Microfluidic Studies on Minimum Miscibility Pressure for n-Decane and CO2
Abstract
:1. Introduction
2. Materials
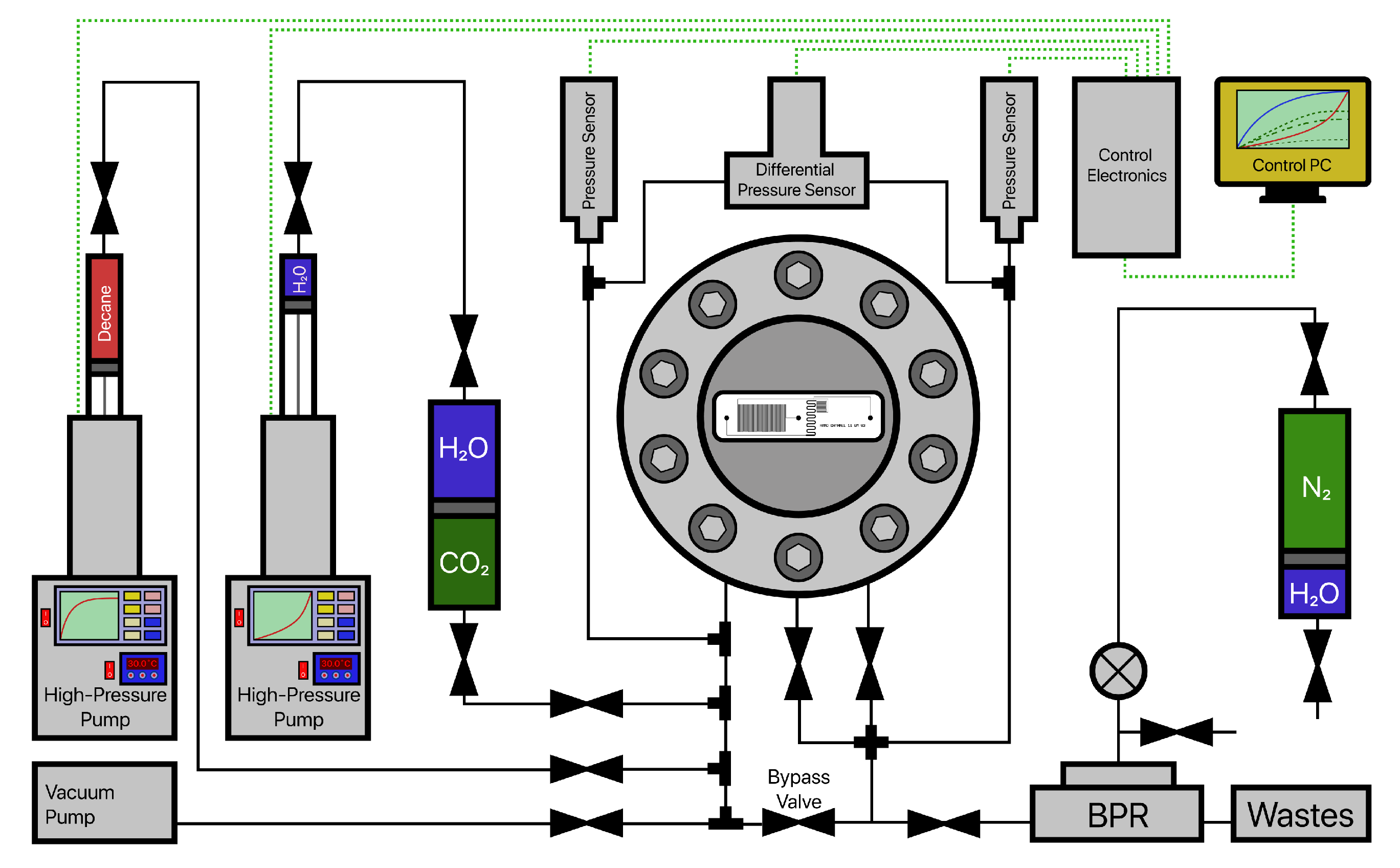
2.1. Microfluidic Assembly
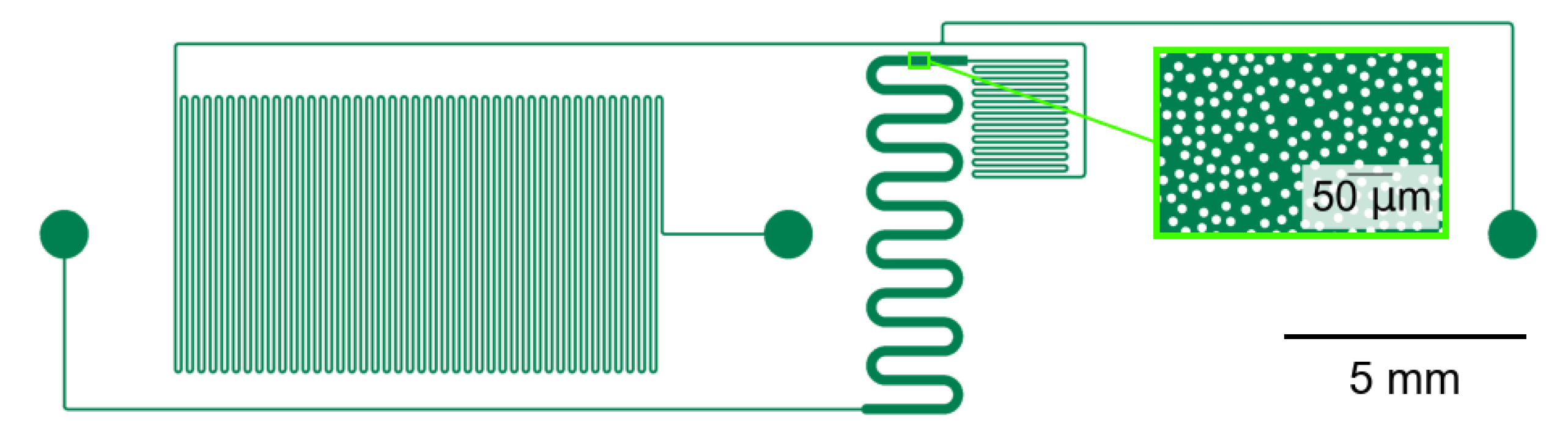
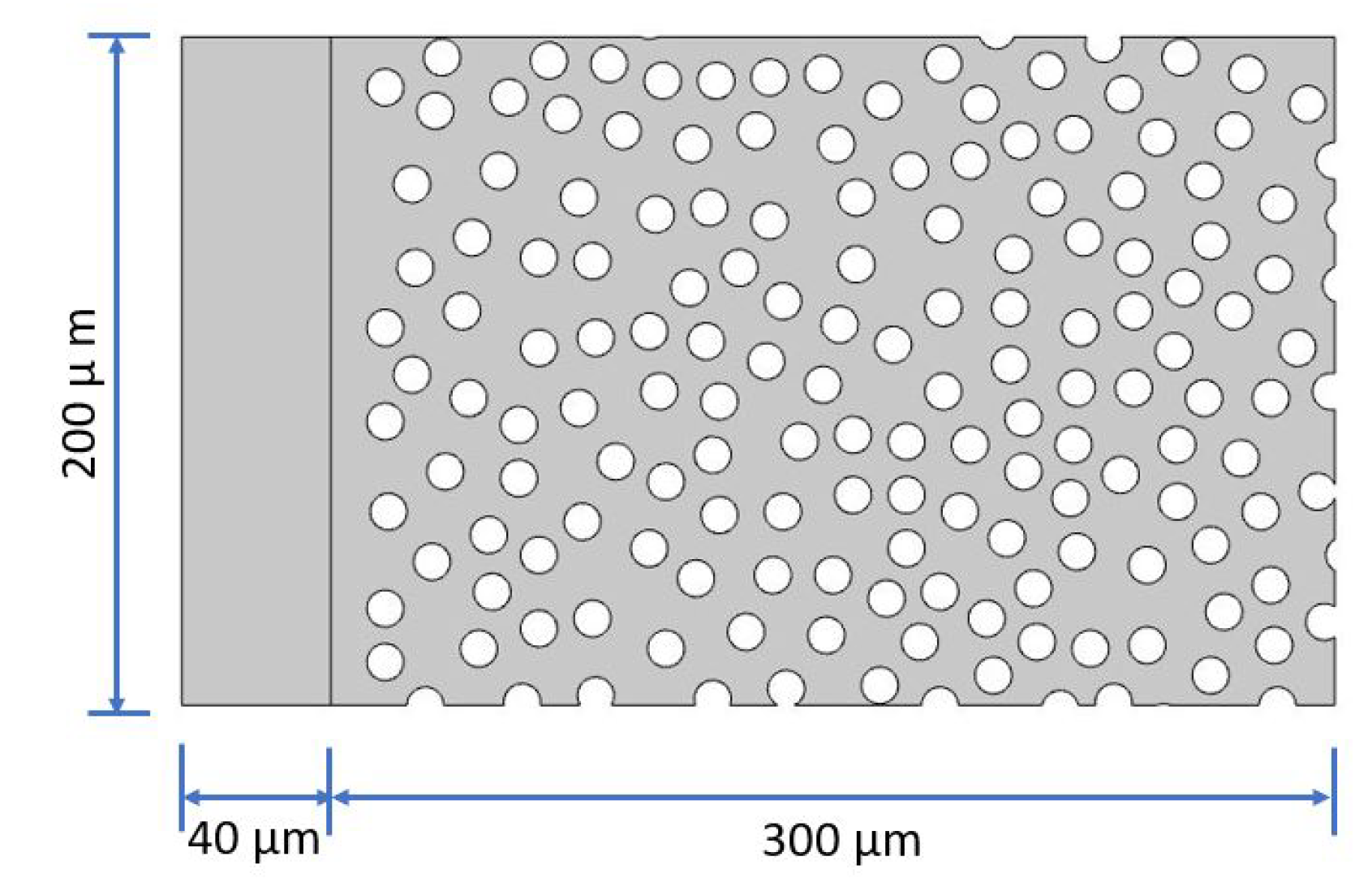
2.2. Microchip Design
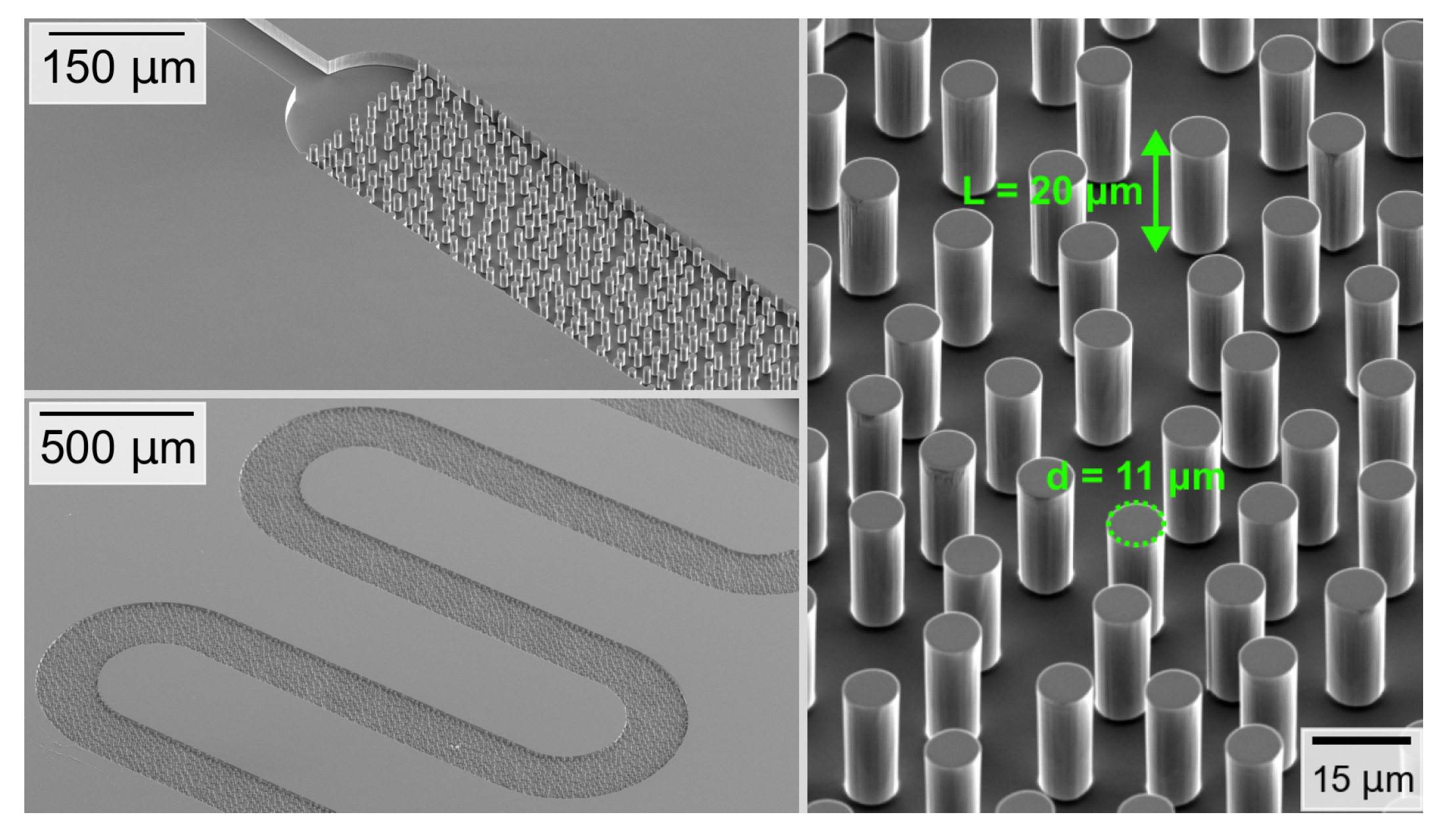
2.3. Manufacturing Process
2.4. Fluids and Fluorescent Additive
3. Methods
3.1. Experimental Procedure
3.2. Numerical Modelling
3.2.1. Governing Equations
Immiscible Flow
Miscible Flow
3.2.2. Computational Domain and Boundary Conditions
4. Results
4.1. Experimental Section
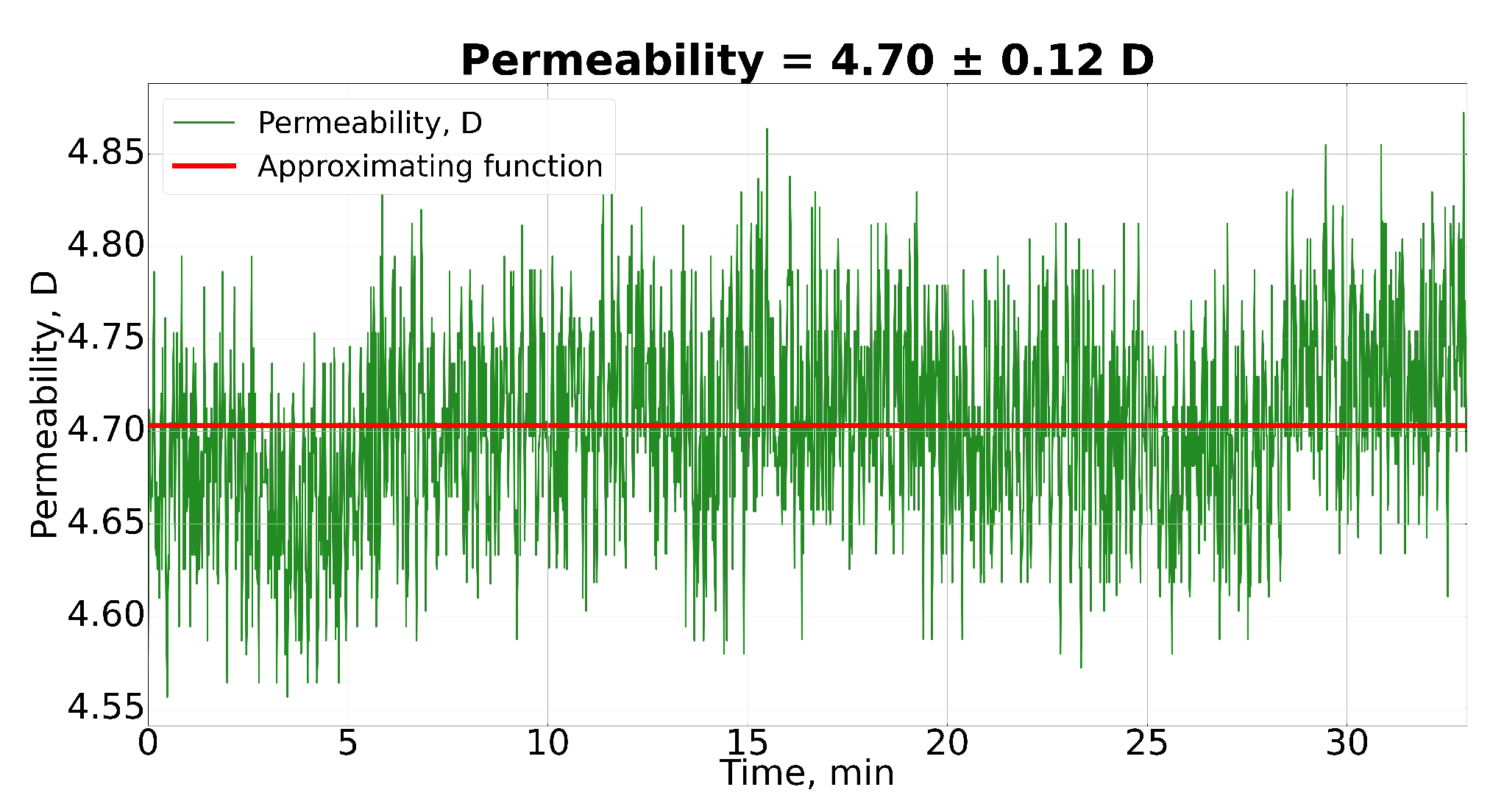
4.1.1. Permeability Determination
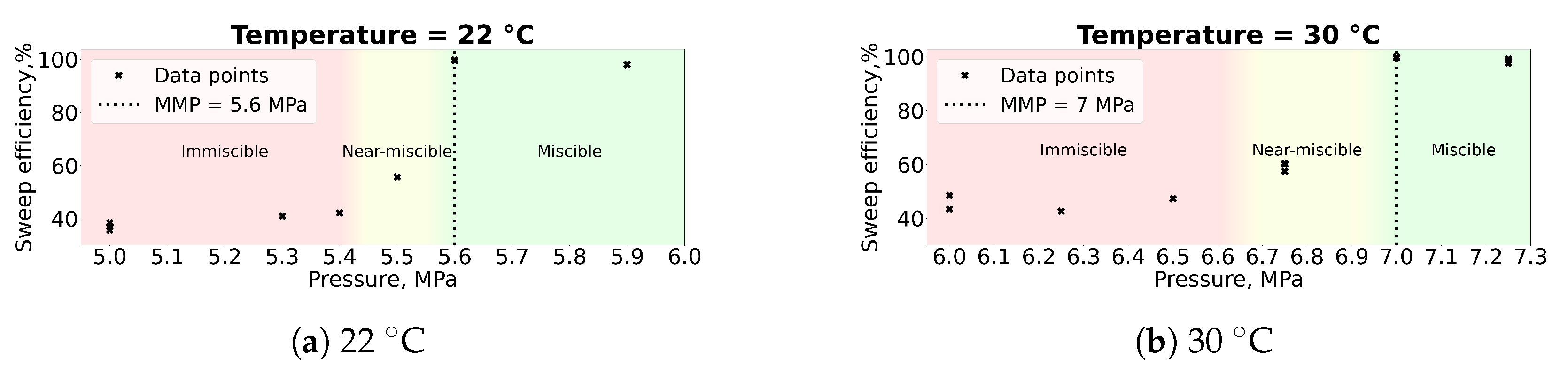
4.1.2. MMP Determination
Raw Data
Video Processing
4.1.3. Final Results
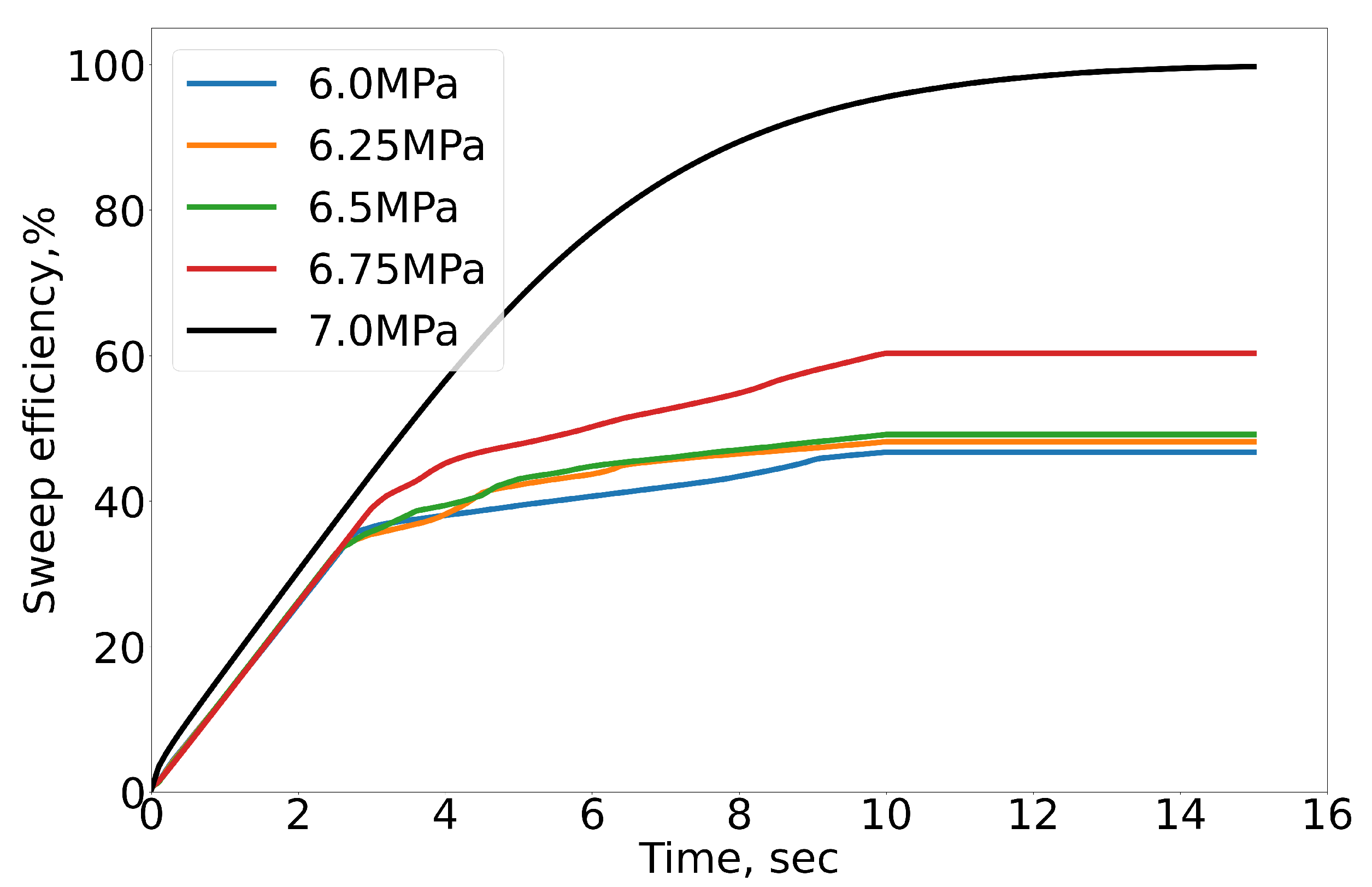
4.2. Results of Numerical Simulation
5. Discussion
- The length of the microfluidic ST is rather long to provide the necessary zone for immiscible displacement while not obscuring the overall impact of miscible displacement.
- The width of the microfluidic channel is modest, which reduces the influence of gravity and fingering effects.
- The injection rate is controlled to be constant and relatively low, but remains unimportant since the system is sufficiently lengthy.
6. Limitations and Future Studies
- The significant reduction in the porous medium’s volume inside the microfluidic chip necessitates precise fluid control, either by flow rate or differential pressure. The microfluidic experiment does not withstand the effects of pulsatile flow.
- Currently, it is not possible to control the composition of the fluid in situ in the microfluidic chip. Moreover, the volumes of the fluids are too small to be analysed by conventional gas chromatographic methods.
- The quality of images obtained from the microfluidic experiment requires a trade-off between magnification and field of view. Our microfluidic model allowed us to visualise the immiscible and miscible fluid flows along with the whole distance of the main tortuous channel.
- The error between the numerically simulated results and the experimental results could be minimised by increasing the dimensions of the simulated volume of the model in future studies, if there is a trade-off between the simulated volume and the computational cost due to an improvement in computational power.
- The presented microfluidic chip geometry allowed us to observe the miscibility zone of C10H22 and CO2 inside the microfluidic channel, but experiments with real fluids using this geometry are required.
- It is necessary to develop a strict experimental procedure in further studies to address the above technological challenges.
7. Conclusions
- For pure C10H22 and CO2 at temperatures of 22 and 30 C, the MMP values of 5.6 and 7 MPa were obtained, respectively.
- The MMP values determined using microfluidic technology are in good agreement with MMP measurements obtained by other methods described in the literature. For instance, the relative difference for 30 C is between 4 and 9%.
- The conducted experiments allowed us to define a mechanism to determine the minimum miscibility pressure, both quantitatively and qualitatively.
- The presented microfluidic technique with a special microfluidic chip with a heterogeneous porous medium, compared to the traditional slim-tube test, required a much smaller fluid volume (1 mL, approx.) and exceeded the maximum of one hour for the preparation and experiment itself.
- The simulation of a section of the entire domain (SV) yielded results in good agreement with the experimental results.
- The sweep efficiency of CO2 increases with increasing confining pressure due to the decreasing IFT between C10H22 and CO2 under the immiscible condition.
- Numerical modelling showed that the convective transport mechanism is dominant over the diffusion mechanism under both miscible and immiscible conditions.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, H.; Cai, J. Screening improved recovery methods in tight-oil formations by injecting and producing through fractures. Int. J. Heat Mass Transf. 2018, 116, 977–993. [Google Scholar] [CrossRef]
- McPhee, C.; Reed, J.; Zubizarreta, I. Core Analysis: A Best Practice Guide; Elsevier BV: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pereponov, D.; Scerbacova, A.; Kazaku, V.; Hajiyev, M.; Tarkhov, M.A.; Shilov, E.; Cheremisin, A. Application of microfluidics to optimize oil and gas field development technologies. Kazakhstan J. Oil Gas Ind. 2023, 1, 57–73. [Google Scholar] [CrossRef]
- Gauteplass, J.; Follesø, H.N.; Graue, A.; Kovscek, A.R.; Fernø, M.A. Visualization of Pore-level Displacement Mechanisms During CO2 Injection and EOR Processes. In Proceedings of the IOR 2013—17th European Symposium on Improved Oil Recovery, Saint Petersburg, Russia, 16–18 April 2013. [Google Scholar] [CrossRef]
- Zhong, J.; Abedini, A.; Xu, L.; Xu, Y.; Qi, Z.; Mostowfi, F.; Sinton, D. Nanomodel visualization of fluid injections in tight formations. Nanoscale 2018, 10, 21994–22002. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Carey, J.W.; Viswanathan, H.S.; Porter, M. Effectiveness of supercritical-CO2 and N2 huff-and-puff methods of enhanced oil recovery in shale fracture networks using microfluidic experiments. Appl. Energy 2018, 230, 160–174. [Google Scholar] [CrossRef]
- Pereponov, D.; Tarkhov, M.; Rykov, A.; Filipov, I.; Zenova, E.; Cheremisin, A.; Shilov, E. Radial and Huff-n-Puff Gas Injection on Microfluidic Chips. In Proceedings of the Gas & Oil Technology Showcase and Conference, Dubai, United Arab Emirates, 13–15 March 2023. [Google Scholar] [CrossRef]
- Howe, A.M.; Clarke, A.; Mitchell, J.; Staniland, J.; Hawkes, L.; Whalan, C. Visualising surfactant enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 449–461. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, F.; Liao, G.; Liu, W.; Su, X.; Feng, Y. In situ micro-emulsification during surfactant enhanced oil recovery: A microfluidic study. J. Colloid Interface Sci. 2022, 620, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Scerbacova, A.; Pereponov, D.; Tarkhov, M.; Kazaku, V.; Rykov, A.; Filippov, I.; Zenova, E.; Krutko, V.; Cheremisin, A.; Shilov, E. Visualization of Surfactant Flooding in Tight Reservoir Using Microfluidics. In Proceedings of the SPE EuropEC—Europe Energy Conference featured at the 84th EAGE Annual Conference & Exhibition, Vienna, Austria, 5–8 June 2023. [Google Scholar] [CrossRef]
- Nilsson, M.A.; Kulkarni, R.; Gerberich, L.; Hammond, R.; Singh, R.; Baumhoff, E.; Rothstein, J.P. Effect of fluid rheology on enhanced oil recovery in a microfluidic sandstone device. J.-Non-Newton. Fluid Mech. 2013, 202, 112–119. [Google Scholar] [CrossRef]
- Lacey, M.; Hollis, C.; Oostrom, M.; Shokri, N. Effects of Pore and Grain Size on Water and Polymer Flooding in Micromodels. Energy Fuels 2017, 31, 9026–9034. [Google Scholar] [CrossRef] [Green Version]
- Conn, C.A.; Ma, K.; Hirasaki, G.J.; Biswal, S.L. Visualizing oil displacement with foam in a microfluidic device with permeability contrast. Lab Chip 2014, 14, 3968–3977. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Xu, K.; Agrawal, D.; Darugar, Q. Hydrophilic Nanoparticle-Based Enhanced Oil Recovery: Microfluidic Investigations on Mechanisms. Energy Fuels 2018, 32, 11243–11252. [Google Scholar] [CrossRef]
- Bryant, R.S.; Douglas, J. Evaluation of Microbial Systems in Porous Media for EOR. SPE Reserv. Eng. 1988, 3, 489–495. [Google Scholar] [CrossRef]
- Gaol, C.L.; Ganzer, L.; Mukherjee, S.; Alkan, H. Parameters govern microbial enhanced oil recovery (MEOR) performance in real-structure micromodels. J. Pet. Sci. Eng. 2021, 205, 13. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mahani, H. Direct insights into the pore-scale mechanism of low-salinity waterflooding in carbonates using a novel calcite microfluidic chip. Fuel 2020, 260, 116374. [Google Scholar] [CrossRef]
- Tetteh, J.T.; Cudjoe, S.E.; Aryana, S.A.; Ghahfarokhi, R.B. Investigation into fluid-fluid interaction phenomena during low salinity waterflooding using a reservoir-on-a-chip microfluidic model. J. Pet. Sci. Eng. 2021, 196, 108074. [Google Scholar] [CrossRef]
- Wan Park, S.; Lee, J.; Yoon, H.; Shin, S. Microfluidic Investigation of Salinity-Induced Oil Recovery in Porous Media during Chemical Flooding. Energy Fuels 2021, 35, 4885–4892. [Google Scholar] [CrossRef]
- De Haas, T.W.; Fadaei, H.; Guerrero, U.; Sinton, D. Steam-on-a-chip for oil recovery: The role of alkaline additives in steam assisted gravity drainage. Lab Chip 2013, 13, 3832. [Google Scholar] [CrossRef]
- De Haas, T.W.; Bao, B.; Ramirez, H.A.; Abedini, A.; Sinton, D. Screening High-Temperature Foams with Microfluidics for Thermal Recovery Processes. Energy Fuels 2021, 35, 7866–7873. [Google Scholar] [CrossRef]
- Mostowfi, F.; Molla, S.; Tabeling, P. Determining phase diagrams of gas–liquid systems using a microfluidic PVT. Lab Chip 2012, 12, 4381–4387. [Google Scholar] [CrossRef]
- Song, W.; Fadaei, H.; Sinton, D. Determination of Dew Point Conditions for CO2 with Impurities Using Microfluidics. Environ. Sci. Technol. 2014, 48, 3567–3574. [Google Scholar] [CrossRef]
- Bao, B.; Riordon, J.; Xu, Y.; Li, H.; Sinton, D. Direct Measurement of the Fluid Phase Diagram. Anal. Chem. 2016, 88, 6986–6989. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.H.; Sieben, V.J.; Kharrat, A.M.; Mostowfi, F. Measurement of Asphaltenes Using Optical Spectroscopy on a Microfluidic Platform. Anal. Chem. 2013, 85, 5153–5160. [Google Scholar] [CrossRef]
- Onaka, Y.; Sato, K. Dynamics of pore-throat plugging and snow-ball effect by asphaltene deposition in porous media micromodels. J. Pet. Sci. Eng. 2021, 207, 109176. [Google Scholar] [CrossRef]
- Hauge, L.; Gauteplass, J.; Høyland, M.; Ersland, G.; Kovscek, A.; Fernø, M. Pore-level hydrate formation mechanisms using realistic rock structures in high-pressure silicon micromodels. Int. J. Greenh. Gas Control 2016, 53, 178–186. [Google Scholar] [CrossRef]
- Kim, M.; Sell, A.; Sinton, D. Aquifer-on-a-Chip: Understanding pore-scale salt precipitation dynamics during CO2 sequestration. Lab Chip 2013, 13, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Elturki, M.; Imqam, A. Application of enhanced oil recovery methods in unconventional reservoirs: A review and data analysis. In Proceedings of the 54th U.S. Rock Mechanics/Geomechanics SymposiumOnePetro, Golden, CO, USA, 28 June–1 July 2020. [Google Scholar]
- Johns, R.T.; Dindoruk, B. Gas Flooding. In Enhanced Oil Recovery Field Case Studies; Elsevier BV: Amsterdam, The Netherlands, 2013; pp. 1–22. [Google Scholar] [CrossRef]
- Alfarge, D.; Alsaba, M.; Wei, M.; Bai, B. Miscible Gases Based EOR in Unconventional Liquids Rich Reservoirs: What We Can Learn. In Proceedings of SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2018. [Google Scholar] [CrossRef]
- Jia, B.; Xian, C.; Tsau, J.S.; Zuo, X.; Jia, W. Status and Outlook of Oil Field Chemistry-Assisted Analysis during the Energy Transition Period. Energy Fuels 2022, 36, 12917–12945. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Ghedan, S. Global Laboratory Experience of CO2-EOR Flooding. In Proceedings of SPE/EAGE Reservoir Characterization and Simulation Conference, Abu Dhabi, United Arab Emirates, 19–21 October 2009. [Google Scholar] [CrossRef]
- Siregar, S.; Hidayaturobbi, A.D.; Wijaya, B.A.; Listiani, S.N.; Adiningrum, T.; Irwan, I.; Pratomo, A.I. Laboratory Experiments on Enhanced Oil Recovery with Nitrogen Injection. ITB J. Eng. Sci. 2007, 39, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Hearn, C.L.; Whitson, C.H. Evaluating miscible and immiscible gas injection in the Safah Field, Oman. In Proceedings of the SPE Reservoir Simulation Symposium, San Antonio, TX, USA, 12–15 February 1995; pp. 335–341. [Google Scholar] [CrossRef]
- Chukwudeme, E.A.; Hamouda, A.A. Enhanced Oil Recovery (EOR) by Miscible CO2 and Water Flooding of Asphaltenic and Non-Asphaltenic Oils. Energies 2009, 2, 714–737. [Google Scholar] [CrossRef] [Green Version]
- Choubineh, N.; Jannesari, H.; Kasaeian, A. Experimental study of the effect of using phase change materials on the performance of an air-cooled photovoltaic system. Renew. Sustain. Energy Rev. 2019, 101, 103–111. [Google Scholar] [CrossRef]
- Luo, E.; Hu, Y.; Wang, J.; Fan, Z.; Hou, Q.; Ma, L.; Dai, S. The effect of impurity on miscible CO2 displacement mechanism. Oil Gas Sci. Technol.–Rev. d’IFP Energies Nouv. 2019, 74, 86. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Luo, W.; Li, S. Effectiveness of miscible and immiscible gas flooding in recovering tight oil from Bakken reservoirs in Saskatchewan, Canada. Fuel 2017, 208, 626–636. [Google Scholar] [CrossRef]
- Li, H.; Qin, J.; Yang, D. An Improved CO2–Oil Minimum Miscibility Pressure Correlation for Live and Dead Crude Oils. Ind. Eng. Chem. Res. 2012, 51, 3516–3523. [Google Scholar] [CrossRef]
- Shilov, E.; Cheremisin, A. Huff-n-Puff Test For Minimum Miscibility Pressure Determination For Heavy Oil. In Proceedings of the Proceedings. EAGE Publications BV, Utrecht, The Netherlands, 21–23 November 2018. [Google Scholar] [CrossRef] [Green Version]
- Shokir, E.M.E.M. CO2–oil minimum miscibility pressure model for impure and pure CO2 streams. J. Pet. Sci. Eng. 2007, 58, 173–185. [Google Scholar] [CrossRef]
- Bougre, E.S.; Gamadi, T.D. Enhanced oil recovery application in low permeability formations by the injections of CO2, N2 and CO2/N2 mixture gases. J. Pet. Explor. Prod. Technol. 2021, 11, 1963–1971. [Google Scholar] [CrossRef]
- Molla, S.H.; Ratulowski, J.; Mostowfi, F.; Gao, J. Microfluidic technique for detection of multi-contact miscibility. U.S. Patent 2020/0290040 A1, 17 September 2020. [Google Scholar]
- Lake, L.W.; Johns, R.; Rossen, B.; Pope, G.A. Fundamentals of Enhanced Oil Recovery; Society of Petroleum Engineers: Richardson, TX, USA, 2014. [Google Scholar]
- Zick, A.A. A Combined Condensing/Vaporizing Mechanism in the Displacement of Oil by Enriched Gases. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 5–8 October 1986. [Google Scholar] [CrossRef]
- Orr, F.; Johns, R.; Dindoruk, B. Development of Miscibility in Four-Component CO2 Floods. SPE Reserv. Eng. 1993, 8, 135–142. [Google Scholar] [CrossRef]
- Dindoruk, B.; Johns, R.; Orr, F.M. Measurement and Modeling of Minimum Miscibility Pressure: A State-of-the-Art Review. SPE Reserv. Eval. Eng. 2021, 24, 367–389. [Google Scholar] [CrossRef]
- Flock, D.L.; Nouar, A. Parametric analysis on the determination of the minimum miscibility pressure in slim tube displacements. J. Can. Pet. Technol. 1984, 23, 80–88. [Google Scholar] [CrossRef]
- Thomas, F.; Zhou, X.; Bennion, D.; Bennion, D. A Comparative Study of RBA, P-x, Multicontact And Slim Tube Results. J. Can. Pet. Technol. 1994, 33, 17–26. [Google Scholar] [CrossRef]
- Christiansen, R.L.; Haines, H.K. Rapid Measurement of Minimum Miscibility Pressure With the Rising-Bubble Apparatus. SPE Reserv. Eng. 1987, 2, 523–527. [Google Scholar] [CrossRef]
- Rao, D.N. A new technique of vanishing interfacial tension for miscibility determination. Fluid Phase Equilibria 1997, 139, 311–324. [Google Scholar] [CrossRef]
- Nguyen, P.; Mohaddes, D.; Riordon, J.; Fadaei, H.; Lele, P.; Sinton, D. Fast Fluorescence-Based Microfluidic Method for Measuring Minimum Miscibility Pressure of CO2 in Crude Oils. Anal. Chem. 2015, 87, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, Y.M.; Al-Hadrami, A.K. The influence of high permeability lenses on immiscible, first- and multi-contact miscible gas injection. J. Pet. Sci. Eng. 2011, 77, 313–325. [Google Scholar] [CrossRef]
- Sharbatian, A.; Abedini, A.; Qi, Z.; Sinton, D. Full Characterization of CO2–Oil Properties On-Chip: Solubility, Diffusivity, Extraction Pressure, Miscibility, and Contact Angle. Anal. Chem. 2018, 90, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, L. Application of spectral fluorescence microscopy for the characterization of Athabasca bitumen vacuum bottoms. Fuel 2000, 79, 769–775. [Google Scholar] [CrossRef]
- De Haas, T.; Pettigrew, A.; Zhao, H.; Garnier, O.F.; Pierobon, S.; Doan-Prevost, J. Measurement of Propane and Butane Diffusion into Heavy Oil using Microfluidics—Is Small Better? In Proceedings of the Abu Dhabi International Petroleum Exhibition &Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Zou, H.; Slim, A.C.; Neild, A. Rapid Characterization of Multiple-Contact Miscibility: Toward a Slim-Tube on a Chip. Anal. Chem. 2019, 91, 13681–13687. [Google Scholar] [CrossRef]
- Zou, H.; Kang, H.; Slim, A.C.; Neild, A. Pore-scale multiple-contact miscibility measurements in a microfluidic chip. Lab Chip 2020, 20, 3582–3590. [Google Scholar] [CrossRef]
- Ungar, F.; Ahitan, S.; Worthing, S.; Abedini, A.; Uleberg, K.; Yang, T. A new fluidics method to determine minimum miscibility pressure. J. Pet. Sci. Eng. 2022, 208, 109415. [Google Scholar] [CrossRef]
- Cheremisin, A.; Shilov, E.; Isupov, A. High-Pressure and High-Temperature Holder for Microfluidic Chip. RU Patent 2764734, 23 July 2021. [Google Scholar]
- Gostick, J.T.; Khan, Z.A.; Tranter, T.G.; Kok, M.D.; Agnaou, M.; Sadeghi, M.; Jervis, R. PoreSpy: A Python Toolkit for Quantitative Analysis of Porous Media Images. J. Open Source Softw. 2019, 4, 1296. [Google Scholar] [CrossRef]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef]
- Follesø, H.N. Fluid Displacements during Multiphase Flow Visualized at the Pore Scale Using Micromodels. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2012. [Google Scholar]
- Bora, R.; Maini, B.; Chakma, A. Flow Visualization Studies of Solution Gas Drive Process in Heavy Oil Reservoirs Using a Glass Micromodel. In Proceedings of the All Days, SPE international Thermal Operations and Heavy Oil Symposium, Bakersfield, CA, USA, 10–12 February 1997. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, Z.; Fan, J.; Jia, J.; Bi, J.; Hu, P.; Wang, Q.; Li, M.; Wei, W.; Wang, D. Pore-Scale Simulations of CO2/Oil Flow Behavior in Heterogeneous Porous Media under Various Conditions. Energies 2021, 14, 533. [Google Scholar] [CrossRef]
- NIST. National Institute of Standards and Technology. Available online: https://www.nist.gov/ (accessed on 24 March 2022).
- Georgiadis, A.; Llovell, F.; Bismarck, A.; Blas, F.J.; Galindo, A.; Maitland, G.C.; Trusler, J.P.M.; Jackson, G. Interfacial tension measurements and modelling of (carbon dioxide+n-alkane) and (carbon dioxide+water) binary mixtures at elevated pressures and temperatures. J. Supercrit. Fluids 2010, 55, 743–754. [Google Scholar] [CrossRef]
- Li, X.; Fan, X. Effect of CO2 phase on contact angle in oil-wet and water-wet pores. Int. J. Greenh. Gas Control 2015, 36, 106–113. [Google Scholar] [CrossRef]
- Bahadori, A. (Ed.) Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Bao, B.; Feng, J.; Qiu, J.; Zhao, S. Direct Measurement of Minimum Miscibility Pressure of Decane and CO2 in Nanoconfined Channels. ACS Omega 2021, 6, 943–953. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Tang, L.; Song, Y.; Zhao, J.; Zhang, Y.; Wang, D.; Yang, M. Minimum miscibility pressure estimation for a CO2/n-decane system in porous media by X-ray CT. Exp. Fluids 2015, 56, 154. [Google Scholar] [CrossRef]
- Song, Y.C.; Zhu, N.J.; Liu, Y.; Zhao, J.F.; Liu, W.G.; Zhang, Y.; Zhao, Y.C.; Jiang, L.L. Magnetic Resonance Imaging Study on the Miscibility of a CO2/n-Decane System. Chin. Phys. Lett. 2011, 28, 096401. [Google Scholar] [CrossRef]
- Ayirala, S.C.; Rao, D.N. Comparative Evaluation of a New MMP Determination Technique. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–26 April 2006; pp. 349–363. [Google Scholar] [CrossRef]
- Elsharkawy, A.M.; Poettmann, F.H.; Christiansen, R.L. Measuring CO2 Minimum Miscibility Pressures: Slim-Tube or Rising-Bubble Method? Energy Fuels 1996, 10, 443–449. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, N.; Zeng, F.; Li, S.; Liu, L. A review of experimental methods for determining the Oil–Gas minimum miscibility pressures. J. Pet. Sci. Eng. 2019, 183, 106366. [Google Scholar] [CrossRef]
- Williams, C.A.; Zana, E.N.; Humphrys, G.E. Use Of The Peng-Robinson Equation Of State To Predict Hydrocarbon Phase Behavior And Miscibility For Fluid Displacement. In Proceedings of the All Days, SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 20–23 April 1980. [Google Scholar] [CrossRef]
- Holm, L.; Josendal, V. Mechanisms of Oil Displacement By Carbon Dioxide. J. Pet. Technol. 1974, 26, 1427–1438. [Google Scholar] [CrossRef]
- Amao, A.M.; Siddiqui, S.; Menouar, H.; Herd, B.L. A New Look at the Minimum Miscibility Pressure (MMP) Determination from Slimtube Measurements. In Proceedings of the All Days, SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar] [CrossRef]
- Wylie, P.L.; Mohanty, K.K. Effect of Wettability on Oil Recovery by Near-Miscible Gas Injection. SPE Reserv. Eval. Eng. 1999, 2, 558–564. [Google Scholar] [CrossRef]
- Agbalaka, C.; Dandekar, A.Y.; Patil, S.L.; Khataniar, S.; Hemsath, J.R. The Effect of Wettability on Oil Recovery: A Review. In Proceedings of the All Days, SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 20–22 October 2008. [Google Scholar] [CrossRef]
- Bikkina, P.; Wan, J.; Kim, Y.; Kneafsey, T.J.; Tokunaga, T.K. Influence of wettability and permeability heterogeneity on miscible CO2 flooding efficiency. Fuel 2016, 166, 219–226. [Google Scholar] [CrossRef] [Green Version]



| Parameter | Unit | Value | ||||
|---|---|---|---|---|---|---|
| Pressure (p) | MPa | 6.0 | 6.25 | 6.5 | 6.75 | 7.0 |
| Density (C10H22) | kg/m | 727.42 | 727.62 | 727.81 | 728.81 | 728.39 |
| Density (CO2) | kg/m | 171.44 | 187.37 | 206.44 | 230.77 | 266.56 |
| Viscosity (C10H22) | mPa.s | 846.67 | 849.14 | 851.54 | 853.98 | 856.43 |
| Viscosity (CO2) | mPa.s | 17.76 | 18.21 | 18.777 | 19.576 | 20.91 |
| Contact angle () a | 26 | 26 | 26 | 26 | - | |
| IFT (F) a | N/m | 0.003 | 0.002 | 0.00105 | 0.0001 | - |
| Diff. coeff. () b | m/s | - | - | - | - | |
| Temperature (T) | K | 303.15 | ||||
| Inlet velocity (u) | m/s | |||||
| Mesh Type | No of Elem. | Min Elem. Quality | Avg Elem. Quality |
|---|---|---|---|
| Fine Mesh | 127845 | 0.02681 | 0.78 |
| Normal Mesh | 53697 | 0.07488 | 0.78 |
| Coarse Mesh | 10494 | 0.03859 | 0.69 |
| T-re, C | MMP, MPa | Method | Source |
|---|---|---|---|
| 20 | 5.4 | Microfluidics | Bao et al. [73] |
| 20 | 5.5 | X-ray CT | Liu et al. [74] |
| 20 | 5.6 | MRI a | Song et al. [75] |
| 22 | 5.6 | Microfluidics | this article |
| 30 | 6.4 | Microfluidics | Bao et al. [73] |
| 30 | 6.7 | X-ray CT | Liu et al. [74] |
| 30 | 6.7 | MRI | Song et al. [75] |
| 30 | 7 | Microfluidics | this article |
| 37.8 | 7.7 | X-ray CT | Liu et al. [74] |
| 37.8 | 7.8 | MRI | Song et al. [75] |
| 37.8 | 8.0 | VIT | Ayirala and Rao [76] |
| 37.8 | 8.3 | Slim-tube (0.95) b | Elsharkawy et al. [77] |
| 37.8 | 8.6 | Slim-tube (BOP) c | Elsharkawy et al. [77] |
| 37.8 | 8.8 | RBA | Elsharkawy et al. [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereponov, D.; Tarkhov, M.; Dorhjie, D.B.; Rykov, A.; Filippov, I.; Zenova, E.; Krutko, V.; Cheremisin, A.; Shilov, E. Microfluidic Studies on Minimum Miscibility Pressure for n-Decane and CO2. Energies 2023, 16, 4994. https://doi.org/10.3390/en16134994
Pereponov D, Tarkhov M, Dorhjie DB, Rykov A, Filippov I, Zenova E, Krutko V, Cheremisin A, Shilov E. Microfluidic Studies on Minimum Miscibility Pressure for n-Decane and CO2. Energies. 2023; 16(13):4994. https://doi.org/10.3390/en16134994
Chicago/Turabian StylePereponov, Dmitrii, Michael Tarkhov, Desmond Batsa Dorhjie, Alexander Rykov, Ivan Filippov, Elena Zenova, Vladislav Krutko, Alexey Cheremisin, and Evgeny Shilov. 2023. "Microfluidic Studies on Minimum Miscibility Pressure for n-Decane and CO2" Energies 16, no. 13: 4994. https://doi.org/10.3390/en16134994









