Thermal Decomposition Processes in Relation to the Type of Organic Matter, Mineral and Maceral Composition of Menilite Shales
Abstract
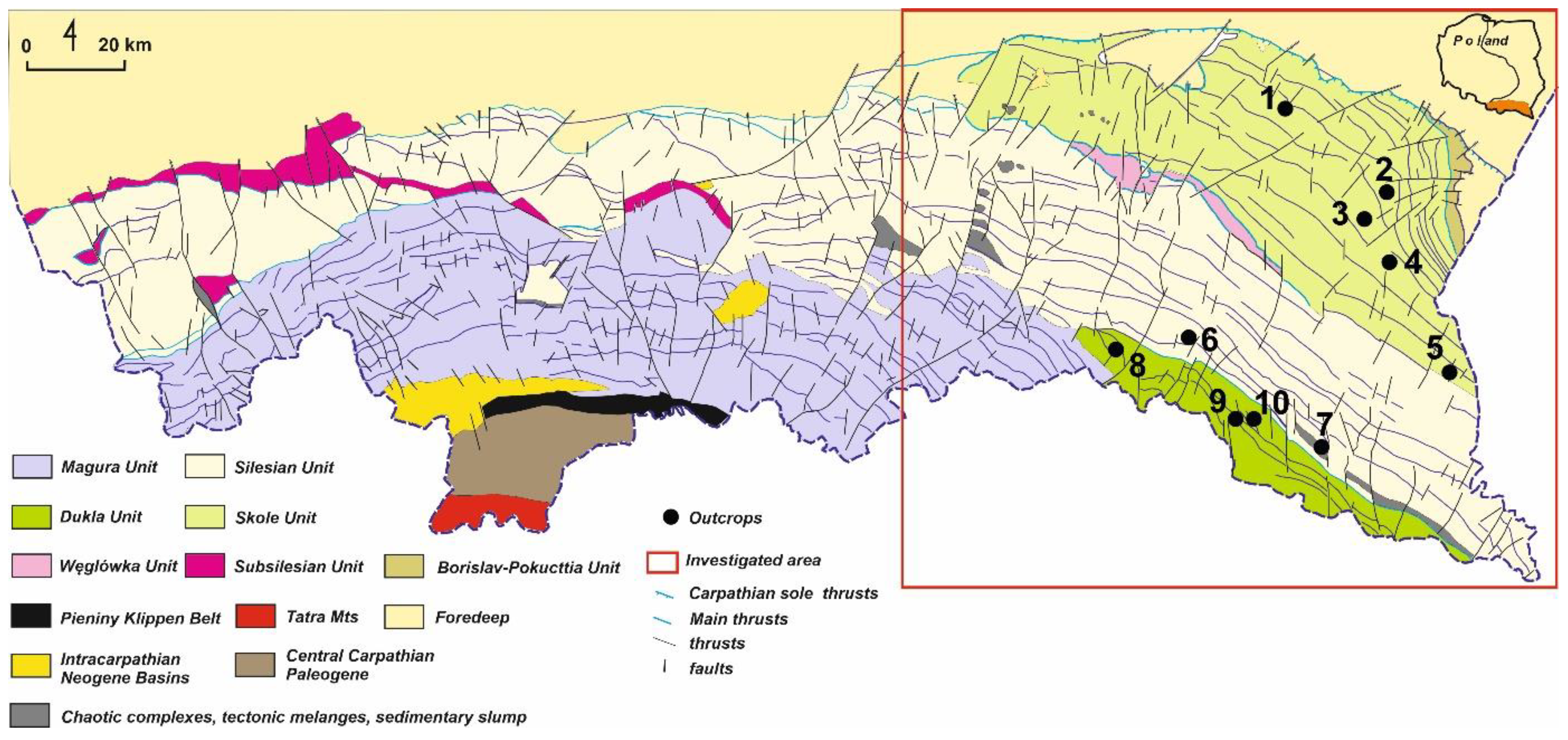
1. Introduction
2. Experimental Methods
3. Results
3.1. Mineral Composition
3.2. Maceral Composition
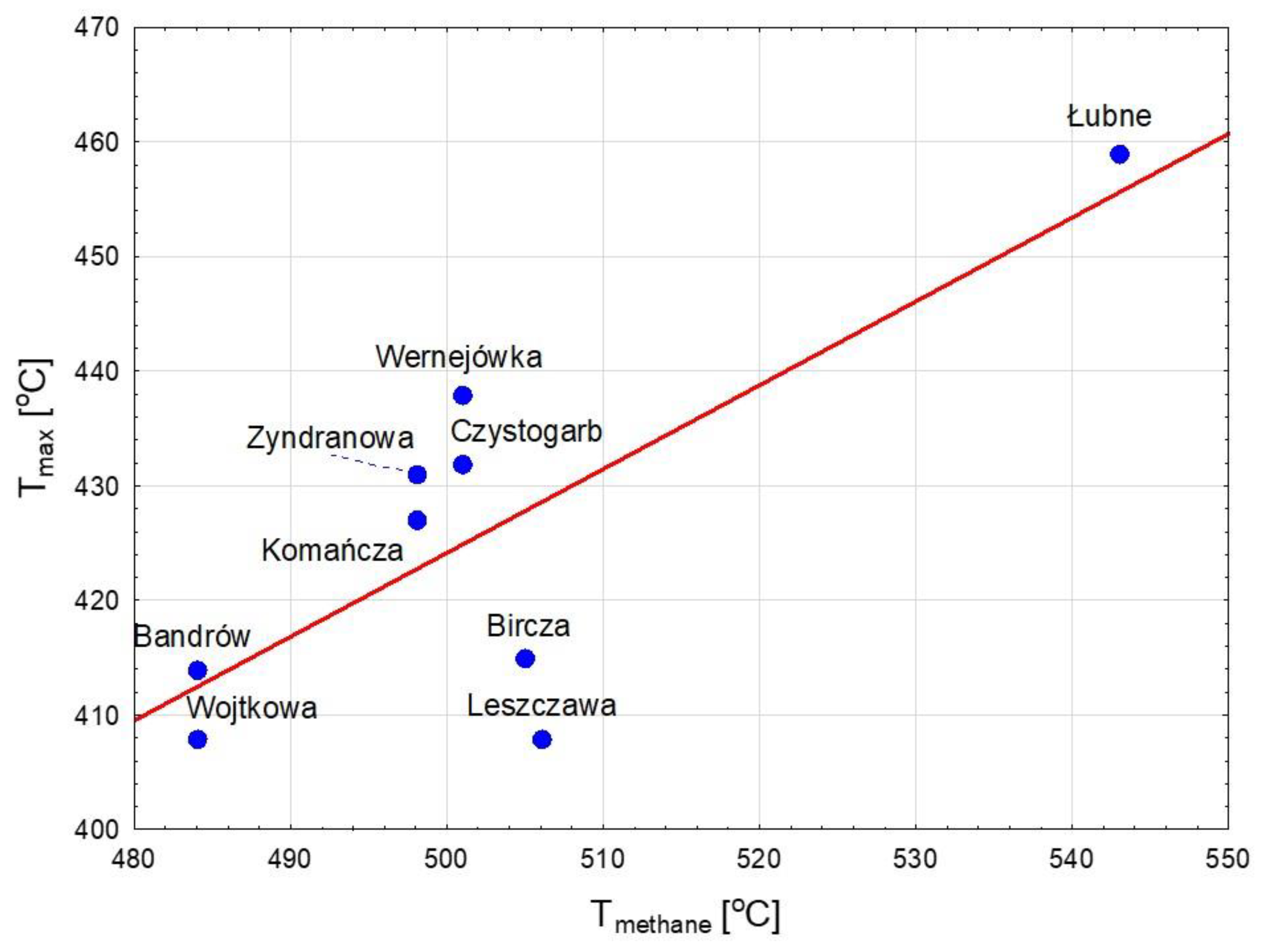
3.3. Hydrocarbon Potential and Thermal Maturity
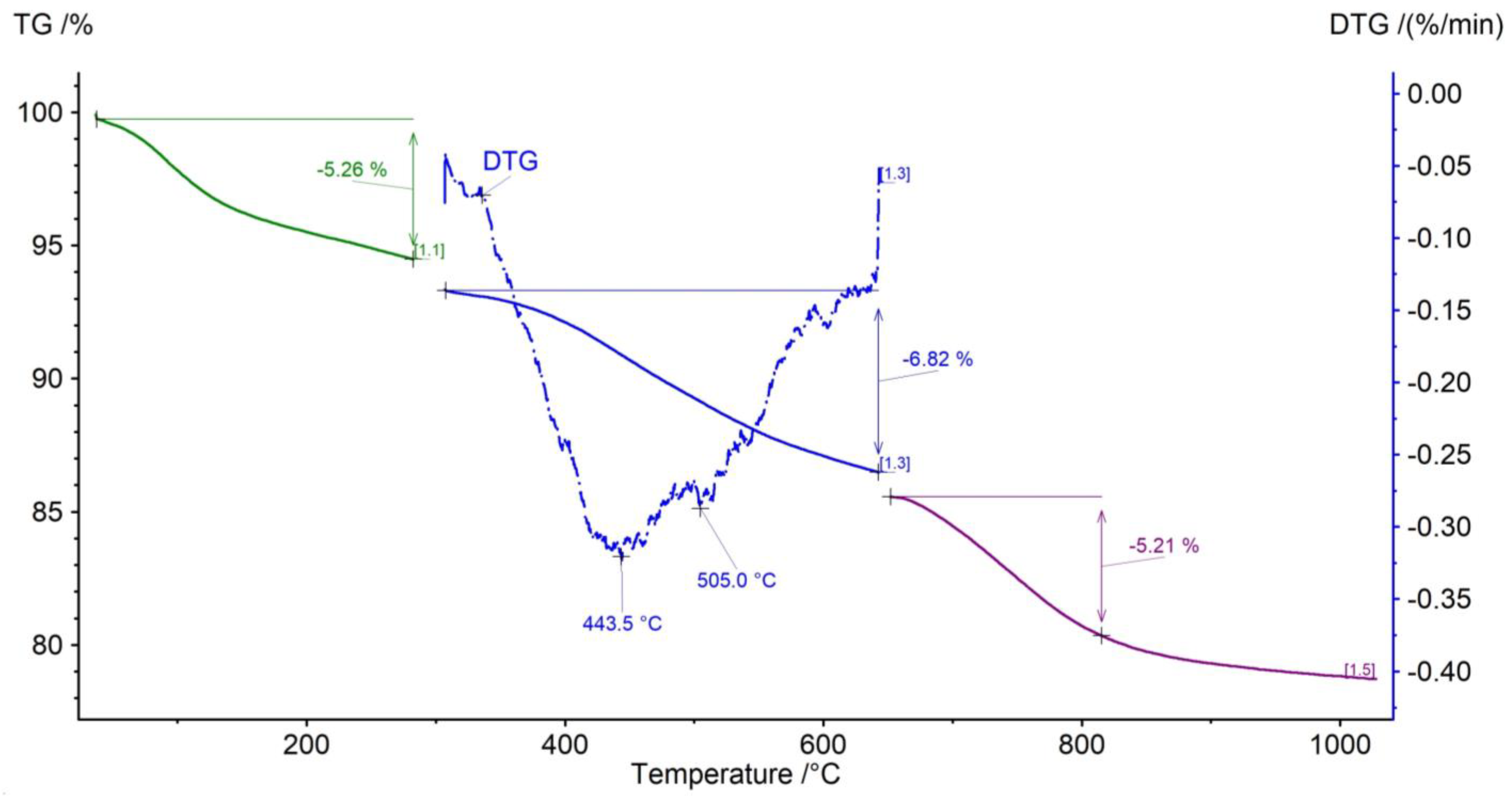
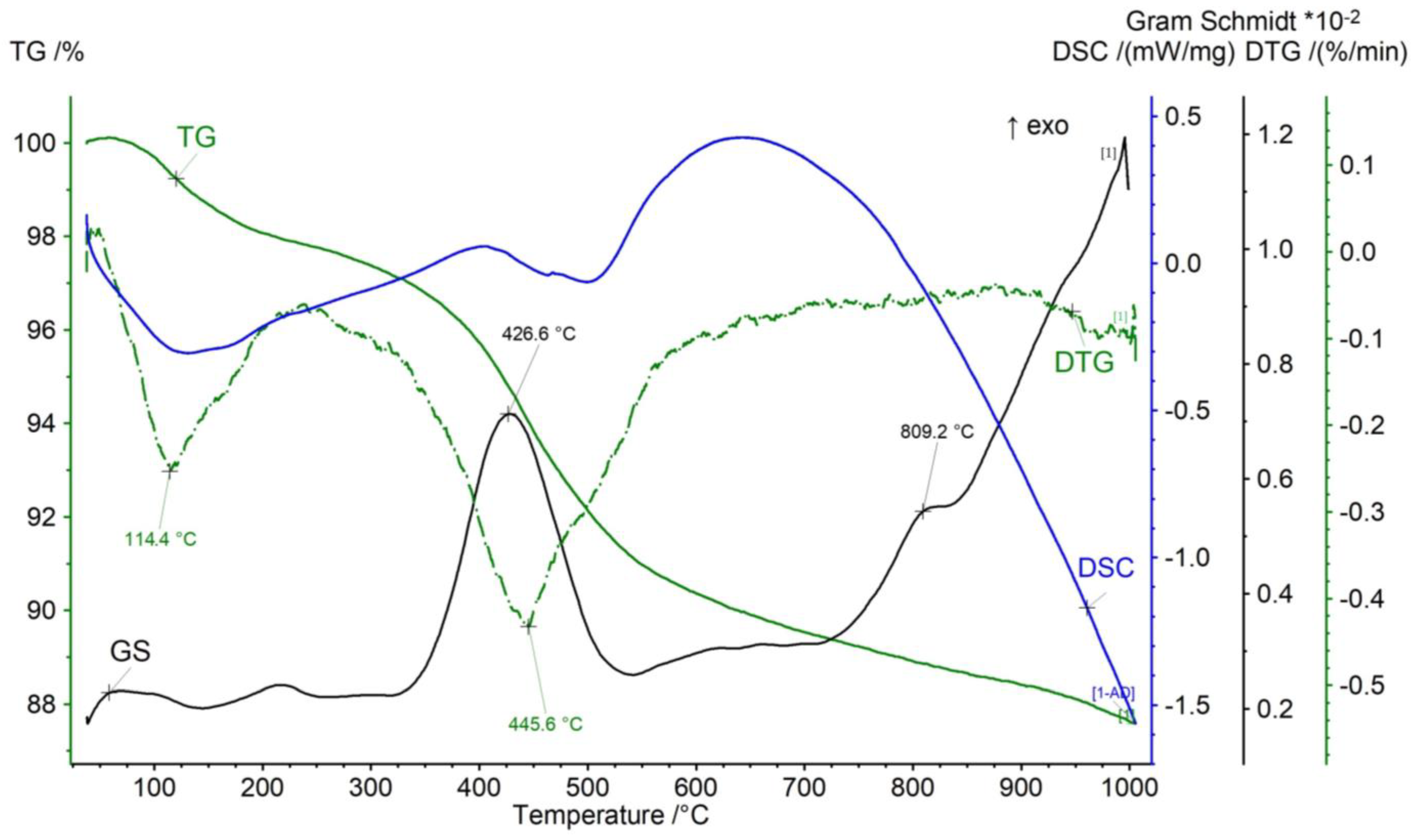
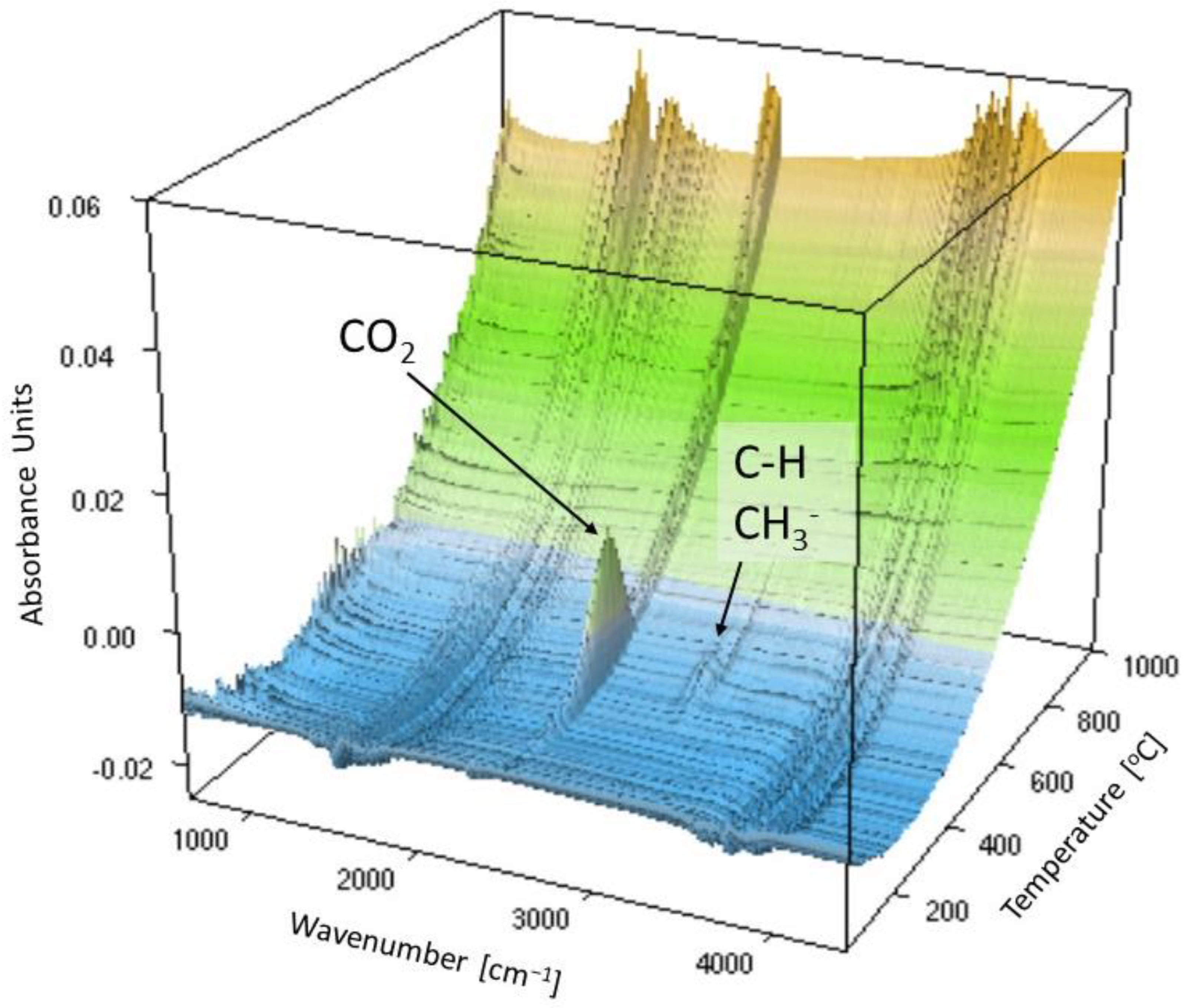
3.4. Thermal Decomposition
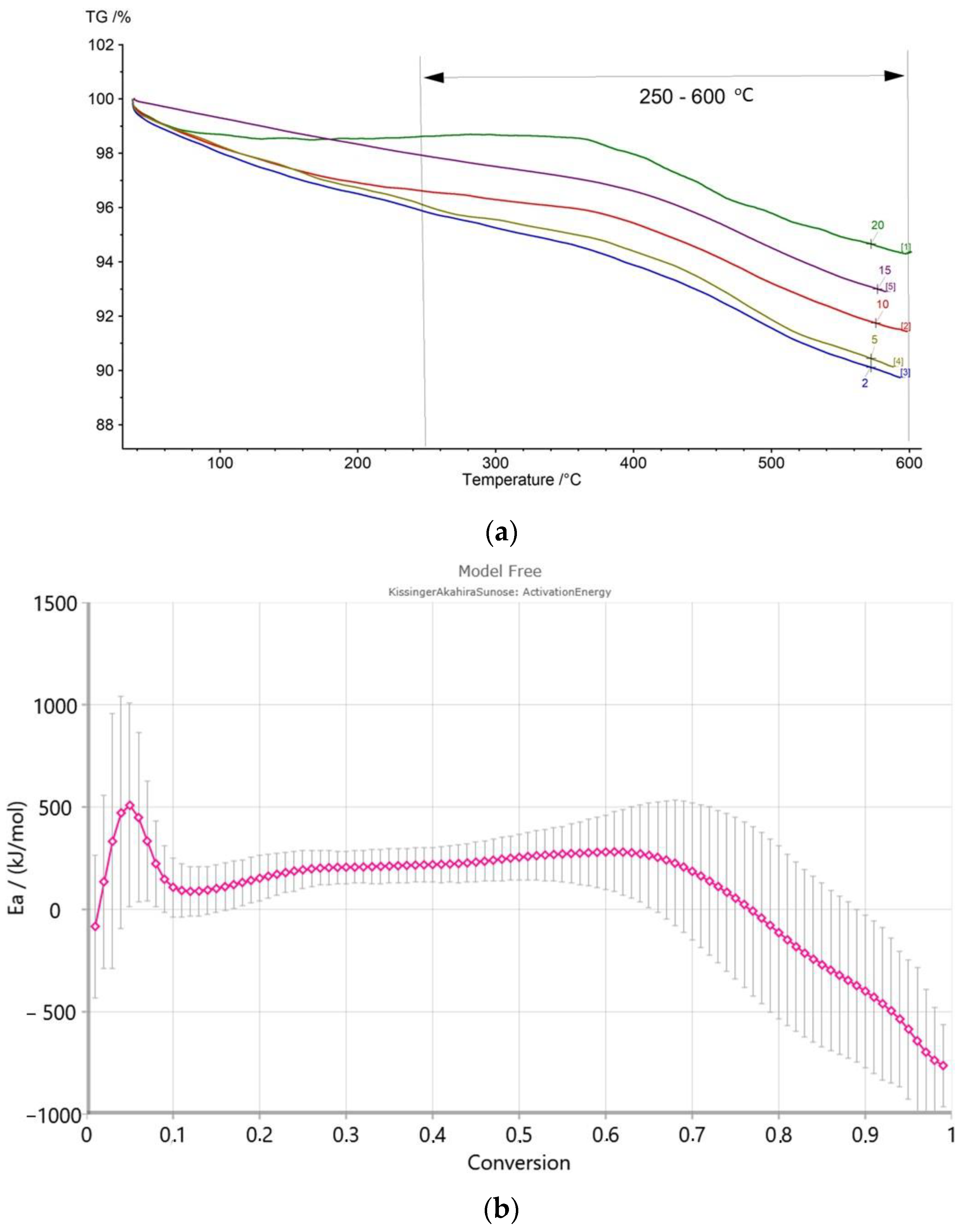
3.5. Kinetic Parameters
4. Discussion
4.1. Organic Matter Decomposition
4.2. Thermal Maturity of the Samples
4.3. Kinetic Parameters
4.4. Mineral and Maceral Composition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ziemianin, K. Petrographic-geochemical characterization of the dispersed organic matter in Menilite shales from the Silesian Unit in the Carpathian Mountains of SE Poland. Naft. Gaz 2017, 11, 835–842. [Google Scholar] [CrossRef]
- Ziemianin, K. Characteristics of dispersed organic matter in the Menilite Beds from the Skole Unit. Naft. Gaz 2018, 9, 636–646. [Google Scholar] [CrossRef]
- Ziemianin, K. Characteristics of dispersed organic matter in the Menilite Beds from the Dukla Unit based on microscopic analysis and Rock-Eval pyrolysis. Naft. Gaz 2019, 6, 303–313. [Google Scholar] [CrossRef]
- Ziemianin, K.; Spunda, K. Maceral composition and maturity of dispersed organic matter in selected lithostratigraphic beds within Dukla Unit. Naft. Gaz 2022, 2, 83–96. [Google Scholar] [CrossRef]
- Koltun, Y.V. Organic matter in oligocene Menilite formation rocks of the Ukrainian Carpathians: Palaeoenvironment and geochemical evolution. Org. Geochem. 1992, 18, 423–430. [Google Scholar] [CrossRef]
- Kruge, M.A.; Mastalerz, M.; Solecki, A.; Stankiewicz, B.A. Organic geochemistry and petrology of oil source rocks, Carpathian Overthrust region, southeastern Poland—Implications for petroleum generation. Org. Geochem. 1996, 24, 897–912. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Więcław, D.; Koltun, Y.V.; Marynowski, L.; Kuśmierek, J.; Dudok, I.V. Organic geochemical study and genetic correlation of natural gas, oil and Menilite source rocks in the area between San and Stryi rivers (Polish and Ukrainian Carpathians). Org. Geochem. 2007, 38, 163–180. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Więcław, D.; Dziadzio, P.; Kowalski, A.; Bilkiewicz, E.; Kosakowski, P. Organic geochemical study of source rocks and natural gas and their genetic correlation in the central part of the Polish Outer Carpathians. Mar. Pet. Geol. 2013, 45, 106–120. [Google Scholar] [CrossRef]
- Kosakowski, P.; Koltun, Y.; Machowski, G.; Papiernik, B. The geochemical characteristics of the Oligocene-lower Miocene menilite formation in the Polish and Ukrainian Outer Carpathians: A review. J. Pet. Geol. 2018, 41, 319–335. [Google Scholar] [CrossRef]
- Kosakowski, P.; Więcław, D.; Kotarba, M.J. Charakterystyka macierzystości wybranych utworów fliszowych w przygranicznej strefie polskich Karpat Zewnętrznych. Geologia 2009, 35, 155–190, (In Polish, with English Abstract). [Google Scholar]
- Wendorff, M.; Rospondek, M.J.; Kluska, B.; Marynowski, L. Organic matter maturity and hydrocarbon potential of the Lower Oligocenen Menilite facies in the Eastern Flysh Carpathians (Tarcău and Vrancea Nappes), Romania. Appl. Geochem. 2017, 78, 295–310. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Qin, J.; Wang, Q.; Zhang, C. Kinetics of the hydrocarbon generation process of marine source rocks in South China. Pet. Explor. Dev. 2010, 37, 174–180. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Mankiewicz, P.J. Evaluation of kinetic uncertainty in numerical models of petroleum generation. AAPG Bull. 2006, 90, 387–403. [Google Scholar] [CrossRef]
- Tissot, B.P.; Pelet, R.; Ungerer, P. Thermal history of sedimentary basins, maturation indices, and kinetics of oil and gas generation. AAPG Bull. 1987, 71, 1445–1466. [Google Scholar]
- Marshall, C.P.; Kannangara, G.S.K.; Wilson, M.A.; Guerbois, J.P.; Hartung-Kagi, B.; Hart, G. Potential of thermogravimetric analysis coupled with mass spectrometry for the evaluation of kerogen in source rocks. Chem. Geol. 2002, 184, 185–194. [Google Scholar] [CrossRef]
- Xinmin, W.; Qing, W.; Chunlei, W. Study of the effect of in situ minerals on the pyrolysis of oil shale in Fushun. China RSC Adv. 2022, 12, 20239–20250. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X.; Liu, J. A TG-FTIR investigation to the catalytic effect of mineral matrix in oil shale on the pyrolysis and combustion of kerogen. Fuel 2013, 104, 307–317. [Google Scholar] [CrossRef]
- Chang, Z.; Chu, M.; Zhang, C.; Bai, S.; Lin, H.; Ma, L. Influence of inherent mineral matrix on the product yield and characterization from Huadian oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 269–276. [Google Scholar] [CrossRef]
- Ariskina, K.A.; Ding, Z.; Abaas, M.; Yuan, C.; Emelianov, D.A.; Chen, Q.; Varfolomeev, M.A. Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR. Energies 2021, 14, 136. [Google Scholar] [CrossRef]
- Karabakan, A.; Yürüm, Y. Effect of the mineral matrix in the reactions of oil shales: 1. Pyrolysis reactions of Turkish Göynük and US Green River oil shales. Fuel 1998, 77, 1303–1309. [Google Scholar] [CrossRef]
- Peters, K.E.; Burnham, A.K.; Walters, C.C. Petroleum generation kinetics: Single versus multiple heating-ramp open-system pyrolysis. AAPG Bull. 2015, 99, 591–616. [Google Scholar] [CrossRef]
- Reynolds, J.G.; Burnham, A.K. Comparison of kinetic analysis of source rocks and kerogen concentrates. Org. Geochem. 1995, 23, 11–19. [Google Scholar] [CrossRef]
- Shindler, A.; Neumann, G.; Rager, A.; Fügline, E.; Blumm, J.; Denner, T. A novel direct coupling of simultaneous thermal analysis (STA) and Fourier transform-infrared (FT-IR) spectroscopy. J. Therm. Anal. Calorim. 2013, 113, 1091–1102. [Google Scholar] [CrossRef]
- Smith, A.L. Applied Infrared Spectroscopy: Fundamentals, Techniques and Analytical Problem-Solving, Series Chemical Analysis; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Commitee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Jankowski, L. Kompleksy chaotyczne Karpat Polskich. In Proceedings of the Conference Materials, Kraków, Poland, 24–27 September 2008; pp. 26–88, (In Polish, with English Abstract). [Google Scholar]
- Jankowski, L.; Probulski, J. Rozwój tektoniczno-basenowy Karpat zewnętrznych na przykładzie budowy geologicznej złóż Grabownica, Strachocina i Łodyna oraz ich otoczenia. Geologia 2011, 37, 555–583, (In Polish, with English abstract). [Google Scholar]
- Labus, M. Pyrite thermal decomposition in source rocks. Fuel 2021, 287, 119529. [Google Scholar] [CrossRef]
- Li, S.; Zhu, R.K.; Cui, J.W.; Luo, Z.; Cui, J.G.; Liu, H.; Li, W.Q. The petrological characteristics and significance of organic-rich shale in the Chang 7 member of the Yanchang Formation, south margin of the Ordos basin, central China. Pet. Sci. 2019, 16, 1255–1269. [Google Scholar] [CrossRef]
- Labus, M.; Matyasik, I. Application of different thermal analysis techniques for the evaluation of petroleum source rocks. J. Therm. Anal. Calorim. 2019, 136, 1185–1194. [Google Scholar] [CrossRef]
- Bennet, B.; Love, G.D. Release of organic nitrogen compounds from kerogen via catalytic hydropyrolysis. Geochem. Trans. 2000, 61, 61. [Google Scholar] [CrossRef]
- You, Y.; Han, X.; Wang, X.; Jiang, X. Evolution of gas and shale oil during oil shale kerogen pyrolysis based on structural characteristics. J. Anal. Appl. Pyrolysis 2019, 138, 203–210. [Google Scholar] [CrossRef]
- Dang, S.T.; Sondergeld, C.H.; Rai, C.S. Study of Kerogen Maturity using Fourier Transform Infrared Spectroscopy (FTIR) and Thermogravimetric Analysis (TGA). In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar] [CrossRef]
- Wood, D.A. Kerogen conversion and thermal maturity modelling of petroleum generation: Integrated analysis applying relevant kerogen kinetics. Mar. Pet. Geol. 2018, 89, 313–329. [Google Scholar] [CrossRef]
- Wood, D.A. Establishing credible reaction-kinetics distributions to fit and explain multi-heating rate S2 pyrolysis peaks of kerogens and shales. Adv. Geo Energy Res. 2019, 3, 1–28. [Google Scholar] [CrossRef]
- Han, J.; Sun, Y.; Guo, W.; Deng, S.; Hou Ch Qu, L.; Li, Q. Non-isothermal thermogravimetric analysis of pyrolysis kinetics of four oil shales using Sestak-Bergen method. J. Therm. Anal. Calorim. 2019, 135, 2287–2296. [Google Scholar] [CrossRef]
- Chi, M.; Xu, X.; Ciu, D.; Zhang, H.; Wang, Q. A TG-FTIR investigation and kinetic analysis of oil shale kerogen pyrolysis using the distributed activation energy model. Oil Shale 2016, 33, 228–247. [Google Scholar] [CrossRef]
- Kok, M.V.; Senguler, I.; Hufnagel, H.; Sonel, N. Thermal and geochemical investigation of Seyitomer oil shale. Thermochim. Acta 2001, 371, 111–119. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Han, X.X.; Li, Q.Y.; Huang, Y.R.; Jiang, X.M. TG-DSC Analysis of Pyrolysis Process of two Chinese Oil Shales. J. Therm. Anal. Calorim. 2014, 116, 511–517. [Google Scholar] [CrossRef]



| Unit | Sample | Quartz | Muscovite | Kaolinite | Montmorillonite | Orthoclase | Clinoptylolite | Chlorite | Pyrite | Calcite | Dolomite | Gypsum | Jarosite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skole | Dynów | 43.26 * | 2.92 | 7.55 | - | - | - | - | - | 44.47 | 1.80 | - | - |
| Bircza | 39.69 | 31.72 | 15.65 | 11.10 | - | 1.84 | - | - | - | - | - | - | |
| Leszczawa G. | 62.38 | 23.41 | - | - | - | - | 10.37 | - | - | - | - | 3.84 | |
| Wojtkowa | 48.12 | 30.67 | 17.28 | - | - | - | - | 3.93 | - | - | - | - | |
| Bandrów | 23.74 | 12.66 | - | - | - | - | 8.17 | 2.95 | 52.48 | - | - | - | |
| Silesian | Wernejówka | 80.54 | 11.61 | 5.79 | - | - | - | - | 2.06 | - | - | - | - |
| Łubne | 90.46 | 5.94 | - | - | - | - | 0.92 | 0.97 | 1.10 | - | - | 0.61 | |
| Dukla | Zyndranowa | 68.33 | 23.83 | 7.84 | - | - | - | - | - | - | - | - | - |
| Czystogarb | 68.22 | 18.49 | 11.24 | - | 2.05 | - | - | - | - | - | - | - | |
| Komańcza | 64.78 | 20.15 | 10.12 | - | - | - | - | 2.67 | - | - | 2.28 | - |
| Vitrinite | Inertinite | Liptinite | Other Components | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Sample | Collotelinite | Vitrodetrinite | Fusinite | Semifusinite | Inertodetrinite | Alginite | Bituminite | Liptodetrinite | Zooclasts | Solid Bitumens | Mineral Matter | Vitrinite Reflectance |
| Skole | Dynów | trace | trace | n.o. | n.o. | n.o. | 3.57 | trace | 2.48 | trace | n.o. | 93.94 | n.m. |
| Bircza | 0.97 | trace | trace | trace | trace | 1.46 | 13.92 | 0.65 | n.o. | n.o. | 83.01 | 0.41 | |
| Leszczawa G. | 5.67 | 0.17 | trace | trace | n.o. | 1.67 | 27.17 | 0.67 | 0.17 | n.o. | 64.5 | 0.27 | |
| Wojtkowa | 0.17 | 0.33 | trace | trace | n.o. | 2.99 | 20.27 | 1.00 | trace | n.o. | 75.25 | 0.33 | |
| Bandrów | 0.63 | 0.16 | trace | trace | trace | 0.32 | 11.36 | 0.32 | trace | n.o. | 87.22 | 0.38 | |
| Silesian | Wernejówka | trace | trace | n.o. | n.o. | trace | 1.10 | 0.78 | 0.47 | trace | n.o. | 97.65 | n.m. |
| Łubne | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | 21.05 | trace | 1.81 | 0.99 | 76.15 | n.m. | |
| Dukla | Zyndranowa | trace | 0.20 | n.o. | n.o. | n.o. | 2.57 | n.o. | 1.38 | n.o. | n.o. | 95.85 | 0.28 |
| Czystogarb | trace | 0.39 | n.o. | n.o. | trace | 4.43 | 1.93 | 1.73 | n.o. | n.o. | 91.52 | 0.28 | |
| Komańcza | trace | trace | trace | n.o. | n.o. | 3.31 | 4.57 | 0.94 | n.o. | n.o. | 91.18 | 0.28 | |
| Unit | Sample | Tmax | S1 | S2 | S3 | PI | PC | RC | TOC | HI | OI | MINC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skole | Dynów | 425 | 0.10 | 4.59 | 1.25 | 0.02 | 0.45 | 0.83 | 1.28 | 359 | 98 | 3.33 |
| Bircza | 415 | 0.35 | 26.42 | 3.50 | 0.01 | 2.44 | 5.94 | 8.38 | 315 | 42 | 0.22 | |
| Leszczawa | 408 | 1.45 | 26.47 | 2.09 | 0.05 | 2.50 | 6.09 | 8.59 | 308 | 24 | 0.25 | |
| Wojtkowa | 408 | 0.24 | 11.56 | 1.50 | 0.02 | 1.10 | 5.38 | 6.48 | 178 | 23 | 0.21 | |
| Bandrów | 414 | 0.26 | 20.92 | 0.97 | 0.01 | 1.84 | 3.34 | 5.18 | 404 | 19 | 5.16 | |
| Silesian | Wernejówka | 438 | 0.15 | 7.63 | 0.20 | 0.02 | 0.67 | 1.59 | 2.26 | 338 | 9 | 0.10 |
| Łubne | 459 | 0.11 | 4.15 | 0.62 | 0.03 | 0.39 | 3.59 | 3.98 | 104 | 16 | 0.12 | |
| Dukla | Zyndranowa | 431 | 0.50 | 10.62 | 1.75 | 0.05 | 1.02 | 2.44 | 3.46 | 307 | 51 | 0.11 |
| Czystogarb | 432 | 0.25 | 12.51 | 1.45 | 0.02 | 1.15 | 2.16 | 3.31 | 378 | 44 | 0.16 | |
| Komańcza | 427 | 0.24 | 6.71 | 1.44 | 0.03 | 0.66 | 1.75 | 2.41 | 278 | 60 | 0.16 |
| Temperature Range | 40–300 °C | 300–650 °C (TTG) | 650–1050 °C | ||
|---|---|---|---|---|---|
| Process | Dehydration | Kerogen pyrolysis | Combustion of residual organic matter (650–775 °C) | Carbonate decomposition (775–850 °C) | Decomposition of the remaining components (850–1050 °C) |
| Sample | Weight loss (%) | Weight loss (%) (TTG) [°C] | Weight loss (%) | ||
| Dynów | 2.38 | 3.70 (TTG n.i.) | 5.72 | 4.17 | 0.37 |
| Bircza | 5.21 | 10.58 (445.6) | 7.87 | - | 0.56 |
| Leszczawa | 5.66 | 11.55 (432.6) | 12.15 | - | - |
| Wojtkowa | 5.24 | 6.80 (441.3, 505.0) | 4.04 | 1.77 | 1.02 |
| Bandrów | 2.77 | 4.48 (519.7) | 6.90 | 5.73 | 0.65 |
| Wernejówka | 1.23 | 3.47 (461.9) | 1.98 | 0.17 | 0.35 |
| Łubne | 0.11 | 2.80 (503.4) | 1.75 | 0.23 | 0.59 |
| Zyndranowa | 3.08 | 4.60 (463.2) | 2.38 | 0.13 | 0.22 |
| Czystogarb | 2.05 | 4.54 (454.0) | 2.34 | 0.11 | 0.30 |
| Komańcza | 1.86 | 5.23 (458.5, 521.6) | 3.00 | 0.45 | 0.95 |
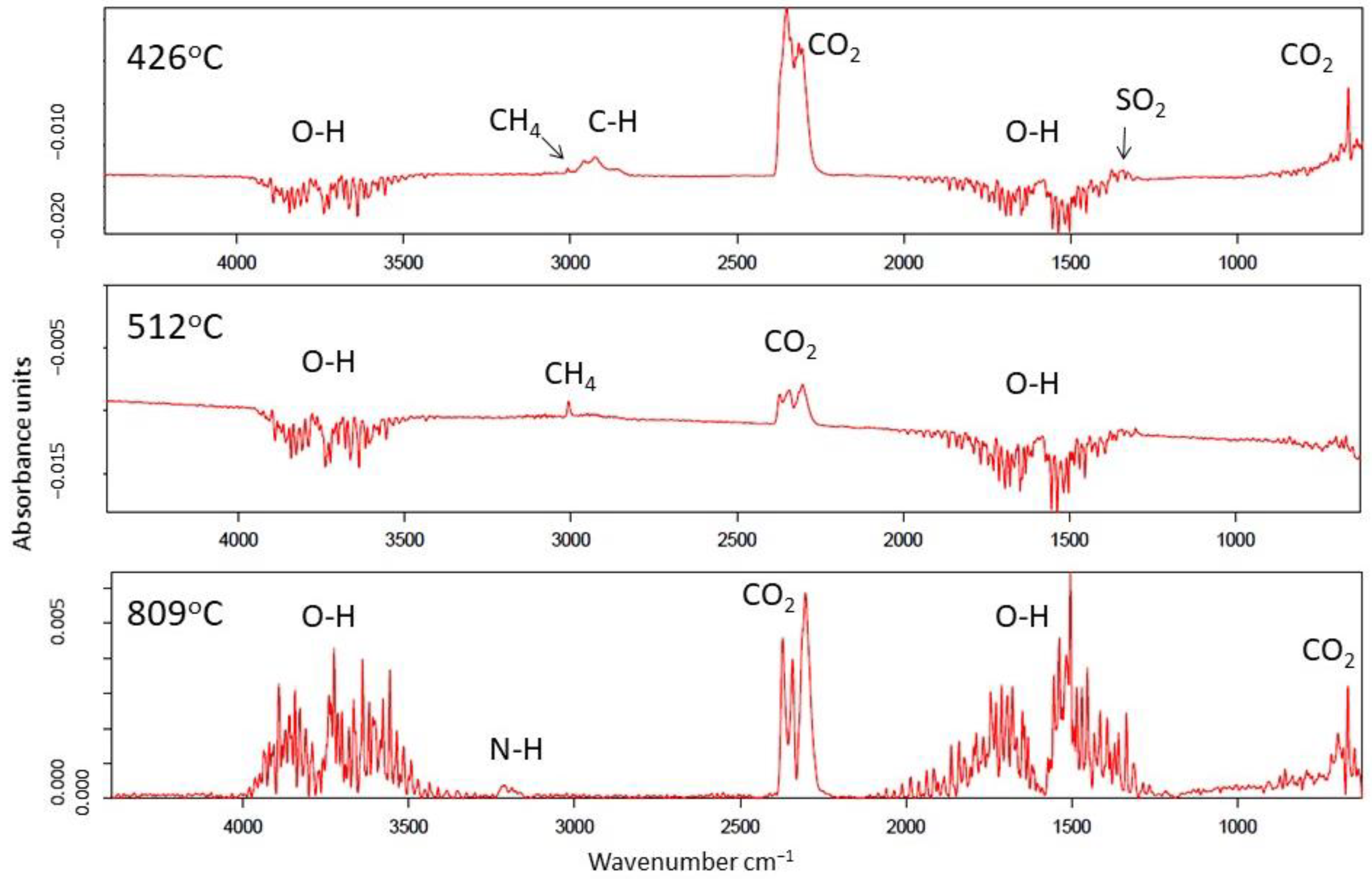
| Temperature Range | 300–500 °C | 500–650 °C |
|---|---|---|
| Sample | Functional Groups | Functional Groups |
| Dynów | C-H SO2 | - |
| Bircza | C-H CH4 SO2 | CH4 |
| Leszczawa | C-H CH4 SO2 | CH4 SO2 |
| Wojtkowa | C-H CH4 SO2 | CH4 SO2 |
| Bandrów | C-H CH4 | CH4 SO2 |
| Wernejówka | C-H CH4 SO2 | - |
| Łubne | C=C SO2 | C=C CH4 SO2 |
| Zyndranowa | C-H CH4 | - |
| Czystogarb | C-H CH4 SO2 | N-H C-H CH4 SO2 |
| Komańcza | C-H CH4 | CH4 SO2 |
| Unit | Sample | Ea | Log A (Log(1/s)) | r2 | |
|---|---|---|---|---|---|
| (kJ/mol) | (kcal/mol) | ||||
| Skole | Dynów | 246 | 59 | 17 | 1.0000 |
| Bircza | 233 | 56 | 15 | 0.9830 | |
| Leszczawa | 109 | 26 | 24 | 0.9892 | |
| Wojtkowa | 134 | 32 | 10 | 0.9998 | |
| Bandrów | 325 | 78 | 10 | 0.9951 | |
| Silesian | Wernejówka | 263 | 63 | 6 | 0.9942 |
| Łubne | 214 | 51 | 19 | 0.9948 | |
| Dukla | Zyndranowa | 125 | 30 | 6 | 1.0000 |
| Czystogarb | 207 | 50 | 18 | 1.0000 | |
| Komańcza | 231 | 55 | 18 | 0.9917 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labus, M.; Matyasik, I.; Ziemianin, K. Thermal Decomposition Processes in Relation to the Type of Organic Matter, Mineral and Maceral Composition of Menilite Shales. Energies 2023, 16, 4500. https://doi.org/10.3390/en16114500
Labus M, Matyasik I, Ziemianin K. Thermal Decomposition Processes in Relation to the Type of Organic Matter, Mineral and Maceral Composition of Menilite Shales. Energies. 2023; 16(11):4500. https://doi.org/10.3390/en16114500
Chicago/Turabian StyleLabus, Małgorzata, Irena Matyasik, and Konrad Ziemianin. 2023. "Thermal Decomposition Processes in Relation to the Type of Organic Matter, Mineral and Maceral Composition of Menilite Shales" Energies 16, no. 11: 4500. https://doi.org/10.3390/en16114500
APA StyleLabus, M., Matyasik, I., & Ziemianin, K. (2023). Thermal Decomposition Processes in Relation to the Type of Organic Matter, Mineral and Maceral Composition of Menilite Shales. Energies, 16(11), 4500. https://doi.org/10.3390/en16114500