Transport Phenomena in a Banded Solid Oxide Fuel Cell Stack—Part 1: Model and Validation
Abstract
1. Introduction
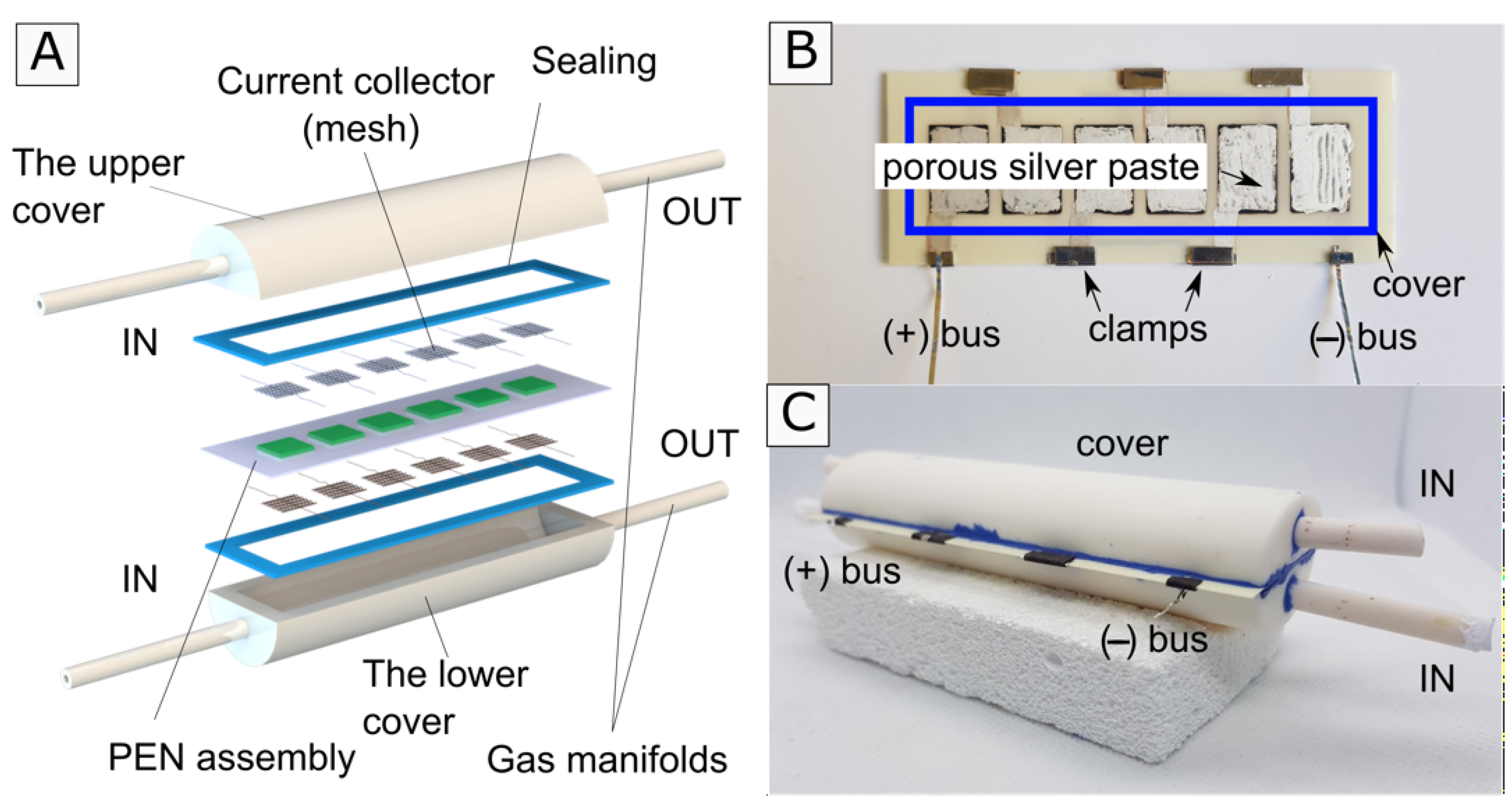
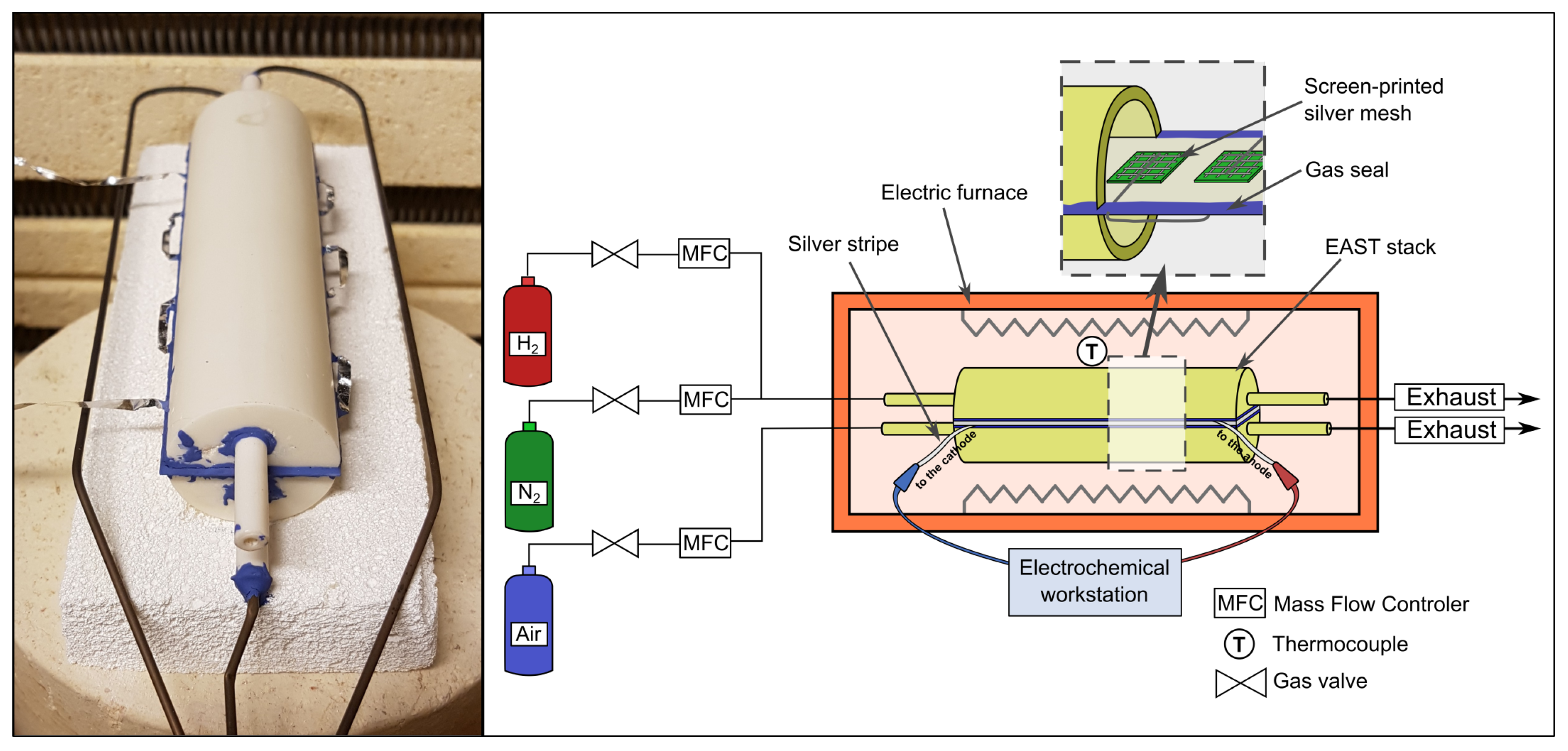
2. Experimental Analysis
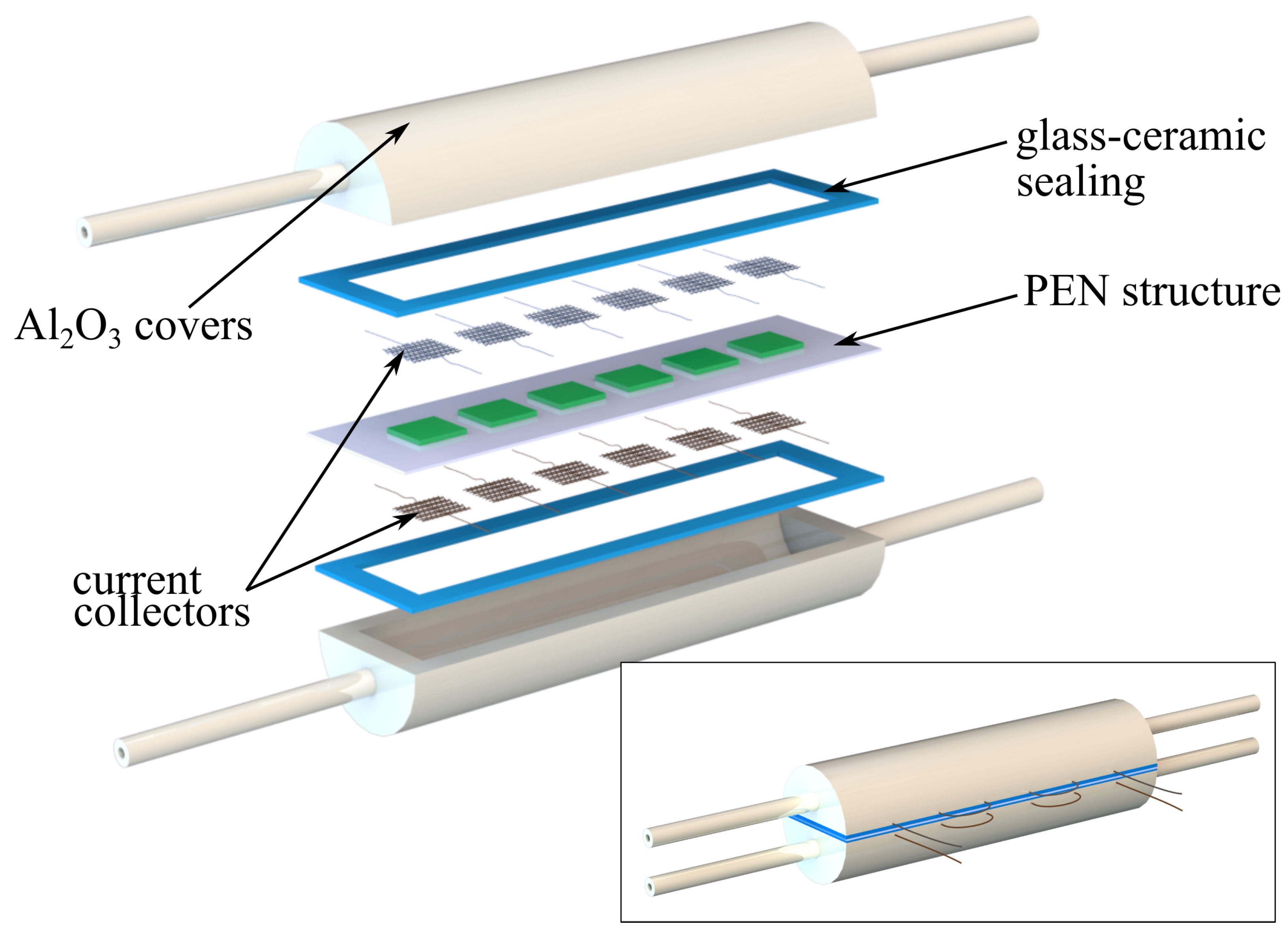
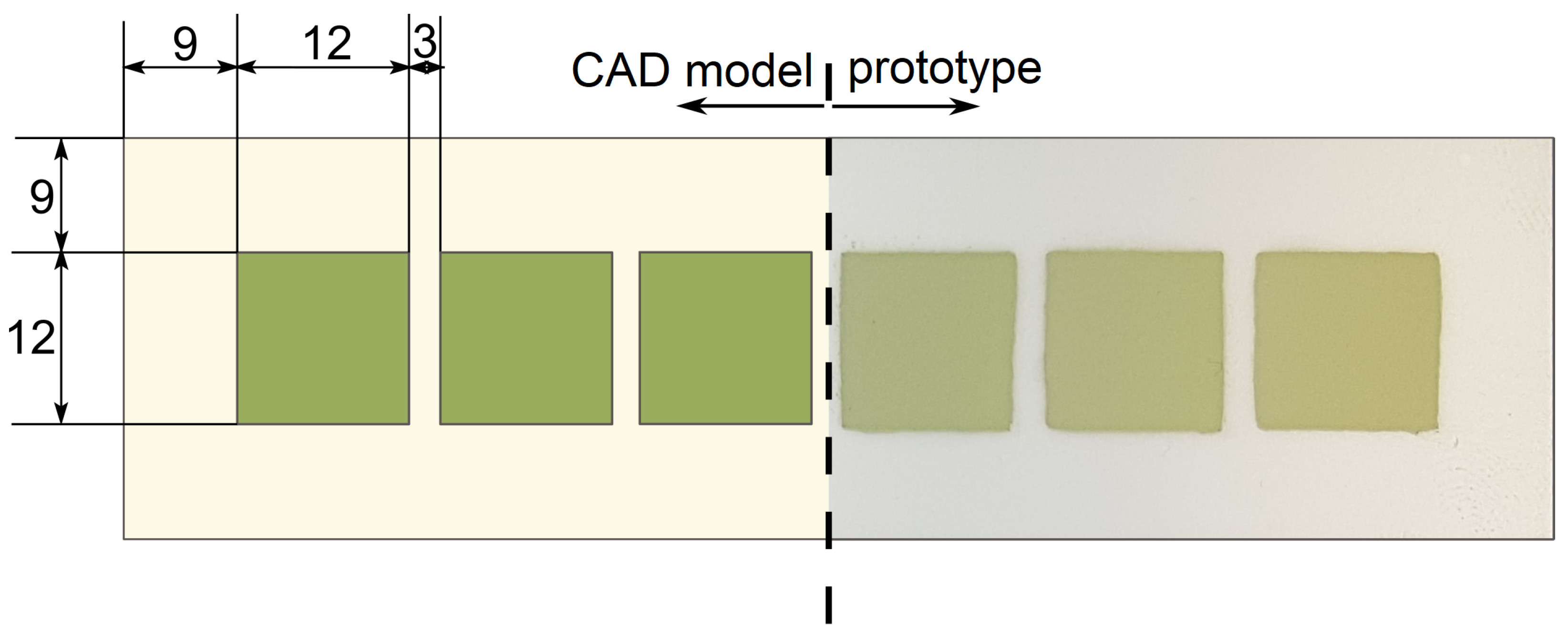
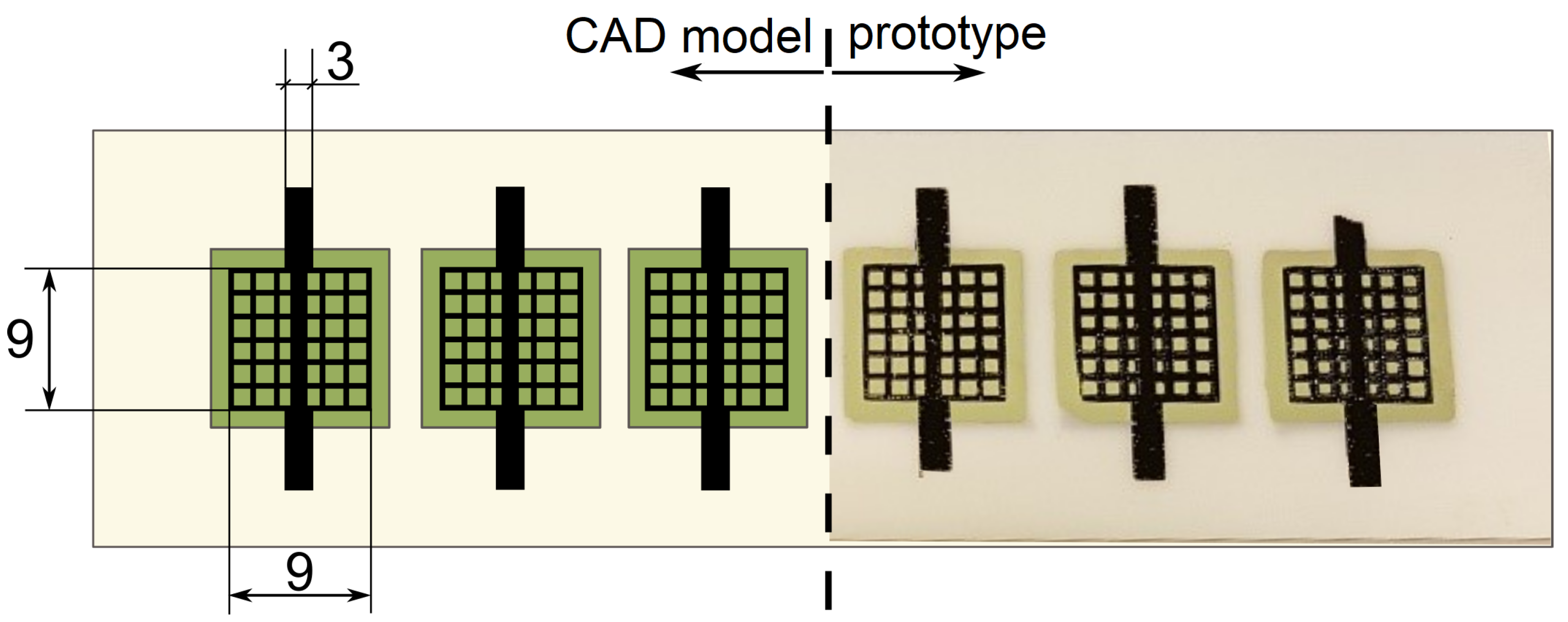
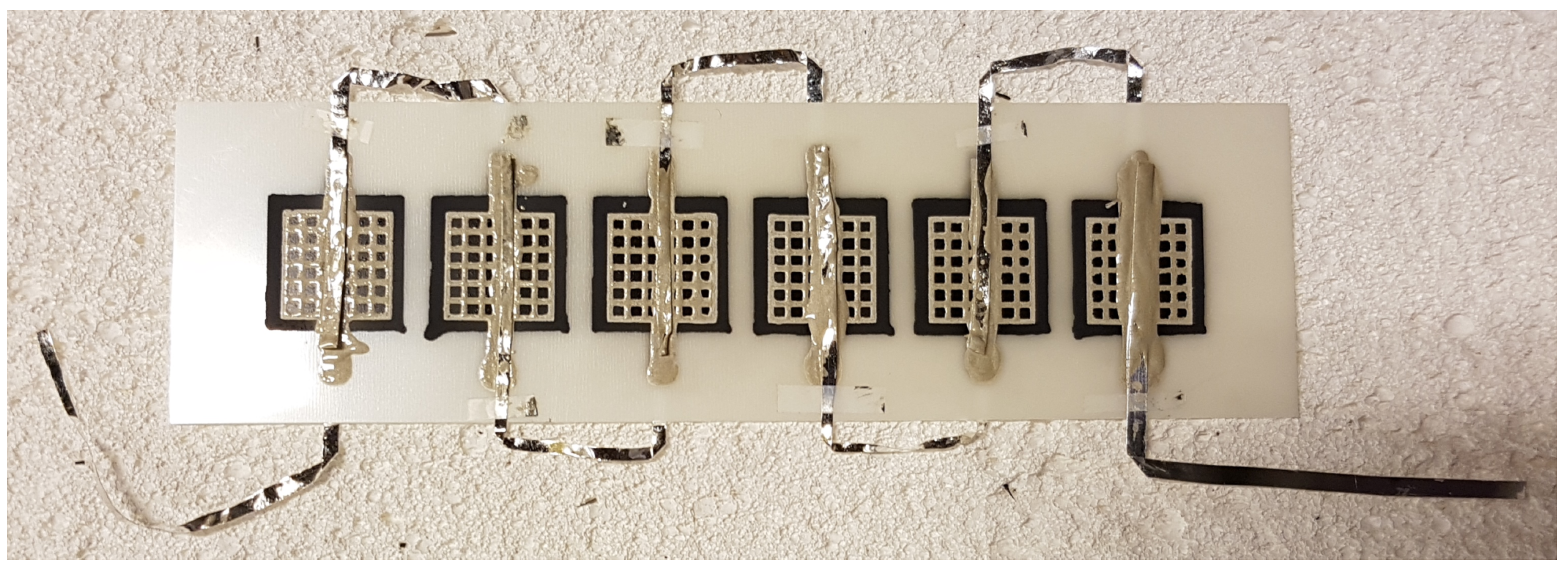
2.1. Stack Preparation
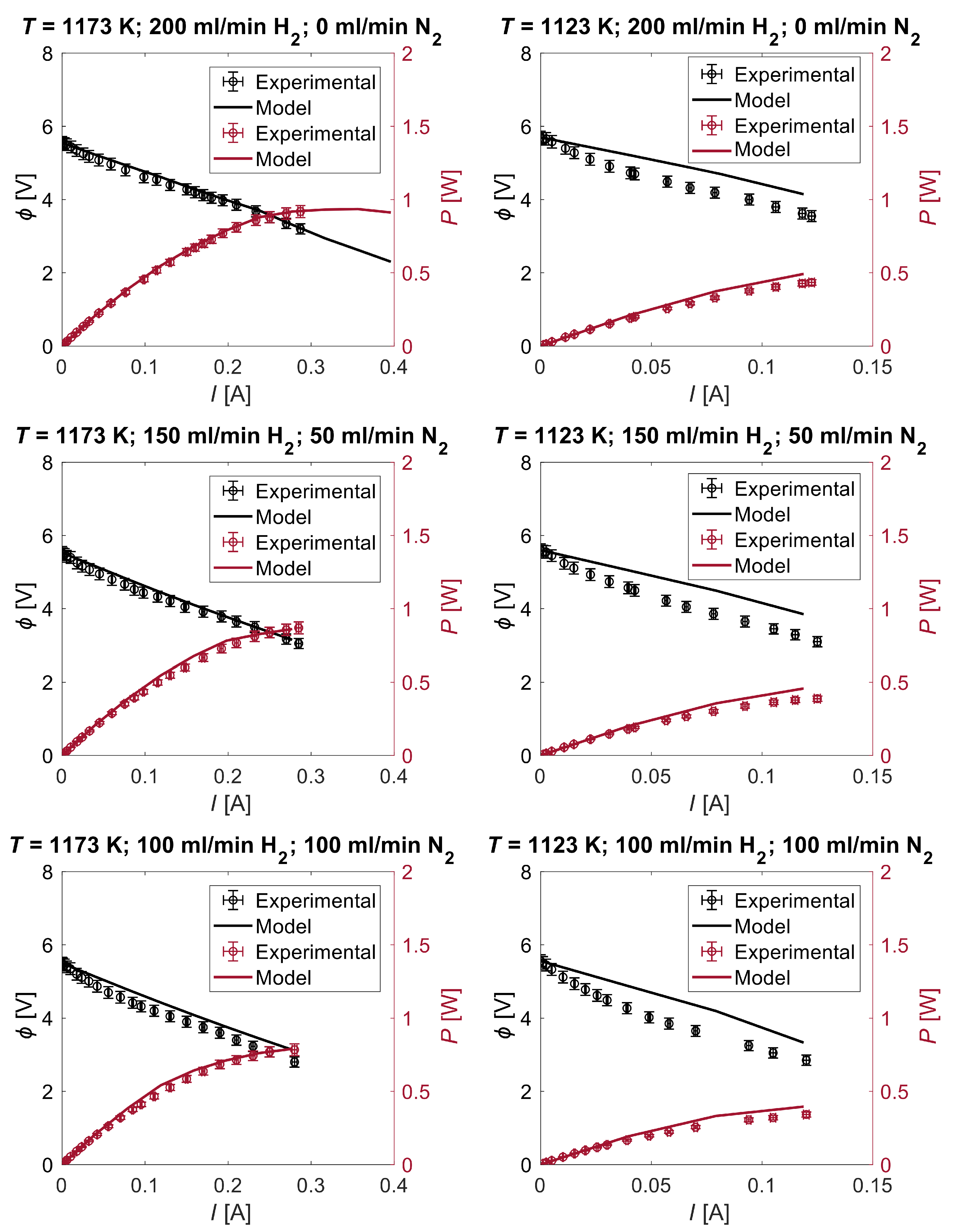
2.2. Electrochemical Testing
3. Mathematical Modeling of Transport Phenomena
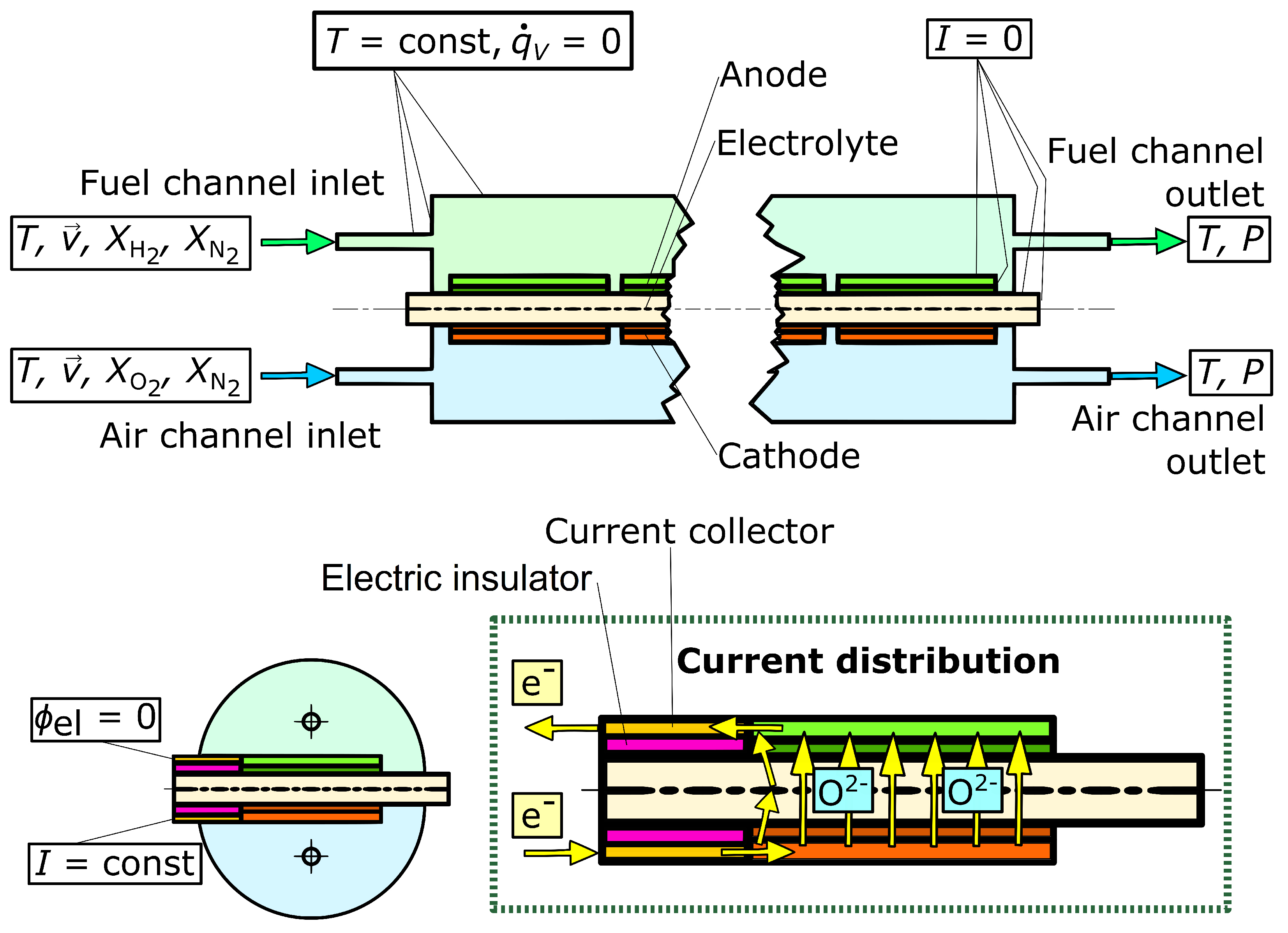
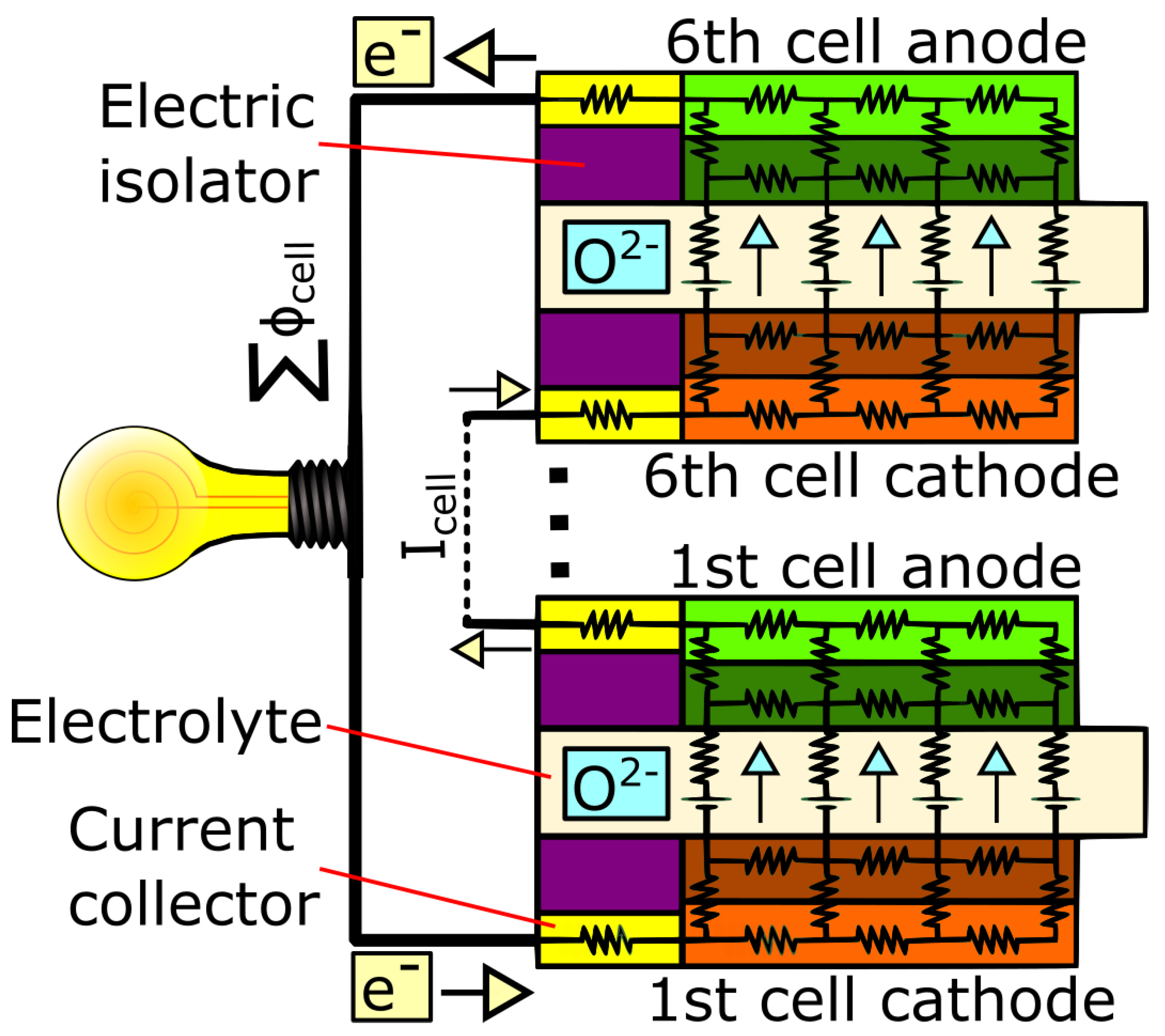
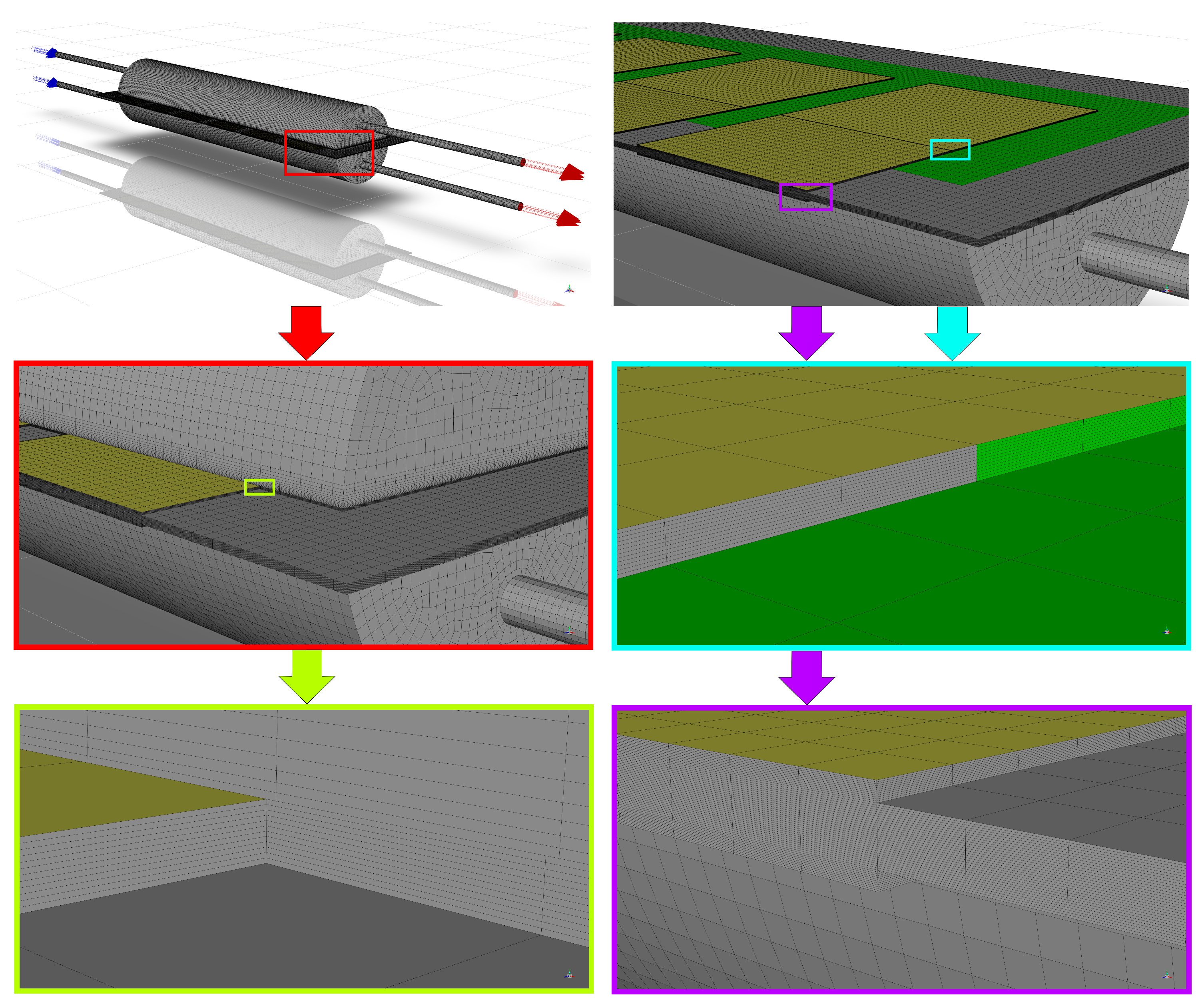
3.1. Computational Domain and Modeling Assumptions
3.2. Mathematical Description of Fluid Zones
3.3. Mathematical Description of Solid and Porous Zones
3.4. Boundary Conditions
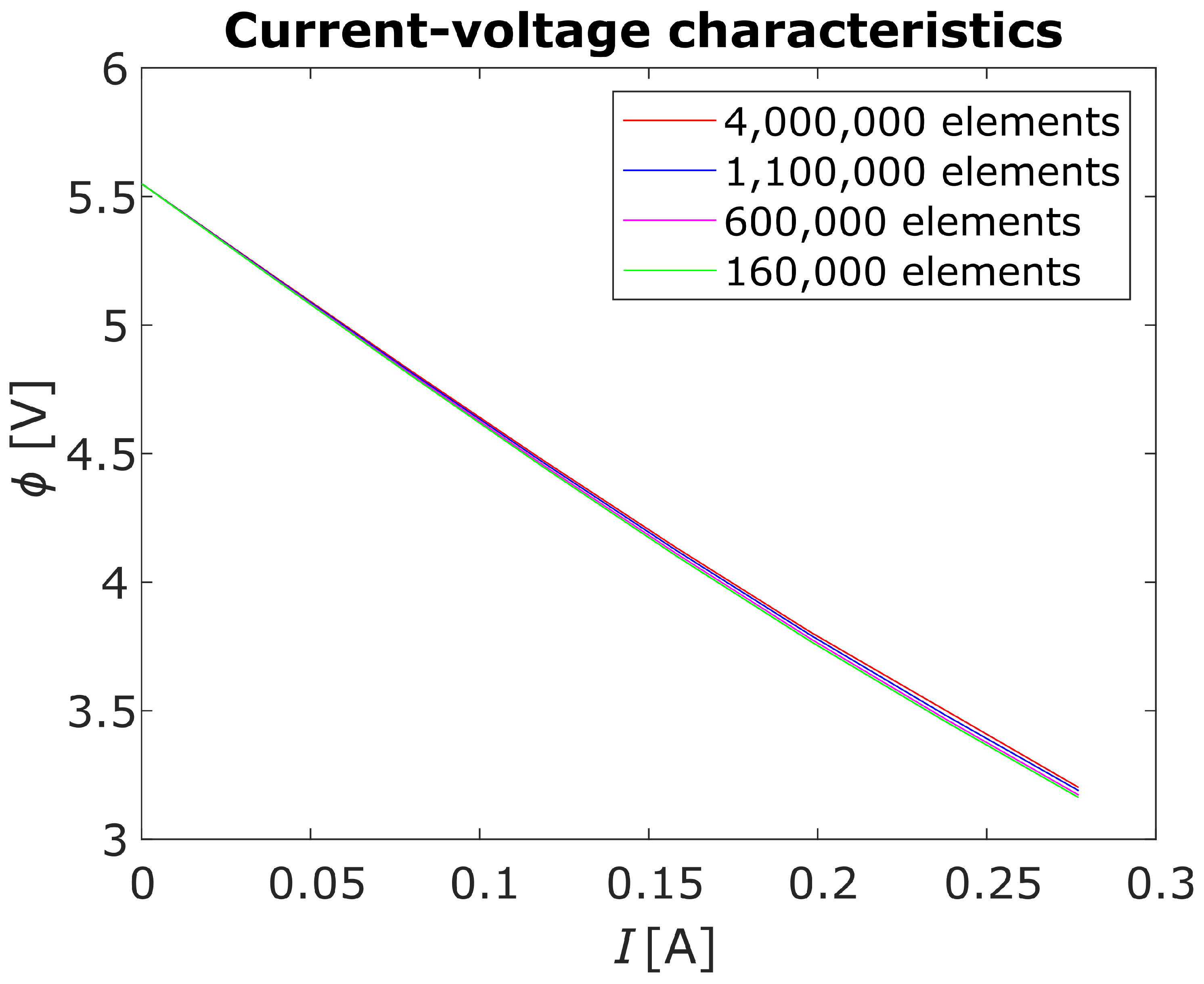
4. Numerical Model
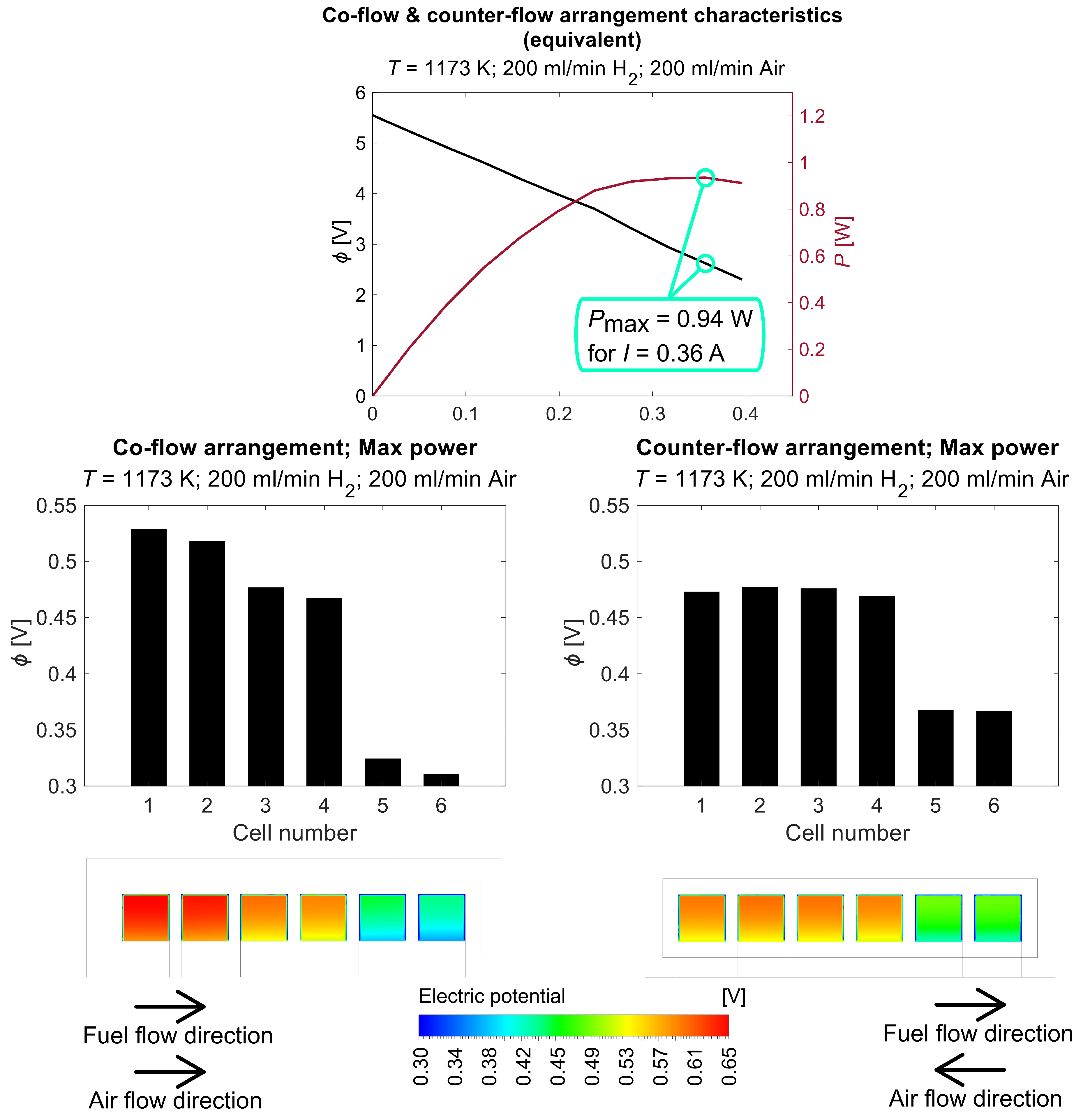
5. Numerical Analysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| C | internal momentum resistance factor | (-) |
| mass diffusion coefficient | () | |
| thermal diffusion coefficient | () | |
| Faraday constant | ( ) | |
| h | specific enthalpy | () |
| enthalpy change | () | |
| I | current | () |
| anode equilibrium exchange current | () | |
| cathode equilibrium exchange current | () | |
| i | current density flux | () |
| j | volumetric transfer current density | () |
| diffusion flux | () | |
| k | thermal conductivity | () |
| M | molar mass | () |
| p | static pressure | () |
| P | power | () |
| volumetric flux of species | () | |
| universal gas constant | ( ) | |
| ohmic resistance | () | |
| source/sink term of momentum | () | |
| source/sink term of mass | () | |
| source/sink term of species rates | () | |
| source/sink term of heat | () | |
| T | temperature | () |
| velocity vector | () | |
| local species concentration | (kmol ) | |
| species mass fraction | (-) | |
| Greek letters | ||
| transfer coefficient | (-) | |
| permeability | () | |
| concentration dependence | (-) | |
| porosity rate | (-) | |
| triple phase boundary length density | () | |
| double phase boundary length density | () | |
| local surface overpotential | () | |
| dynamic viscosity | () | |
| chemical potential of species i | () | |
| density | () | |
| conductivity | () | |
| stress tensor | () | |
| electric potential | () | |
| actual potential of an appropriate phase | () | |
| Sub- and superscripts | ||
| anodic | ||
| anode | ||
| cathodic | ||
| cathode | ||
| double phase boundary | ||
| effective value | ||
| electronic | ||
| equilibrium | ||
| i | reaction component | |
| ionic | ||
| reaction | ||
| reference value | ||
| solid | ||
| thermal | ||
| triple phase boundary | ||
| Abbreviations | ||
| 3D | three-dimensional | |
| CAD | computer aided design | |
| CFD | computational fluid dynamics | |
| OCV | open circuit voltage | |
| SOFC | solid oxide fuel cell | |
References
- Buchaniec, S.; Sciazko, A.; Mozdzierz, M.; Brus, G. A Novel Approach to the Optimization of a Solid Oxide Fuel Cell Anode Using Evolutionary Algorithms. IEEE Access 2019, 7, 34361–34372. [Google Scholar] [CrossRef]
- Prokop, T.A.; Berent, K.; Iwai, H.; Szmyd, J.S. A Three-Dimensional Numerical Assessment of Heterogeneity Impact on a Solid Oxide Fuel Cell’s Anode Performance. Catalysts 2018, 8, 503. [Google Scholar] [CrossRef]
- Koncewicz, W.; Moździerz, M.; Brus, G. A fast Gaussian process-based method to evaluate carbon deposition during hydrocarbons reforming. Int. J. Hydrogen Energy 2023, 48, 11666–11679. [Google Scholar] [CrossRef]
- Mukerjee, S.; Leah, R.; Selby, M.; Stevenson, G.; Brandon, N.P. Chapter 9—Life and Reliability of Solid Oxide Fuel Cell-Based Products: A Review. In Solid Oxide Fuel Cell Lifetime and Reliability; Brandon, N.P., Ruiz-Trejo, E., Boldrin, P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 173–191. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. A review on recent advances and trends in symmetrical electrodes for solid oxide cells. J. Power Sources 2022, 520, 230852. [Google Scholar] [CrossRef]
- Brus, G. High-Temperature Solid Oxide Fuel Cell Stack. Polish Patent PL 234427, 28 February 2020. [Google Scholar]
- Isenberg, A. Energy Conversion via Solid Oxide Electrolyte Electrochemical Cells at High Temperatures. Solid State Ionics 1981, 3–4, 431–437. [Google Scholar] [CrossRef]
- Gardner, F.; Day, M.; Brandon, N.; Pashley, M.; Cassidy, M. SOFC Technology Development at Rolls-Royce. J. Power Sources 2000, 86, 122–129. [Google Scholar] [CrossRef]
- Haberman, B.; Young, J. Three-Dimensional Simulation of Chemically Reacting Gas Flows in the Porous Support Structure of an Integrated-Planar Solid Oxide Fuel Cell. Int. J. Heat Mass Transf. 2004, 47, 3617–3629. [Google Scholar] [CrossRef]
- Koi, M.; Yamashita, S.; Matsuzaki, Y. Development of Segmented-in-series Cell-stacks with Flat-tubular Substrates. ECS Trans. 2007, 7, 235–243. [Google Scholar] [CrossRef]
- Badding, M.; Bouton, W.; Brown, J.; Kester, L.; Pollard, S.; Tanner, C.W.; Tepesch, P. Ultra-Low Mass Planar SOFC Design. ECS Trans. 2011, 35, 465–471. [Google Scholar] [CrossRef]
- Kim, M.W.; Son, M.J.; Park, H.; Park, J.Y.; Lim, H.T. Experimental Investigation of In-plane Performance Variation on Anode Supported Solid Oxide Fuel Cells Using Segmented Cathodes and Reference Electrodes. Fuel Cells 2020, 20, 212–219. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Timurkutluk, C.; Mat, M.D.; Kaplan, Y. A Review on Cell/Stack Designs for High Performance Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2016, 56, 1101–1121. [Google Scholar] [CrossRef]
- Ghorbani, B.; Vijayaraghavan, K. A review study on software-based modeling of hydrogen-fueled solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 13700–13727. [Google Scholar] [CrossRef]
- Ghorbani, B.; Vijayaraghavan, K. 3D and simplified pseudo-2D modeling of single cell of a high temperature solid oxide fuel cell to be used for online control strategies. Int. J. Hydrogen Energy 2018, 43, 9733–9748. [Google Scholar] [CrossRef]
- Tikiz, I.; Taymaz, I.; Pehlivan, H. CFD modelling and experimental validation of cell performance in a 3-D planar SOFC. Int. J. Hydrogen Energy 2019, 44, 15441–15455. [Google Scholar] [CrossRef]
- Dong, S.K.; Jung, W.N.; Rashid, K.; Kashimoto, A. Design and numerical analysis of a planar anode-supported SOFC stack. Renew. Energy 2016, 94, 637–650. [Google Scholar] [CrossRef]
- Pirasaci, T. Non-uniform, multi-stack solid oxide fuel cell (SOFC) system design for small system size and high efficiency. J. Power Sources 2019, 426, 135–142. [Google Scholar] [CrossRef]
- Pianko-oprych, P.; Zinko, T. Computational fluid dynamics calculation of a planar solid oxide fuel cell design running on syngas. Chem. Process. Eng. 2017, 38, 513–521. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Yue, D.; Yang, G.; Ye, S.; He, C.; Wang, W.; Yuan, J.; Huang, N.; Equipment, T. Three-dimensional CFD modeling of transport phenomena in a cross-flow anode-supported planar SOFC. Energies 2014, 7, 80–98. [Google Scholar] [CrossRef]
- Wei, S.S.; Wang, T.H.; Wu, J.S. Numerical modeling of interconnect flow channel design and thermal stress analysis of a planar anode-supported solid oxide fuel cell stack. Energy 2014, 69, 553–561. [Google Scholar] [CrossRef]
- Pianko-oprych, P.; Zinko, T. Simulation of the steady-state behaviour of a new design of a single planar Solid Oxide Fuel Cell. Pol. J. Chem. Technol. 2016, 1, 64–71. [Google Scholar] [CrossRef]
- Li, A.; Song, C.; Lin, Z. A multiphysics fully coupled modeling tool for the design and operation analysis of planar solid oxide fuel cell stacks. Appl. Energy 2017, 190, 1234–1244. [Google Scholar] [CrossRef]
- Chelmehsara, M.E.; Mahmoudimehr, J. Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrogen Energy 2018, 43, 15521–15530. [Google Scholar] [CrossRef]
- Beale, S.B.; Choi, H.w.; Pharoah, J.G.; Roth, H.K.; Jasak, H.; Hyup, D. Open-source computational model of a solid oxide fuel cell. Comput. Phys. Commun. 2016, 200, 15–26. [Google Scholar] [CrossRef]
- Nishida, R.T.; Beale, S.B.; Pharoah, J.G.; Haart, L.G.J.D.; Blum, L. Three-dimensional computational fluid dynamics modelling and experimental validation of the Jülich Mark-F solid oxide fuel cell stack. J. Power Sources 2018, 373, 203–210. [Google Scholar] [CrossRef]
- Andersson, M.; Paradis, H.; Yuan, J.; Sundén, B. Modeling Analysis of Different Renewable Fuels in an Anode Supported SOFC. J. Fuel Cell Sci. Technol. 2011, 8, 031013. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Berent, K.; Kimijima, S.; Szmyd, J.S.; Brus, G. A Multiscale Approach to the Numerical Simulation of the Solid Oxide Fuel Cell. Catalysts 2019, 9, 253. [Google Scholar] [CrossRef]
- Chalusiak, M.; Wróbel, M.; Moździerz, M.; Berent, K.; Szmyd, J.; Kimijima, S.; Brus, G. A numerical analysis of unsteady transport phenomena in a Direct Internal Reforming Solid Oxide Fuel Cell. Int. J. Heat Mass Transf. 2019, 131, 1032–1051. [Google Scholar] [CrossRef]
- Costamagna, P.; Selimovic, A.; Del Borghi, M.; Agnew, G. Electrochemical Model of the Integrated Planar Solid Oxide Fuel Cell (IP-SOFC). Chem. Eng. J. 2004, 102, 61–69. [Google Scholar] [CrossRef]
- Son, B.W.; Park, S.J.; Lee, S.B.; Lim, T.H.; Song, R.H.; Lee, J.W. A Tubular Segmented-in-Series Solid Oxide Fuel Cell with Metallic Interconnect Films: A Performance Study through Mathematical Simulations. Curr. Appl. Phys. 2013, 13, 1906–1913. [Google Scholar] [CrossRef]
- Cui, D.; Ji, Y.; Chang, C.; Wang, Z.; Xiao, X.; Li, Y. Influence of structure size on voltage uniformity of flat tubular segmented-in-series solid oxide fuel cell. J. Power Sources 2020, 460, 228092. [Google Scholar] [CrossRef]
- Ahmed, S.; McPheeters, C.; Kumar, R. Thermal-hydraulic model of a monolithic solid oxide fuel cell. J. Electrochem. Soc. 1991, 138, 2712–2718. [Google Scholar] [CrossRef]
- Li, X. Principles of Fuel Cells; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; Series in Computational Methods in Mechanics and Thermal Sciences, Hemisphere; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar] [CrossRef]
- Le, A.; Beale, S.; Pharoah, J. Validation of a Solid Oxide Fuel Cell Model on the International Energy Agency Benchmark Case with Hydrogen Fuel. Fuel Cells 2014, 15, 27–41. [Google Scholar] [CrossRef]


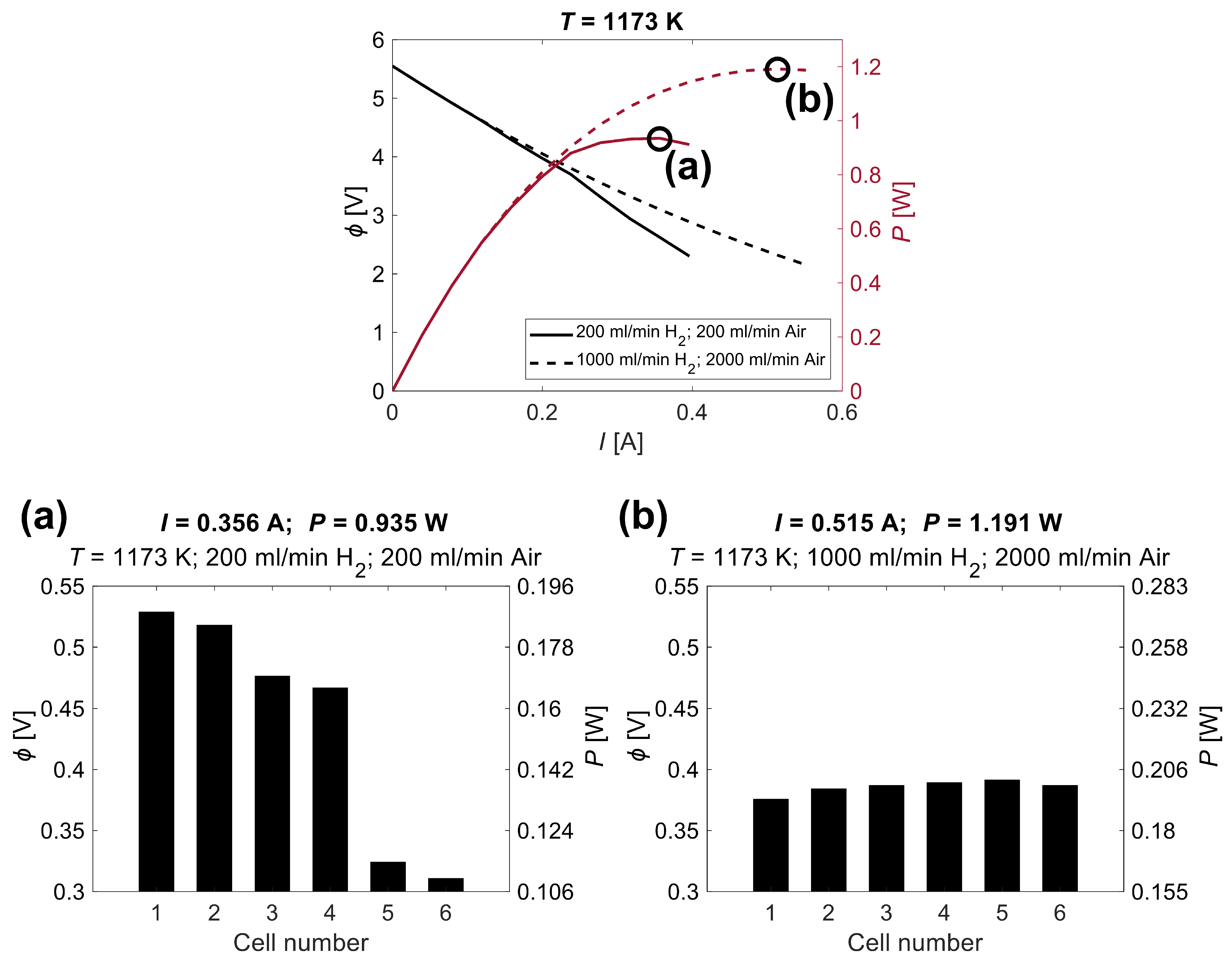
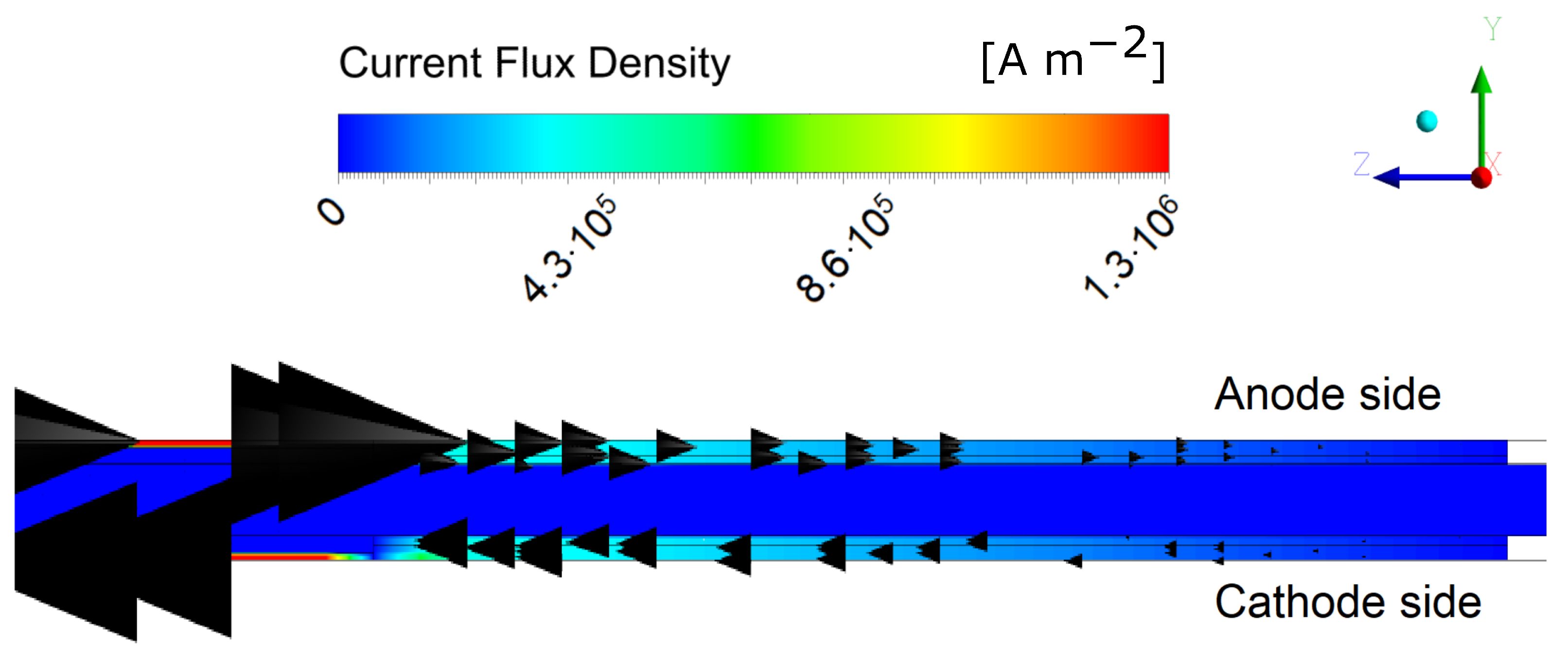
| Parameter | Symbol | Value | |
|---|---|---|---|
| Anode | Cathode | ||
| For T = 1173 K | |||
| Anode equilibrium exchange current | [] | [28] | - |
| Cathode equilibrium exchange current | [] | - | 220 [28] |
| Concentration dependence | [-] | 0.05 * | 0.05 * |
| Electric conductivity | [] | 42,941 [28] | 22,560 [28] |
| For T = 1123 K | |||
| Anode equilibrium exchange current | [] | [28] | - |
| Cathode equilibrium exchange current | [] | - | 149 [28] |
| Concentration dependence | [-] | 1 * | 1 * |
| Electric conductivity | [] | 43,838 [28] | 21,399 [28] |
| Reference concentration | [kmol ] | [20] | [20] |
| Anode side transfer rate | [-] | 1.4 [28] | 1.2 [28] |
| Cathode side transfer rate | [-] | 0.8 [28] | 1 [28] |
| Porosity | [-] | 0.48 ** | 0.39 ** |
| Triple phase boundary length density | [] | [28] | - |
| Double phase boundary length density | [] | - | [28] |
| Density | [] | 6220 [18] | 5300 [19] |
| Specific heat | [] | 450 [18] | 607 [19] |
| Thermal conductivity | k [] | 6.23 [18] | 10 [19] |
| Parameter | Symbol | Value | |
| Electrolyte | Current Collector | ||
| Electric conductivity | [] | * | * |
| Thermal conductivity | k [] | 2 *** | 100 *** |
| Parameter | Value | |||
|---|---|---|---|---|
| Number of elements | ||||
| Mass conservation () | ||||
| Time per iteration () | 1.05 | 2.14 | 3.68 | 17.45 |
| Parameter | Spatial Discretization | Underrelaxation Factor |
|---|---|---|
| Gradient | Green-Gauss Node Based | 0.3 |
| Pressure | Second Order | 1 |
| Density | First Order Upwind | 1 |
| Momentum | First Order Upwind | 0.7 |
| Hydrogen | First Order Upwind | 1 |
| Oxygen | First Order Upwind | 1 |
| Water Vapor | First Order Upwind | 1 |
| Energy | First Order Upwind | 1 |
| Electric Potential | First Order Upwind | 1 |
| Ionic Potential | First Order Upwind | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śreniawski, K.K.; Chalusiak, M.; Moździerz, M.; Szmyd, J.S.; Brus, G. Transport Phenomena in a Banded Solid Oxide Fuel Cell Stack—Part 1: Model and Validation. Energies 2023, 16, 4511. https://doi.org/10.3390/en16114511
Śreniawski KK, Chalusiak M, Moździerz M, Szmyd JS, Brus G. Transport Phenomena in a Banded Solid Oxide Fuel Cell Stack—Part 1: Model and Validation. Energies. 2023; 16(11):4511. https://doi.org/10.3390/en16114511
Chicago/Turabian StyleŚreniawski, Karol K., Maciej Chalusiak, Marcin Moździerz, Janusz S. Szmyd, and Grzegorz Brus. 2023. "Transport Phenomena in a Banded Solid Oxide Fuel Cell Stack—Part 1: Model and Validation" Energies 16, no. 11: 4511. https://doi.org/10.3390/en16114511
APA StyleŚreniawski, K. K., Chalusiak, M., Moździerz, M., Szmyd, J. S., & Brus, G. (2023). Transport Phenomena in a Banded Solid Oxide Fuel Cell Stack—Part 1: Model and Validation. Energies, 16(11), 4511. https://doi.org/10.3390/en16114511







