Subcooling Effect on PCM Solidification: A Thermostat-like Approach to Thermal Energy Storage
Abstract
1. Introduction
- As a heat storage material, in which case they are used to store heat from a certain source, e.g., the sun, power plants, or to control temperature fluctuations, for instance with IT equipment.
- As a cold storage material, in which case they are used to store cold energy from a refrigeration system and release it when needed.
- Slow thermal transients: one of the biggest disadvantages of latent heat storage is that the time required for fusion/solidification generally exceeds that required for specific applications. This limits the amount of heat that can be stored.
- High costs: another significant disadvantage of latent heat storage is its high cost. This is because PCMs are generally expensive.
- Phase change handling: another drawback of latent heat storage is that it can be difficult to manage PCM volume expansion during phase change.
1.1. Subcooling: Literature Review
1.2. Aims and Originality
2. Materials and Methods
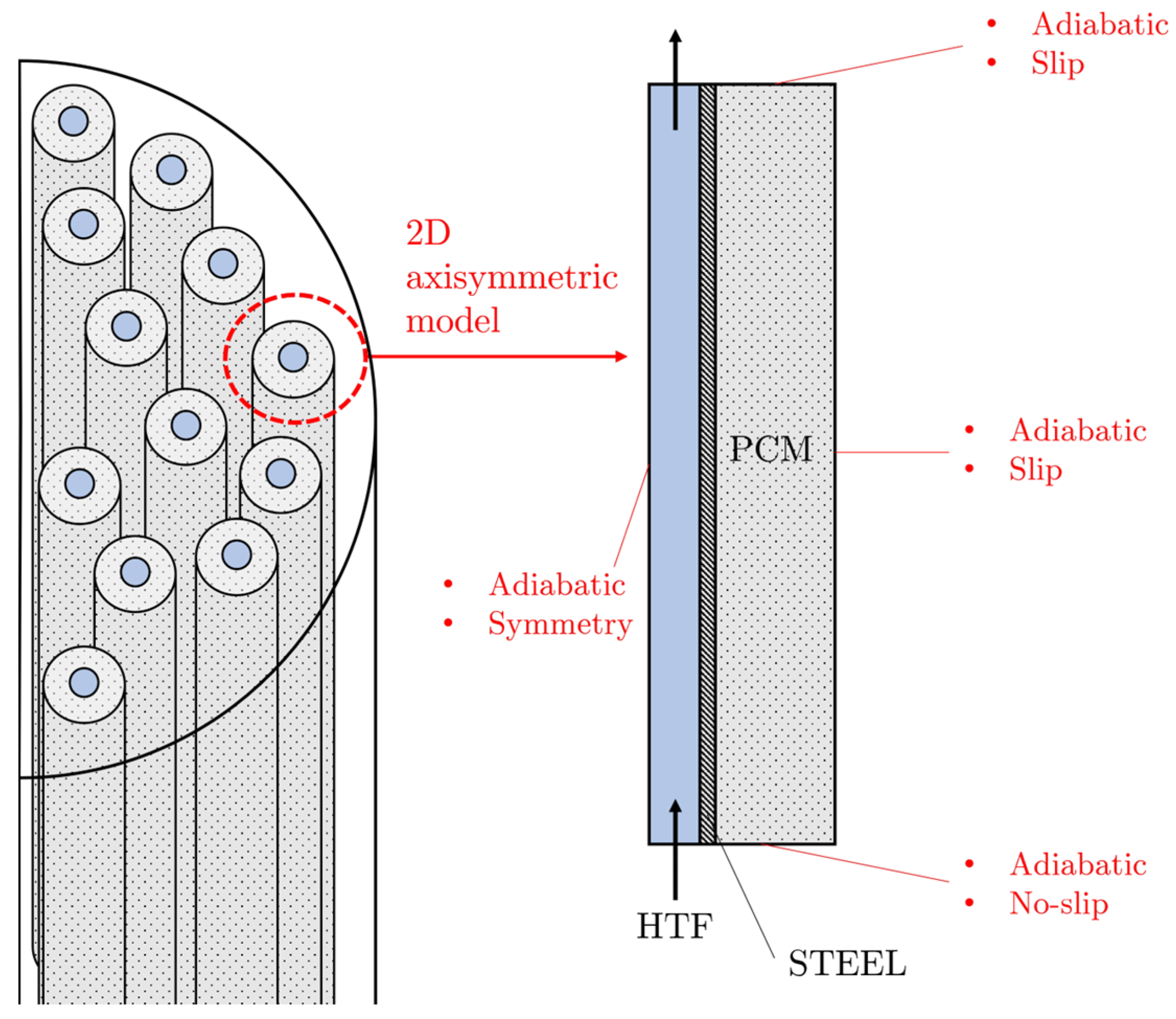
2.1. Latent Heat Thermal Energy Storage: Shell-and-Tube Heat Exchanger
2.2. Conduction-Based Model
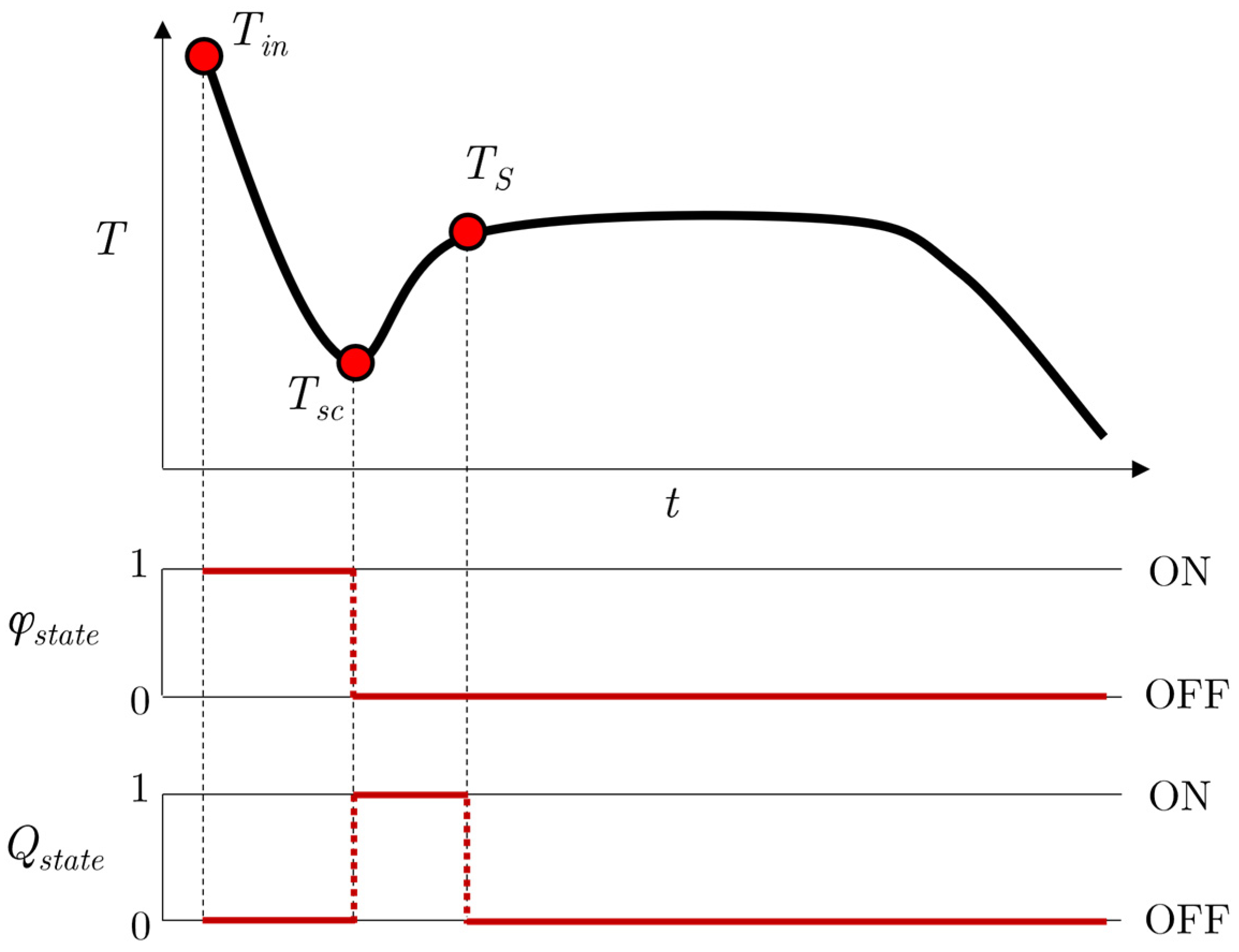
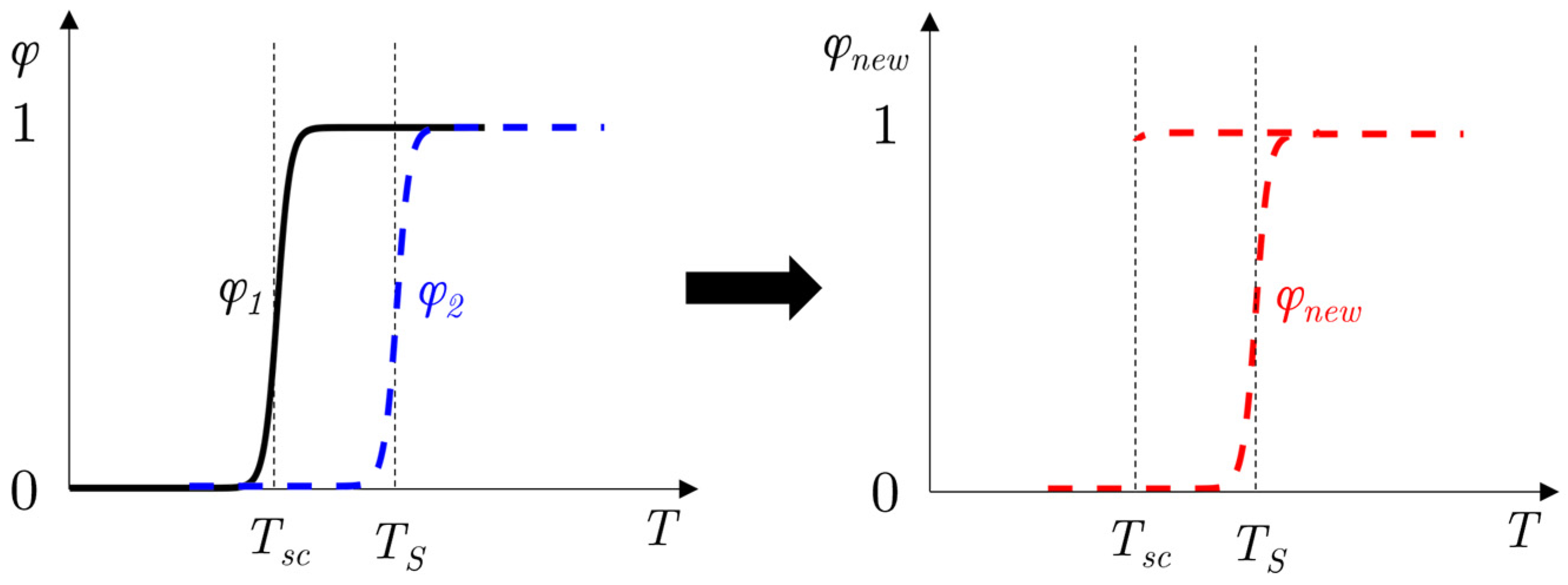
2.3. Analogy with Thermostat
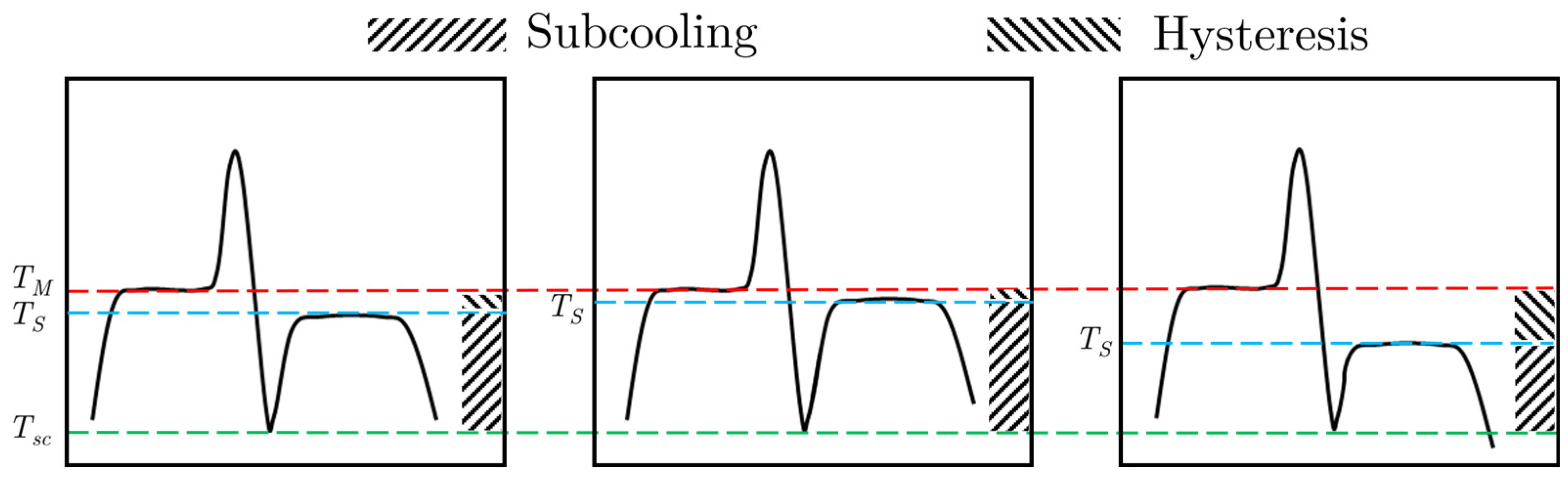
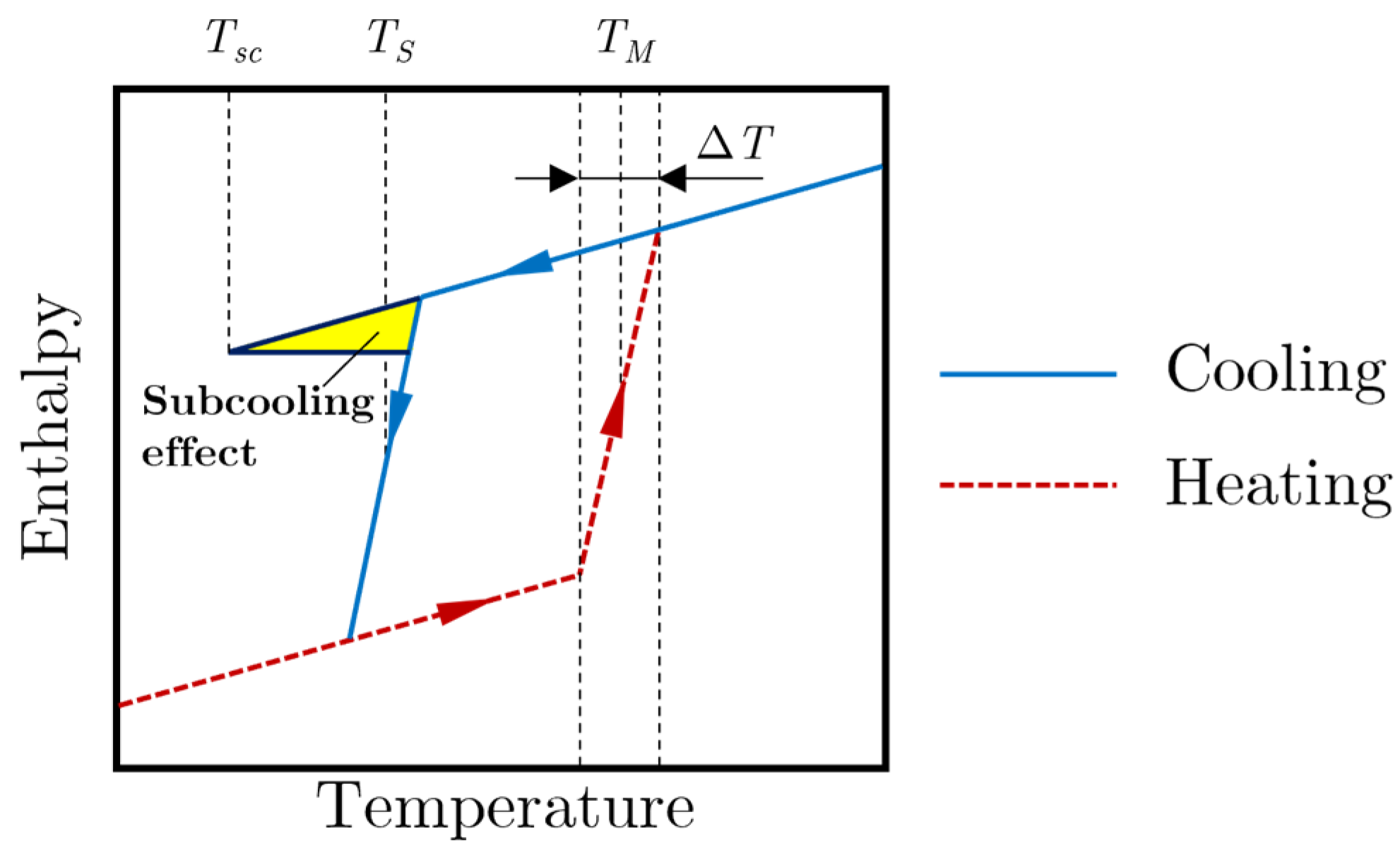
2.4. Subcooling Model
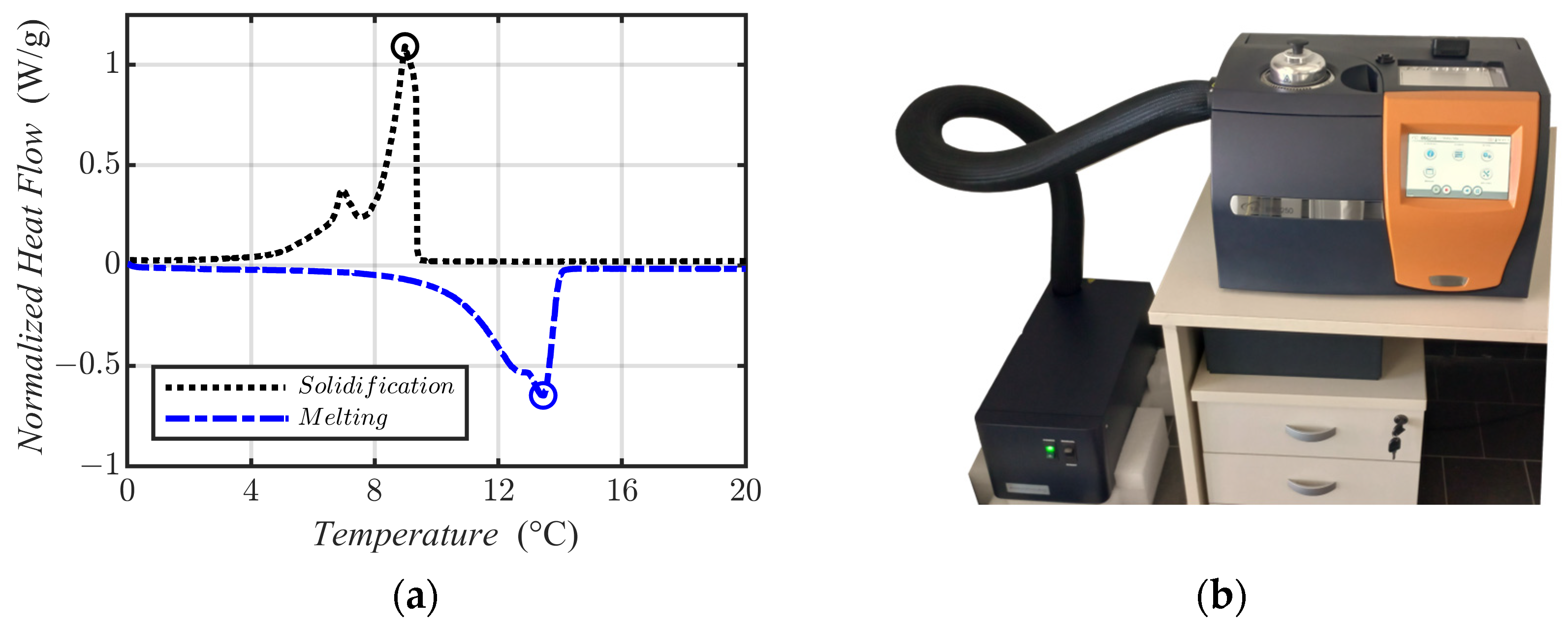
2.5. DSC-Based Model
- isotherm at 40 °C (2 min);
- ramp from 40 °C to 0 °C, at −0.5 °C/min;
- isotherm at 0 °C (2 min);
- ramp from 0 °C to 40 °C at +0.5 °C/min.
3. Results
3.1. Experimental Results
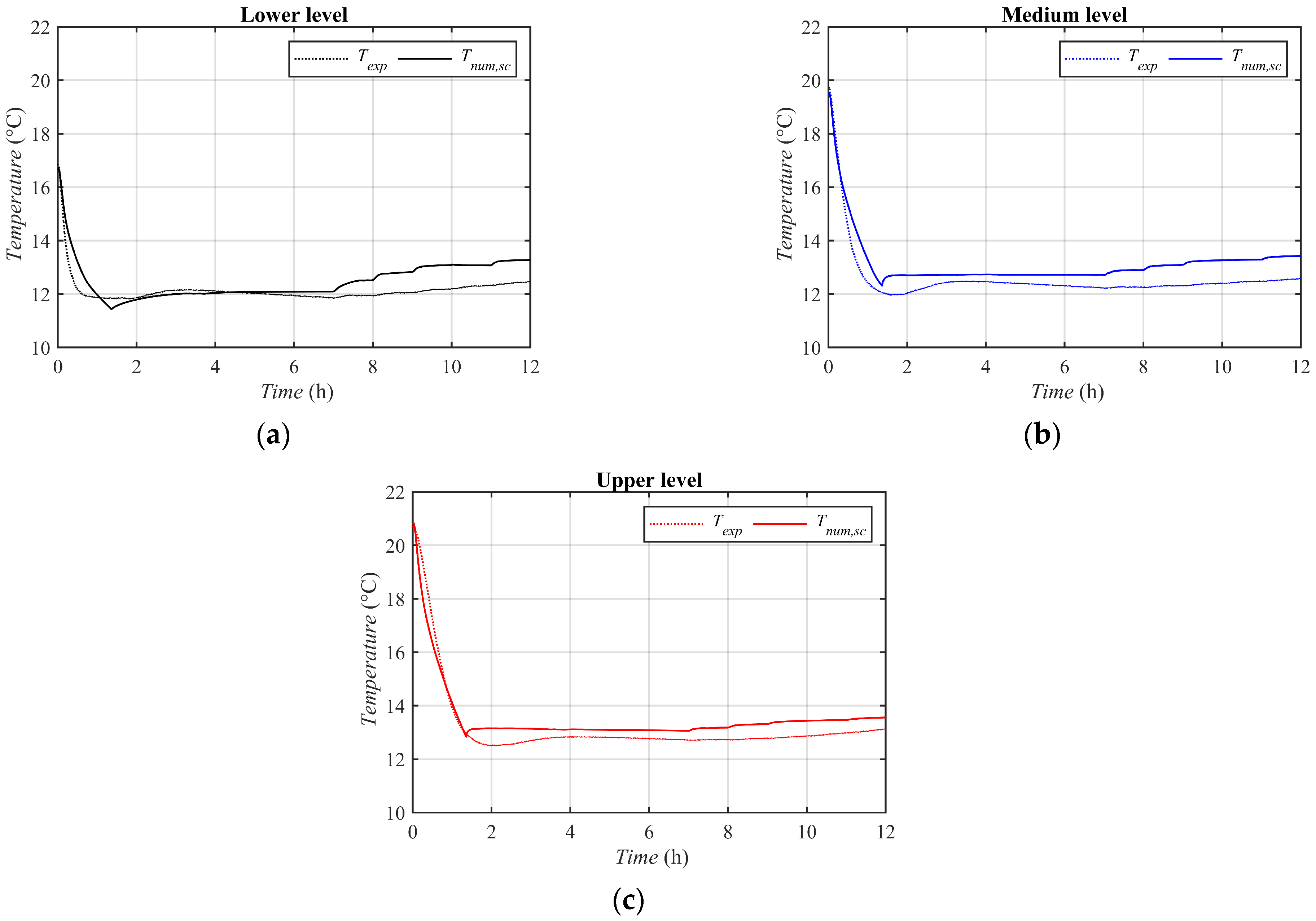
3.2. Numerical Results: First Model
3.3. Numerical Results: Second Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| c | specific heat (J/kg/K) |
| g | gravitational acceleration (m/s2) |
| h | enthalpy (J/kg/K) |
| k | thermal conductivity (W/m/K) |
| L | heat storage tank height (m) |
| Lf | latent heat of fusion (J/kg) |
| p | pressure (Pa) |
| Q | heat source (W/m3) |
| s | thickness (m) |
| t | time (s) |
| T | temperature (K) |
| Greek Symbols | |
| Δ | interval |
| µ | dynamic viscosity (Pa s) |
| ρ | density (kg/m3) |
| φ | melt fraction |
| Subscripts | |
| eff | effective |
| exp | experimental |
| HTF | heat transfer fluid |
| in | inlet |
| init | initial |
| int | internal |
| L | liquid |
| m | mean |
| M | melting |
| num | numerical |
| out | outlet |
| S | solid |
| sc | subcooling |
| state | state variable |
| t | tube |
| Acronyms | |
| LHTES | latent heat thermal energy storage |
| MAE | mean absolute error |
| PCM | phase change material |
| RMSE | root mean square error |
| STHE | shell-and-tube heat exchanger |
References
- Cabeza, L.; Mehling, H. Heat and Cold Storage with PCM: An up to Date Introduction into Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-642-08807-0. [Google Scholar]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent Thermal Energy Storage Technologies and Applications: A Review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Rostami, S.; Afrand, M.; Shahsavar, A.; Sheikholeslami, M.; Kalbasi, R.; Aghakhani, S.; Shadloo, M.S.; Oztop, H.F. A Review of Melting and Freezing Processes of PCM/Nano-PCM and Their Application in Energy Storage. Energy 2020, 211, 118698. [Google Scholar] [CrossRef]
- Bianco, N.; Busiello, S.; Iasiello, M.; Mauro, G.M. Finned Heat Sinks with Phase Change Materials and Metal Foams: Pareto Optimization to Address Cost and Operation Time. Appl. Therm. Eng. 2021, 197, 117436. [Google Scholar] [CrossRef]
- Bianco, N.; Caliano, M.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Thermal Analysis of Micro-Encapsulated Phase Change Material (MEPCM)-Based Units Integrated into a Commercial Water Tank for Cold Thermal Energy Storage. Energy 2023, 266, 126479. [Google Scholar] [CrossRef]
- NematpourKeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Enhancing PCMs Thermal Conductivity: A Comparison among Porous Metal Foams, Nanoparticles and Finned Surfaces in Triplex Tube Heat Exchangers. Appl. Therm. Eng. 2022, 212, 118623. [Google Scholar] [CrossRef]
- Iasiello, M.; Mameli, M.; Filippeschi, S.; Bianco, N. Metal Foam/PCM Melting Evolution Analysis: Orientation and Morphology Effects. Appl. Therm. Eng. 2021, 187, 116572. [Google Scholar] [CrossRef]
- Dinesh, B.V.S.; Bhattacharya, A. Effect of Foam Geometry on Heat Absorption Characteristics of PCM-Metal Foam Composite Thermal Energy Storage Systems. Int. J. Heat Mass Transf. 2019, 134, 866–883. [Google Scholar] [CrossRef]
- Abdi, M.; Chaib, K.; Menouer, A.; Benferhat, S. A Natural Convection Conjugate Heat Transfer of Nano-Encapsulated Phase Change Materials (NEPCMs) in an Inclined Blocked Square Enclosure. Numer. Heat Transf. Part A Appl. 2022, 1–22. [Google Scholar] [CrossRef]
- Abderrahmane, A.; Qasem, N.A.A.; Mourad, A.; Al-Khaleel, M.; Said, Z.; Guedri, K.; Younis, O.; Marzouki, R. Enhancing the Melting Process of Shell-and-Tube PCM Thermal Energy Storage Unit Using Modified Tube Design. Nanomaterials 2022, 12, 3078. [Google Scholar] [CrossRef]
- González-Peña, D.; Alonso-deMiguel, I.; Díez-Mediavilla, M.; Alonso-Tristán, C. Experimental Analysis of a Novel PV/T Panel with PCM and Heat Pipes. Sustainability 2020, 12, 1710. [Google Scholar] [CrossRef]
- Moench, S.; Dittrich, R. Influence of Natural Convection and Volume Change on Numerical Simulation of Phase Change Materials for Latent Heat Storage. Energies 2022, 15, 2746. [Google Scholar] [CrossRef]
- Soni, M.K.; Tamar, N.; Bhattacharyya, S. Numerical Simulation and Parametric Analysis of Latent Heat Thermal Energy Storage System. J. Therm. Anal. Calorim. 2020, 141, 2511–2526. [Google Scholar] [CrossRef]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and Numerical Analysis of a Phase Change Material-Based Shell-and-Tube Heat Exchanger for Cold Thermal Energy Storage. J. Energy Storage 2022, 56, 105975. [Google Scholar] [CrossRef]
- Que, L.; Zhang, X.; Ji, J.; Gao, L.; Xie, W.; Liu, L.; Ding, X. Numerical Simulation and Experimental Research Progress of Phase Change Hysteresis: A Review. Energy Build. 2021, 253, 111402. [Google Scholar] [CrossRef]
- Shamseddine, I.; Pennec, F.; Biwole, P.; Fardoun, F. Supercooling of Phase Change Materials: A Review. Renew. Sustain. Energy Rev. 2022, 158, 112172. [Google Scholar] [CrossRef]
- Biswas, K.; Shukla, Y.; Desjarlais, A.; Rawal, R. Thermal Characterization of Full-Scale PCM Products and Numerical Simulations, Including Hysteresis, to Evaluate Energy Impacts in an Envelope Application. Appl. Therm. Eng. 2018, 138, 501–512. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, R.; Heiselberg, P.K.; Johra, H. Modeling PCM Phase Change Temperature and Hysteresis in Ventilation Cooling and Heating Applications. Energies 2020, 13, 6455. [Google Scholar] [CrossRef]
- Kuznik, F.; Virgone, J. Experimental Investigation of Wallboard Containing Phase Change Material: Data for Validation of Numerical Modeling. Energy Build. 2009, 41, 561–570. [Google Scholar] [CrossRef]
- Barz, T.; Sommer, A. Modeling Hysteresis in the Phase Transition of Industrial-Grade Solid/Liquid PCM for Thermal Energy Storages. Int. J. Heat Mass Transf. 2018, 127, 701–713. [Google Scholar] [CrossRef]
- Günther, E.; Huang, L.; Mehling, H.; Dötsch, C. Subcooling in PCM Emulsions—Part 2: Interpretation in Terms of Nucleation Theory. Thermochim. Acta 2011, 522, 199–204. [Google Scholar] [CrossRef]
- Taylor, R.A.; Tsafnat, N.; Washer, A. Experimental Characterisation of Sub-Cooling in Hydrated Salt Phase Change Materials. Appl. Therm. Eng. 2016, 93, 935–938. [Google Scholar] [CrossRef]
- Azad, M.; Groulx, D.; Donaldson, A. Natural Convection Onset during Melting of Phase Change Materials: Part I—Effects of the Geometry, Stefan Number, and Degree of Subcooling. Int. J. Therm. Sci. 2021, 170, 107180. [Google Scholar] [CrossRef]
- Vikram, M.P.; Kumaresan, V.; Christopher, S.; Velraj, R. Experimental Studies on Solidification and Subcooling Characteristics of Water-Based Phase Change Material (PCM) in a Spherical Encapsulation for Cool Thermal Energy Storage Applications. Int. J. Refrig. 2019, 100, 454–462. [Google Scholar] [CrossRef]
- Faucheux, M.; Muller, G.; Havet, M.; LeBail, A. Influence of Surface Roughness on the Supercooling Degree: Case of Selected Water/Ethanol Solutions Frozen on Aluminium Surfaces. Int. J. Refrig. 2006, 29, 1218–1224. [Google Scholar] [CrossRef]
- Ribezzo, A.; Falciani, G.; Bergamasco, L.; Fasano, M.; Chiavazzo, E. An Overview on the Use of Additives and Preparation Procedure in Phase Change Materials for Thermal Energy Storage with a Focus on Long Term Applications. J. Energy Storage 2022, 53, 105140. [Google Scholar] [CrossRef]
- Thonon, M.; Fraisse, G.; Zalewski, L.; Pailha, M. Analytical Modelling of PCM Supercooling Including Recalescence for Complete and Partial Heating/Cooling Cycles. Appl. Therm. Eng. 2021, 190, 116751. [Google Scholar] [CrossRef]
- Bony, J.; Citherlet, S. Numerical Model and Experimental Validation of Heat Storage with Phase Change Materials. Energy Build. 2007, 39, 1065–1072. [Google Scholar] [CrossRef]
- Günther, E.; Mehling, H.; Hiebler, S. Modeling of Subcooling and Solidification of Phase Change Materials. Model. Simul. Mater. Sci. Eng. 2007, 15, 879–892. [Google Scholar] [CrossRef]
- Uzan, A.Y.; Kozak, Y.; Korin, Y.; Harary, I.; Mehling, H.; Ziskind, G. A Novel Multi-Dimensional Model for Solidification Process with Supercooling. Int. J. Heat Mass Transf. 2017, 106, 91–102. [Google Scholar] [CrossRef]
- Jin, X.; Hu, H.; Shi, X.; Zhou, X.; Yang, L.; Yin, Y.; Zhang, X. A New Heat Transfer Model of Phase Change Material Based on Energy Asymmetry. Appl. Energy 2018, 212, 1409–1416. [Google Scholar] [CrossRef]
- Davin, T.; Lefez, B.; Guillet, A. Supercooling of Phase Change: A New Modeling Formulation Using Apparent Specific Heat Capacity. Int. J. Therm. Sci. 2020, 147, 106121. [Google Scholar] [CrossRef]
- Brent, A.D.; Voller, V.R.; Reid, K.J. Enthalpy-Porosity Technique for Modeling Convection-Diffusion Phase Change: Application to the Melting of a Pure Metal. Numer. Heat Transf. 1988, 13, 297–318. [Google Scholar] [CrossRef]
- Jin, X.; Hu, H.; Shi, X.; Zhang, X. Energy Asymmetry in Melting and Solidifying Processes of PCM. Energy Convers. Manag. 2015, 106, 608–614. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, N.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Subcooling Effect on PCM Solidification: A Thermostat-like Approach to Thermal Energy Storage. Energies 2023, 16, 4834. https://doi.org/10.3390/en16124834
Bianco N, Fragnito A, Iasiello M, Mauro GM, Mongibello L. Subcooling Effect on PCM Solidification: A Thermostat-like Approach to Thermal Energy Storage. Energies. 2023; 16(12):4834. https://doi.org/10.3390/en16124834
Chicago/Turabian StyleBianco, Nicola, Andrea Fragnito, Marcello Iasiello, Gerardo Maria Mauro, and Luigi Mongibello. 2023. "Subcooling Effect on PCM Solidification: A Thermostat-like Approach to Thermal Energy Storage" Energies 16, no. 12: 4834. https://doi.org/10.3390/en16124834
APA StyleBianco, N., Fragnito, A., Iasiello, M., Mauro, G. M., & Mongibello, L. (2023). Subcooling Effect on PCM Solidification: A Thermostat-like Approach to Thermal Energy Storage. Energies, 16(12), 4834. https://doi.org/10.3390/en16124834









