Flexible Solid-State Lithium-Ion Batteries: Materials and Structures
Abstract
1. Introduction
2. Materials for Flexible Solid-State Lithium-Ion Batteries
2.1. Flexible Electrode Materials
2.1.1. Carbon Nanotube
2.1.2. Graphene
2.1.3. Carbon Fiber/Carbon Fiber Cloth
2.1.4. Conductive Polymers
2.1.5. Challenges and Summary of Flexible Electrodes
2.2. Flexible Electrolyte Materials
3. Structural Design of Flexible Solid-State Lithium-Ion Batteries
3.1. 1D Fiber-Shaped FLIBs
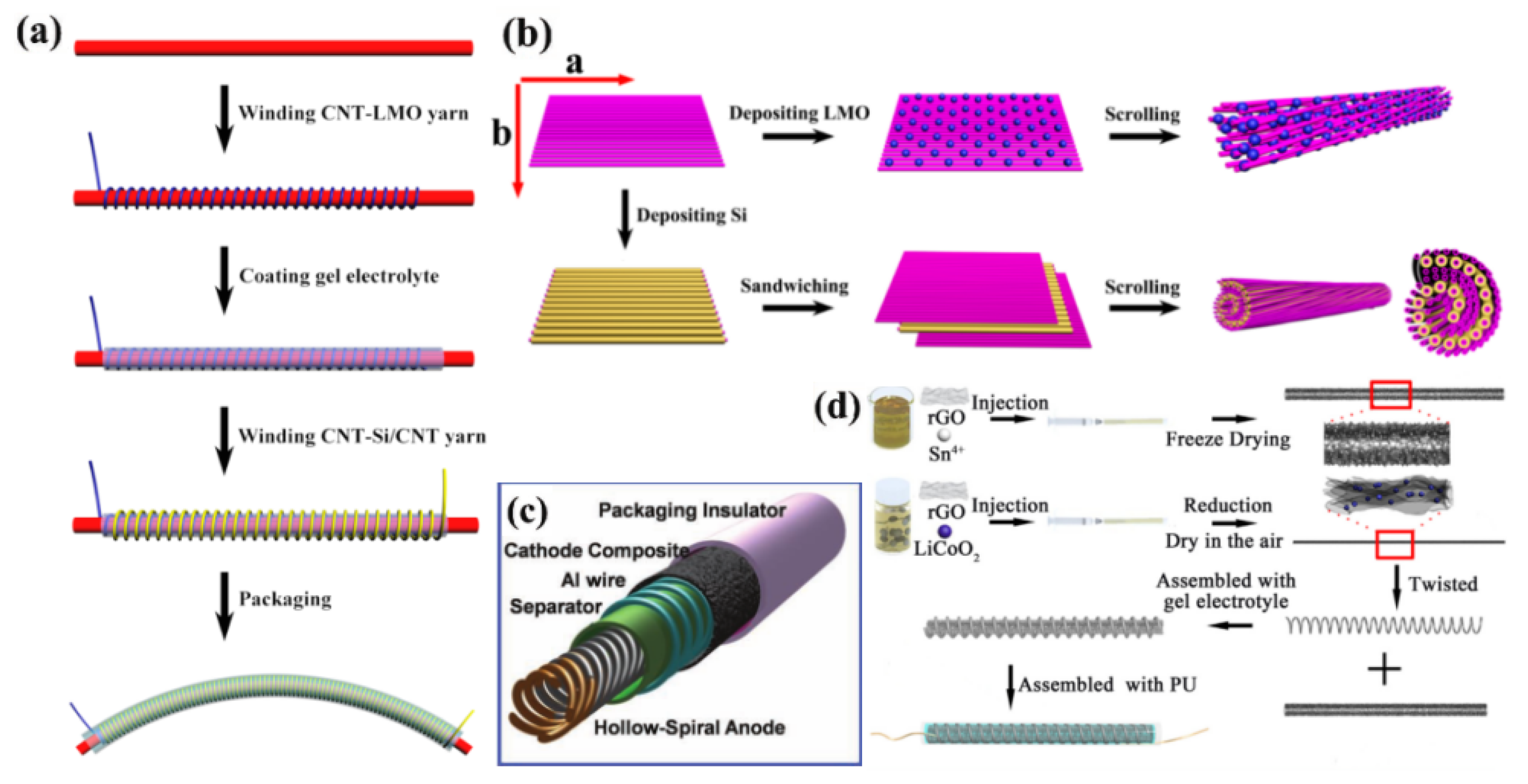
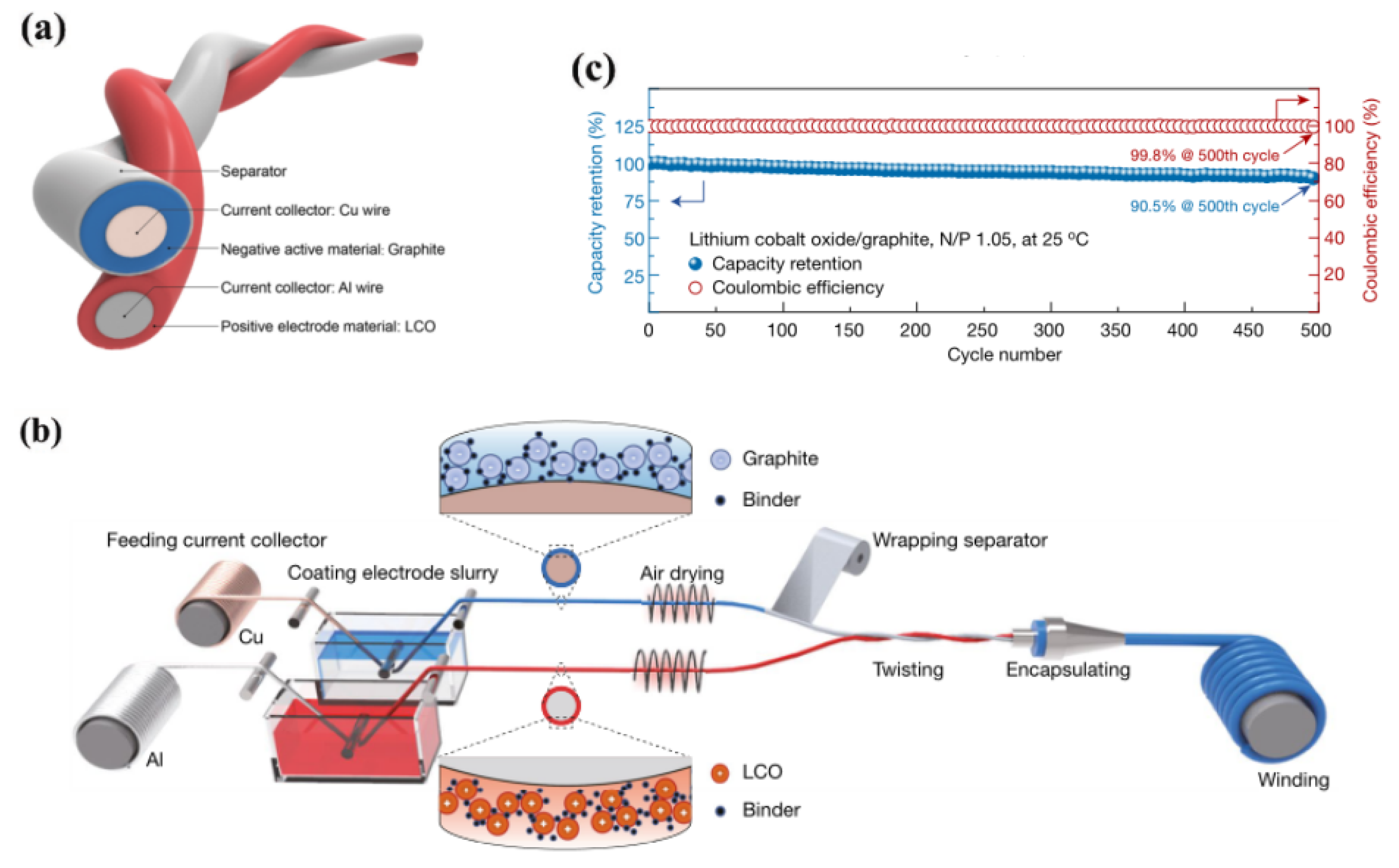
3.1.1. WEFLIBs
3.1.2. HEFLIBs
3.1.3. PEFLIBs
3.1.4. CEFLIBs
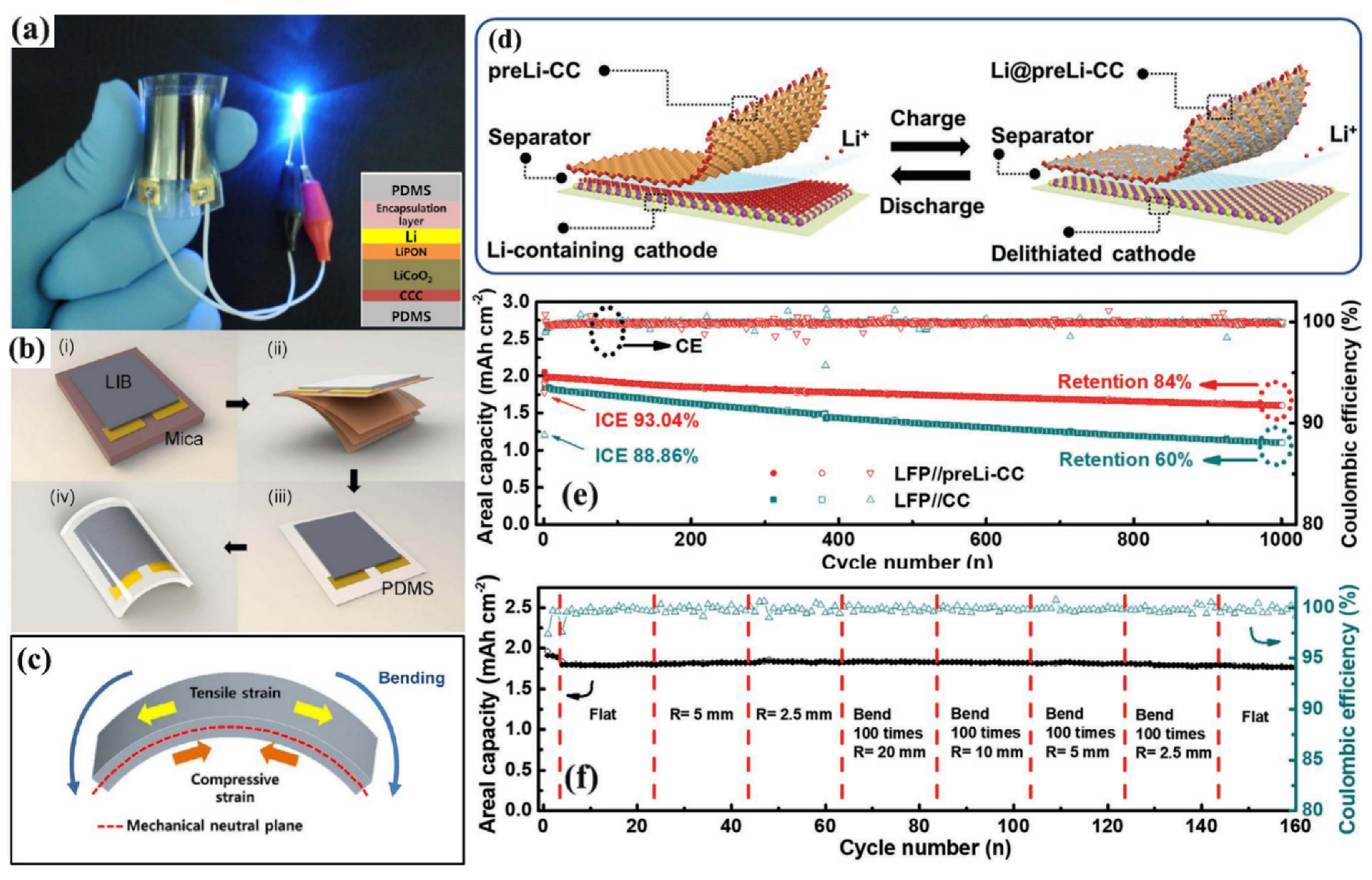
3.2. 2D Film-Shaped FLIBs
3.2.1. UFFLIBs
3.2.2. GPFLIBs
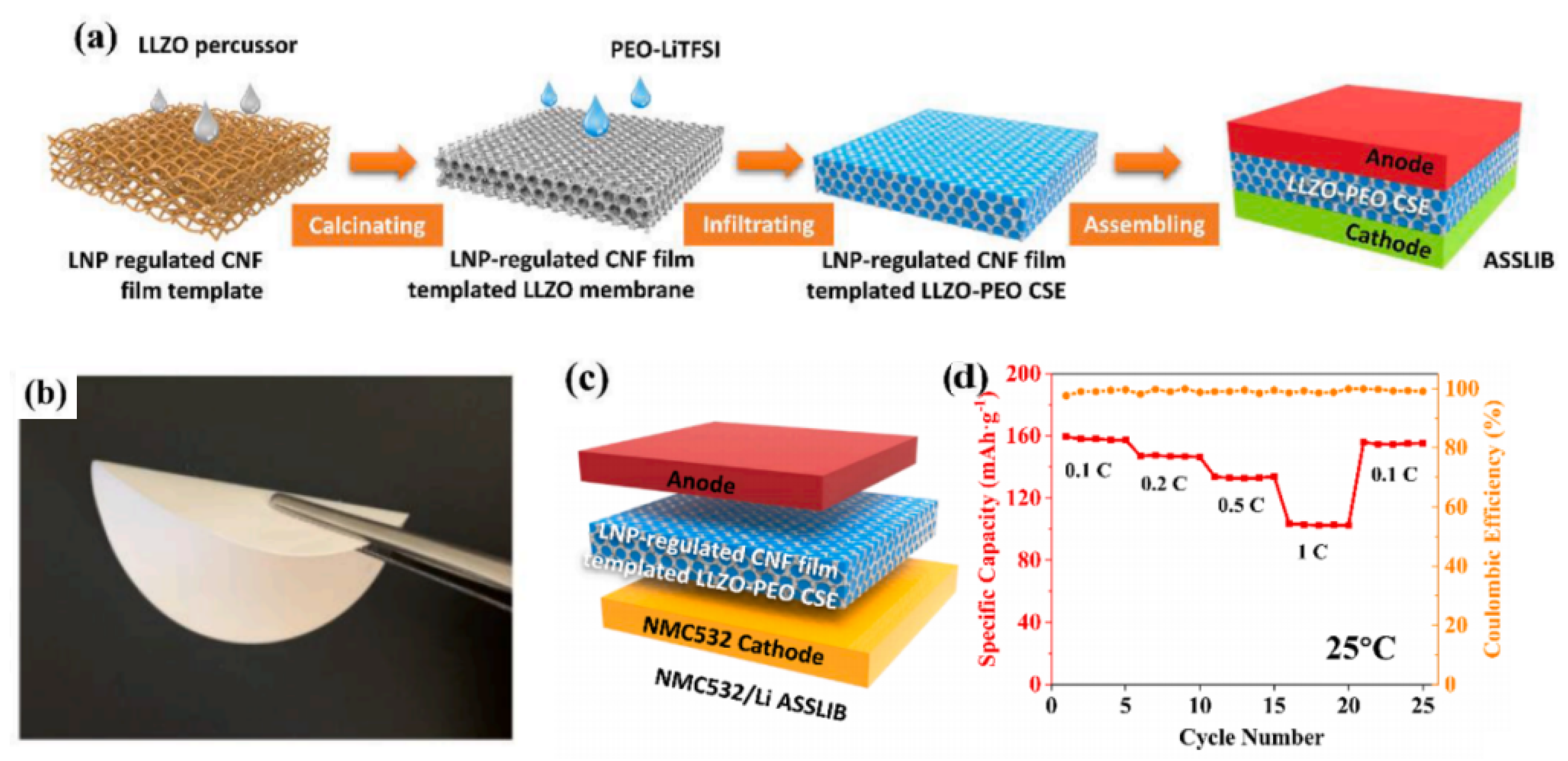
3.2.3. IPFLIBs
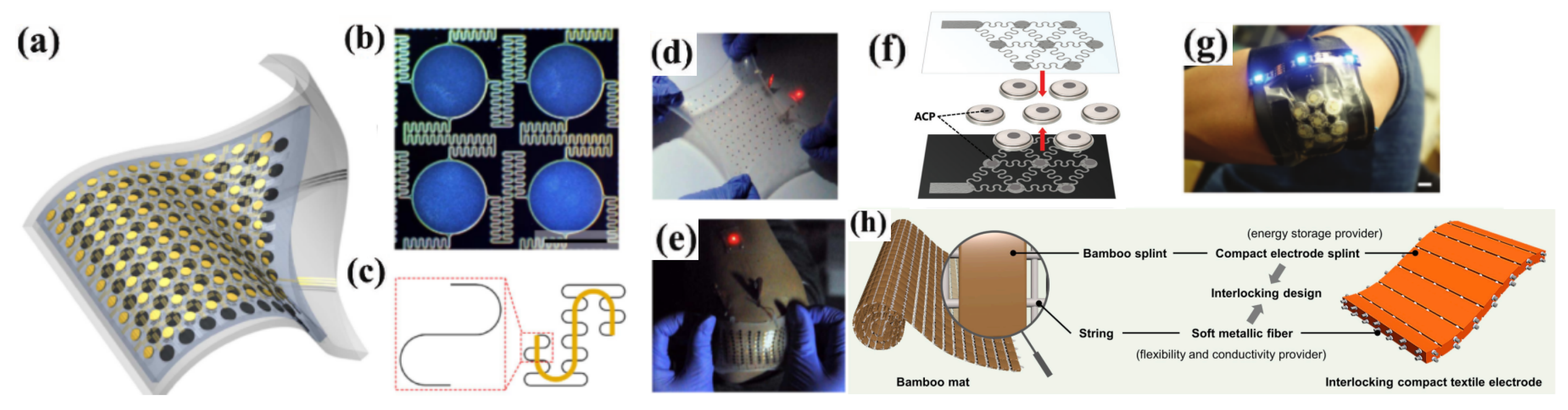
3.2.4. CPFLIBs
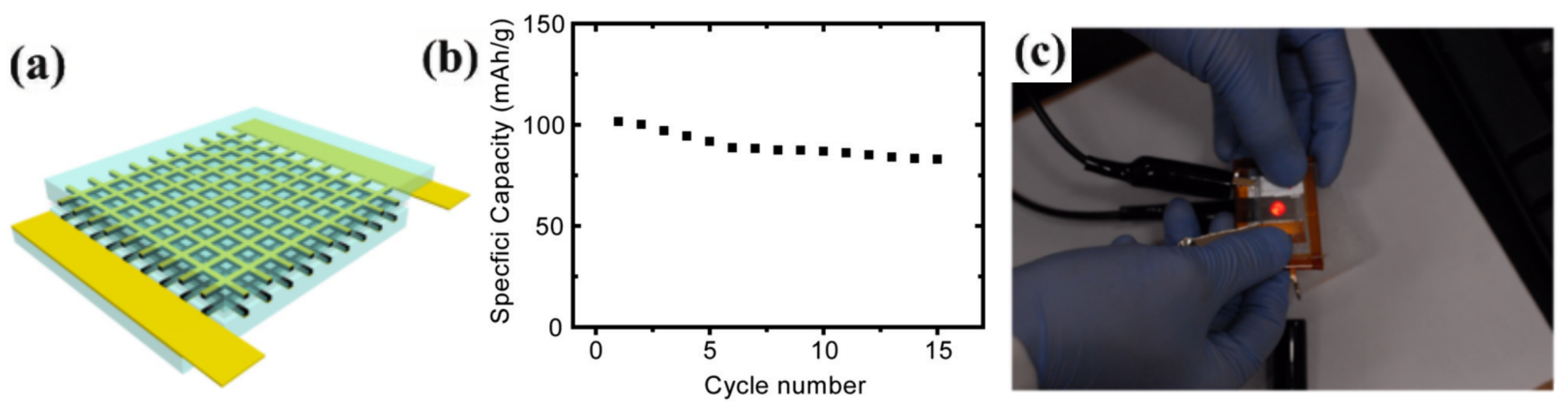
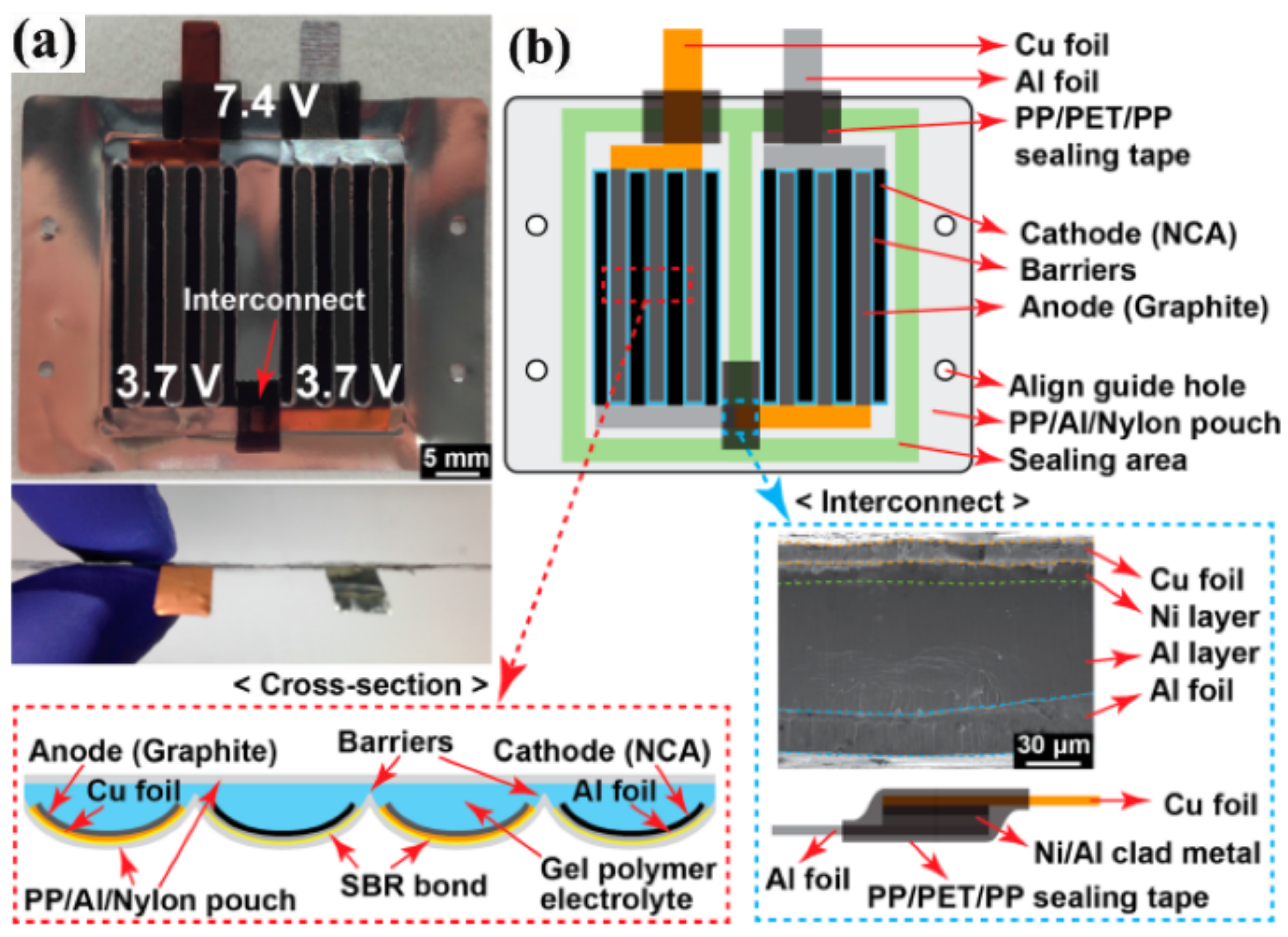
3.3. 3D FLIBs



4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamada, T.; Tsubata, Y. Recent progress and future perspectives of light emitting polymers for full-color display. J. Synth. Org. Chem. Jpn. 2012, 70, 473–479. [Google Scholar] [CrossRef]
- Pang, C.; Lee, C.; Suh, K.Y. Recent advances in flexible sensors for wearable and implantable devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Sumboja, A.; Foo, C.Y.; Wang, X.; Lee, P.S. Large areal mass, flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv. Mater. 2013, 25, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhang, Y.; Zhong, Q.; Hu, Q.; Hu, B.; Wang, Z.L.; Zhou, J. Fiber-based generator for wearable electronics and mobile medication. ACS Nano 2014, 8, 6273–6280. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Baek, D.H.; Jung, H.C.; Song, J.H.; Moon, J.H.; Hong, S.W.; Kim, I.Y.; Lee, S.H. Solderable and electroplatable flexible electronic circuit on a porous stretchable elastomer. Nat. Commun. 2012, 3, 977. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.S.; Noh, J.; Lee, I.; Kim, H.J.; Choi, S.; Seo, J.; Jeon, S.; Kim, T.S.; Lee, J.Y.; et al. Wearable textile battery rechargeable by solar energy. Nano Lett. 2013, 13, 5753–5761. [Google Scholar] [CrossRef]
- Sun, D.m.; Timmermans, M.Y.; Tian, Y.; Nasibulin, A.G.; Kauppinen, E.I.; Kishimoto, S.; Mizutani, T.; Ohno, Y. Flexible high-performance carbon nanotube integrated circuits. Nat. Nanotechnol. 2011, 6, 156–161. [Google Scholar] [CrossRef]
- Qian, G.; Liao, X.; Zhu, Y.; Pan, F.; Chen, X.; Yang, Y. Designing flexible lithium-ion batteries by structural engineering. ACS Energy Lett. 2019, 4, 690–701. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Zeng, L.; Qiu, L.; Cheng, H.M. Towards the practical use of flexible lithium ion batteries. Energy Storage Mater. 2019, 23, 434–438. [Google Scholar] [CrossRef]
- Chang, J.; Huang, Q.; Zheng, Z. A figure of merit for flexible batteries. Joule 2020, 4, 1346–1349. [Google Scholar] [CrossRef]
- Gu, Z.; Li, W.; Miao, Y.; Chen, Y.; Xia, X.; Chen, G.; Liu, H. Influencing factors and behavior mechanism of the initial coulombic efficiency of silicon/graphite composites in lithium-ion batteries. Electrochim. Acta 2021, 366, 137424. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Myung, S. A flexible approach to mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.; Wang, Y.; Jiao, L. 1D nanomaterials: Design, synthesis, and applications in sodium–ion batteries. Small 2018, 14, 1703086. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, Q.; Cao, Y.; Fang, D.; Xu, W.; Jiang, M.; Huang, J.; Liu, H.; Fan, X. Polypyrrole nanotube arrays on carbonized cotton textile for aqueous sodium battery. Org. Electron. 2017, 46, 211–217. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Salunke, P.; Lee, L.; Head, E.; Mallik, N.; Yun, Y.; Pendyala, C.; Schulz, M.J.; Shanov, V.N. Extremely Long Multiwall Carbon Nanotube Arrays for Spinning Yarn. In Proceedings of the APS March Meeting Abstracts, Louisiana, OR, USA, 10–14 March 2008; p. C1.160. [Google Scholar]
- Tian, R.; Alcala, N.; O’Neill, S.J.; Horvath, D.V.; Coelho, J.; Griffin, A.J.; Zhang, Y.; Nicolosi, V.; O’Dwyer, C.; Coleman, J.N. Quantifying the effect of electronic conductivity on the rate performance of nanocomposite battery electrodes. ACS Appl. Energy Mater. 2020, 3, 2966–2974. [Google Scholar] [CrossRef]
- Yan, J.; Huang, H.; Zhang, J.; Liu, Z.; Yang, Y. A study of novel anode material CoS2 for lithium ion battery. J. Power Sources 2005, 146, 264–269. [Google Scholar] [CrossRef]
- Feng, C.; Ma, J.; Li, H.; Zeng, R.; Guo, Z.; Liu, H. Synthesis of molybdenum disulfide (MoS2) for lithium ion battery applications. Mater. Res. Bull. 2009, 44, 1811–1815. [Google Scholar] [CrossRef]
- Tang, S.; Yang, L.; Liu, J.; Fichou, D. Carbon coated hierarchical porous MoO2 nanoflowers as high-performance anodes in lithium-ion batteries. Mater. Res. Bull. 2018, 102, 277–281. [Google Scholar] [CrossRef]
- Dominko, R.; Arčon, D.; Mrzel, A.; Zorko, A.; Cevc, P.; Venturini, P.; Gaberscek, M.; Remskar, M.; Mihailovic, D. Dichalcogenide nanotube electrodes for Li-ion batteries. Adv. Mater. 2002, 14, 1531–1534. [Google Scholar] [CrossRef]
- Ding, S.; Chen, J.S.; Lou, X.W. Glucose-assisted growth of MoS2 nanosheets on CNT backbone for improved lithium storage properties. Chem. Eur. J. 2011, 17, 13142–13145. [Google Scholar] [CrossRef] [PubMed]
- Ban, C.; Wu, Z.; Gillaspie, D.T.; Chen, L.; Yan, Y.; Blackburn, J.L.; Dillon, A.C. Nanostructured Fe3O4/swnt electrode: Binder-free and high-rate li-ion anode. Adv. Mater. 2010, 22, E145–E149. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon nanotubes–the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Huang, Y.H.; Goodenough, J.B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem. Mater. 2008, 20, 7237–7241. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Yang, K.; Liu, X.; Chen, Y.; Lao, Z.; Mai, K.; Zhang, Z. Polymer Molecular Engineering Enables Rapid Electron/Ion Transport in Ultra-Thick Electrode for High-Energy-Density Flexible Lithium-Ion Battery. Adv. Funct. Mater. 2021, 31, 2100434. [Google Scholar] [CrossRef]
- Amin, K.; Meng, Q.; Ahmad, A.; Cheng, M.; Zhang, M.; Mao, L.; Lu, K.; Wei, Z. A carbonyl compound-based flexible cathode with superior rate performance and cyclic stability for flexible lithium-ion batteries. Adv. Mater. 2018, 30, 1703868. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Y.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Li, J.; Wang, J. Ultrastretchable carbon nanotube composite electrodes for flexible lithium-ion batteries. Nanoscale 2018, 10, 19972–19978. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yun, S.D.; Nyamaa, O.; Yang, J.H.; Huh, S.C.; Jeong, H.M.; Nam, T.H.; Ryu, Y.J.; Noh, J.P. Free-Standing Li4Ti5O12/Carbon Nanotube Electrodes for Flexible Lithium-Ion Batteries. Energies 2022, 15, 8585. [Google Scholar] [CrossRef]
- Zhang, P.; Qiu, J.; Zheng, Z.; Liu, G.; Ling, M.; Martens, W.; Wang, H.; Zhao, H.; Zhang, S. Free-standing and bendable carbon nanotubes/TiO2 nanofibres composite electrodes for flexible lithium ion batteries. Electrochim. Acta 2013, 104, 41–47. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Feng, S.; Han, Y.; Gao, L.; Hue Ly, T.; Xu, Z.; Lu, Y. Elastic straining of free-standing monolayer graphene. Nat. Commun. 2020, 11, 284. [Google Scholar] [CrossRef]
- Cui, T.; Mukherjee, S.; Sudeep, P.M.; Colas, G.; Najafi, F.; Tam, J.; Ajayan, P.M.; Singh, C.V.; Sun, Y.; Filleter, T. Fatigue of graphene. Nat. Mater. 2020, 19, 405–411. [Google Scholar] [CrossRef]
- Qi, X.; Wang, F.; Xie, H.; Mao, L.; Mao, J. Ultra-high capacity dual-ion batteries realized by few-layered reduced graphene oxide and cathode structure design. J. Mater. Sci. 2021, 56, 10555–10564. [Google Scholar] [CrossRef]
- Hu, Z.; Zhengquan, Z.; Mao, J. Silicon and reduced graphene oxide employed as additives to enhance the performances of artificial graphite anode for lithium-ion battery. Fullerenes Nanotub. Carbon Nanostructures 2019, 27, 887–894. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Xiao, X.; Wu, S.; Zhong, G.; Xu, K.; Wei, Z.; Su, W.; Lu, X. γ-MnO2 nanorods/graphene composite as efficient cathode for advanced rechargeable aqueous zinc-ion battery. J. Energy Chem. 2020, 43, 182–187. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Qi, S.; Ge, W.; Ma, W.; Zhao, Y.; Huang, R.; Xu, L.; Qian, Y. Ultrahigh-areal-capacity battery anodes enabled by free-standing vanadium nitride@ N-doped carbon/graphene architecture. ACS Appl. Mater. Interfaces 2020, 12, 49607–49616. [Google Scholar] [CrossRef]
- Wang, H.; Lu, S.; Chen, Y.; Han, L.; Zhou, J.; Wu, X.; Qin, W. Graphene/Co 9 S 8 nanocomposite paper as a binder-free and free-standing anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23677–23683. [Google Scholar] [CrossRef]
- Xie, Y.; Ao, J.; Zhang, L.; Shao, Y.; Zhang, H.; Cheng, S.; Wang, X. Multi-functional bilayer carbon structures with micrometer-level physical encapsulation as a flexible cathode host for high-performance lithium-sulfur batteries. Chem. Eng. J. 2023, 451, 139017. [Google Scholar] [CrossRef]
- Han, M.; Lin, Z.; Ji, X.; Mu, Y.; Li, J.; Yu, J. Growth of flexible and porous surface layers of vertical graphene sheets for accommodating huge volume change of silicon in lithium-ion battery anodes. Mater. Today Energy 2020, 17, 100445. [Google Scholar] [CrossRef]
- Gao, Z.; Song, N.; Zhang, Y.; Li, X. Cotton-textile-enabled, flexible lithium-ion batteries with enhanced capacity and extended lifespan. Nano Lett. 2015, 15, 8194–8203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Li, G.; Luo, D.; Liu, J.; Zhang, Y.; Wang, X.; Shui, L.; Chen, Z. Aligned sulfur-deficient ZnS1- x nanotube arrays as efficient catalyzer for high-performance lithium/sulfur batteries. Nano Energy 2021, 84, 105891. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@ NC heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium–sulfur batteries. ACS Nano 2021, 15, 7114–7130. [Google Scholar] [CrossRef]
- He, J.; Bhargav, A.; Manthiram, A. High-energy-density, long-life lithium–sulfur batteries with practically necessary parameters enabled by low-cost Fe–Ni nanoalloy catalysts. ACS Nano 2021, 15, 8583–8591. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Rui, K.; Wu, X.; Wu, M.; Zhang, J. Enhanced performance of lithium sulfur battery with polypyrrole warped mesoporous carbon/sulfur composite. J. Power Sources 2014, 254, 353–359. [Google Scholar] [CrossRef]
- Yu, Y.; Zhan, Z.; Tang, P.; Xu, Q.; Fan, Q.; Wang, W.; Shen, K. Promoting polysulfide redox kinetics by Co9S8 nanoparticle-embedded in N-doped carbon nanotube hollow polyhedron for lithium sulfur batteries. J. Alloys Compd. 2021, 869, 159306. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Cheng, B.; Yu, J. Hollow carbon spheres and their hybrid nanomaterials in electrochemical energy storage. Adv. Energy Mater. 2019, 9, 1803900. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Lei, Z.; Sun, K.; Zhu, S. MXene enabled binder-free FeOF cathode with high volumetric and gravimetric capacities for flexible lithium ion batteries. Electrochim. Acta 2022, 423, 140595. [Google Scholar] [CrossRef]
- Du, Y.T.; Kan, X.; Yang, F.; Gan, L.Y.; Schwingenschlogl, U. MXene/graphene heterostructures as high-performance electrodes for Li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 32867–32873. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, D.; Sheng, J.; Han, Z.; Sun, C.; Tan, J.; Gao, R.; Lv, W.; Xu, X.; Wei, G.; et al. Freestanding and sandwich MXene-based cathode with suppressed lithium polysulfides shuttle for flexible lithium–sulfur batteries. Nano Lett. 2022, 22, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Goe, A. Carbon fibres: Production, properties and potential use. Mater. Sci. Res. India 2017, 14, 52–57. [Google Scholar] [CrossRef]
- Hagberg, J. Carbon Fibres for Multifunctional Lithium-Ion Batteries. Ph.D. Thesis, Kungliga Tekniska högskolan, Stockholm, Sverige, 2018. [Google Scholar]
- Zhang, X.; Gao, M.; Wang, W.; Liu, B.; Li, X. Encapsulating MoO2 nanocrystals into flexible carbon nanofibers via electrospinning for high-performance lithium storage. Polymers 2020, 13, 22. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Z.; Hu, X.; Liu, X.; Wang, H. Silicon in Hollow Carbon Nanospheres Assembled Microspheres Cross-linked with N-doped Carbon Fibers toward a Binder Free, High Performance, and Flexible Anode for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101487. [Google Scholar] [CrossRef]
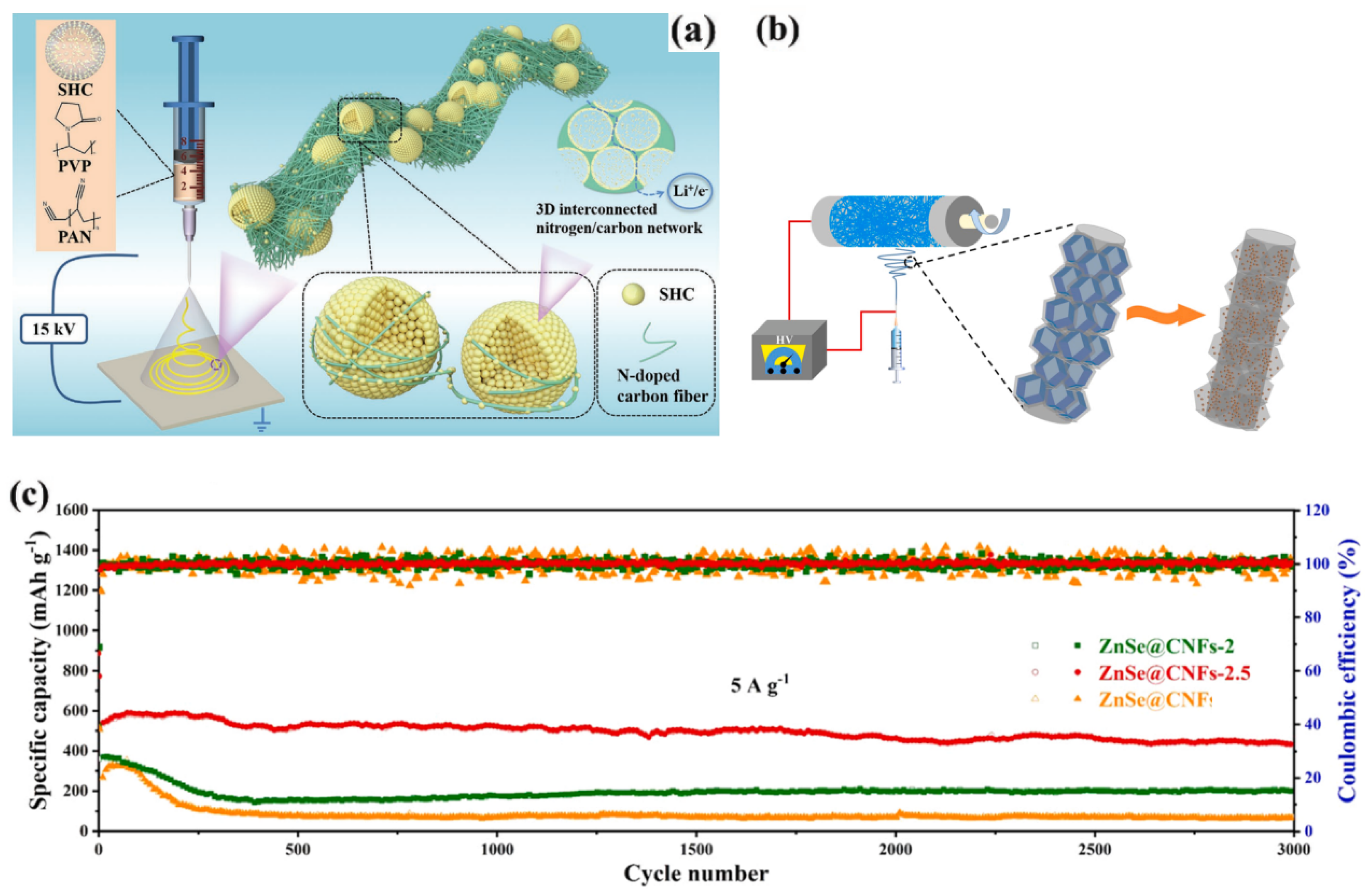
- Zhang, T.; Qiu, D.; Hou, Y. Free-standing and consecutive ZnSe@ carbon nanofibers architectures as ultra-long lifespan anode for flexible lithium-ion batteries. Nano Energy 2022, 94, 106909. [Google Scholar] [CrossRef]
- Li, Y.; Lei, D.; Yang, S.; Chen, J.; Zhao, Z.; Guo, J.; Xiang, M.; Liu, X.; Bai, W. Facile preparation of flexible porous carbon fibers as self-supporting sulfur cathode hosts for high-performance Li–S batteries. Dalton Trans. 2022, 51, 16206–16214. [Google Scholar] [CrossRef]
- Lu, H.Y.; Zhang, X.H.; Wan, F.; Liu, D.S.; Fan, C.Y.; Xu, H.M.; Wang, G.; Wu, X.L. Flexible P-doped carbon cloth: Vacuum-sealed preparation and enhanced Na-storage properties as binder-free anode for sodium ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 12518–12527. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Yu, C.; Wu, H.B.; Wang, G.; Dong, Q.; Qiu, J.; Eychmüller, A.; Lou, X.W. A flexible TiO2 (B)-based battery electrode with superior power rate and ultralong cycle life. Adv. Mater. 2013, 25, 3462–3467. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ni, M.; Chen, M.; Xia, H. Low-temperature synthesized self-supported single-crystalline LiCoO2 nanoflake arrays as advanced 3D cathodes for flexible lithium-ion batteries. J. Mater. Chem. A 2019, 7, 6187–6196. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lin, C.; Zhao, X.S. Lithium titanate cuboid arrays grown on carbon fiber cloth for high-rate flexible lithium-ion batteries. Small 2019, 15, 1902183. [Google Scholar] [CrossRef]
- Storan, D.; Ahad, S.A.; Forde, R.; Kilian, S.; Adegoke, T.E.; Kennedy, T.; Geaney, H.; Ryan, K.M. Silicon nanowire growth on carbon cloth for flexible Li-ion battery anodes. Mater. Today Energy 2022, 27, 101030. [Google Scholar] [CrossRef]
- Mi, H.; Zhang, X.; Yang, S.; Ye, X.; Luo, J. Polyaniline nanofibers as the electrode material for supercapacitors. Mater. Chem. Phys. 2008, 112, 127–131. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Duan, X.; Liu, D.; Guo, J.; Liu, P. Crosslinked polyaniline nanorods with improved electrochemical performance as electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 12323–12329. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, S.; Jiang, W. Electrochemical properties of PANI as single electrode of electrochemical capacitors in acid electrolytes. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Kumar, M.; Angaiah, S. Synthesis of polythiophene and its carbonaceous nanofibers as electrode materials for asymmetric supercapacitors. Adv. Mater. Res. 2014, 938, 151–157. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; He, Y.; Zhou, K.; Ji, H.; Shi, W.; Sun, Y.; Wu, T.; Ge, D. Preparation of Nanocomposites of Polypyrrole-metal (or Metal Oxide) on the Surface of V2O5 Coated Cathode through a One-step Method. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; Volume 611, p. 012037. [Google Scholar]
- Liao, Y.; Liang, K.; Ren, Y.; Huang, X. Fabrication of SiOx-G/PAA-PANi/Graphene composite with special cross-doped conductive hydrogels as anode materials for lithium ion batteries. Front. Chem. 2020, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ma, L.; Zhang, Y.; Zheng, X.; Shi, Y.; Zeng, X.; Wang, X.; Wei, L. A flexible and conductive binder with strong adhesion for high performance silicon-based lithium-ion battery anode. ChemElectroChem 2020, 7, 1992–2000. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.M.; Chen, Z. practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 2017, 164, A1731. [Google Scholar] [CrossRef]
- Agrawal, R.; Pandey, G. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. J. Phys. D: Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Hallinan, D.T., Jr.; Balsara, N.P. Polymer electrolytes. Annu. Rev. Mater. Res. 2013, 43, 503–525. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, T.; Wang, S.; Wang, C.; Li, D.; Xia, Y. Alginate Fiber-Grafted Polyetheramine-Driven High Ion-Conductive and Flame-Retardant Separator and Solid Polymer Electrolyte for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 56780–56789. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V aqueous Li-ion batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Kato, A.; Yamamoto, M.; Utsuno, F.; Higuchi, H.; Takahashi, M. Lithium-ion-conductive sulfide polymer electrolyte with disulfide bond-linked PS4 tetrahedra for all-solid-state batteries. Commun. Mater. 2021, 2, 112. [Google Scholar] [CrossRef]
- OV, S.; Elsin Abraham, S.; Ramaswamy, M. Free-Standing and Flexible Garnet-PVDF Ceramic Polymer Electrolyte Membranes for Solid-State Batteries. Energy Fuels 2023, 37, 2401–2409. [Google Scholar]
- Grewal, M.S.; Tanaka, M.; Kawakami, H. Fabrication and characterizations of soft and flexible Poly (dimethylsiloxane)-incorporated network polymer electrolyte membranes. Polymer 2020, 186, 122045. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
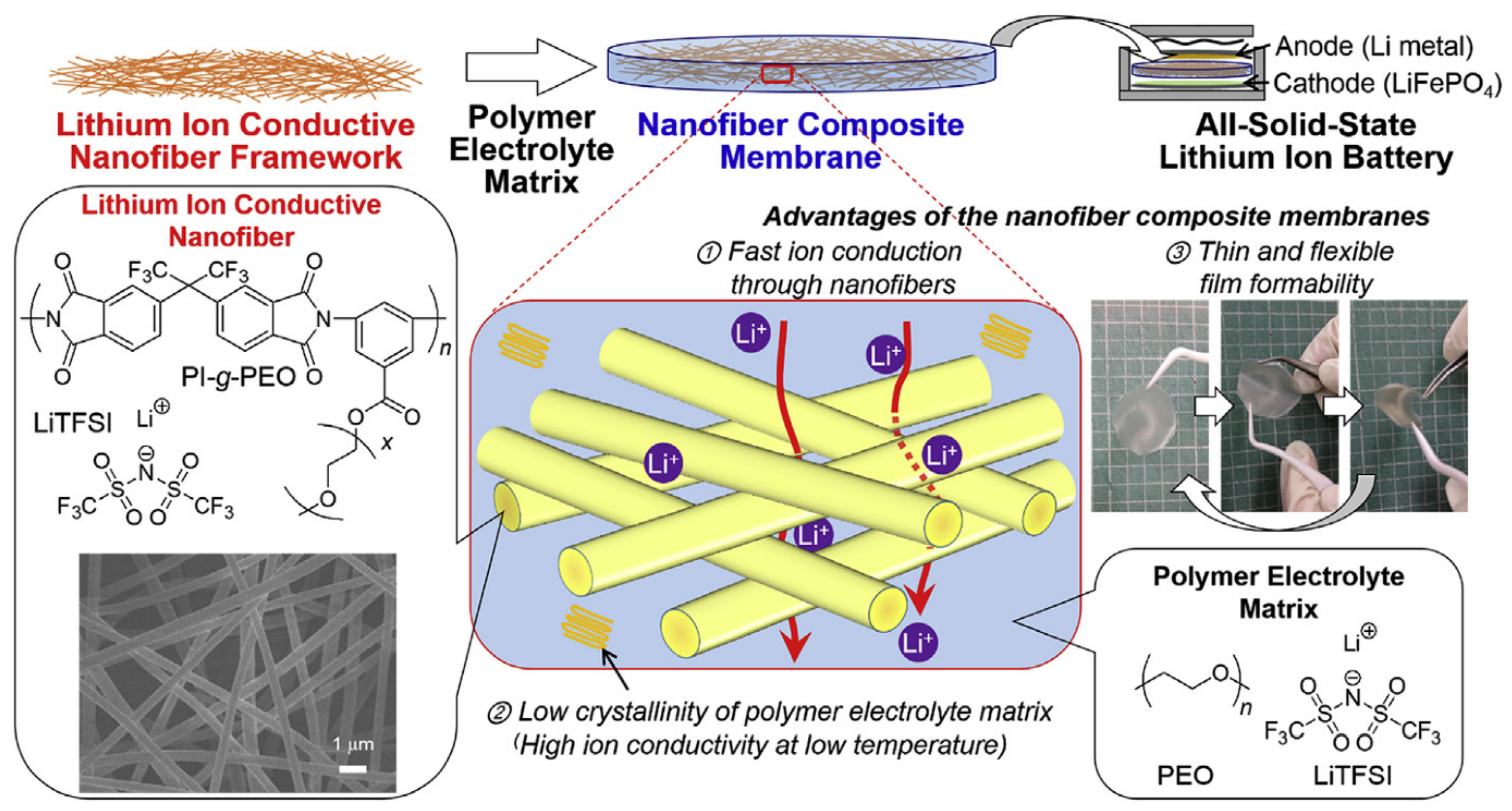
- Watanabe, T.; Inafune, Y.; Tanaka, M.; Mochizuki, Y.; Matsumoto, F.; Kawakami, H. Development of all-solid-state battery based on lithium ion conductive polymer nanofiber framework. J. Power Sources 2019, 423, 255–262. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building better batteries in the solid state: A review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Chen, G. Status and prospect of garnet/polymer solid composite electrolytes for all-solid-state lithium batteries. J. Energy Chem. 2020, 50, 154–177. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Zhang, H.; An, X.; Long, Y.; Cao, H.; Cheng, Z.; Liu, H.; Ni, Y. A thin and flexible solid electrolyte templated by controllable porous nanocomposites toward extremely high performance all-solid-state lithium-ion batteries. Chem. Eng. J. 2021, 425, 130632. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef]
- Liang, L.; Chen, X.; Yuan, W.; Chen, H.; Liao, H.; Zhang, Y. Highly conductive, flexible, and nonflammable double-network poly (ionic liquid)-based ionogel electrolyte for flexible lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 25410–25420. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, M.; Liu, C.; Li, G.; Lu, X.; Cai, H.; Liu, C.; Lai, W.Y. Highly stretchable multifunctional polymer ionic conductor with high conductivity based on organic-inorganic dual networks. Chem. Eng. J. 2022, 440, 135824. [Google Scholar] [CrossRef]
- Wei, D.; Shen, W.; Xu, T.; Li, K.; Yang, L.; Zhou, Y.; Zhong, M.; Yang, F.; Xu, X.; Wang, Y.; et al. Ultra-flexible and foldable gel polymer lithium–ion batteries enabling scalable production. Mater. Today Energy 2022, 23, 100889. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Ock, J.y.; Motoyoshi, R.; Li, S.; Ueno, K.; Dokko, K.; Tsuzuki, S.; Watanabe, M. LiNi0. 5Mn1. 5O4-Hybridized Gel Polymer Cathode and Gel Polymer Electrolyte Containing a Sulfolane-Based Highly Concentrated Electrolyte for the Fabrication of a 5 V Class of Flexible Lithium Batteries. ACS Omega 2022, 7, 17732–17740. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Santiago, A.; Judez, X.; Garbayo, I.; Coca Clemente, J.A.; Morant-Miñana, M.C.; Villaverde, A.; González-Marcos, J.A.; Zhang, H.; Armand, M.; et al. Safe, Flexible, and High-Performing Gel-Polymer Electrolyte for Rechargeable Lithium Metal Batteries. Chem. Mater. 2021, 33, 8812–8821. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, L.; Luo, S.; Zhao, E.; Saito, N. A non-flammable, flexible and UV-cured gel polymer electrolyte with crosslinked polymer network for dendrite-suppressing lithium metal batteries. Ionics 2022, 28, 3743–3759. [Google Scholar] [CrossRef]
- Fu, K.K.; Cheng, J.; Li, T.; Hu, L. Flexible batteries: From mechanics to devices. ACS Energy Lett. 2016, 1, 1065–1079. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Woo, S.W.; Jung, H.R.; Yu, H.K.; Kim, K.; Oh, B.H.; Ahn, S.; Lee, S.Y.; Song, S.W.; Cho, J.; et al. Cable-type flexible lithium ion battery based on hollow multi-helix electrodes. Adv. Mater. 2012, 24, 5192–5197. [Google Scholar] [CrossRef]
- Koo, M.; Park, K.I.; Lee, S.H.; Suh, M.; Jeon, D.Y.; Choi, J.W.; Kang, K.; Lee, K.J. Bendable inorganic thin-film battery for fully flexible electronic systems. Nano Lett. 2012, 12, 4810–4816. [Google Scholar] [CrossRef]
- Cheng, Q.; Song, Z.; Ma, T.; Smith, B.B.; Tang, R.; Yu, H.; Jiang, H.; Chan, C.K. Folding paper-based lithium-ion batteries for higher areal energy densities. Nano Lett. 2013, 13, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Liu, N.; Zhang, Z.; Su, J.; Li, L.; Xiong, L.; Gao, Y. All-fiber-based quasi-solid-state lithium-ion battery towards wearable electronic devices with outstanding flexibility and self-healing ability. Nano Energy 2018, 51, 425–433. [Google Scholar] [CrossRef]
- He, J.; Lu, C.; Jiang, H.; Han, F.; Shi, X.; Wu, J.; Wang, L.; Chen, T.; Wang, J.; Zhang, Y.; et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 2021, 597, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hoshide, T.; Zheng, Y.; Hou, J.; Wang, Z.; Li, Q.; Zhao, Z.; Ma, R.; Sasaki, T.; Geng, F. Flexible lithium-ion fiber battery by the regular stacking of two-dimensional titanium oxide nanosheets hybridized with reduced graphene oxide. Nano Lett. 2017, 17, 3543–3549. [Google Scholar] [CrossRef]
- Xie, C.; Chang, J.; Shang, J.; Wang, L.; Gao, Y.; Huang, Q.; Zheng, Z. Hybrid lithium-ion/metal electrodes enable long cycle stability and high energy density of flexible batteries. Adv. Funct. Mater. 2022, 32, 2203242. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Cho, J.; Lee, J.; Huang, X.; Jia, L.; Fan, J.A.; Su, Y.; Su, J.; Zhang, H.; et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013, 4, 1543. [Google Scholar] [CrossRef]
- Yin, L.; Seo, J.K.; Kurniawan, J.; Kumar, R.; Lv, J.; Xie, L.; Liu, X.; Xu, S.; Meng, Y.S.; Wang, J. Highly Stable Battery Pack via Insulated, Reinforced, Buckling-Enabled Interconnect Array. Small 2018, 14, 1800938. [Google Scholar] [CrossRef]
- Kim, J.S.; Ko, D.; Yoo, D.J.; Jung, D.S.; Yavuz, C.T.; Kim, N.I.; Choi, I.S.; Song, J.Y.; Choi, J.W. A half millimeter thick coplanar flexible battery with wireless recharging capability. Nano Lett. 2015, 15, 2350–2357. [Google Scholar] [CrossRef]
- Ahmad, S.; Copic, D.; George, C.; De Volder, M. Hierarchical assemblies of carbon nanotubes for ultraflexible Li-ion batteries. Adv. Mater. 2016, 28, 6705–6710. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.y.; Lu, B.; Song, Y.; Qiao, Y.; Guo, X.; Ao, S.; Zhang, J.; Fang, D.; Bao, Y. Crocodile skin inspired rigid-supple integrated flexible lithium ion batteries with high energy density and bidirectional deformability. Energy Storage Mater. 2022, 47, 149–157. [Google Scholar] [CrossRef]
- Mo, F.; Liang, G.; Huang, Z.; Li, H.; Wang, D.; Zhi, C. An overview of fiber-shaped batteries with a focus on multifunctionality, scalability, and technical difficulties. Adv. Mater. 2020, 32, 1902151. [Google Scholar] [CrossRef]
- Weng, W.; Sun, Q.; Zhang, Y.; Lin, H.; Ren, J.; Lu, X.; Wang, M.; Peng, H. Winding aligned carbon nanotube composite yarns into coaxial fiber full batteries with high performances. Nano Lett. 2014, 14, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Weng, W.; Ren, J.; Qiu, L.; Zhang, Z.; Chen, P.; Chen, X.; Deng, J.; Wang, Y.; Peng, H. Twisted aligned carbon nanotube/silicon composite fiber anode for flexible wire-shaped lithium-ion battery. Adv. Mater. 2014, 26, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wang, L.; Lo, C.M.; Zhao, Y.; Jiao, Y.; Zheng, G.; Peng, H. A fiber-shaped aqueous lithium ion battery with high power density. J. Mater. Chem. A 2016, 4, 9002–9008. [Google Scholar] [CrossRef]
- Khudiyev, T.; Grena, B.; Loke, G.; Hou, C.; Jang, H.; Lee, J.; Noel, G.H.; Alain, J.; Joannopoulos, J.; Xu, K.; et al. Thermally drawn rechargeable battery fiber enables pervasive power. Mater. Today 2022, 52, 80–89. [Google Scholar] [CrossRef]
- Yadav, A.; De, B.; Singh, S.K.; Sinha, P.; Kar, K.K. Facile development strategy of a single carbon-fiber-based all-solid-state flexible lithium-ion battery for wearable electronics. ACS Appl. Mater. Interfaces 2019, 11, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Weng, W.; Ren, J.; Peng, H. A cable-shaped lithium sulfur battery. Adv. Mater. 2016, 28, 491–496. [Google Scholar] [CrossRef]
- Liu, Y.; Gorgutsa, S.; Santato, C.; Skorobogatiy, M. Flexible, solid electrolyte-based lithium battery composed of LiFePO4 cathode and Li4Ti5O12 anode for applications in smart textiles. J. Electrochem. Soc. 2012, 159, A349. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; La Mantia, F.; Yang, Y.; Cui, Y. Thin, flexible secondary Li-ion paper batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, L.; Mao, X.; Yang, Y.; Yan, L.; Zeng, S.; Zhao, K.; Huang, Q.a.; Liu, M.; Liu, X.; et al. Electronic synergy to boost the performance of NiCoP-NWs@ FeCoP-NSs anodes for flexible lithium-ion batteries. Nanoscale 2022, 14, 8398–8408. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Du, L.; Han, B.; Xu, H.; Meng, H.; Zeng, H.; Zheng, Z.; Deng, Y. Biobased Self-Growing Approach toward Tailored, Integrated High-Performance Flexible Lithium-Ion Battery. Nano Lett. 2022, 22, 9327–9334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jeong, S.; Hu, L.; Wu, H.; Lee, S.W.; Cui, Y. Transparent lithium-ion batteries. Proc. Natl. Acad. Sci. USA 2011, 108, 13013–13018. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wen, S.; Hu, H.; Tang, Q.; Chi, S.S.; Wang, J.; Huang, W.; Yang, Y.; Wang, C.; Deng, Y.; et al. Bamboo mat-inspired interlocking compact textile electrodes for high-energy-density flexible lithium-ion full batteries. Energy Storage Mater. 2023, 55, 388–396. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Song, M.S.; Bao, Z.; Cui, Y. Stretchable lithium-ion batteries enabled by device-scaled wavy structure and elastic-sticky separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Song, Z.; Ma, T.; Tang, R.; Cheng, Q.; Wang, X.; Krishnaraju, D.; Panat, R.; Chan, C.K.; Yu, H.; Jiang, H. Origami lithium-ion batteries. Nat. Commun. 2014, 5, 3140. [Google Scholar] [CrossRef]
- Li, N.; Chen, H.; Yang, S.; Yang, H.; Jiao, S.; Song, W.L. Bidirectional planar flexible snake-origami batteries. Adv. Sci. 2021, 8, 2101372. [Google Scholar] [CrossRef]
- Meng, Q.; Zhu, J.; Kang, C.; Xiao, X.; Ma, Y.; Huo, H.; Zuo, P.; Du, C.; Lou, S.; Yin, G. Kirigami-Inspired Flexible Lithium-Ion Batteries via Transformation of Concentrated Stress into Segmented Strain. Small 2022, 18, 2204745. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Lv, C.; An, Y.; Liang, M.; Ma, T.; He, D.; Zheng, Y.J.; Huang, S.Q.; Yu, H.; et al. Kirigami-based stretchable lithium-ion batteries. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Hong, G.; Chen, Y.; Chen, J.; Chen, H.; Song, W.L.; Fang, D. Customized kirigami electrodes for flexible and deformable lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 12, 780–788. [Google Scholar] [CrossRef]
- Qian, G.; Zhu, B.; Liao, X.; Zhai, H.; Srinivasan, A.; Fritz, N.J.; Cheng, Q.; Ning, M.; Qie, B.; Li, Y.; et al. Bioinspired, spine-like, flexible, rechargeable lithium-ion batteries with high energy density. Adv. Mater. 2018, 30, 1704947. [Google Scholar] [CrossRef]
- Chen, A.; Guo, X.; Yang, S.; Liang, G.; Li, Q.; Chen, Z.; Huang, Z.; Yang, Q.; Han, C.; Zhi, C. Human joint-inspired structural design for a bendable/foldable/stretchable/twistable battery: Achieving multiple deformabilities. Energy Environ. Sci. 2021, 14, 3599–3608. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Bai, W.; Chen, X.; Zhang, Z.; Fang, X.; Weng, W.; Wang, Y.; Peng, H. Elastic and wearable wire-shaped lithium-ion battery with high electrochemical performance. Angew. Chem. 2014, 126, 7998–8003. [Google Scholar] [CrossRef]
- Meng, Q.; Kang, C.; Zhu, J.; Xiao, X.; Ma, Y.; Huo, H.; Zuo, P.; Du, C.; Lou, S.; Yin, G. DNA Helix Structure Inspired Flexible Lithium-Ion Batteries with High Spiral Deformability and Long-Lived Cyclic Stability. Nano Lett. 2022, 22, 5553–5560. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Nam, S.; Oh, M.; Lee, H.J.; Jang, B.; Hyun, S. Bioinspired, Shape-Morphing Scale Battery for Untethered Soft Robots. Soft Robot. 2022, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]







| Electrode | Electrolyte | Bending Angle | Elongation Rate | Curling Radius | |
|---|---|---|---|---|---|
| Traditional LIBs | rigidity | rigidity | × | × | × |
| FSLIBs | flexible | flexible solid-state | 180 | 240% | 0.3 mm |
| No. | Material | Specific Capacity | Cycling Performance | Maximum Deformation | Deformation Cycle | Ref. |
|---|---|---|---|---|---|---|
| 1 | FeO/SWCNT | 1000 mAh/g | 800 mAh/g (at 100 cycles) | N/A | N/A | [27] |
| 2 | LFP/CNT/EVA | 166 mAh/g | 120 mAh/g (at 60 cycles) | N/A | 60 | [30] |
| 3 | PDHBQS/SWCNTs | 156 mAh/g (at 250 mA/g) | 136 mAh/g (at 250 mA/g, 500 cycles) | r = 210 mm | 2000 | [31] |
| 4 | RGO/CoS | 1415 mAh/g | 573 mAh/g (at 545 mA/g, 500 cycles) | N/A | N/A | [42] |
| 5 | VGSs/Si | 1754 mAh/g | 1403.5 mAh/g (at 2A/g, 500 cycles) | N/A | N/A | [44] |
| 6 | ACT/NiS −graphene | 1710 mAh/g (at 17 mA/g) | 1016 mAh/g (at 170 mA/g, 400 cycles) | N/A | N/A | [45] |
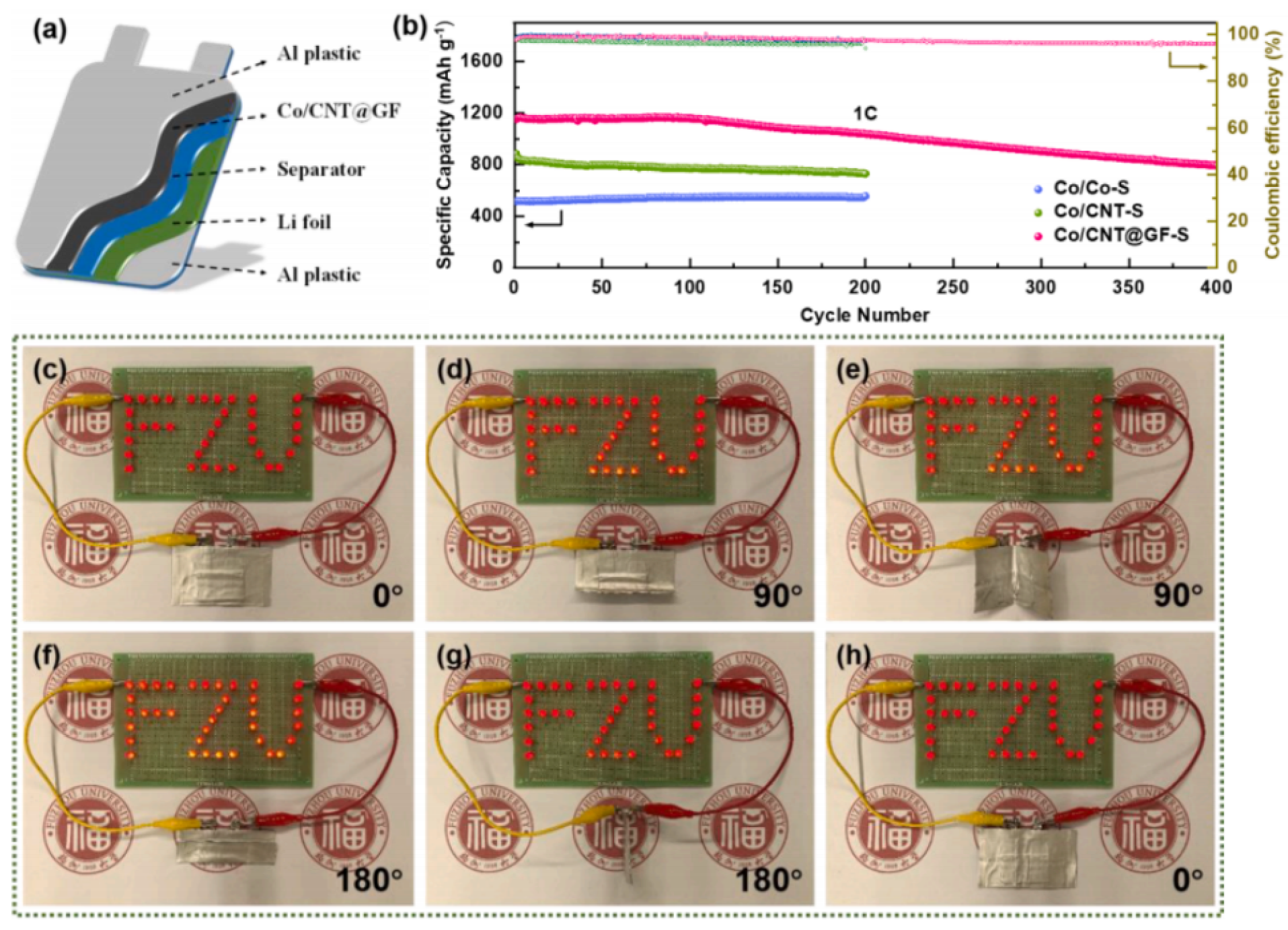
| 7 | Co/CNT@GF | 1043 mAh/g (at 1675 mA/g) | 793 mAh/g (at 1675 mA/g, 400 cycles) | deg = 180 | N/A | [43] |
| 8 | SHCM/NCF | 1800 mAh/g (at 1 A/g) | 1442 mAh/g (at 1 A/g, 800 cycles) | deg = 180 | N/A | [59] |
| 9 | ZnSe@CNFs | 547.6 mAh/g (at 5 A/g) | 426.1 mAh/g (at 5 A/g, 3000 cycles) | deg = 180 | N/A | [60] |
| 10 | LTO@CFC | 105.8 mAh/g (at 50 C) | 118 mAh/g (at 10 C, 500 cycles) | deg = 180 | 200 | [63] |
| 11 | SiOx-G/PAAPANi/ graphene | 1126 mAh/g (at 500 mA/g) | 842.3 mAh/g (at 500 mA/g, 100 cycles) | N/A | N/A | [29] |
| 12 | Si | 2026 mAh/g | 1620.8 mAh/g | ɛ = 243.7% | N/A | [74] |
| No. | Design Principle | Electrode Materials | Specific Capacity | Cylability and Defprmability | Ref. |
|---|---|---|---|---|---|
| 1 | 1D fiber-shaped FLIB | LiCoO@rGO cathode, SnO@rGO anode | 82.6 mAh/g | 50.3% capacity retention after being cut and healed for 5 times during 50 cycles, bendable | [105] |
| 2 | 1D fiber-shaped FLIB | LCO cathode, graphite anode | 85.69 Wh/kg | 80% capacity retention after 100,000 bending cycles. | [106] |
| 3 | 1D fiber-shaped FLIB | LiMnO/CNT cathode, PI/CNT anode | 123 mAh/g | 90% capacity retention after 200 cycles.bendable, foldable, and rollable | [107] |
| 4 | 2D film-shaped FLIB | LiCoO cathode, Li metal anode | 106 Ah/cm | 98.4% capacity retention after 100 cycles, r = 3.1 mm | [103] |
| 5 | 2D film-shaped FLIB | LiFePO cathode, preLi-CC anode | 1.91 mAh/cm | 84% capacity retention after 1000 cycles, r = 3.1 mm | [108] |
| 6 | 2D film-shaped FLIB | LiCoO cathode, LiTiO anode | 1.1 mAh/cm | 59% capacity retention after 20 cycles, bendable, ɛ = 300% | [109] |
| 7 | 2D film-shaped FLIB | LCO−ICTE cathode, Gr−ICTE anode | 4.5 mAh/cm | 90% capacity retention after 150 cycles, r = 4.0 mm | [110] |
| 8 | 2D film-shaped FLIB | Graphite cathode, NCA anode | 130 mAh/g | 87.5% capacity retentions after 50 cycles at 25C, r = 5 mm | [111] |
| 9 | 3D FLIB | CNT−LNCO cathode, CNT−FeO anode | 120 mAh/g (at 2 C ) | 70% capacity retentions after 500 cycles at 1 C, r = 0.3 mm | [112] |
| 10 | 3D FLIB | LiCoO2cathode, graphite anode | 144.2 mAh/g (at 0.5 C ) | 70% capacity retentions after stretching and bending at 90° and 180° for 10,000 times each, and 100 charge/discharge cycles | [113] |
| 11 | 3D FLIB | LiNiCoMnO-based cathode, graphite-based anode | 164.24 mAh/g (at 0.5 C) | 92.3% capacity retentions undergo 30,000 times bending and after 200 charge/discharge cycles | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; He, T. Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies 2023, 16, 4549. https://doi.org/10.3390/en16124549
Deng R, He T. Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies. 2023; 16(12):4549. https://doi.org/10.3390/en16124549
Chicago/Turabian StyleDeng, Ru, and Tian He. 2023. "Flexible Solid-State Lithium-Ion Batteries: Materials and Structures" Energies 16, no. 12: 4549. https://doi.org/10.3390/en16124549
APA StyleDeng, R., & He, T. (2023). Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies, 16(12), 4549. https://doi.org/10.3390/en16124549








