Investigation to Enhance Solid Fuel Quality in Torrefaction of Cow Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Solid Fuel Preparation
2.3. Characterization of Solid Fuel
3. Results
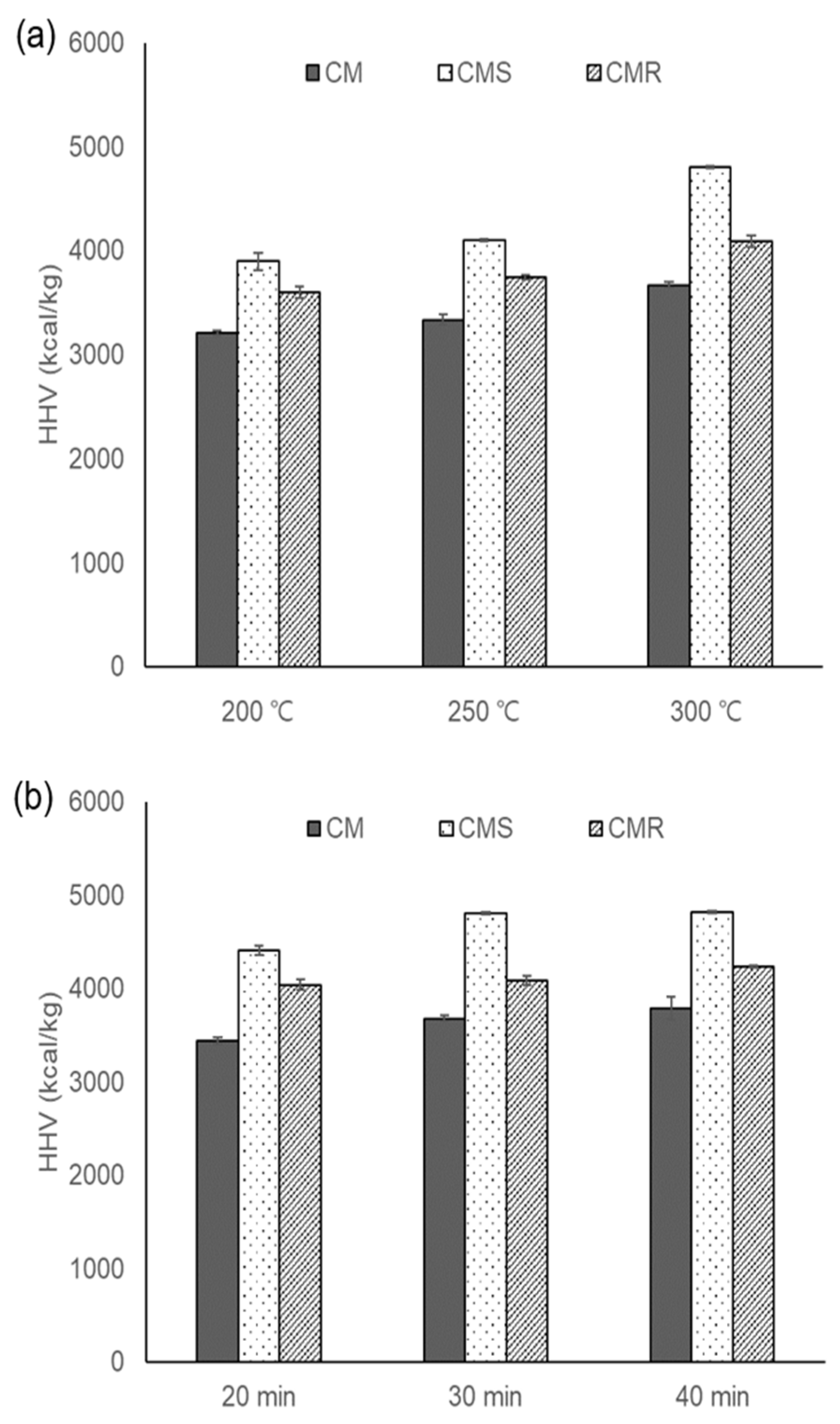
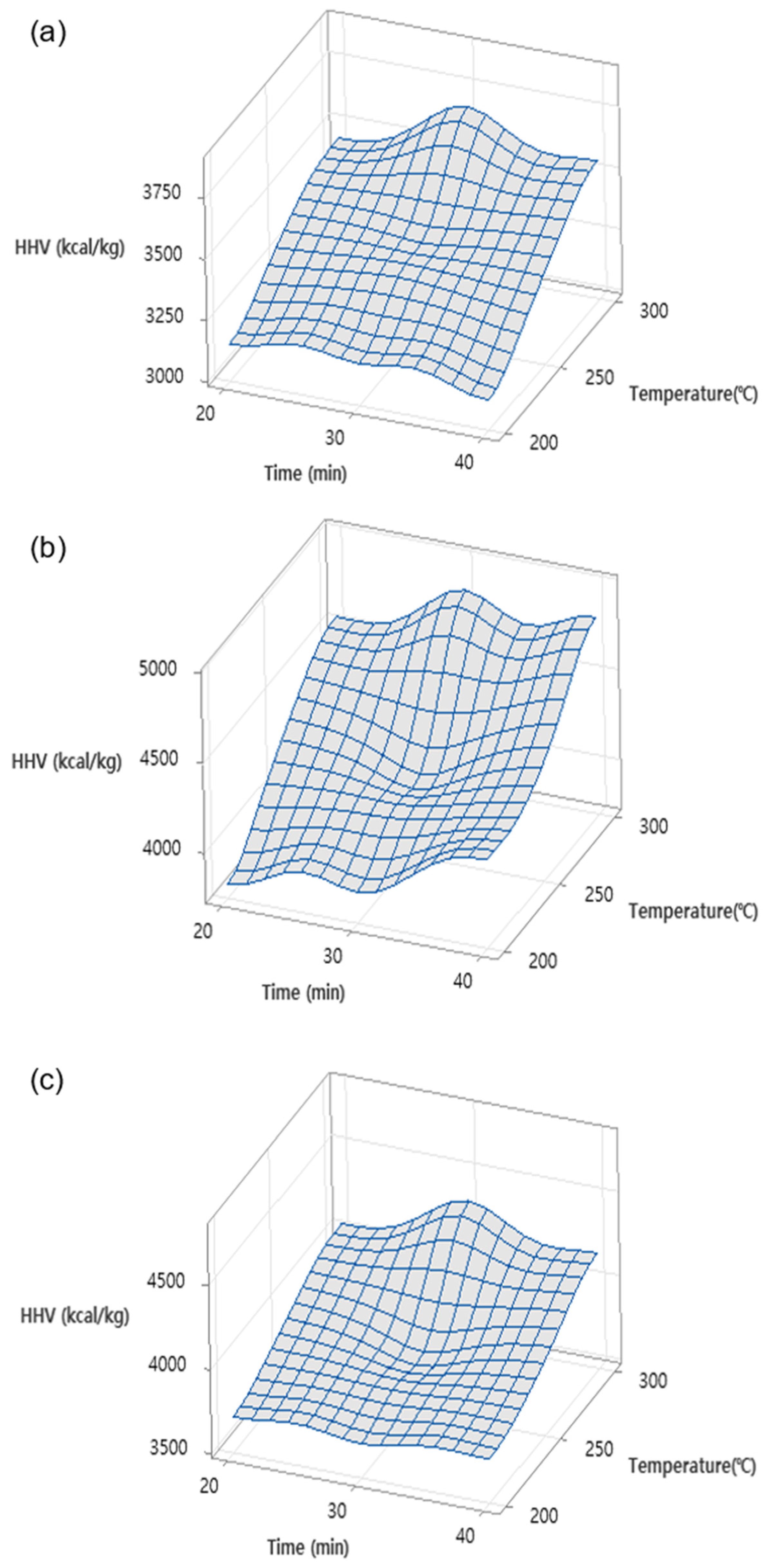
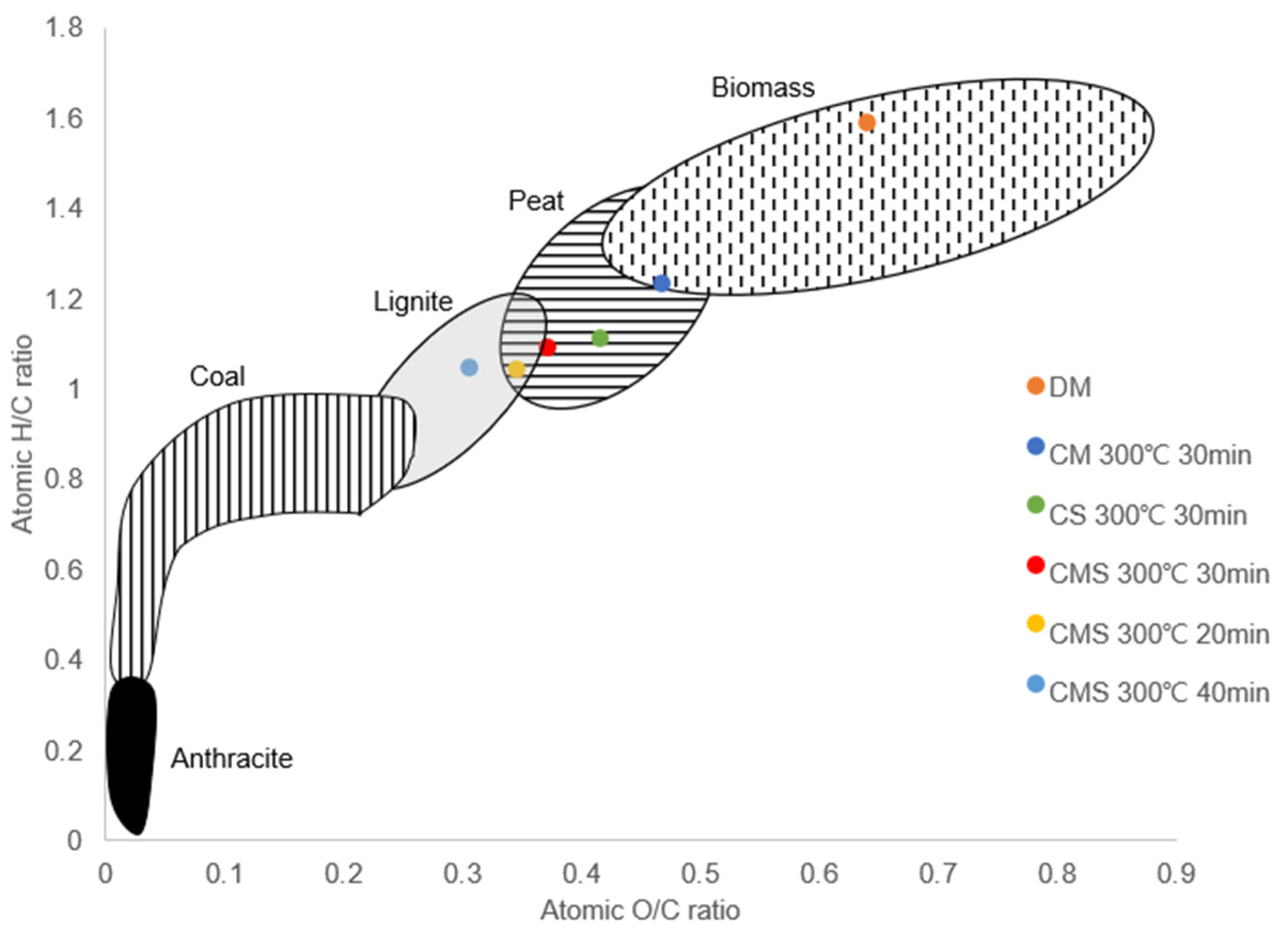
3.1. Effects of Torrefaction Temperature and Time
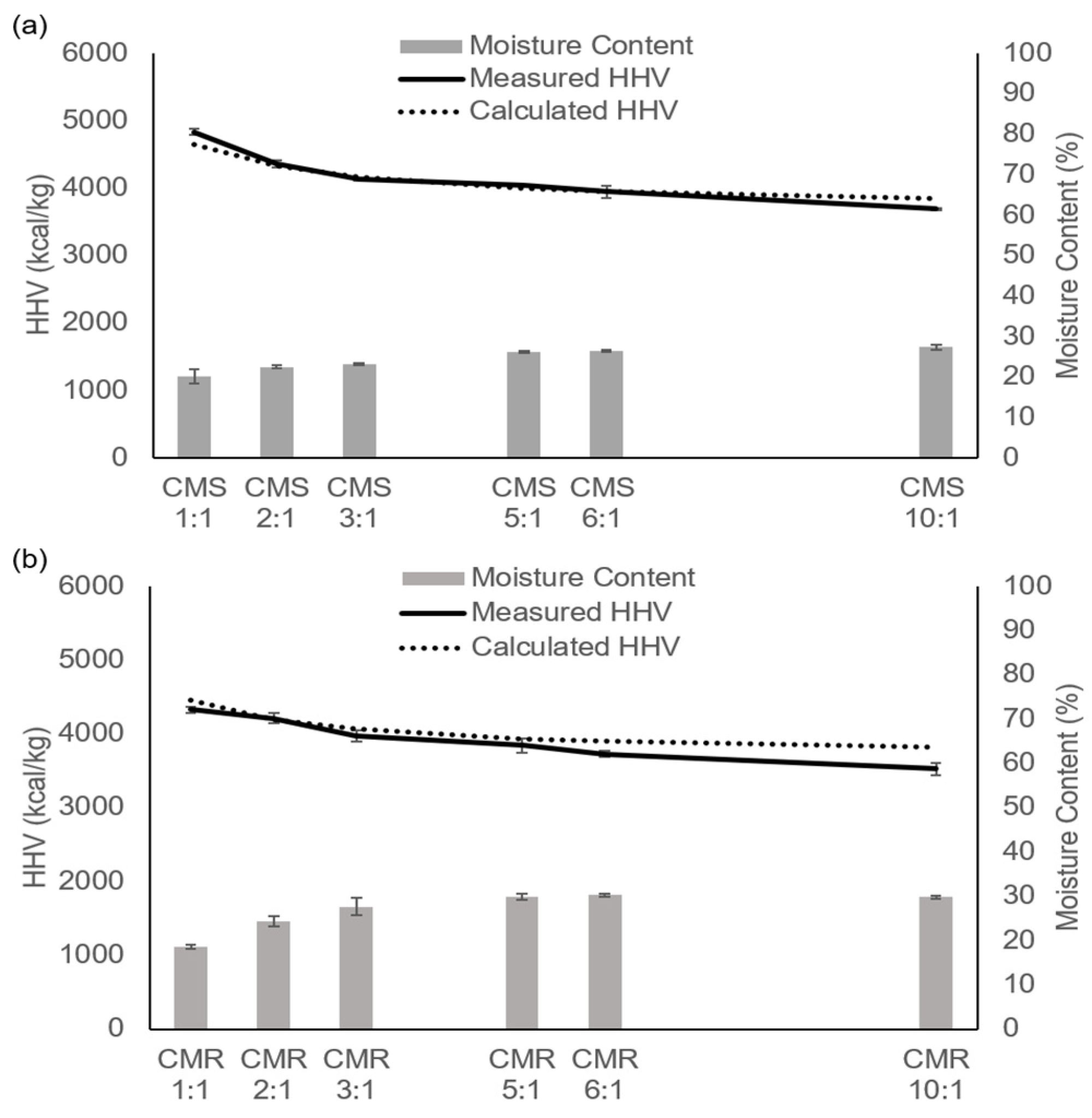
3.2. Effects of Mixing Ratio of Cow Manure with Sawdust or Rice Straw
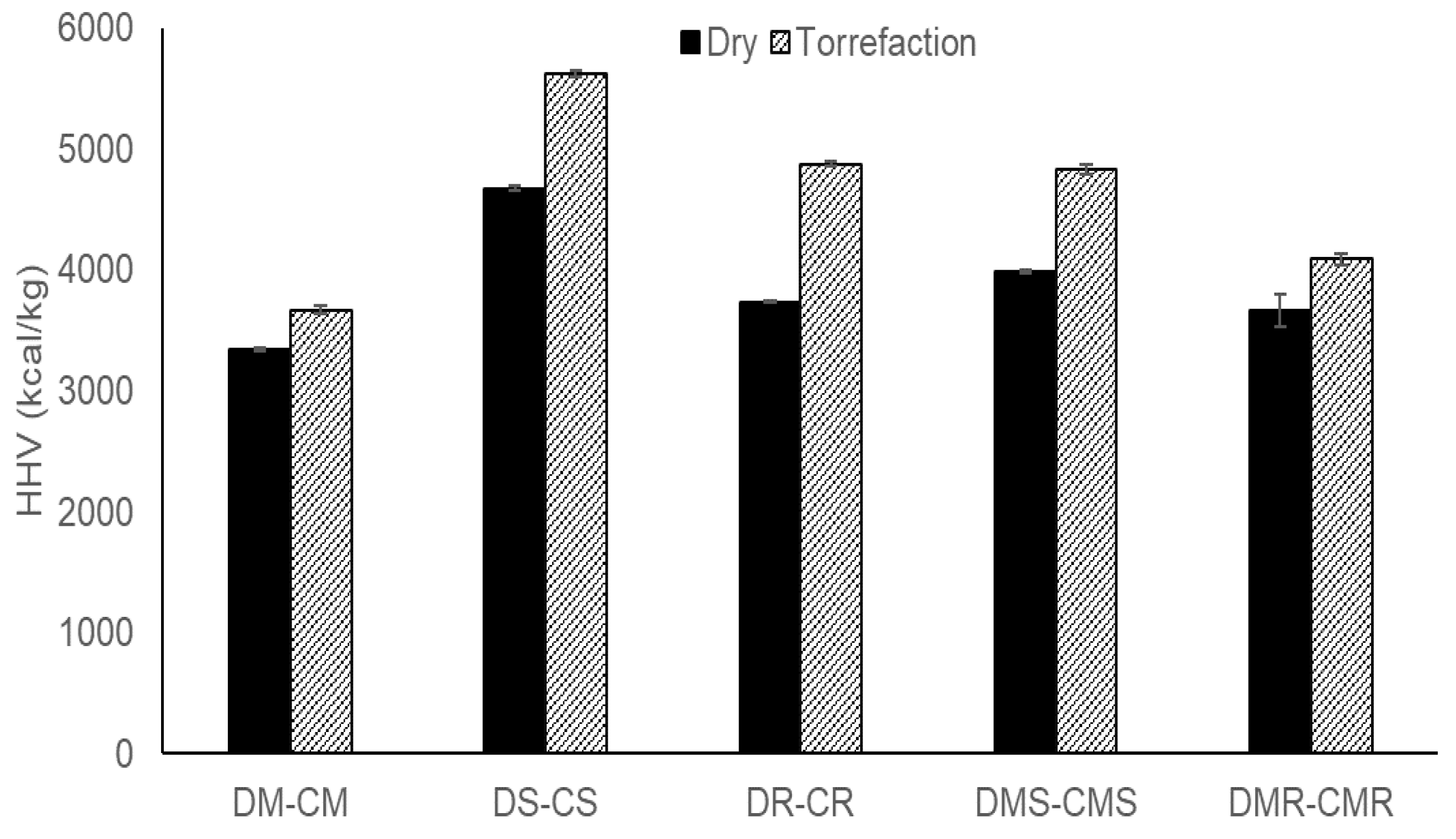
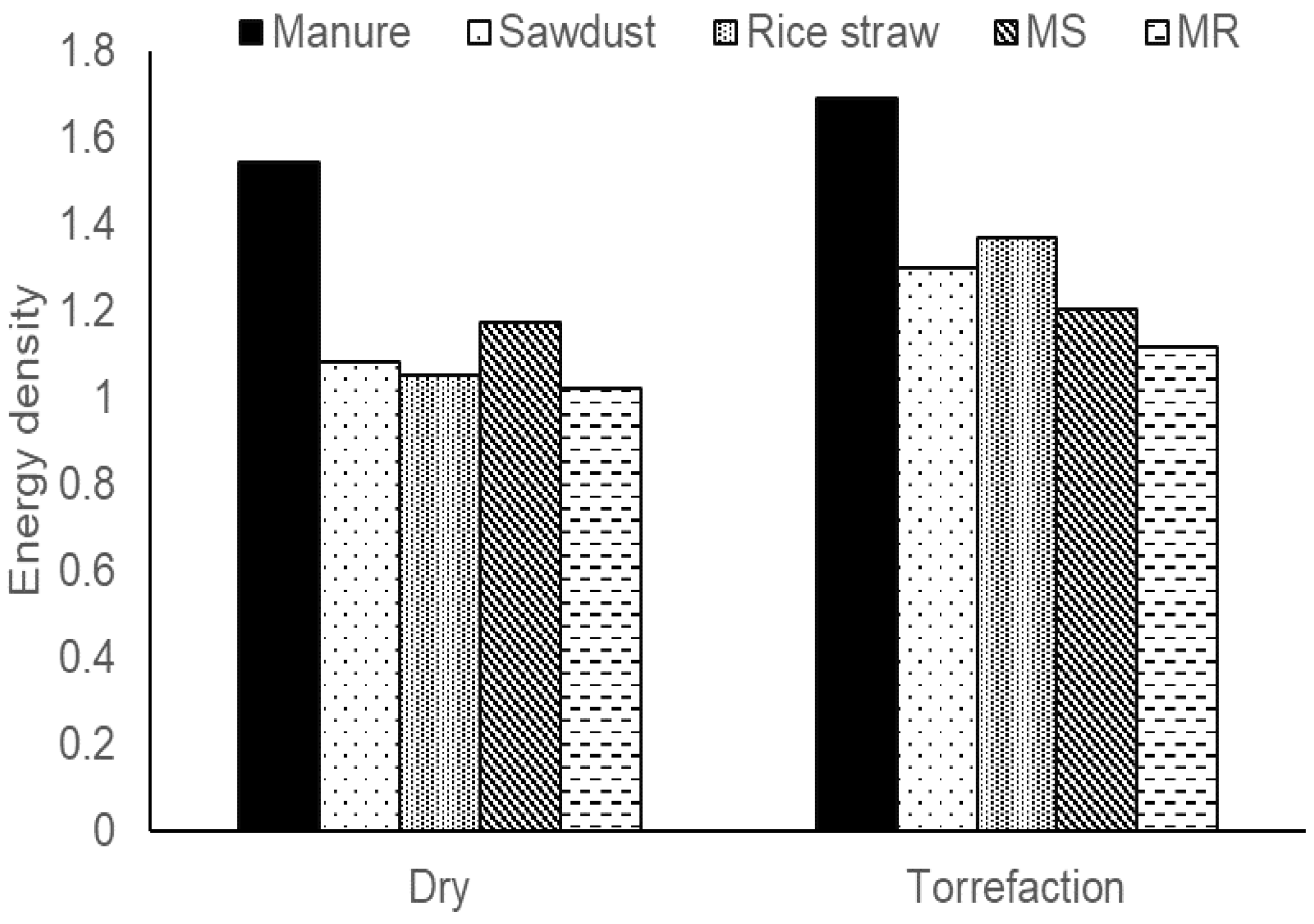
3.3. Comparison of Simple Drying and Torrefaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iwuozor, K.O.; Emenike, E.C.; Aniagor, C.O.; Iwuchukwu, F.U.; Ibitogbe, E.M.; Okikiola, T.B.; Omuku, P.E.; Adeniyi, A.G. Removal of pollutants from aqueous media using cow dung-based adsorbents. Curr. Res. Green Sustain. Chem. 2022, 5, 100300. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Thangaraj, R.; Ravindran, B.; Woong, S.; Karmegam, N. Centrality of cattle solid wastes in vermicomposting technology e A cleaner resource recovery and biowaste recycling option for agricultural and environmental sustainability. Environ. Pollut. 2021, 268, 115688. [Google Scholar] [CrossRef]
- Ministry of Agriculture, food and Rural Affairs. Survey on Livestock Manure. 2023. Available online: https://www.korea.kr/briefing/pressReleaseView.do?newsId=156559757 (accessed on 31 May 2023).
- Liu, W.; Zeng, D.; She, L.; Su, W.; He, D.; Wu, G.; Ma, X.; Jiang, S.; Jiang, C.; Ying, G. Science of the Total Environment Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef] [PubMed]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. [Google Scholar] [CrossRef]
- Oshita, K.; Toda, S.; Takaoka, M.; Kanda, H.; Fujimori, T.; Matsukawa, K.; Fujiwara, T. Solid fuel production from cattle manure by dewatering using liquefied dimethyl ether. Fuel 2015, 159, 7–14. [Google Scholar] [CrossRef]
- Leclerc, A.; Laurent, A. Framework for estimating toxic releases from the application of manure on agricultural soil: National release inventories for heavy metals in 2000–2014. Sci. Total Environ. 2017, 590–591, 452–460. [Google Scholar] [CrossRef]
- Reinelt, T.; Delre, A.; Westerkamp, T.; Holmgren, M.A.; Liebetrau, J.; Scheutz, C. Comparative use of different emission measurement approaches to determine methane emissions from a biogas plant. Waste Manag. 2017, 68, 173–185. [Google Scholar] [CrossRef]
- Batzias, F.A.; Sidiras, D.K.; Spyrou, E.K. Evaluating livestock manures for biogas production: A GIS based method. Renew. Energy 2005, 30, 1161–1176. [Google Scholar] [CrossRef]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Ali, M.M.; Ndongo, M.; Bilal, B.; Yetilmezsoy, K.; Youm, I.; Bahramian, M. Mapping of biogas production potential from livestock manures and slaughterhouse waste: A case study for African countries. J. Clean. Prod. 2020, 256, 120499. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Lim, B.R.; Lee, K.; Noh, S.Y.; Park, K.Y. Characteristics of Microalgal Growth on Anaerobic Effluent of Animal Waste. J. Korean Soc. Water Qual. 2008, 24, 306–310. [Google Scholar]
- Bagher, A.M. Advantages and disadvantages of biogas energy. Bull. Adv. Sci. Res. 2015, 1, 132–135. [Google Scholar]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers. Manag. 2014, 78, 815–821. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Xu, Z.X.; Song, H.; Li, P.J.; He, Z.X.; Wang, Q.; Wang, K.; Duan, P.G. Hydrothermal carbonization of sewage sludge: Effect of aqueous phase recycling. Chem. Eng. J. 2020, 387, 123410. [Google Scholar] [CrossRef]
- Papanikola, K.; Papadopoulou, K.; Tsiliyannis, C.; Fotinopoulou, I.; Katsiampoulas, A.; Chalarakis, E.; Georgiopoulou, M.; Rontogianni, V.; Michalopoulos, I.; Mathioudakis, D.; et al. Food residue biomass product as an alternative fuel for the cement industry. Environ. Sci. Pollut. Res. 2019, 26, 35555–35564. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Li, L.; Hu, Z.F.; Guo, P.; Jiang, Y. The catalytic pyrolysis of food waste by microwave heating. Bioresour. Technol. 2014, 166, 45–50. [Google Scholar] [CrossRef]
- Nepal, R.; Kim, H.J.; Poudel, J.; Oh, S.C. A study on torrefaction of spent coffee ground to improve its fuel properties. Fuel 2022, 318, 123643. [Google Scholar] [CrossRef]
- Kang, S.B.; Oh, H.Y.; Kim, J.J.; Choi, K.S. Characteristics of spent coffee ground as a fuel and combustion test in a small boiler (6.5 kW). Renew. Energy 2017, 113, 1208–1214. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Bae, D.; Park, K.Y. Characterizations of biochar from hydrothermal carbonization of exhausted coffee residue. J. Mater. Cycles Waste Manag. 2017, 19, 1036–1043. [Google Scholar] [CrossRef]
- Soroush, S.; Ronsse, F.; Verberckmoes, A.; Verpoort, F.; Park, J.; Wu, D.; Heynderickx, P.M. Production of solid hydrochar from waste seaweed by hydrothermal carbonization: Effect of process variables. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Jang, D.H. Moon Surface Analysis and Heavy Metal Adsorption Evaluation of Chemically Modified Biochar Derived from Starfish (Asterina pectinifera). J. Korean Soc. Water Environ. 2022, 38, 82–94. [Google Scholar]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Garba, M.U.; Moritiwon Oloruntoba, J.; Isah, A.G.; Alhassan, M. Production of Solid Fuel From Rice Straw Through Torrefaction Process. Int. J. Sci. Eng. Investig. 2014, 4, 37. [Google Scholar]
- Chen, X.; Lin, Q.; He, R.; Zhao, X.; Li, G. Hydrochar production from watermelon peel by hydrothermal carbonization. Bioresour. Technol. 2017, 241, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, E.; Lee, J.; Lee, Y.-G.; Chon, K. Comparative Evaluation of Methylene Blue and Humic Acids Removal Efficiency Using Rice Husk Derived Biochars and Powdered Activated Carbon. J. Korean Soc. Water Environ. 2021, 37, 483–492. [Google Scholar]
- Dai, L.; Tan, F.; Wu, B.; He, M.; Wang, W.; Tang, X.; Hu, Q.; Zhang, M. Immobilization of phosphorus in cow manure during hydrothermal carbonization. J. Environ. Manag. 2015, 157, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Toufiq Reza, M.; Freitas, A.; Yang, X.; Hiibel, S.; Lin, H.; Coronella, C.J. Coronella Hydrothermal Carbonization (HTC) of Cow Manure: Carbon and Nitrogen Distributions in HTC Products. Am. Inst. Chem. Eng. 2016, 35, 1002–1011. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.W.; Skrivan, W.; Lotfi, S. Identification of Optimal Binders for Torrefied Biomass Pellets. Energies 2023, 16, 3390. [Google Scholar] [CrossRef]
- Waheed, A.; Naqvi, S.R.; Ali, I. Co-Torrefaction Progress of Biomass Residue/Waste Obtained for High-Value Bio-Solid Products. Energies 2022, 15, 8297. [Google Scholar] [CrossRef]

| Cow Manure | Sawdust | Rice Straw | |
|---|---|---|---|
| Moisture content (%) | 31.09 ± 2.19 | 9.47 ± 0.82 | 7.84 ± 0.09 |
| Volatile solids (%) | 23.62 ± 3.29 | 6.56 ± 2.04 | 23.99 ± 3.17 |
| Fixed solids (%) | 40.78 ± 1.65 | 83.96 ± 0.67 | 68.11 ± 3.78 |
| HHV 1 (kcal/kg) | 2168.65 ± 18.2 | 4322.78 ± 29.18 | 3562.54 ± 62.58 |
| Sample | Temp. (°C) | Time (min) | Mass Yield (%) | Moisture (%) | Ash (%) | Volatile (%) | Fixed Carbon (%) |
|---|---|---|---|---|---|---|---|
| CM | 200 | 30 | 64.04 | 1.78 ± 1.21 | 28.69 ± 0.11 | 69.22 ± 3.00 | 0.31 |
| 250 | 30 | 64.47 | 1.8 ± 0.76 | 28.65 ± 0.31 | 69.42 ± 0.95 | 0.12 | |
| 300 | 30 | 52.30 | 1.39 ± 0.41 | 39.21 ± 0.82 | 52.83 ± 1.60 | 6.56 | |
| 300 | 20 | 56.10 | 1.99 ± 0.12 | 36.22 ± 0.11 | 58.22 ± 2.76 | 3.57 | |
| 300 | 40 | 46.43 | 1.36 ± 0.99 | 42.71 ± 0.14 | 44.10 ± 0.92 | 11.83 | |
| CMS (1:1) | 200 | 30 | 79.95 | 2.1 ± 0.3 | 11.12 ± 0.49 | 79.28 ± 2.27 | 7.51 |
| 250 | 30 | 76.62 | 1.47 ± 0.53 | 11.73 ± 0.49 | 71.80 ± 1.19 | 15.00 | |
| 300 | 30 | 52.80 | 2.02 ± 0.23 | 17.87 ± 0.11 | 56.14 ± 1.89 | 23.97 | |
| 300 | 20 | 55.46 | 2.34 ± 0.24 | 16.80 ± 0.08 | 65.78 ± 0.92 | 15.09 | |
| 300 | 40 | 51.52 | 2.23 ± 0.97 | 21.55 ± 0.26 | 47.29 ± 0.15 | 28.93 | |
| CMR (1:1) | 200 | 30 | 76.69 | 1.34 ± 0.25 | 20.45 ± 0.39 | 73.66 ± 0.08 | 4.55 |
| 250 | 30 | 74.52 | 1.9 ± 0.23 | 20.51 ± 0.001 | 71.29 ± 0.08 | 6.31 | |
| 300 | 30 | 50.33 | 1.18 ± 0.23 | 31.51 ± 0.69 | 52.30 ± 1.07 | 15.01 | |
| 300 | 20 | 49.21 | 1.08 ± 0.12 | 29.86 ± 0.33 | 59.61 ± 0.35 | 9.45 | |
| 300 | 40 | 48.99 | 1.04 ± 0.07 | 30.13 ± 0.67 | 49.98 ± 1.88 | 18.85 |
| Mixing Ratio | 1:1 | 2:1 | 3:1 | 5:1 | 6:1 | 10:1 |
|---|---|---|---|---|---|---|
| CMS | 52.8 ± 9.01% | 53.98 ± 1.94% | 50.68 ± 1.17% | 51.06 ± 0.58% | 52.69 ± 0.55% | 51.67 ± 0.60% |
| CMR | 50.58 ± 4.68% | 49.93 ± 2.17% | 52.66 ± 0.58% | 52.44 ± 1.45% | 51.47 ± 0.39% | 49.83 ± 0.66% |
| Mixing Ratio | 1:1 | 2:1 | 5:1 | 10:1 |
|---|---|---|---|---|
| Moisture | 2.02 ± 0.23 | 0.85 ± 0.04 | 1.95 ± 0.34 | 1.39 ± 0.61 |
| Ash | 17.87 ± 0.11 | 26.74 ± 0.22 | 32.10 ± 0.85 | 36.10 ± 0.68 |
| Volatile | 56.14 ± 1.89 | 56.12 ± 3.89 | 53.10 ± 2.35 | 53.10 ± 1.29 |
| Fixed carbon | 23.97 | 16.29 | 12.06 | 9.50 |
| Mixing Ratio | 1:1 | 2:1 | 5:1 | 10:1 |
|---|---|---|---|---|
| Moisture | 1.29 ± 0.15 | 1.90 ± 0.62 | 1.27 ± 0.71 | 1.39 ± 0.36 |
| Ash | 27.78 ± 0.54 | 32.27 ± 0.31 | 35.99 ± 0.65 | 38.96 ± 0.47 |
| Volatile | 57.17 ± 0.15 | 55.06 ± 0.48 | 53.34 ± 0.11 | 51.64 ± 0.78 |
| Fixed carbon | 13.77 | 10.77 | 9.40 | 8.01 |
| Sample | Mass Yield (%) | Moisture (%) | Ash (%) | Volatile (%) | Fixed Carbon (%) |
|---|---|---|---|---|---|
| DM | 69.17 | 3.71 ± 0.34 | 28.57 ± 0.40 | 67.43 ± 0.76 | 0.47 |
| CM | 52.3 | 1.39 ± 0.41 | 39.21 ± 0.82 | 52.83 ± 1.60 | 6.56 |
| DS | 84.11 | 1.25 ± 0.1 | 0.82 ± 0.03 | 88.29 ± 0.28 | 9.65 |
| CS | 62.11 | 2.00 ± 0.28 | 0.98 ± 0.002 | 82.91 ± 0.11 | 14.11 |
| DR | 79.48 | 3.16 ± 0.43 | 10.79 ± 1.75 | 85.78 ± 0.45 | 0.27 |
| CR | 54.34 | 2.83 ± 0.42 | 20.49 ± 0.15 | 59.04 ± 0.42 | 17.64 |
| DMS | 82.13 | 2.23 ± 0.12 | 13.34 ± 0.11 | 76.56 ± 0.25 | 7.86 |
| CMS | 52.8 | 2.02 ± 0.23 | 17.87 ± 0.11 | 56.14 ± 1.89 | 23.07 |
| DMR | 81.08 | 2.39 ± 0.06 | 20.05 ± 0.03 | 73.22 ± 0.19 | 4.34 |
| CMR | 49.06 | 0.91 ± 0.14 | 29.71 ± 0.22 | 50.39 ± 0.64 | 18.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Chae, C.; Kim, H.; Kwon, H.; Kim, J.; Kim, I. Investigation to Enhance Solid Fuel Quality in Torrefaction of Cow Manure. Energies 2023, 16, 4505. https://doi.org/10.3390/en16114505
Hong J, Chae C, Kim H, Kwon H, Kim J, Kim I. Investigation to Enhance Solid Fuel Quality in Torrefaction of Cow Manure. Energies. 2023; 16(11):4505. https://doi.org/10.3390/en16114505
Chicago/Turabian StyleHong, Jiseok, Changwon Chae, Hyunjoong Kim, Hyeokjun Kwon, Jisu Kim, and Ijung Kim. 2023. "Investigation to Enhance Solid Fuel Quality in Torrefaction of Cow Manure" Energies 16, no. 11: 4505. https://doi.org/10.3390/en16114505
APA StyleHong, J., Chae, C., Kim, H., Kwon, H., Kim, J., & Kim, I. (2023). Investigation to Enhance Solid Fuel Quality in Torrefaction of Cow Manure. Energies, 16(11), 4505. https://doi.org/10.3390/en16114505






