Combustion Characteristics of N-Butanol/N-Heptane Blend Using Reduced Chemical Kinetic Mechanism
Abstract
:1. Introduction
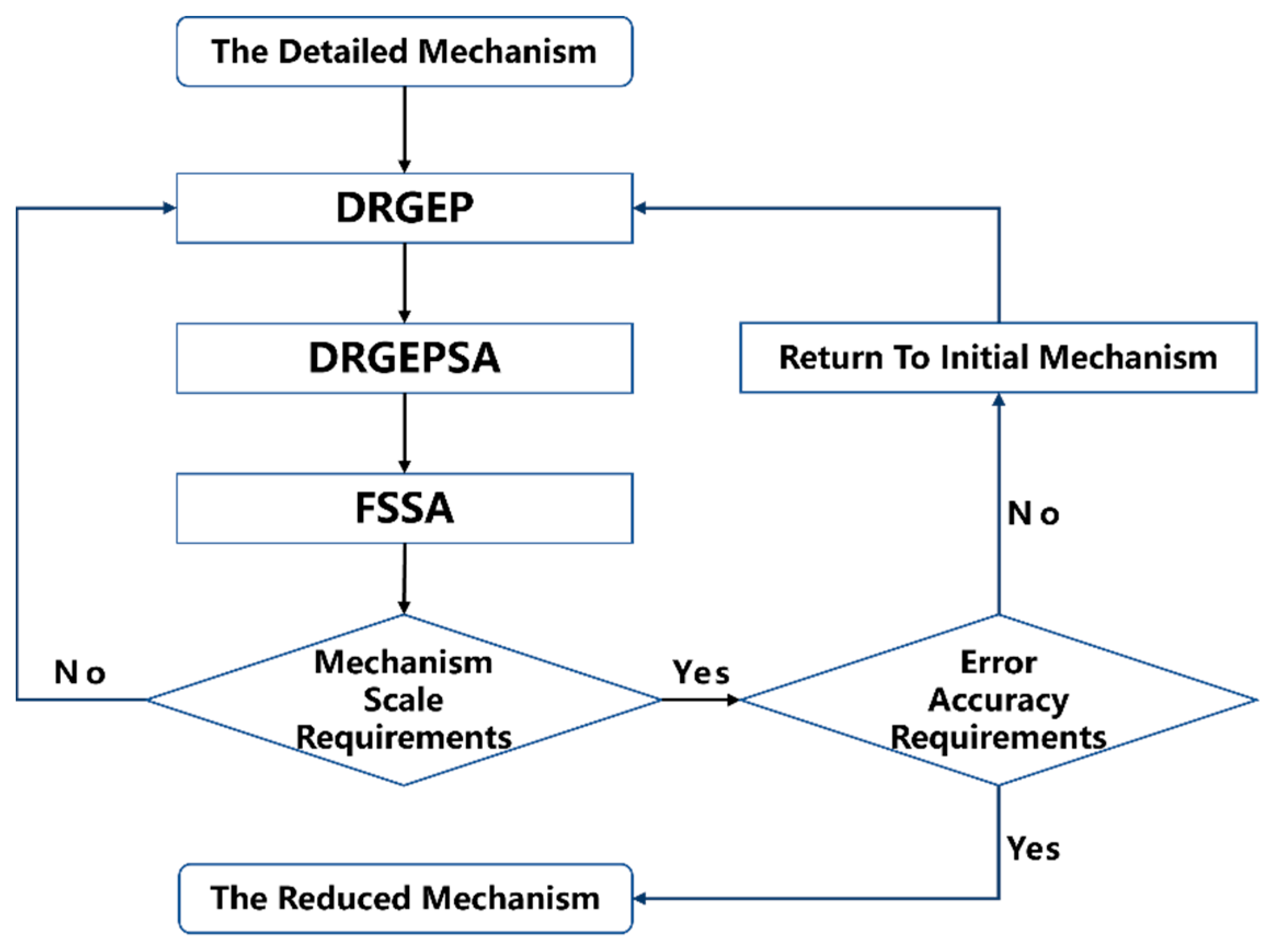
2. Mechanism Reduction Method
2.1. Direct Relationship Graph Method Based on Error Propagation
2.2. Direct Relationship Graph Method for Coupled Error Propagation and Sensitivity Analysis
2.3. Total Material Sensitivity Analysis
3. Reduction of the Chemical Reaction Mechanism of Oxygenated Fuels
3.1. Mechanism Reduction for N-Heptane
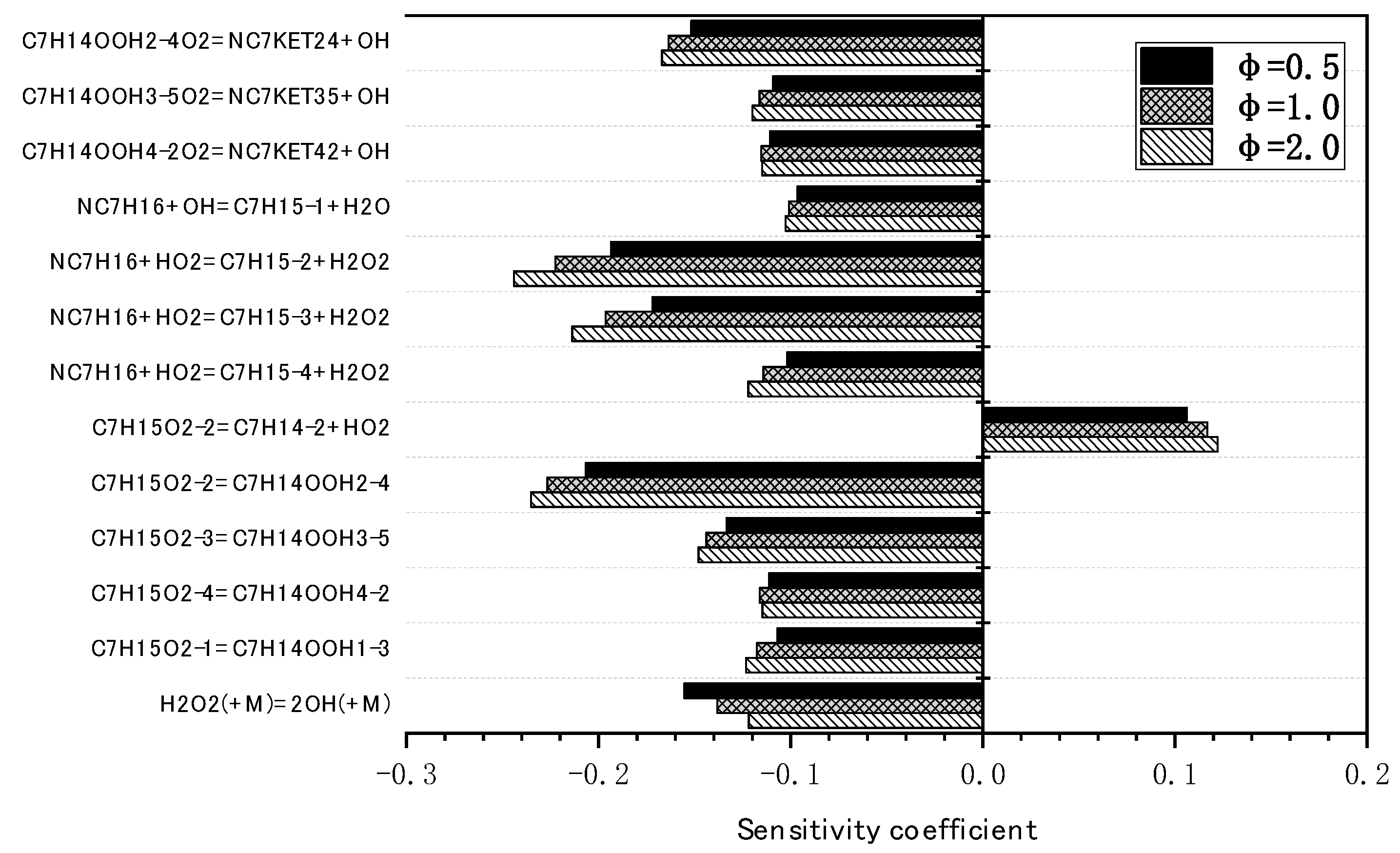
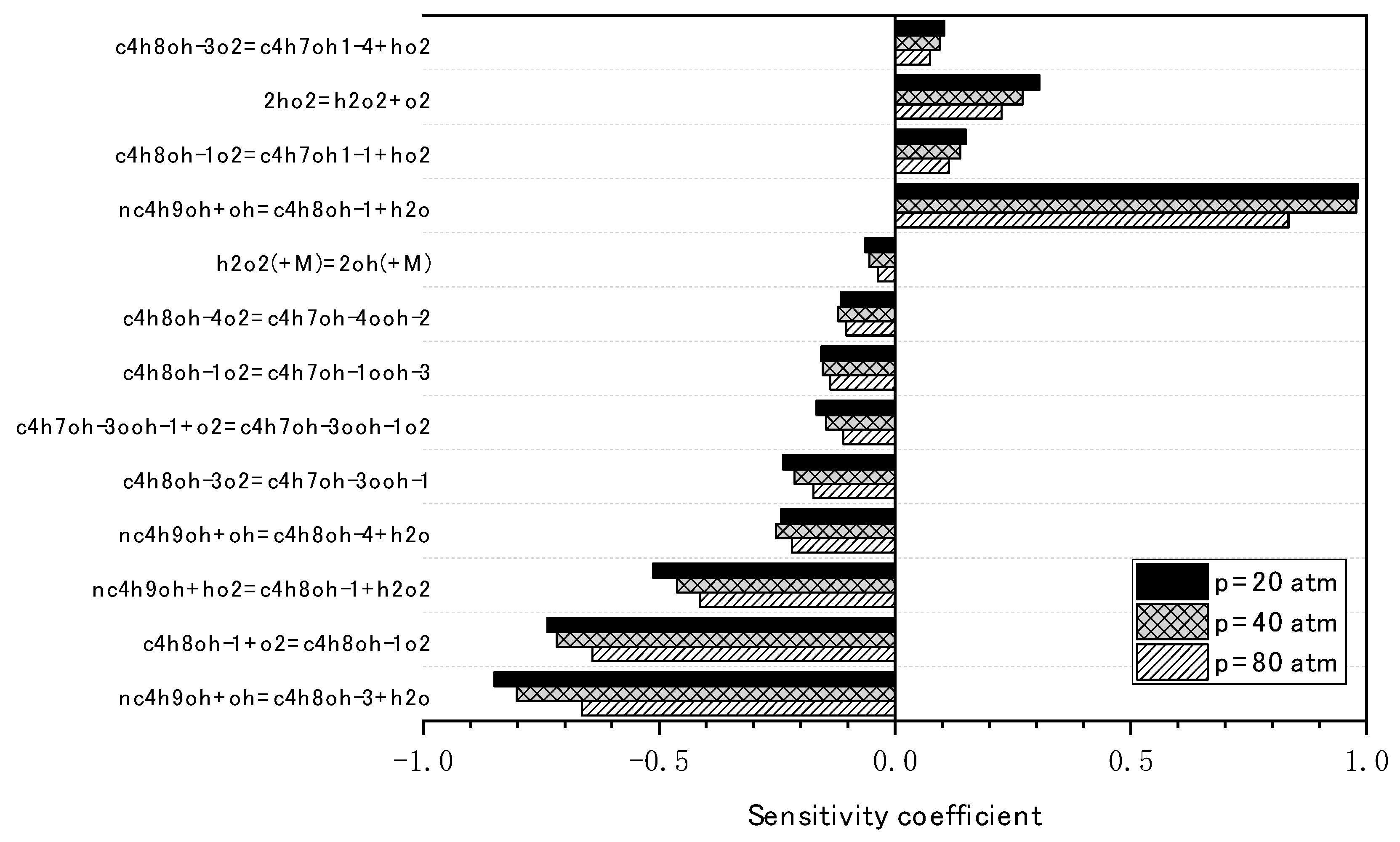
3.2. Mechanism Reduction for N-Butanol
4. Reduced Mechanism Validation
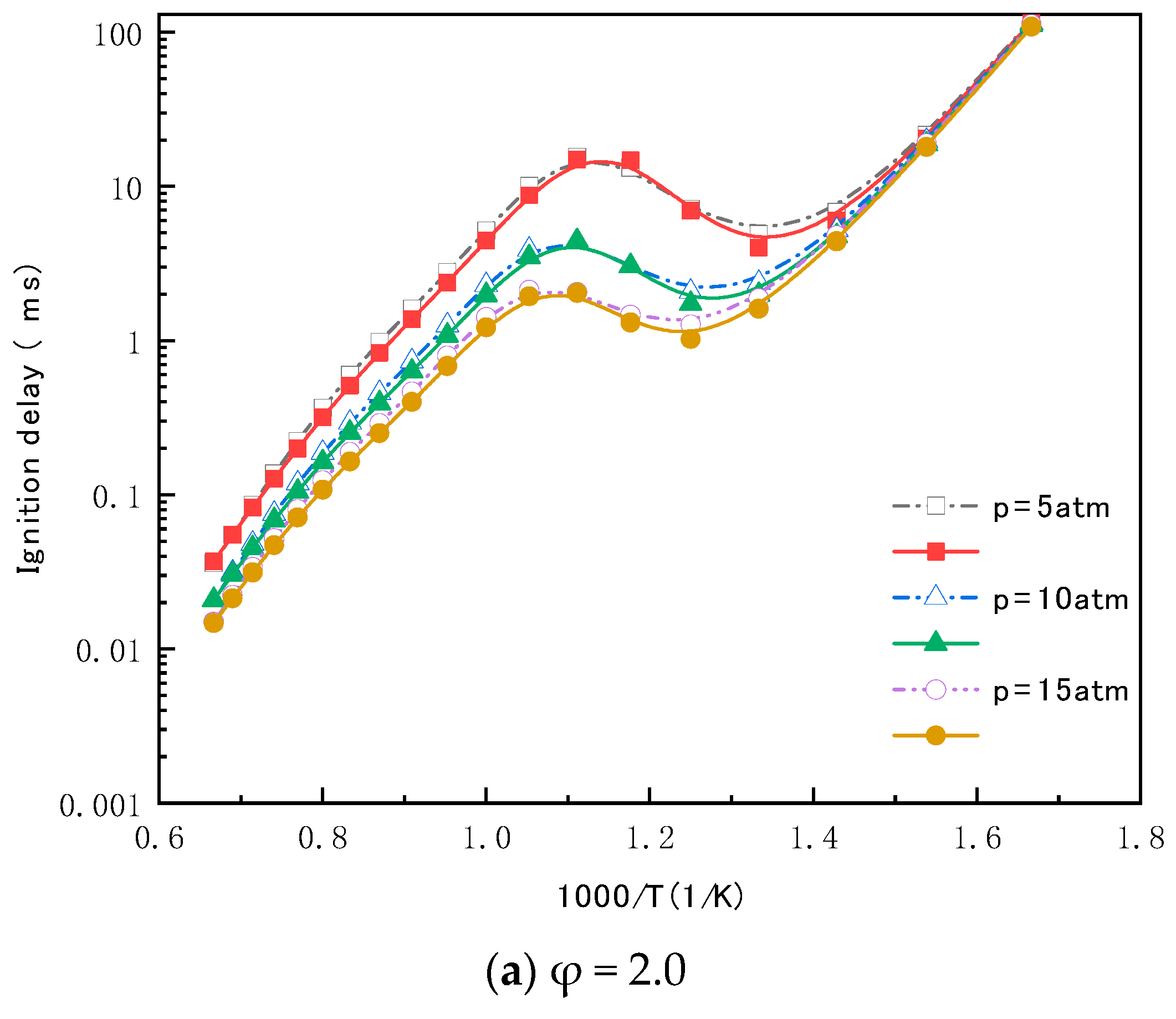
4.1. Ignition Delay Time Validation of the Reduced Mechanism for N-Heptane
4.2. Validation of N-Heptane Reduced Mechanism Species Profiles
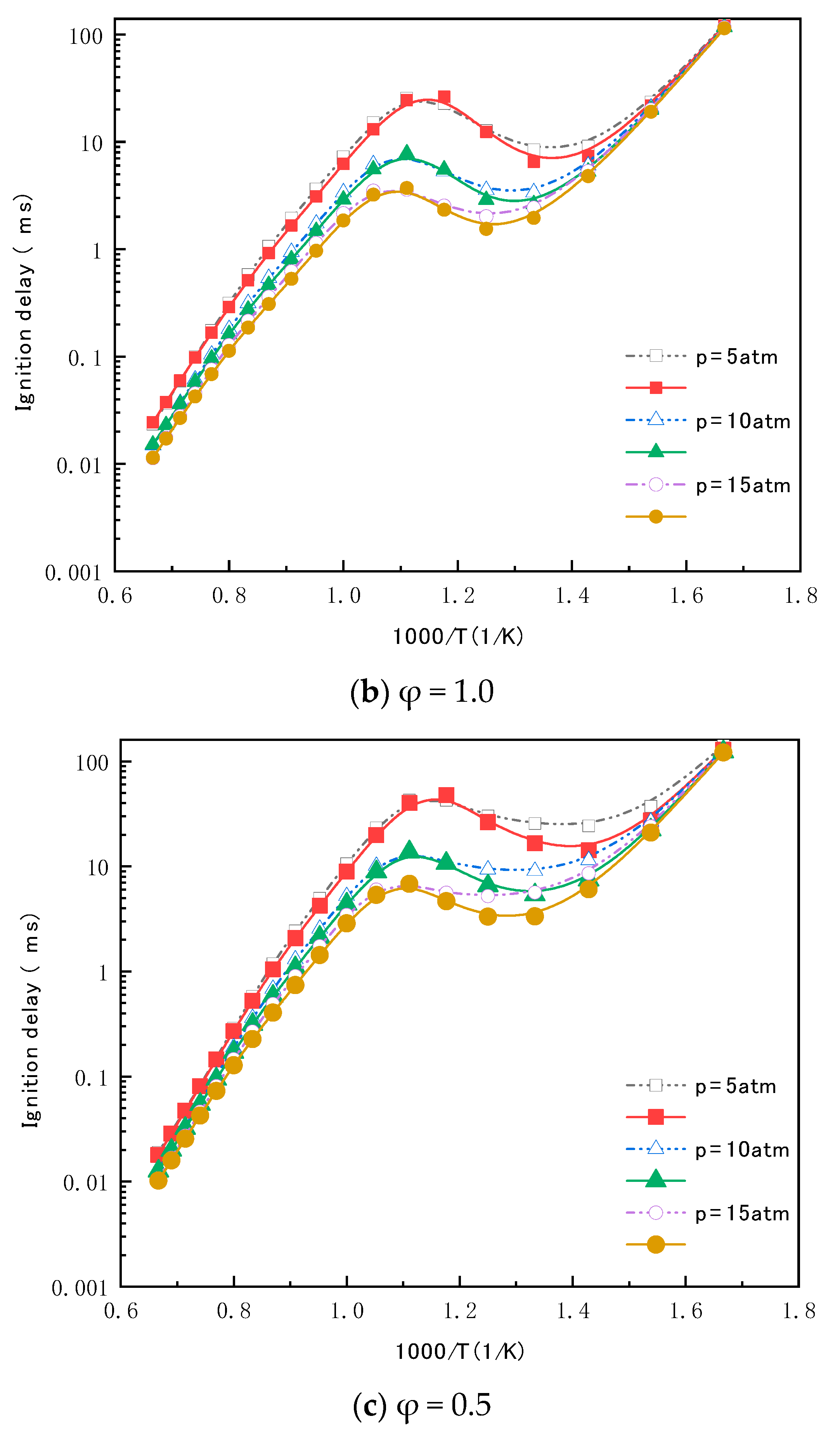
4.3. Ignition Delay Time Validation of the Reduced Mechanism for N-Butanol
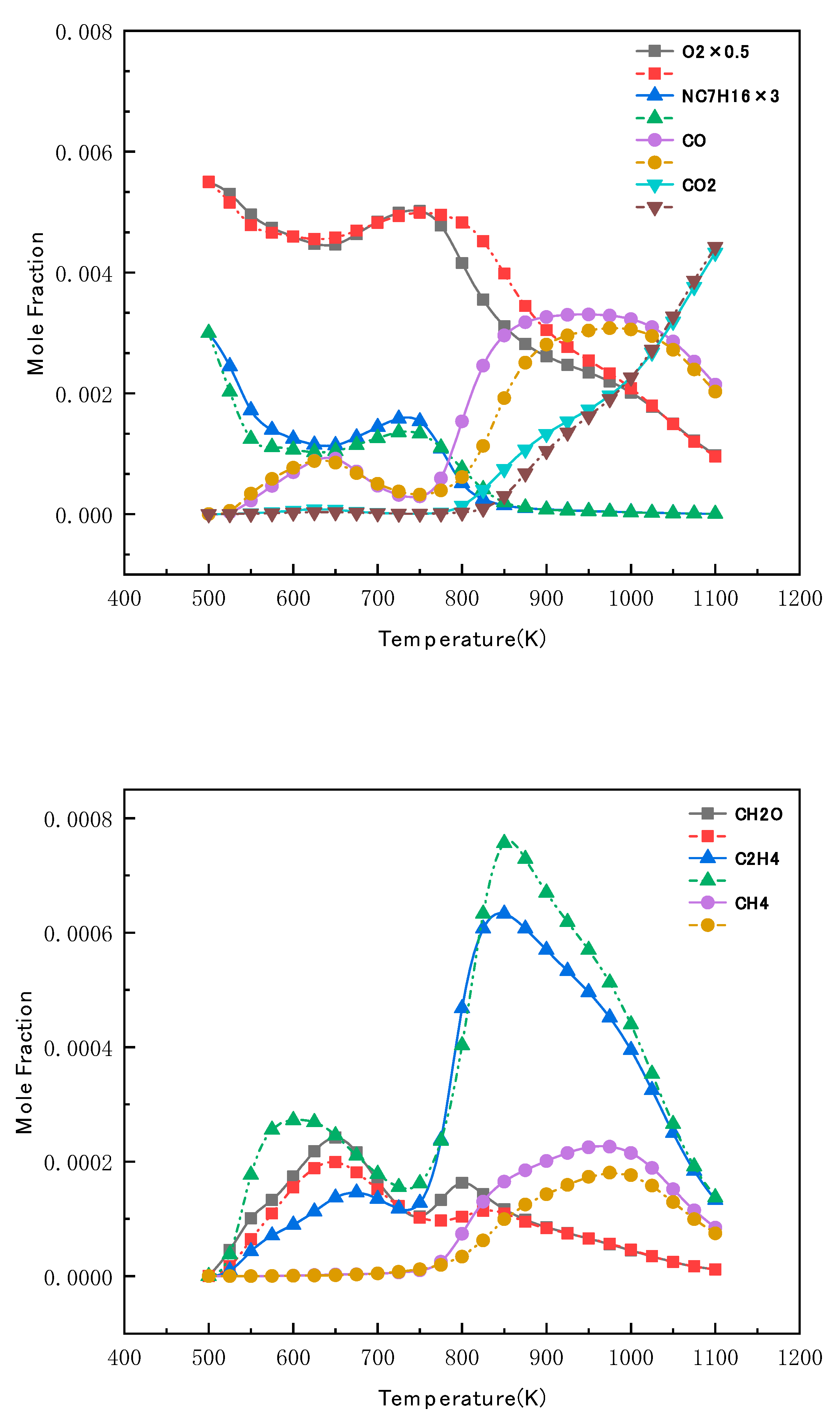
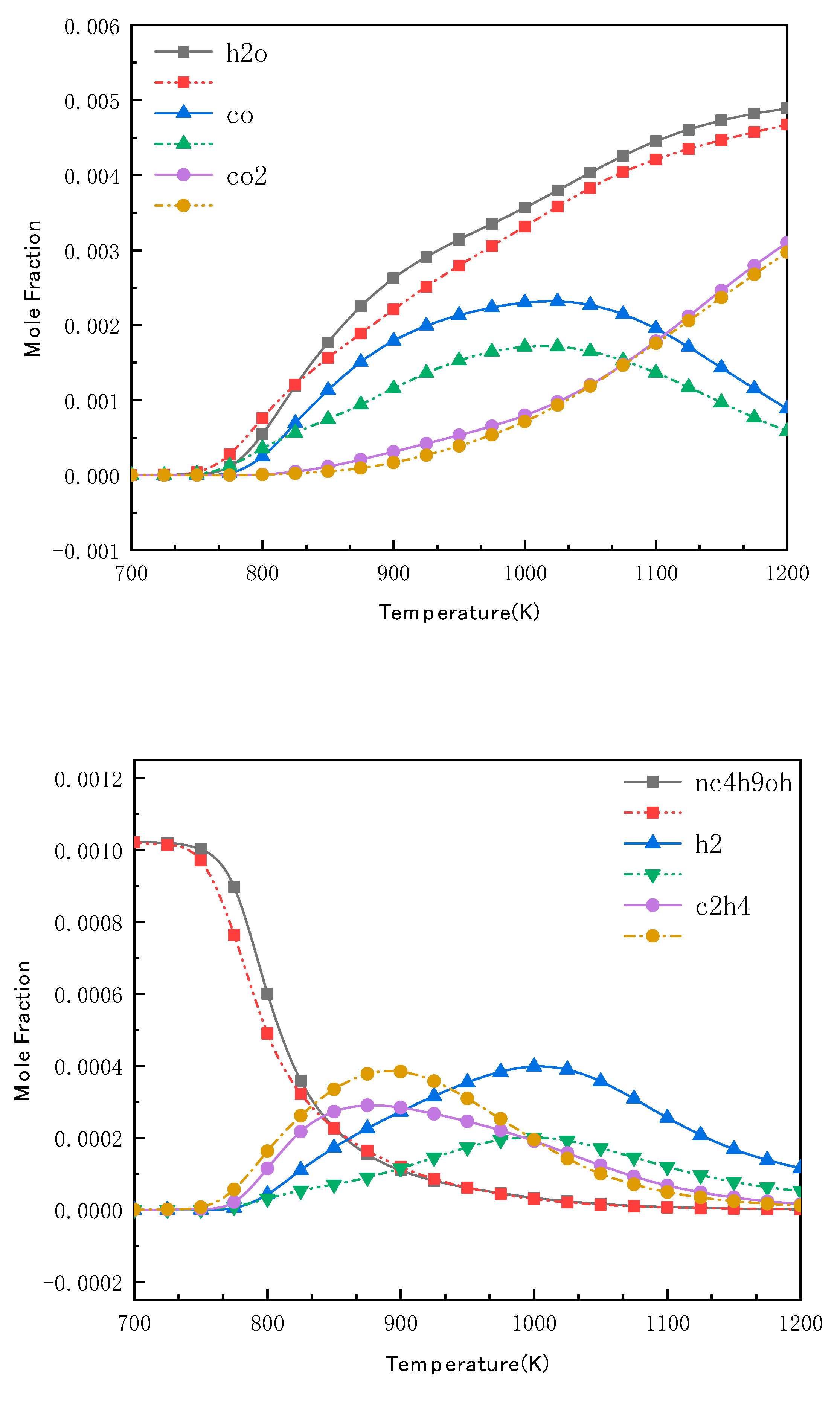
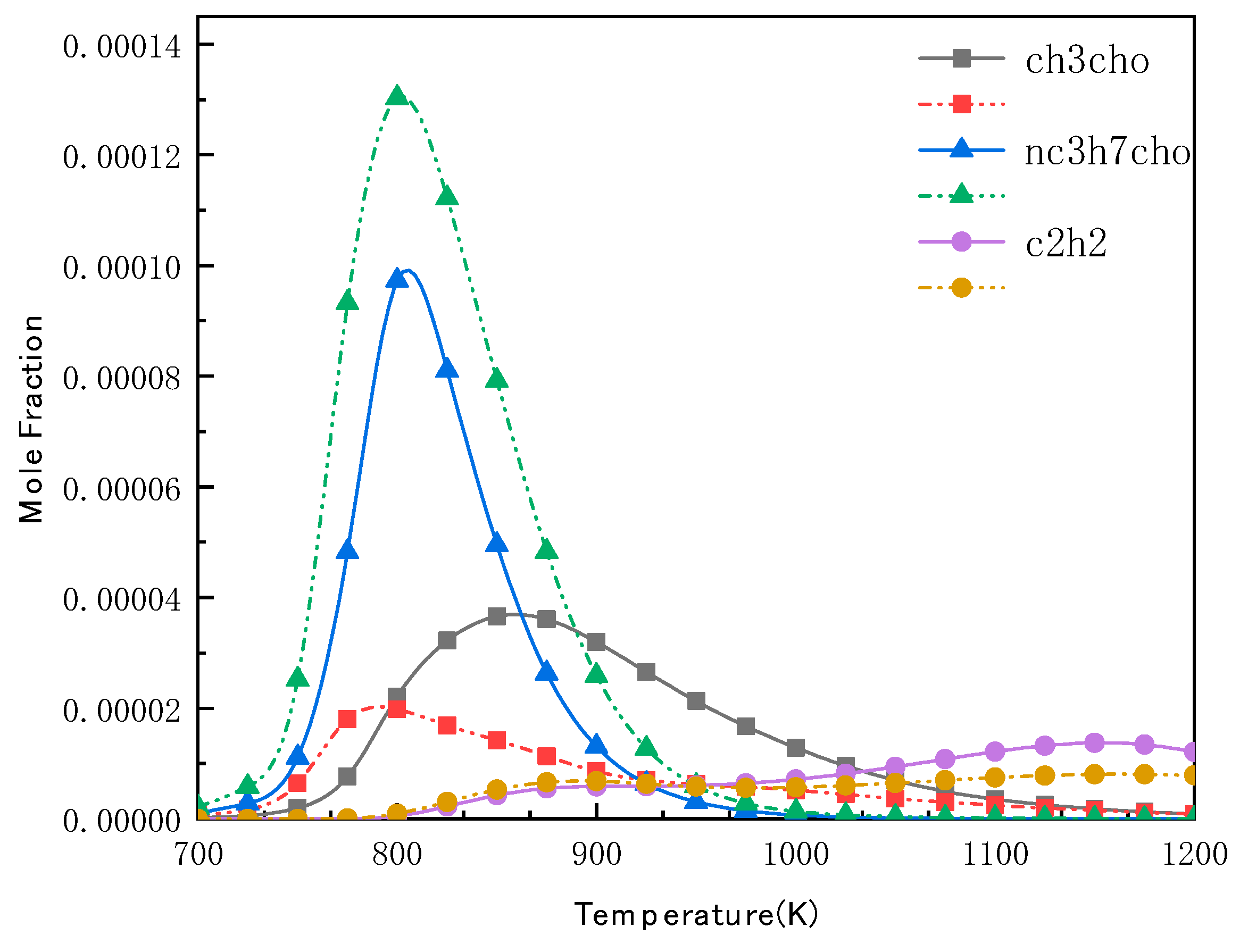
4.4. Validation of N-Butanol Reduced Mechanism Species Profiles
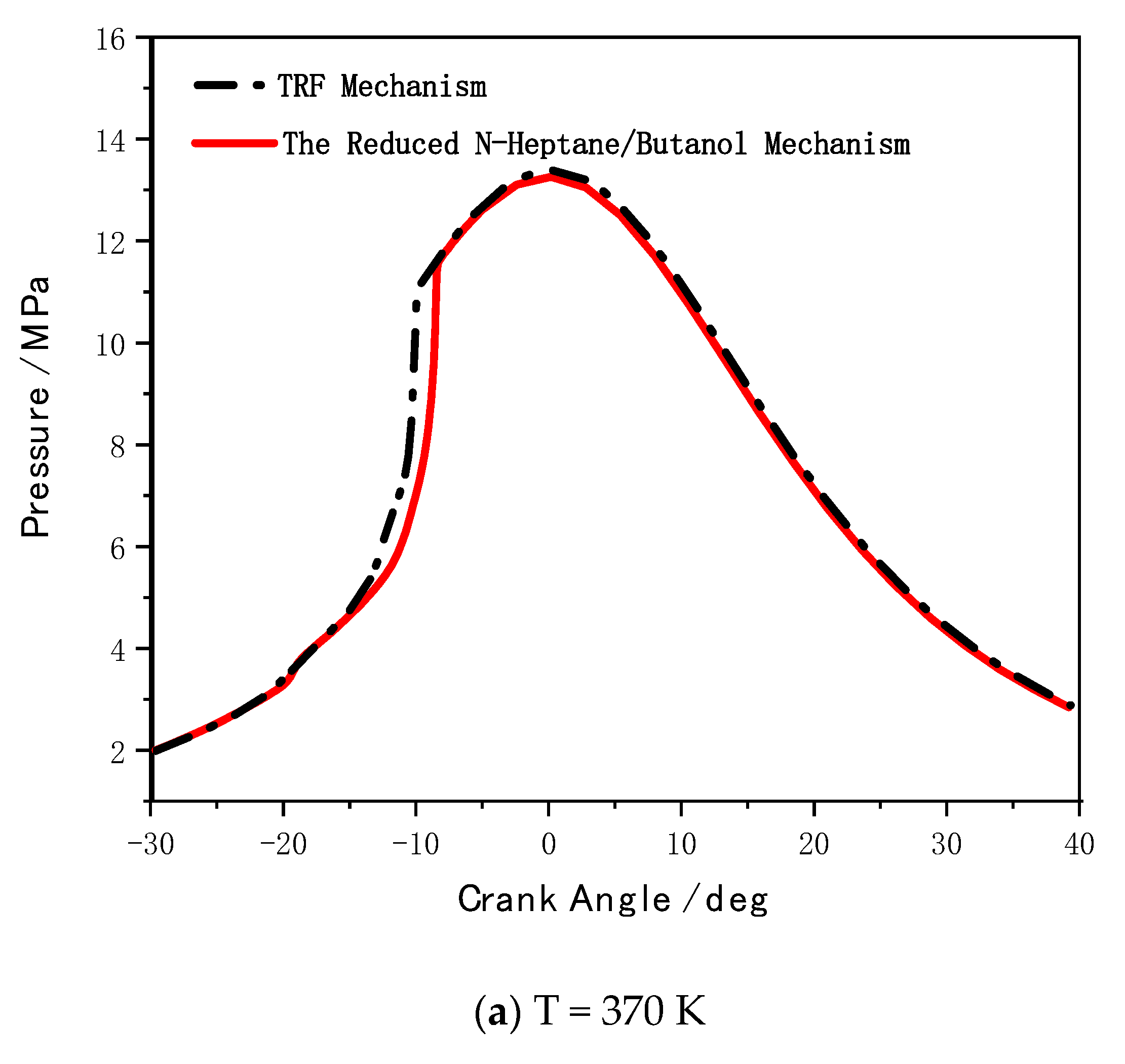
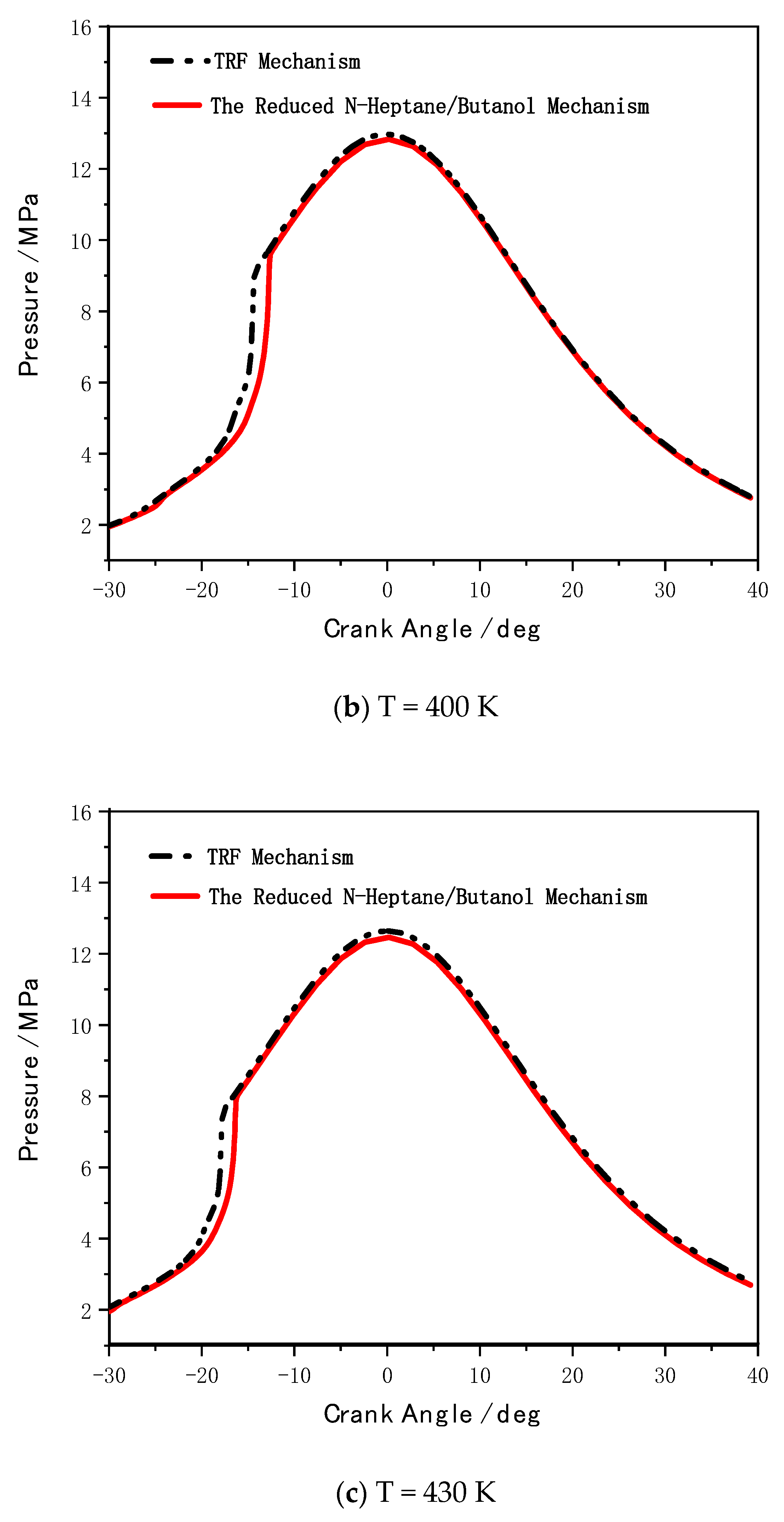
5. Mechanism Coupling and HCCI Combustion Validation
6. Conclusions
- Complete the detailed mechanism simplification of n-heptane
- 2.
- Complete the detailed mechanism simplification of n-butanol
- 3.
- Verification of n-heptane simplification mechanism
- 4.
- n-butanol simplification mechanism verification
- 5.
- Simplified mechanism coupling and HCCI model verification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Song, C.; Tao, Y.; Lv, G.; Dong, S. Diesel soot oxidation during the late combustion phase. Combust. Flame 2011, 158, 446–451. [Google Scholar] [CrossRef]
- Li, H.; Song, C.; Lv, G.; Pang, H.; Qiao, Y. Assessment of the impact of post-injection on exhaust pollutants emitted from a diesel engine fueled with biodiesel. Renew. Energy 2017, 114, 924–933. [Google Scholar] [CrossRef]
- Prabhahar, M.; Alharbi, S.A.; Almoallim, H.S.; Prakash, S.; Kumar, M.S.; Surendrababu, K.; Sendilvelan, S.; Bhaskar, K.; Maroušek, J.; Anderson, A. Trade-off characteristic between soot and oxides of nitrogen emission with pre-injected diesel and biodiesel in a SICI-CIDI engine. Fuel 2023, 331, 125624. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, C.; Lyu, G.; Li, Y.; Liu, Y.; Wang, C.; Song, C. An analysis of the in-cylinder soots generated from the main- and post-injection combustion in diesel engines. Proc. Combust. Inst. 2022, 39, 939–947. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Xu, W.; Ma, Y.; Tan, D.; Peng, Q.; Tan, Y.; Chen, L. Soot formation mechanism of modern automobile engines and methods of reducing soot emissions: A review. Fuel Process. Technol. 2022, 235, 107373. [Google Scholar]
- Thakar, R.; Lahane, S.; Bhosle, S. Experimental investigation to study combustion and emission characteristics of diesel engine by application of EGR and heated intake air. Mater. Today Proc. 2023, 72, 687–693. [Google Scholar] [CrossRef]
- Mahla, S.K.; Dhir, A.; Gill, K.J.; Cho, H.M.; Lim, H.C.; Chauhan, B.S. Influence of EGR on the simultaneous reduction of NOx-smoke emissions trade-off under CNG-biodiesel dual fuel engine. Energy 2018, 152, 303–312. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Shu, G.; Zhou, L. Ammonia-hydrogen engine with reactivity-controlled turbulent jet ignition (RCTJI). Fuel 2023, 348, 128580. [Google Scholar] [CrossRef]
- Zhang, D. Investigation on Hot Surface Assisted Ignition Combustion Process of Electrical Control Injection Natural Gas Engine. Trans. Chin. Soc. Agric. Mach. 2008. [Google Scholar]
- Mahla, S.K.; Ardebili, S.M.S.; Sharma, H.; Dhir, A.; Goga, G.; Solmaz, H. Determination and utilization of optimal diesel/n-butanol/biogas derivation for small utility dual fuel diesel engine. Fuel 2021, 289, 119913. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The effect of ammonia addition on the low-temperature autoignition of n-heptane: An experimental and modeling study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Eryilmaz, T.; Arslan, M. A comparative analysis of the engine performance, exhaust emissions and combustion behaviors of a compression ignition engine fuelled with biodiesel/diesel/1-butanol (C4 alcohol) and biodiesel/diesel/n-pentanol (C5 alcohol) fuel blends. Energy 2018, 165, 1332–1351. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S.; Kanapkienė, I. The individual effects of cetane number oxygen content or fuel properties on the ignition delay combustion characteristics and cyclic variation of a turbocharged CRDI diesel engine-part 1. Energy Convers. Manag. 2017, 148, 1003–1027. [Google Scholar] [CrossRef]
- Deng, S.; Koch, J.A.; Mueller, M.E.; Law, C.K. Sooting limits of non-premixed n-heptane, n-butanol, and methyl butanoate flames: Experimental determination and mechanistic analysis. Fuel 2014, 136, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lyu, G.; Song, C.; Qiao, Y. Effects of Biodiesel Addition on the Physical Properties and Reactivity of the Exhaust Soot Particles from Diesel Engine. Energies 2020, 13, 4206. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Song, J.-T. Experimental study of co-combustion ratio on fuel consumption and emissions of NG–diesel dual-fuel heavy-duty engine equipped with a common rail injection system. J. Energy Inst. 2016, 89, 578–585. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.; Zheng, C.; Zhang, B. Chemical kinetics model construction and path analysis of biodiesel alternative mixture. J. Phys. Chem. 2014, 30, 217–226. [Google Scholar]
- Xin, Y.; Sheen, D.A.; Wang, H.; Law, C.K. Skeletal reaction model generation, uncertainty quantification and minimization: Combustion of butane. Combust. Flame 2014, 161, 3031–3039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qi, Q.; Sun, S. Reduced construction of combustion reaction mechanism of biodiesel substitute. J. Combust. Sci. Technol. 2021, 27, 635–643. [Google Scholar]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a waste cooking oil biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Prince, J.C.; Williams, F.A.; Ovando, G.E. A short mechanism for the low-temperature ignition of n-heptane at high pressures. Fuel 2015, 149, 138–142. [Google Scholar] [CrossRef]
- Xu, H.; Yao, C.; Xu, G. Chemical kinetic mechanism and a skeletal model for oxidation of n-heptane/methanol fuel blends. Fuel 2012, 93, 625–631. [Google Scholar] [CrossRef]
- Hatim, M. Experimental validation of a kinetic multi-component mechanism in a wide HCCI engine operating range for mixtures of n-heptane, iso-octane and toluene:Influence of EGR parameters. Energy Convers. Manag. 2008, 49, 2956–2965. [Google Scholar]
- Bernardi, M.S.; Pelucchi, M.; Stagni, A.; Sangalli, L.M.; Cuoci, A.; Frassoldati, A.; Secchi, P.; Faravelli, T. Curve matching, a generalized framework for models/experiments comparison: An application to n- heptane combustion kinetic mechanisms. Combust. Flame 2016, 168, 186–203. [Google Scholar] [CrossRef]
- Curran, H.; Gaffuri, P.; Pitz, W.; Westbrook, C. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Pelucchi, M.; Bissoli, M.; Cavallotti, C.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Ranzi, E.; Stagni, A. Improved kinetic model of the low-temperature oxidation of n-heptane. Energy Fuels 2014, 28, 7178–7193. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Lee, C.-F.L.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Liang, Y.; Yu, L.; Lu, X. Combustion reaction kinetics of biodiesel/n-butanol blends:Experiments in an ultrahigh-pressure rapid compression machine. Combust. Flame 2022, 245, 112313. [Google Scholar] [CrossRef]
- Han, J.; Somers, L.M.T. Comparative investigation of ignition behavior of butanol isomers using constant volume combustion chamber under diesel-engine like conditions. Fuel 2021, 304, 121347. [Google Scholar] [CrossRef]
- Wakale, A.B.; Banerjee, S.; Banerjee, R. Estimation of NOx and soot emission from a constant volume n-butanol/n-dodecane blended spray using unsteady flamelet model based on n-dodecane/n-butanol/NOx/PAH chemistry. J. Energy Inst. 2020, 93, 1868–1882. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, J.; Liu, D.; An, M.; Zhang, W.; Zhang, X. Development of a reduced n-butanol mechanism with combined reduction methods. Fuel 2017, 187, 403–416. [Google Scholar] [CrossRef]
- Li, Y.; Tang, W.; Abubakar, S.; Wu, G. Construction of a compact skeletal mechanism for acetone–n–butanol–ethanol (ABE)/diesel blends combustion in engines using a decoupling methodology. Fuel Process. Technol. 2020, 209, 106526. [Google Scholar] [CrossRef]
- Valentino, G.; Corcione, F.E.; Iannuzzi, S.E.; Serra, S. Experimental study on performance and emissions of a high speed diesel engine fuelled with n-butanol diesel blends under premixed low temperature combustion. Fuel 2012, 92, 295–307. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, J.; Zhu, Z.; Wei, H.; Lv, D.; Zhang, P.; Sun, H. Development and validation of a new reduced diesel-n-butanol blends mechanism for engine applications. Energy Convers. Manag. 2017, 149, 553–563. [Google Scholar] [CrossRef]
- Cai, Y.; Jia, M.; Xu, G.; Li, Y.; Wang, T. Feasibility study of the combustion strategy of n-butanol/diesel dual direct injection (DI2) in a compression-ignition engine. Fuel 2021, 289, 119865. [Google Scholar] [CrossRef]
- Wan, Z.; Shi, L.; Chen, D.; Li, P.; Zhang, C. Experimental and kinetic study of the effects of n-butanol addition on toluene reference fuel auto-ignition. Fuel 2023, 337, 126858. [Google Scholar] [CrossRef]
- Xiao, J.; Jia, M.; Chang, Y.; Li, Y.; Xu, Z.; Xu, G.; Liu, H.; Wang, T. Numerical optimization and comparative study of n-butanol concentration stratification combustion and n-butanol/diesel reactivity stratification combustion for advanced compression ignition (CI) engine. Fuel 2018, 213, 83–93. [Google Scholar] [CrossRef]
- Alam, F.E.; Liu, Y.; Avedisian, C.; Dryer, F.; Farouk, T. n-Butanol droplet combustion: Numerical modeling and reduced gravity experiments. Proc. Combust. Inst. 2015, 35, 1693–1700. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zheng, M. n-Butanol reactivity modulation with early fuel injections for low NOx compression ignition combustion. Fuel 2019, 242, 243–254. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Vranckx, S.; Yasunaga, K.; Mehl, M.; Oßwald, P.; Metcalfe, W.K.; Westbrook, C.K.; Pitz, W.J.; Kohse-Höinghaus, K.; Fernandes, R.X.; et al. A comprehensive chemical kinetic combustion model for the four butanol isomers. Combust. Flame 2012, 159, 2028–2055. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. Toward accommodating realistic fuel chemistry in large-scale computations. Prog. Energy Combust. 2009, 35, 192–215. [Google Scholar] [CrossRef]
- Pepiot-Desjardins, P.; Pitsch, H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms. Combust Flame 2008, 154, 67–81. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Gou, X.; Ju, Y. A path flux analysis method for the reduction of detailed chemical kinetic mechanisms. Combust. Flame 2010, 157, 1298–1307. [Google Scholar] [CrossRef]
- Niemeyer, K.E.; Sung, C.J.; Raju, M.P. Skeletal mechanism generation for surrogate fuels using directed relation graph with error propagation and sensitivity analysis. Combust Flame 2010, 157, 1760–1770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Simulation Study on the Mechanism of CNG/Diesel Reactivity Controlled Compression Ignition Reaction; Jilin University: Changchun, China, 2016. [Google Scholar]
- Wang, H. Multi-Dimensional Numerical Simulation and Experimental Research on Diesel Engine Soot Generation Mechanism; Tianjin University: Changchun, China, 2012. [Google Scholar]
- Zhang, J.; Wei, L.; Man, X.; Jiang, X.; Zhang, Y.; Hu, E.; Huang, Z. Experimental and modeling study of n-butanol oxidation at high temperature. Energy Fuels 2012, 26, 3368–3380. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, G. A new skeletal mechanism for diesel-n-butanol blends combustion in engine. Fuel 2020, 264, 0016–2361. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Zheng, Z.; Yao, M. Development of a reduced n-butanol/biodiesel mechanism for a dual fuel engine. Fuel 2015, 157, 87–96. [Google Scholar] [CrossRef]
- Heufer, K.A.; Fernandes, R.X.; Olivier, H.; Beeckmann, J.; Röhl, O.; Peters, N. Shock tube investigations of ignition delays of n-butanol at elevated pressures between 770 and 1250 K. Pro. Combust. Inst. 2011, 33, 359–366. [Google Scholar] [CrossRef]
- He, B.Q.; Liu, M.B.; Yuan, J.; Zhao, H. Combustion and Emission Characteristics of a HCCI Engine Fuelled with n-Butanol-Gasoline Blends. Fuel 2013, 108, 668–674. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for com-bustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]


| Engine type | 6 sylinders, 4-stroke, turbo-charged |
| Bore × stroke | 105 mm × 125 mm |
| Engine displacement | 6.5 L |
| Compression ratio | 16:1 |
| Intake valve close timing | −133 deg. CA ATDC |
| Exhaust valve open timing | 125 deg. CA ATDC |
| Engine speed | 1400 r/min |
| IMEP | 4.3 bar |
| Intake air temperature | 373 K |
| Initial pressure | 1.8 bar |
| EGR ratio | 0 |
| Equivalent ratio | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Wang, F.; Pei, Y.; Yang, J.; An, D.; Hao, H. Combustion Characteristics of N-Butanol/N-Heptane Blend Using Reduced Chemical Kinetic Mechanism. Energies 2023, 16, 4768. https://doi.org/10.3390/en16124768
Zhang D, Wang F, Pei Y, Yang J, An D, Hao H. Combustion Characteristics of N-Butanol/N-Heptane Blend Using Reduced Chemical Kinetic Mechanism. Energies. 2023; 16(12):4768. https://doi.org/10.3390/en16124768
Chicago/Turabian StyleZhang, Defu, Fang Wang, Yiqiang Pei, Jiankun Yang, Dayang An, and Hongbin Hao. 2023. "Combustion Characteristics of N-Butanol/N-Heptane Blend Using Reduced Chemical Kinetic Mechanism" Energies 16, no. 12: 4768. https://doi.org/10.3390/en16124768




