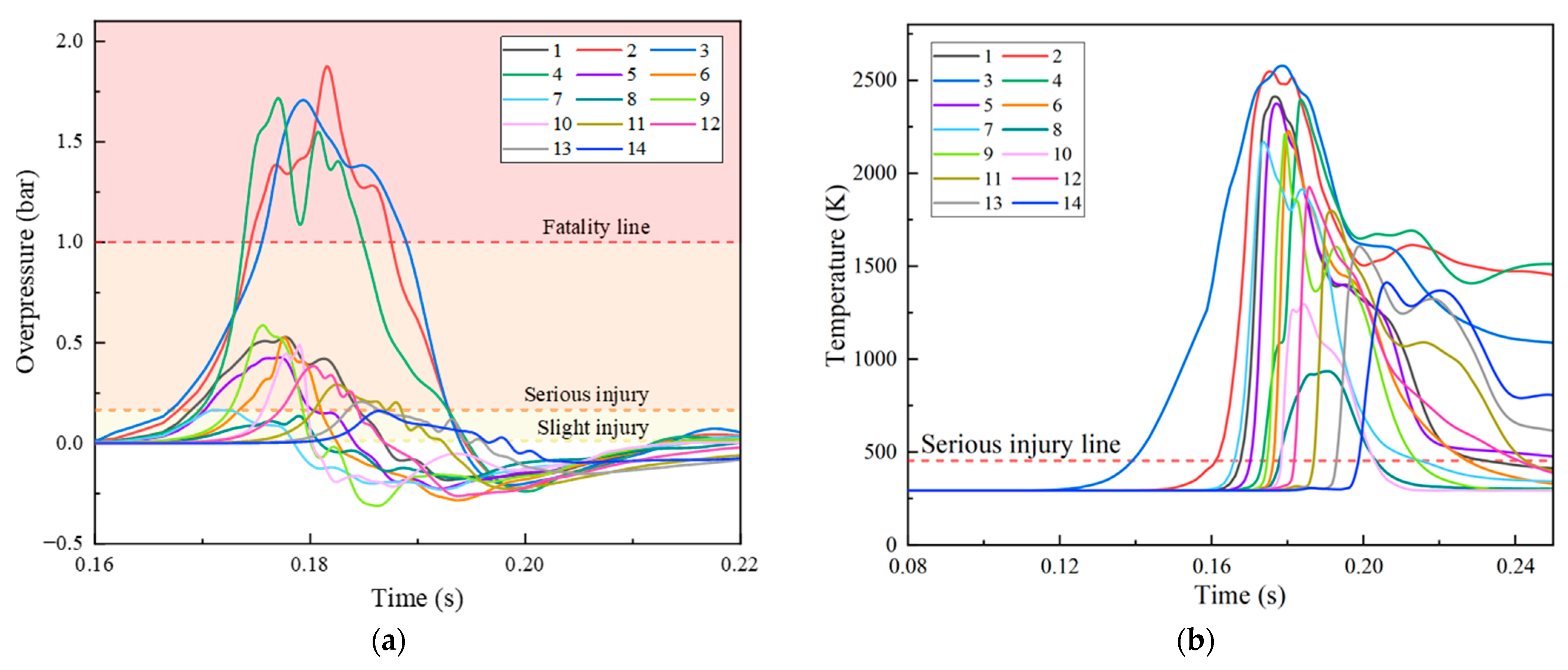
3. The Explosion Characteristics without Protective Walls
4. The Protective Effects of the Protective Walls
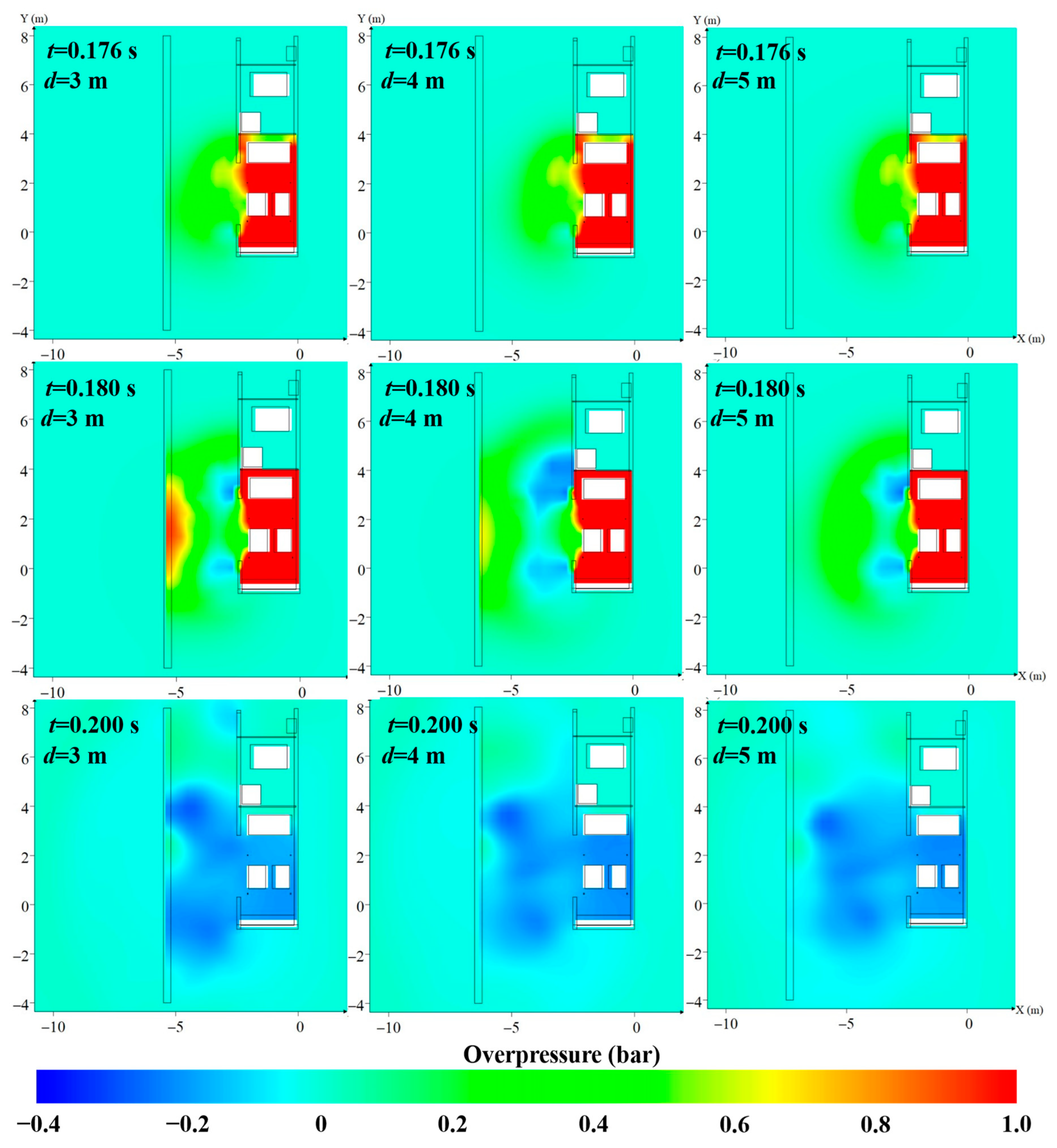
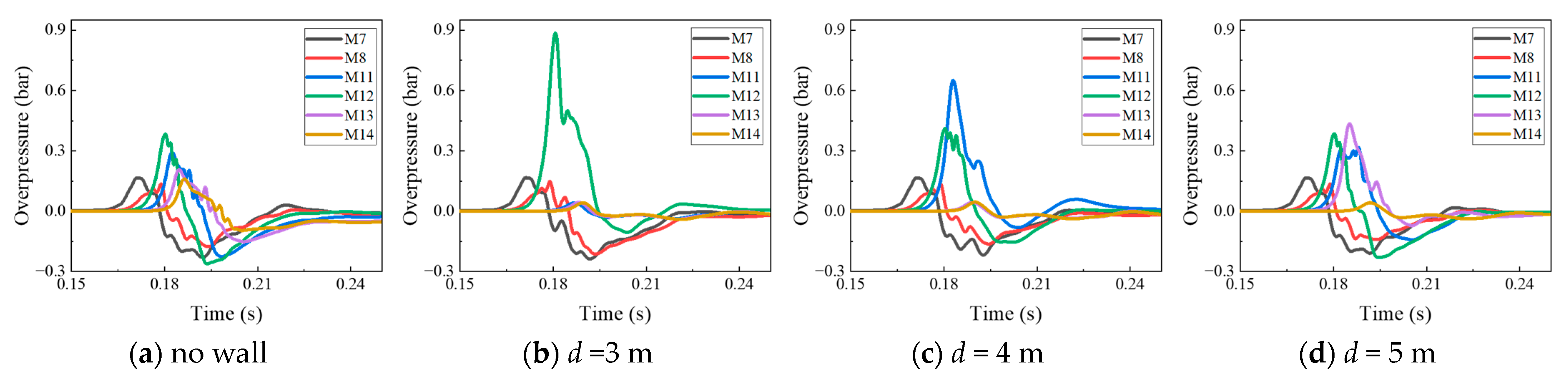
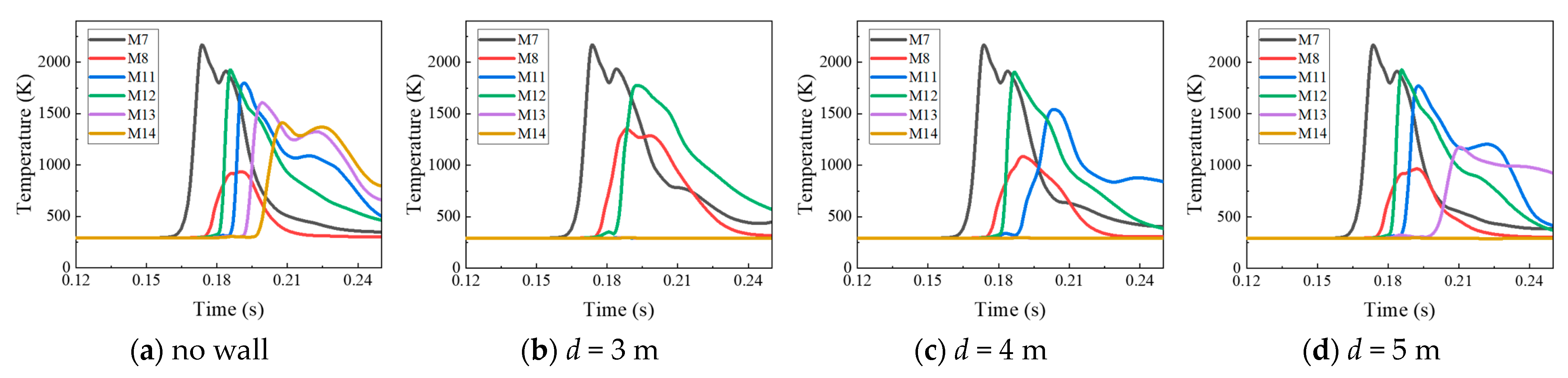
4.1. The Protective Effects of the Protective Walls with Various Distances
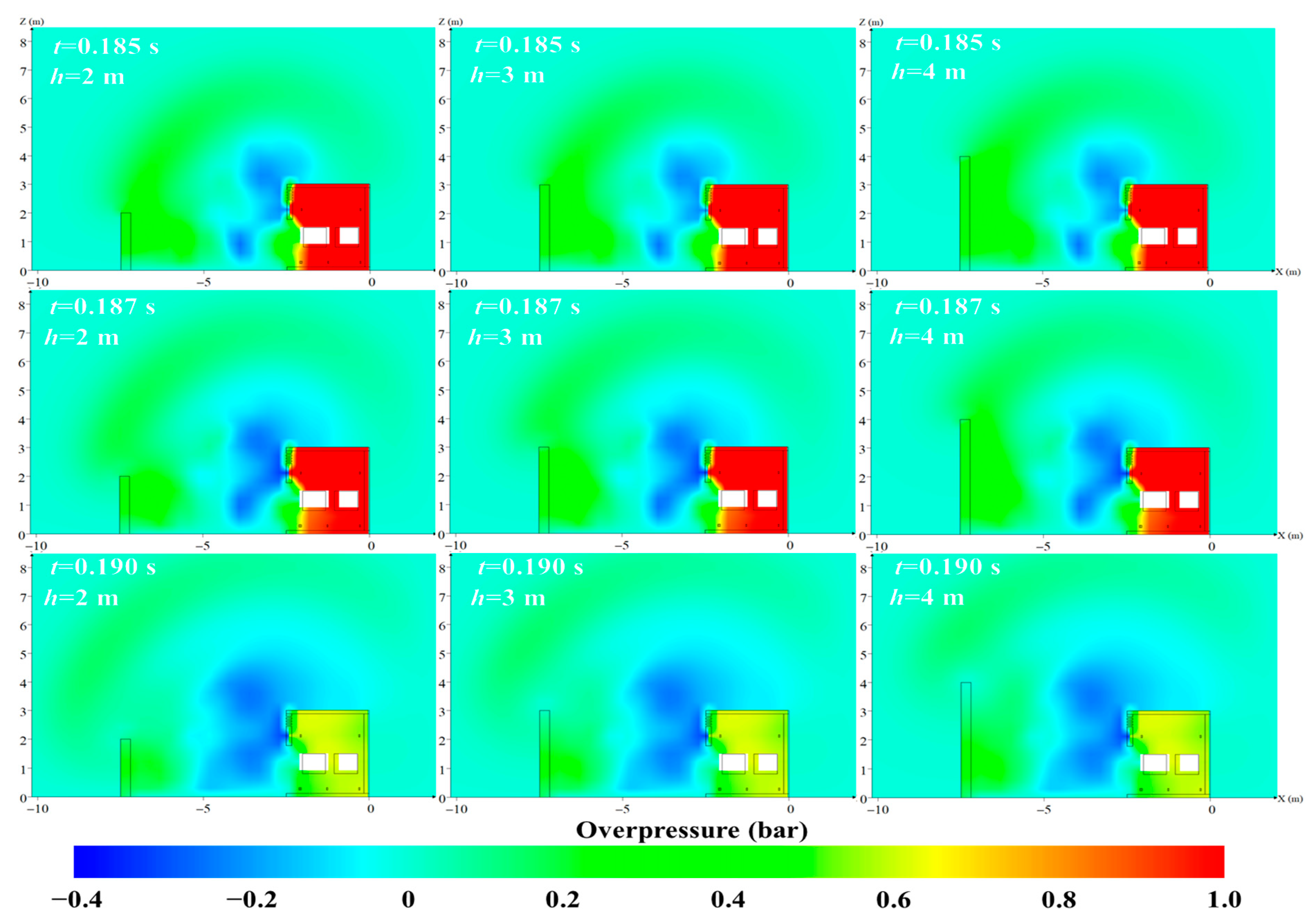
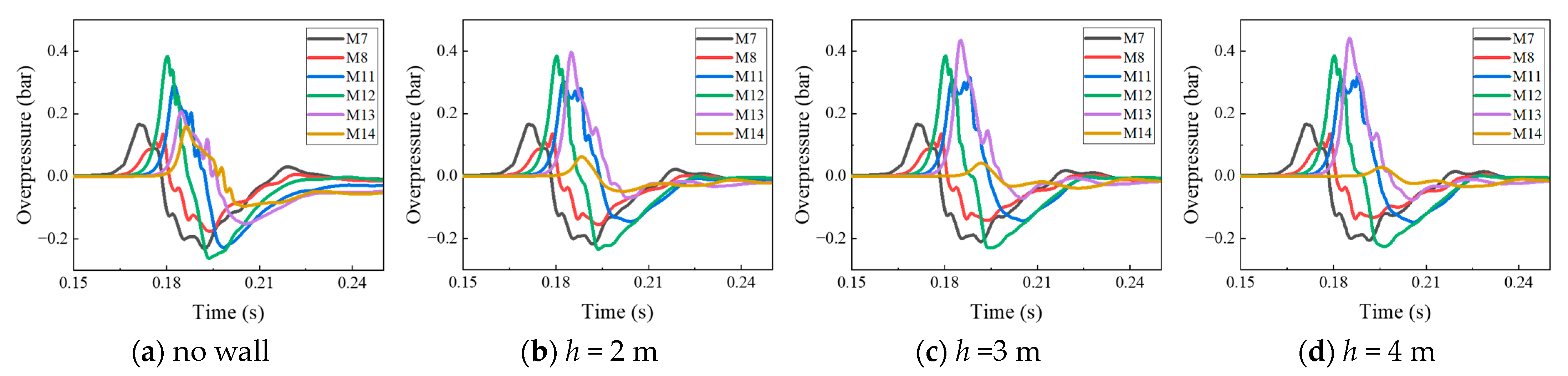
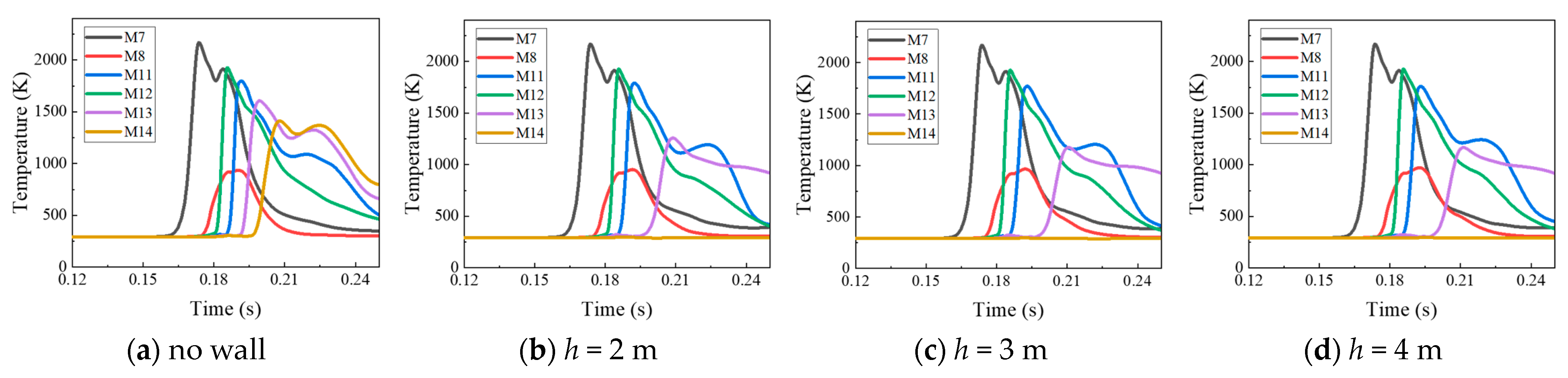
4.2. The Protective Effects of the Protective Walls with Various Heights
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noyan, O.F.; Hasan, M.M.; Pala, N. A Global Review of the Hydrogen Energy Eco-System. Energies 2023, 16, 1484. [Google Scholar] [CrossRef]
- Mneimneh, F.; Ghazzawi, H.; Abu Hejjeh, M.; Manganelli, M.; Ramakrishna, S. Roadmap to Achieving Sustainable Development via Green Hydrogen. Energies 2023, 16, 1368. [Google Scholar] [CrossRef]
- Samsun, R.C.; Rex, M.; Antoni, L.; Stolten, D. Deployment of Fuel Cell Vehicles and Hydrogen Refueling Station Infrastructure: A Global Overview and Perspectives. Energies 2022, 15, 4975. [Google Scholar] [CrossRef]
- Hu, Q.C.; Zhang, X.H.; Hao, H. A review of hydrogen-air cloud explosions: The fundamentals, overpressure prediction methods, and influencing factors. Int. J. Hydrogen Energy 2023, 48, 13705–13730. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, Y.C.; Gao, W. Experimental investigation on unconfined hydrogen explosion with different ignition height. Int. J. Hydrogen Energy 2023, 48, 20112–20123. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, Y.C.; Gao, W. Experimental research on unconfined hydrogen explosion of different gas scale and the overpressure prediction method. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Jiang, Y.T.; Li, Y.C.; Zhou, Y.H.; Jiang, H.P.; Zhang, K.; Gao, Z.H.; Gao, W. Investigation on unconfined hydrogen cloud explosion with external turbulence. Int. J. Hydrogen Energy 2021, 47, 8658–8670. [Google Scholar] [CrossRef]
- Zhuang, C.J.; Zhang, L.J.; Tao, G.; Zhang, Y.Q.; Huang, H.; Wang, Z.R. Effect of concentration, obstacles, and ignition location on the explosion overpressure of hydrogen-air in a closed-vessel. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Zhou, N.; Mei, Y.; Li, X.; Chen, B.; Huang, W.Q.; Zhao, H.J.; Yuan, X.J. Numerical simulation of the influence of vent conditions on the characteristics of hydrogen explosion in confined space. Combust. Theory Model. 2022, 26, 241–259. [Google Scholar] [CrossRef]
- Xing, H.; Yu, R.; Xu, G.; Li, X.; Qiu, Y.; Wang, D.; Li, B.; Xie, L. Theoretical and Experimental Investigation of Explosion Characteristics of Hydrogen Explosion in a Closed Vessel. Energies 2022, 15, 8630. [Google Scholar] [CrossRef]
- Dai, T.K.; Zhang, B.; Liu, H. On the explosion characteristics for central and end-wall ignition in hydrogen-air mixtures: A comparative study. Int. J. Hydrogen Energy 2021, 46, 30861–30869. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.J.; Liu, X.Y.; Chen, Y. Explosion venting of rich hydrogen-air mixtures in a small cylindrical vessel with two symmetrical vents. Int. J. Hydrogen Energy 2017, 42, 7644–7650. [Google Scholar] [CrossRef]
- Luo, X.J.; Wang, C.J.; Rui, S.C.; Wan, Y.; Zhang, Z.; Li, Q. Effects of ignition location, obstacles, and vent location on the vented hydrogen-air deflagrations with low vent burst pressure in a 20-foot container. Fuel 2020, 280, 118677. [Google Scholar] [CrossRef]
- Russo, P.; De Marco, A.; Parisi, F. Assessment of the Damage from Hydrogen Pipeline Explosions on People and Buildings. Energies 2020, 13, 5051. [Google Scholar] [CrossRef]
- To, C.W.; Chow, W.K.; Cheng, F.M. Simulation of Possible Fire and Explosion Hazards of Clean Fuel Vehicles in Garages. Sustainability 2021, 13, 12537. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Xiao, G.; Cui, T.; Ba, Q.; Li, X. Numerical Study on Protective Measures for a Skid-Mounted Hydrogen Refueling Station. Energies 2023, 16, 910. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhang, Q. Effect of vent size on vented hydrogen-air explosion. Int. J. Hydrogen Energy 2018, 43, 17788–17799. [Google Scholar] [CrossRef]
- Pang, L.; Li, G.Y.; Yang, K.; Hu, Q.R. Characteristics of external explosions induced by vented hydrogen deflagration. Int. J. Hydrogen Energy 2023, 48, 18129–18140. [Google Scholar] [CrossRef]
- Rokhy, H.; Mostofi, T.M. Tracking the explosion characteristics of the hydrogen-air mixture near a concrete barrier wall using CESE IBM FSI solver in LS-DYNA incorporating the reduced chemical kinetic model. Int. J. Impact Eng. 2023, 172, 104401. [Google Scholar] [CrossRef]
- Yu, X.; Yan, W.J.; Liu, Y.; Zhou, P.G.; Li, B.; Wang, C.J. The flame mitigation effect of vertical barrier wall in hydrogen refueling stations. Fuel 2022, 315, 123265. [Google Scholar] [CrossRef]
- Xu, R.Z.; Chen, L.; Fang, Q.; Zheng, Y.Z.; Li, Z.; Cao, M.J. Protective effects of gabion wall against blast waves from large TNT-equivalent explosions. Eng. Struct. 2021, 249, 113389. [Google Scholar] [CrossRef]
- Nian, X.Z.; Xie, Q.M.; Kong, X.L.; Yao, Y.K.; Huang, K. Experimental and numerical study on protective effect of RC blast wall against air shock wave. Def. Technol. 2022. [Google Scholar] [CrossRef]
- Bleyer, A.; Taveau, J.; Djebaïli-Chaumeix, N.; Paillard, C.E.; Bentaïb, A. Comparison between FLACS explosion simulations and experiments conducted in a PWR Steam Generator casemate scale down with hydrogen gradients. Nucl. Eng. Des. 2012, 245, 189–196. [Google Scholar] [CrossRef]
- Su, B.; Luo, Z.M.; Wang, T.; Liu, L. Experimental and numerical evaluations on characteristics of vented methane explosion. J. Cent. South Univ 2020, 27, 2382–2393. [Google Scholar] [CrossRef]
- Vyazmina, E.; Jallais, S. Validation and recommendations for FLACS CFD and engineering approaches to model hydrogen vented explosions: Effects of concentration, obstruction vent area and ignition position. Int. J. Hydrogen Energy 2016, 41, 15101–15109. [Google Scholar] [CrossRef]
- Hjertager, B.H. Computer Simulation of Turbulent Reactive Gas Dynamics. Model. Identif. Control (MIC) 1984, 5, 211–236. [Google Scholar] [CrossRef]
- FLACS-CFD Technical Manuals. Available online: https://www.gexcon.com/wp-content/uploads/2023/02/FLACS-CFD-22.2-manual.pdf (accessed on 9 April 2023).
- Treadwell, I. Effects of blasts on the human body. Nurs RSA 1989, 4, 32–36. Available online: https://pubmed.ncbi.nlm.nih.gov/2733763/ (accessed on 9 April 2023).
- Kashkarov, S.; Li, Z.Y.; Molkov, V. Blast wave from a hydrogen tank rupture in a fire in the open: Hazard distance nomograms. Int. J. Hydrogen Energy 2020, 45, 2429–2446. [Google Scholar] [CrossRef]



| Scenario | Distance of Protective Wall from the Container (m) | Height of Protective Wall (m) |
|---|---|---|
| case 1 | No protective walls | No protective walls |
| case 2 | 3 | 3 |
| case 3 | 4 | 3 |
| case 4 | 5 | 3 |
| case 5 | 5 | 4 |
| case 6 | 5 | 2 |
| Overpressure (bar) | Injury to the Human Body |
|---|---|
| <0.0135 | No harm |
| 0.0135–0.165 | Slight injury |
| 0.165–1.0 | Serious injury |
| >1.0 | Fatality |
| Overpressure (bar) | Damage to Buildings |
|---|---|
| <0.048 | No damage |
| 0.048–0.069 | Minor damage |
| 0.069–0.345 | Partial demolition |
| >0.345 | Destruction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, L.; Wu, Q.; Zhang, Y.; Zhang, J.; Li, X.; Ba, Q. The Effect of Explosions on the Protective Wall of a Containerized Hydrogen Fuel Cell System. Energies 2023, 16, 4477. https://doi.org/10.3390/en16114477
Liu M, Zhang L, Wu Q, Zhang Y, Zhang J, Li X, Ba Q. The Effect of Explosions on the Protective Wall of a Containerized Hydrogen Fuel Cell System. Energies. 2023; 16(11):4477. https://doi.org/10.3390/en16114477
Chicago/Turabian StyleLiu, Min, Leiqi Zhang, Qiliang Wu, Yunpeng Zhang, Jiaxin Zhang, Xuefang Li, and Qingxin Ba. 2023. "The Effect of Explosions on the Protective Wall of a Containerized Hydrogen Fuel Cell System" Energies 16, no. 11: 4477. https://doi.org/10.3390/en16114477
APA StyleLiu, M., Zhang, L., Wu, Q., Zhang, Y., Zhang, J., Li, X., & Ba, Q. (2023). The Effect of Explosions on the Protective Wall of a Containerized Hydrogen Fuel Cell System. Energies, 16(11), 4477. https://doi.org/10.3390/en16114477








