Experimental Investigation on Deformation and Permeability of Clayey–Silty Sediment during Hydrate Dissociation by Depressurization
Abstract
:1. Introduction
2. Experiments
2.1. Experimental Materials
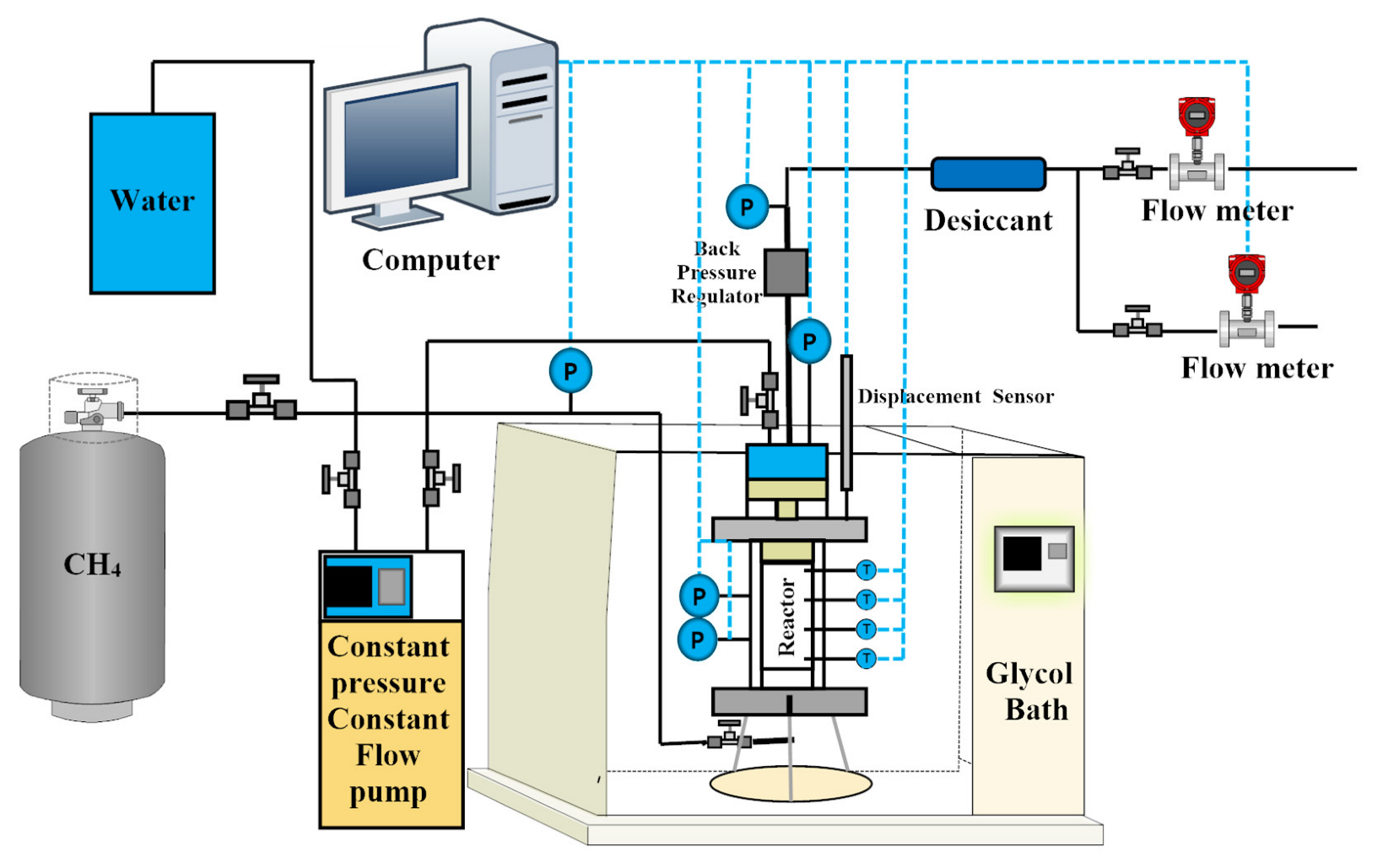
2.2. Experimental Apparatus
2.3. Experimental Procedure
2.3.1. Gas Hydrate In Situ Formation Stage
2.3.2. Depressurization Decomposition Stage
3. Results and Discussion
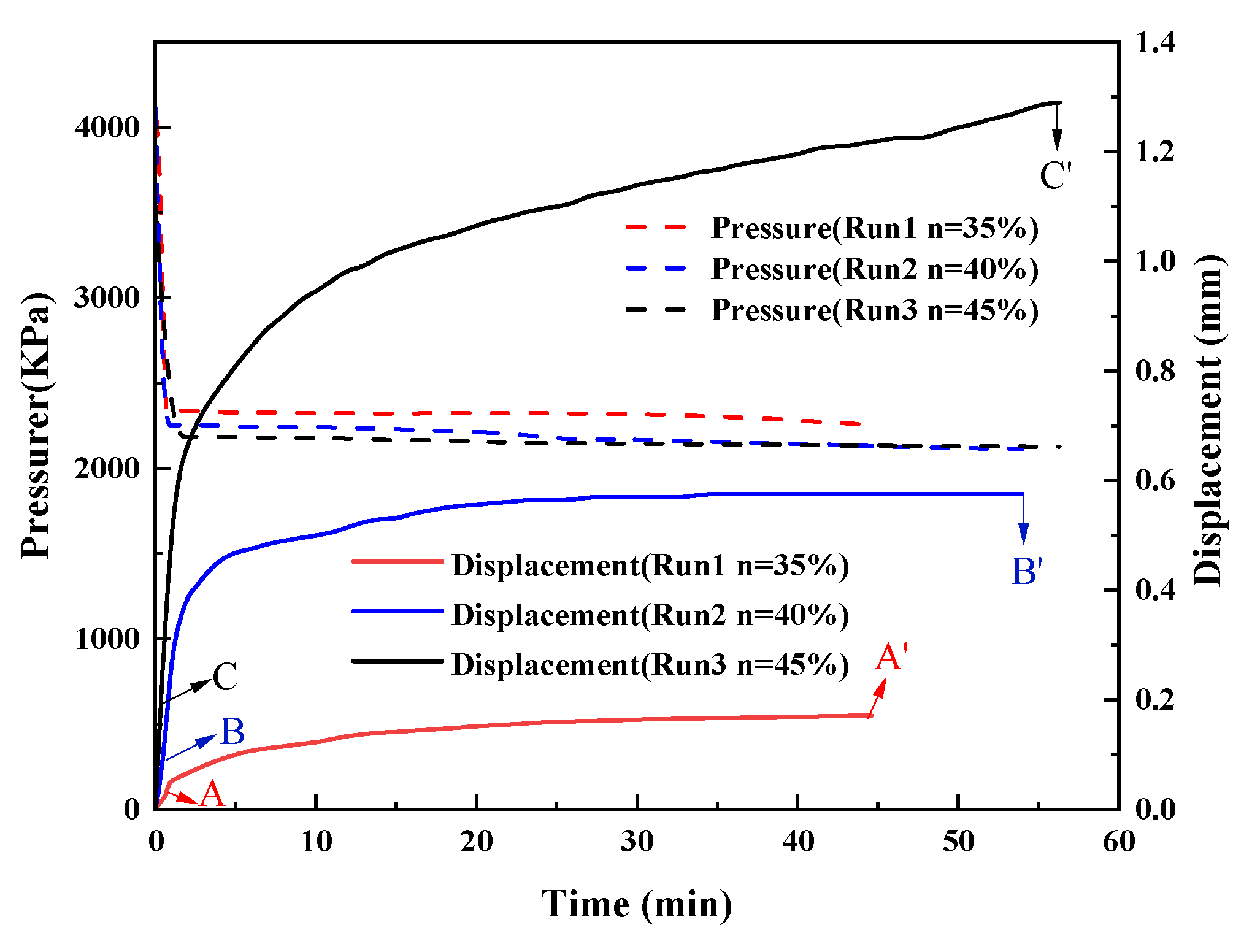
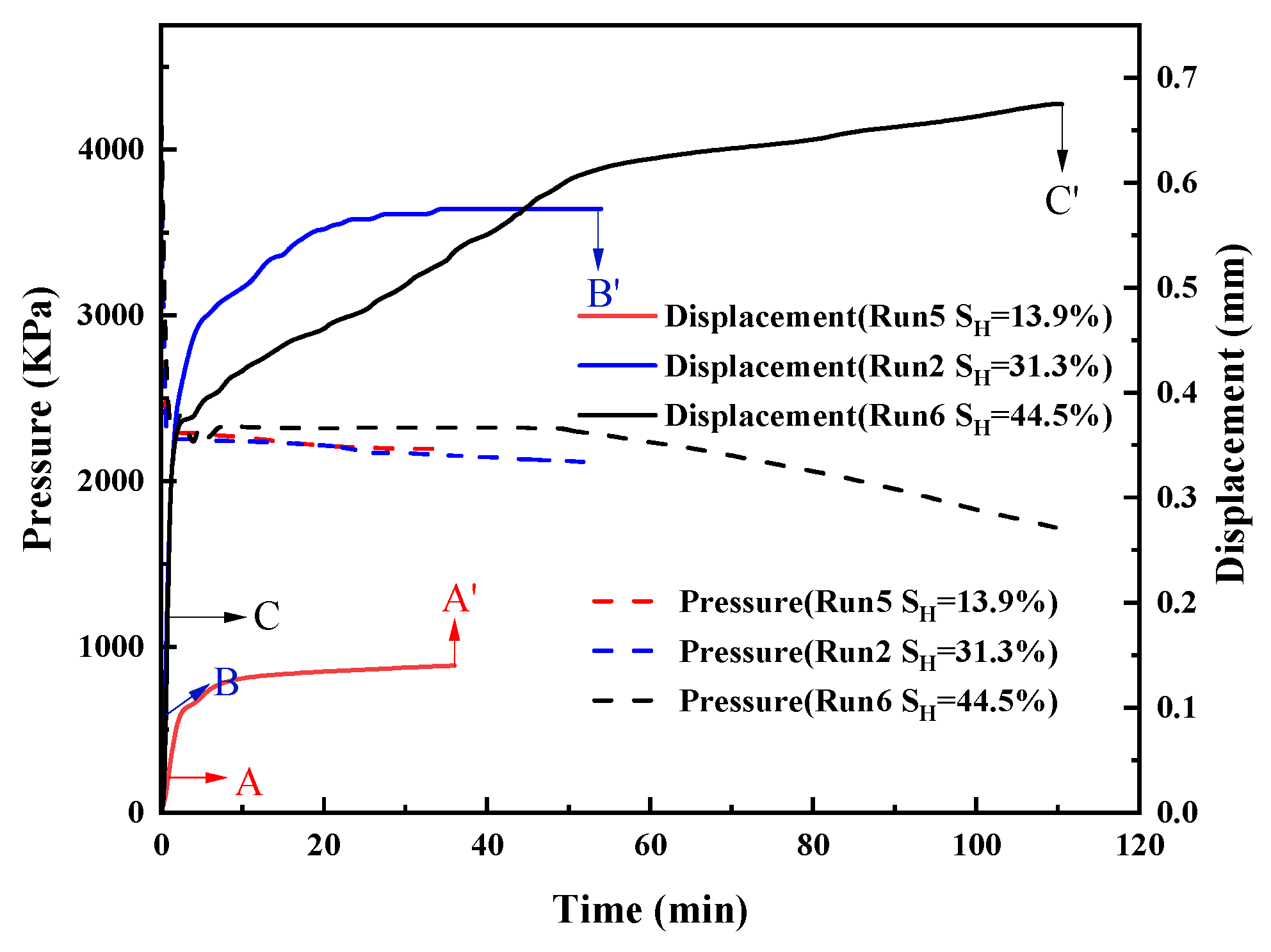
3.1. Sediment Displacement
3.1.1. Effect of Sediment Porosity
3.1.2. Effect of Hydrate Saturation
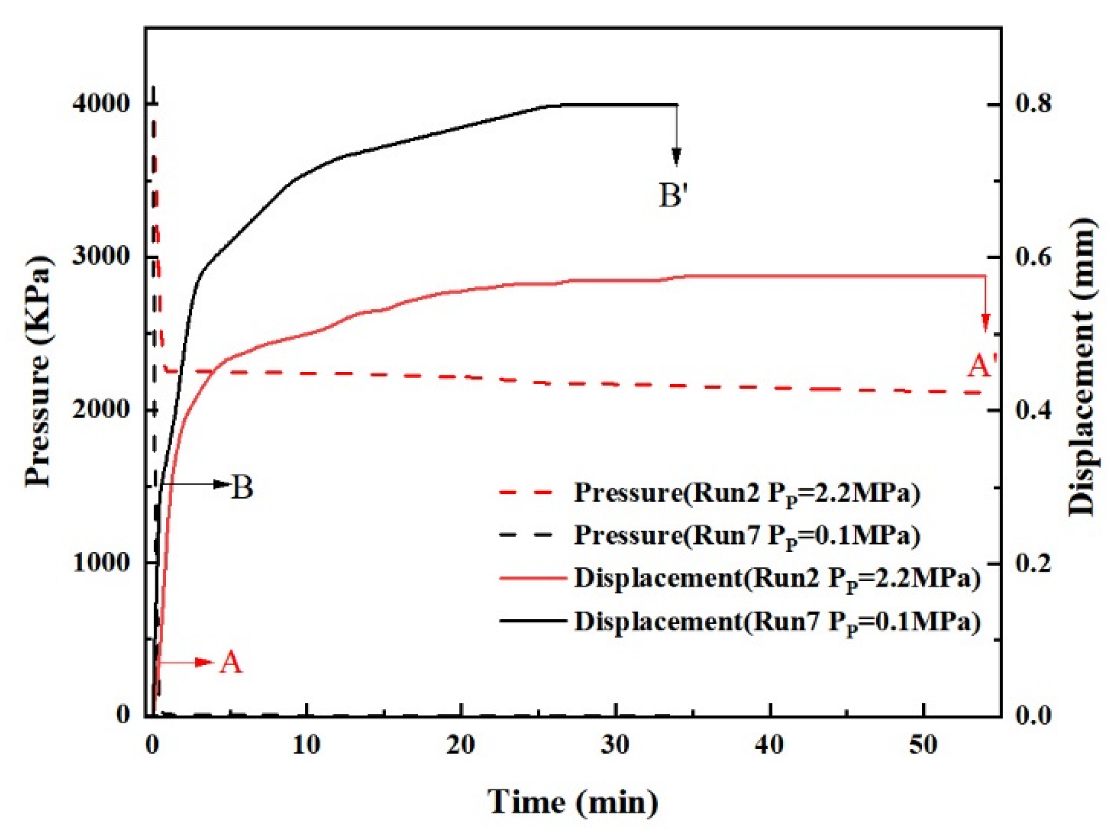
3.1.3. Effect of Production Pressure
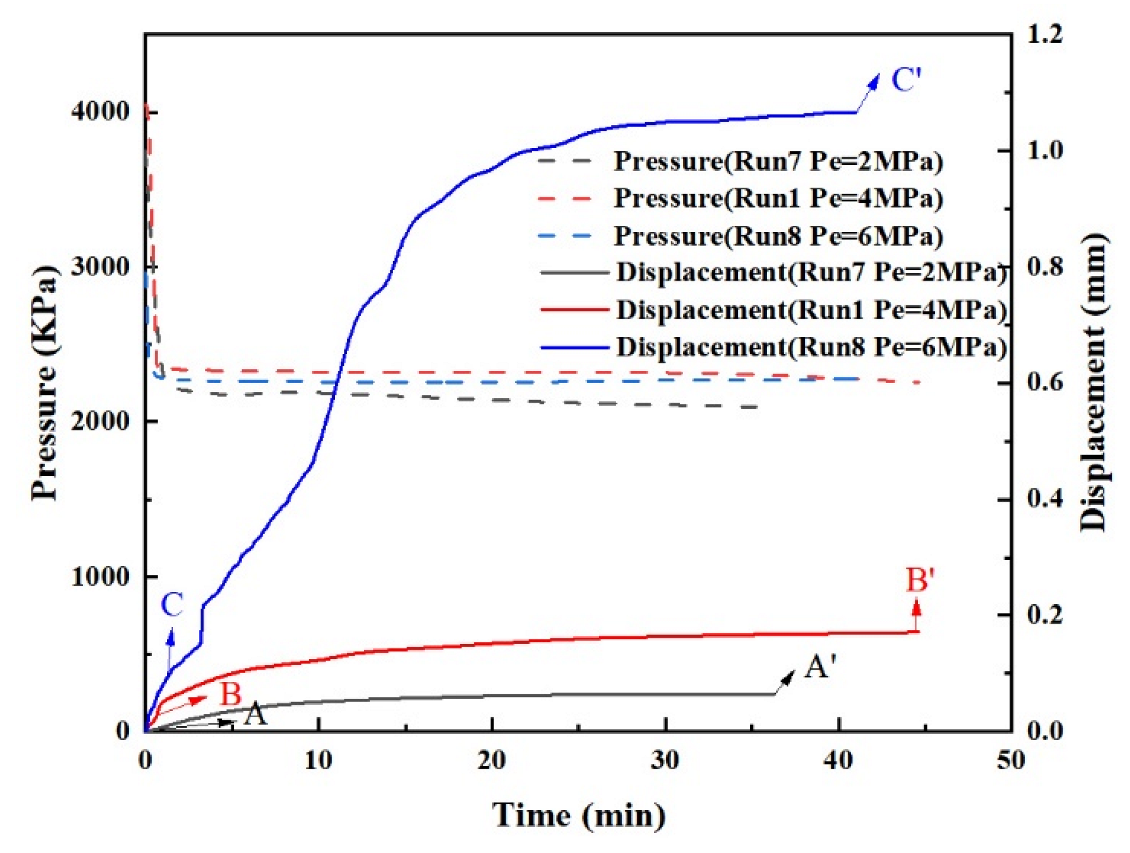
3.1.4. Effect of Effective Pressure
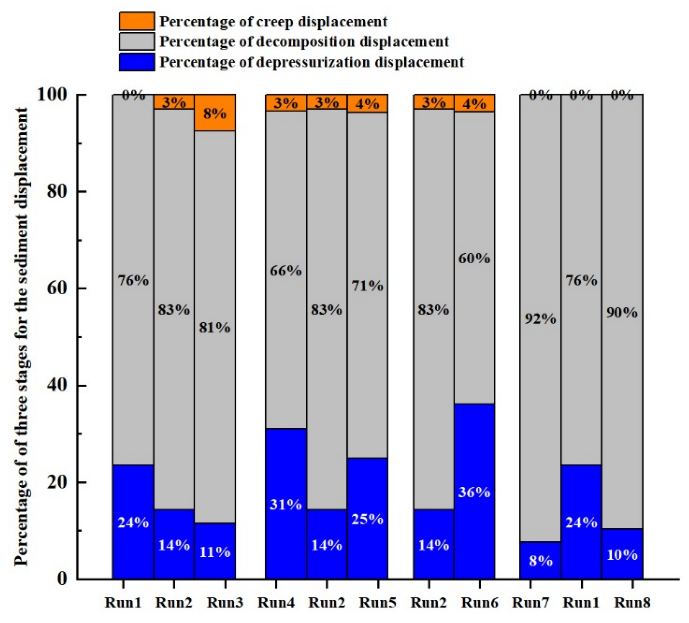
3.1.5. Displacement during Different Periods
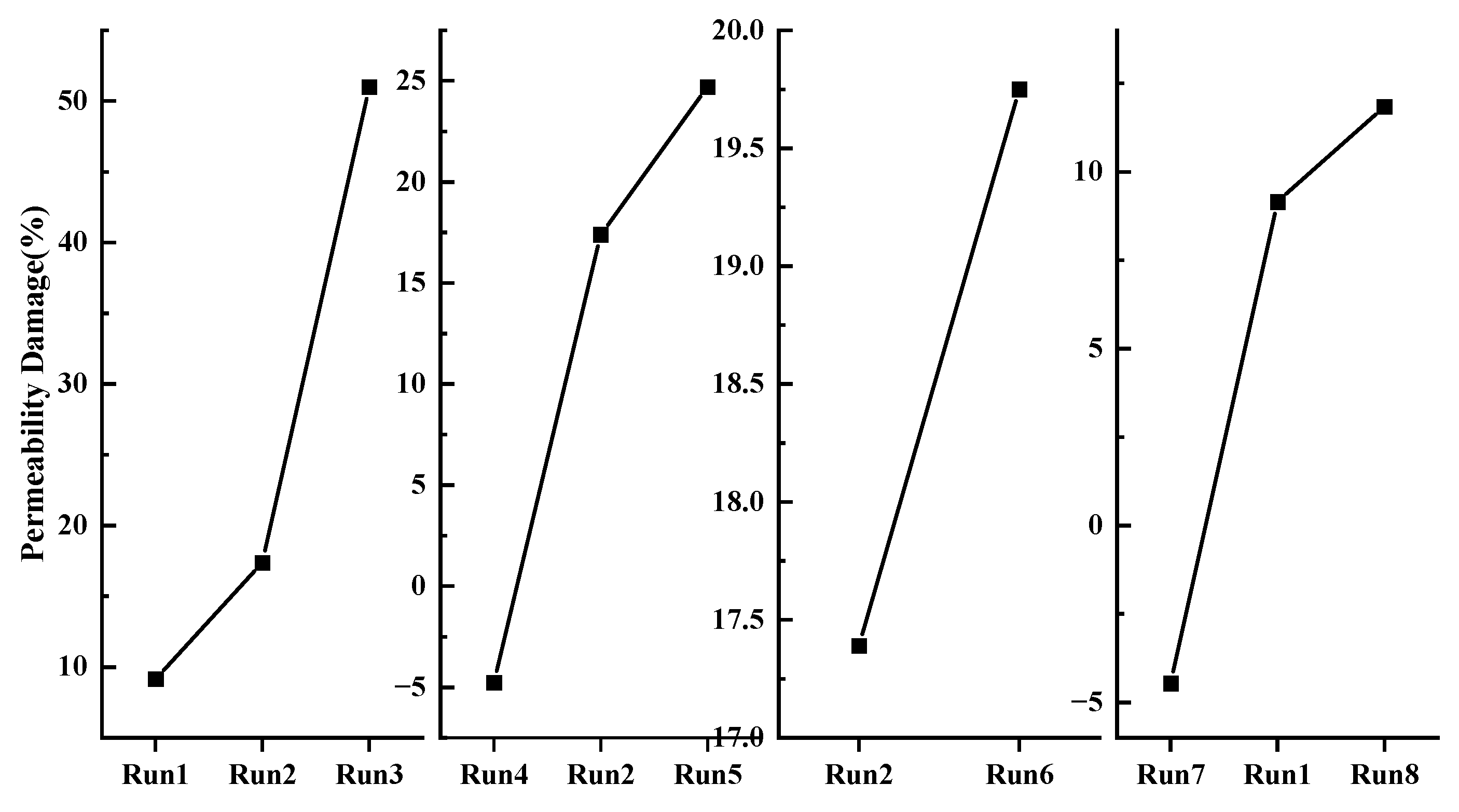
3.2. Sediment Permeability
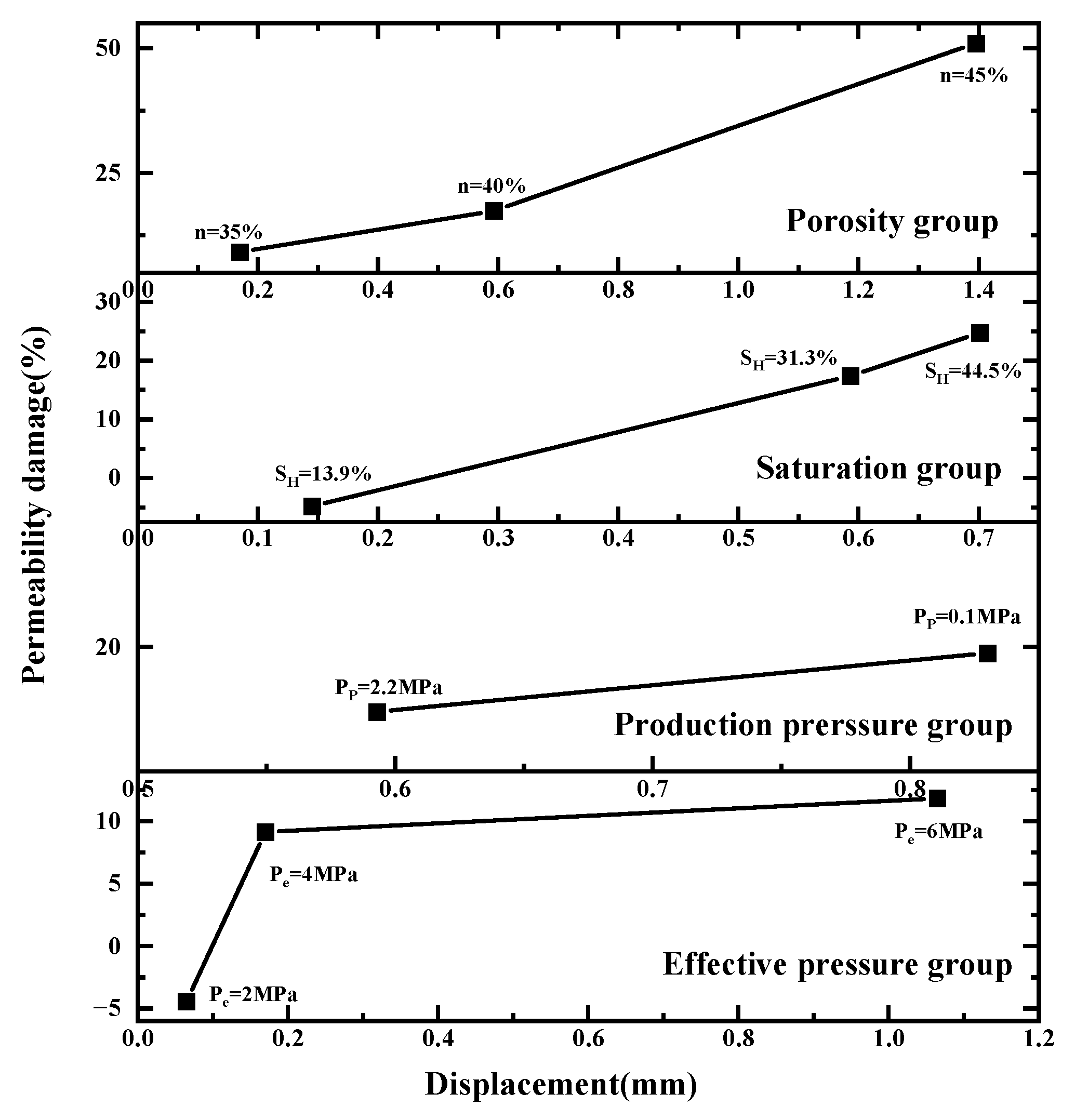
3.3. Relationship between Displacement and Permeability Damage
4. Conclusions
- The total displacement of sediments can be divided into three stages, namely, the displacement in the depressurization stage, the displacement in the process of hydrate decomposition, and the creep displacement. The sediments deform rapidly and the displacement is small in the depressurization stage. In the process of hydrate decomposition, the sediments deform slowly and the displacement is maximum. The creep stage has the slowest deformation and the smallest displacement.
- The deformation of sediments continues at the end of hydrate decomposition when the initial sediments porosity and hydrate saturation are higher. When the hydrate saturation is lower, the sediment displacement reaches the stable state earlier. Furthermore, the deformation of the sediments is larger when the effective pressure in the process of hydrate decomposition is larger.
- Sediment displacement and permeability damage increase correspondingly with the increase in sediment porosity, hydrate saturation, depressurization range, and effective pressure. Moreover, sediment displacement is positively correlated with permeability damage. However, no permeability damage occurs in the sediments when the hydrate saturation and effective pressure are low.
- In this study, it is believed that there is no wall flow between the rigid wall of the reactor and the sample, and the accuracy of permeability is ensured through multiple tests of permeability under different flow conditions. However, in future research, it is best to use an experimental setup with a flexible wall to ensure that the fluid does not flow on the wall.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Ren, S.R.; Liu, Y.J.; Liu, Y.X.; Zhang, W.D. Acoustic velocity and electrical resistance of hydrate bearing sediments. J. Pet. Sci. Eng. 2010, 70, 52–56. [Google Scholar] [CrossRef]
- Lu, S.M. A global survey of gas hydrate development and reserves: Specifically in the marine field (Retracted article. See vol. 64, pg. 851, 2016). Renew. Sustain. Energy Rev. 2015, 41, 884–900. [Google Scholar] [CrossRef]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Pet. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Li, Y.; Liu, W.; Liu, Y.; Wang, D.; Song, Y. Microstructure Evolution of Hydrate-Bearing Sands During Thermal Dissociation and Ensued Impacts on the Mechanical and Seepage Characteristics. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019103. [Google Scholar] [CrossRef]
- Mel’nikov, N.N.; Kalashnik, A.I. Geodynamic aspects of the development of offshore oil and gas deposits: Case study of Barents region. Water Resour. 2011, 38, 896–905. [Google Scholar] [CrossRef]
- Sun, X.; Luo, T.; Wang, L.; Wang, H.; Song, Y.; Li, Y. Numerical simulation of gas recovery from a low-permeability hydrate reservoir by depressurization. Appl. Energy 2019, 250, 7–18. [Google Scholar] [CrossRef]
- Yun, T.S.; Santamarina, J.C.; Ruppel, C. Mechanical properties of sand, silt, and clay containing tetrahydrofuran hydrate. J. Geophys. Res.-Sol. Ea. 2007, 112. [Google Scholar] [CrossRef] [Green Version]
- Masui, A.; Haneda, H.; Ogata, Y.; Aoki, K. Effects of Methane Hydrate Formation on Shear Strength of Synthetic Methane Hydrate sediments. In Proceedings of the Fifteenth (2005) International Offshore and Polar Engineering Conference, Seoul, Republic of Korea, 19–24 June 2005; pp. 364–369. [Google Scholar]
- Yoneda, J.; Jin, Y.; Katagiri, J.; Tenma, N. Strengthening mechanism of cemented hydrate-bearing sand at microscales. Geophys. Res. Lett. 2016, 43, 7442–7450. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Masui, A.; Sakamoto, Y.; Aoki, K.; Tenma, N.; Yamaguchi, T. Triaxial compressive properties of artificial methane-hydrate-bearing sediment. J. Geophys. Res-Sol. Ea. 2011, 116. [Google Scholar] [CrossRef]
- Jiang, M.J.; Peng, D.; Ooi, J.Y. DEM investigation of mechanical behavior and strain localization of methane hydrate bearing sediments with different temperatures and water pressures. Eng. Geol. 2017, 223, 92–109. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, Y.; Yu, F.; Liu, W.; Wang, R. Effect of confining pressure on mechanical behavior of methane hydrate-bearing sediments. Pet. Explor. Dev. 2011, 38, 637–640. [Google Scholar] [CrossRef]
- Nariai, H.; Yamane, K.; Aya, I. Strength abnormarility of CO2 hydrate membrane at deep ocean storage site. In Proceedings of the international symposium DEEP SEA & CO2, Tsukuba, Japan, 18–22 January 2000; pp. 1–2. [Google Scholar]
- Ohmura, R.; Shigetomi, T.; Mori, Y.H. Bending tests on clathrate hydrate single crystals. Philos. Mag. A-Phys. Condens. Matter Struct. Defects Mech. Prop. 2002, 82, 1725–1740. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.H.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A.; Song, Y.C. Mechanical behavior of gas-saturated methane hydrate-bearing sediments. J. Geophys. Res-Sol. Ea. 2013, 118, 5185–5194. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, M.; Yoneda, J.; Yoshimoto, N.; Nakata, Y. Mechanical and dissociation properties of methane hydrate-bearing sand in deep seabed. Soils. Found. 2013, 53, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, M.; Li, Y.H.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A. Effects of dissociation on the shear strength and deformation behavior of methane hydrate-bearing sediments. Mar. Pet. Geol. 2014, 51, 52–62. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, X.; Feng, J.C.; Li, X.S.; Zhang, Y. Sediment deformation and strain evaluation during methane hydrate dissociation in a novel experimental apparatus. Appl. Energy 2020, 262, 114397. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.L.; Jia, Y.G.; Duan, Z.B. Permeability anisotropy of methane hydrate-bearing sands: Insights from CT scanning and pore network modelling. Comput. Geotech. 2020, 123, 103568. [Google Scholar] [CrossRef]
- Lijith, K.P.; Malagar, B.R.C.; Singh, D.N. A comprehensive review on the geomechanical properties of gas hydrate bearing sediments. Mar. Pet. Geol. 2019, 104, 270–285. [Google Scholar] [CrossRef]
- Nimblett, J.; Ruppel, C. Permeability evolution during the formation of gas hydrates in marine sediments. J. Geophys. Res.-Sol. Ea. 2003, 108. [Google Scholar] [CrossRef]
- Lu, S.M.; McMechan, G.A. Estimation of gas hydrate and free gas saturation, concentration, and distribution from seismic data. Geophysics 2002, 67, 582–593. [Google Scholar] [CrossRef]
- Liu, X.L.; Flemings, P.B. Dynamic multiphase flow model of hydrate formation in marine sediments. J. Geophys. Res.-Sol. Ea. 2007, 112. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Yao, Y.; Sun, X.; Wen, Z.; Ma, Y. Permeability change with respect to different hydrate saturation in clayey-silty sediments. Energy 2022, 254, 124417. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Chen, Z.Y. Fluid flow mechanisms and heat transfer characteristics of gas recovery from gas-saturated and water-saturated hydrate reservoirs. Int. J. Heat Mass Transfer. 2018, 118, 1115–1127. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental and modeling analyses of scaling criteria for methane hydrate dissociation in sediment by depressurization. Appl. Energy 2016, 181, 299–309. [Google Scholar] [CrossRef]
- Delli, M.L.; Grozic, J.L.H. Experimental determination of permeability of porous media in the presence of gas hydrates. J. Pet. Sci. Eng. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Wu, D.M.; Li, X.S.; Lv, Q.N.; Li, C.; Zhang, Y. Experimental measurement and mathematical model of permeability with methane hydrate in quartz sands. Appl. Energy 2017, 202, 282–292. [Google Scholar] [CrossRef]
- Li, G.; Xu, Z.-L.; Li, X.-S.; Shen, P.-F.; Lv, Q.-N. Permeability investigation and hydrate migration of hydrate? Bearing silty sands and silt. J. Nat. Gas Sci. Eng. 2021, 89, 103891. [Google Scholar] [CrossRef]
- Moridis, G.J. TOUGH+HYDRATE v1.2 User’s Manual: A code for the simulation of system behavior in hydrate-bearing geologic media. Stereochem. Stereophys. Behav. Macrocycles 2014, 10. Available online: https://escholarship.org/uc/item/3mk82656 (accessed on 21 August 2022).
- Shen, P.; Li, G.; Li, B.; Li, X. Coupling effect of porosity and hydrate saturation on the permeability of methane hydrate-bearing sediments. Fuel 2020, 269, 117425. [Google Scholar] [CrossRef]
- Shen, P.; Li, G.; Li, B.; Li, X.; Liang, Y.; Lv, Q. Permeability measurement and discovery of dissociation process of hydrate sediments. J. Nat. Gas Sci. Eng. 2020, 75, 103155. [Google Scholar] [CrossRef]
- Liu, Z.C.; Kim, J.; Lei, L.; Ning, F.L.; Dai, S. Tetrahydrofuran Hydrate in Clayey SedimentsLaboratory Formation, Morphology, and Wave Characterization. J. Geophys. Res.-Sol. Ea. 2019, 124, 3307–3319. [Google Scholar] [CrossRef]
- Okwananke, A.; Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Bukhanov, B.; Istomin, V.; Cheremisin, A. Experimental study of gas permeation through gas hydrate-bearing sediments. In Proceedings of the 9th International Conference of Gas Hydrate, Denver, CO, USA, 25–30 June 2017. [Google Scholar]
- Ning, F.L.; Fang, X.Y.; Liu, Z.C.; Yu, Y.J.; Li, Y.L.; Wang, L.J.; Lu, H.F.; Sun, J.X.; Cao, X.X.; Shi, H.X. Sand production behaviors during gas recovery from sandy and clayey-silty hydrate-bearing sediments: A comparative analysis. Energy Sci. Eng. 2022, 10, 2224–2238. [Google Scholar] [CrossRef]
- Liu, C.L.; Ye, Y.G.; Meng, Q.G.; He, X.L.; Lu, H.L.; Zhang, J.; Liu, J.; Yang, S.X. The Characteristics of Gas Hydrates Recovered from Shenhu Area in the South China Sea. Mar. Geol. 2012, 307, 22–27. [Google Scholar] [CrossRef]
- Mohan, K.K.; Vaidya, R.N.; Reed, M.G.; Fogler, H.S. Water Sensitivity of Sandstones Containing Swelling and Non-Swelling Clays. Colloids Surf. A 1993, 73, 237–254. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.R.; Li, Y.H.; Sun, X.; Wu, P.; Zheng, J.A. Experimental study on the effect of methane hydrate decomposition on gas phase permeability of clayey sediments. Appl. Energy 2018, 230, 1304–1310. [Google Scholar] [CrossRef]
- Jing, M.X.; Yuan, X.L. Experiment research on core stress sensitivity of carbonate rock. Nat. Gas Ind. 2002, 20, 114–117. (In Chinese) [Google Scholar]
- Zhu, Y.M.; Chen, C.; Luo, T.T.; Song, Y.C.; Li, Y.H. Creep behaviours of methane hydrate-bearing sediments. Environ. Geotech. 2022, 9, 199–209. [Google Scholar] [CrossRef]
- Lyu, X.; Li, Q.; Ge, Y.; Zhu, J.; Zhou, S.; Fu, Q. Fundamental characteristics of gas hydrate-bearing sediments in the Shenhu area, South China Sea. Front Energy 2020, 15, 367–373. [Google Scholar] [CrossRef]
- Li, J.F.; Ye, J.L.; Qin, X.W.; Qiu, H.J.; Wu, N.Y.; Lu, H.L.; Xie, W.W.; Lu, J.A.; Peng, F.; Xu, Z.Q.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Li, Y.H.; Wu, Z.R.; Liu, W.G.; Luo, T.T.; Song, Y.C. Effect of reformation of gas hydrate on the gas phase permeability of montmorillonite. Enrgy Proced. 2019, 158, 5269–5274. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Guo, X.; Wei, R.; Yu, T.; Xu, L.; Sun, L.; Yang, L. Gas production behavior from hydrate-bearing fine natural sediments through optimized step-wise depressurization. Appl. Energy 2020, 260, 114275. [Google Scholar] [CrossRef]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Ebinuma, T. Basic research on the mechanical behavior of methane hydrate-sediments mixture. Soils. Found. 2005, 45, 75–85. [Google Scholar]
- Miyazaki, K.; Tenma, N.; Aoki, K.; Yamaguchi, T. A Nonlinear Elastic Model for Triaxial Compressive Properties of Artificial Methane-Hydrate-Bearing Sediment Samples. Energies 2012, 5, 4057–4075. [Google Scholar] [CrossRef] [Green Version]
- Ebinuma, T.; Kamata, Y.; Minagawa, H.; Ohmura, R.; Nagao, J.; Narita, H. Mechanical properties of sandy sediment containing methane hydrate. In Proceedings of the International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005. [Google Scholar]
- Mody, F.K.; Hale, A.H. Borehole-stability model to couple the mechanics and chemistry of drilling-fluid shale interactions. J. Pet. Technol. 1993, 45, 1093–1101. [Google Scholar] [CrossRef]
- Liu, G.F.; Miao, X.Q. Influence factor analysis on hydrations swelling of clay minerals. Pet. Drill. Tech. 2009, 37, 81–84. [Google Scholar]
- Wu, Z.R. Study on Seepage Characteristics of Methane Hydrate Sedimennts under Effective Stress. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2020. [Google Scholar]
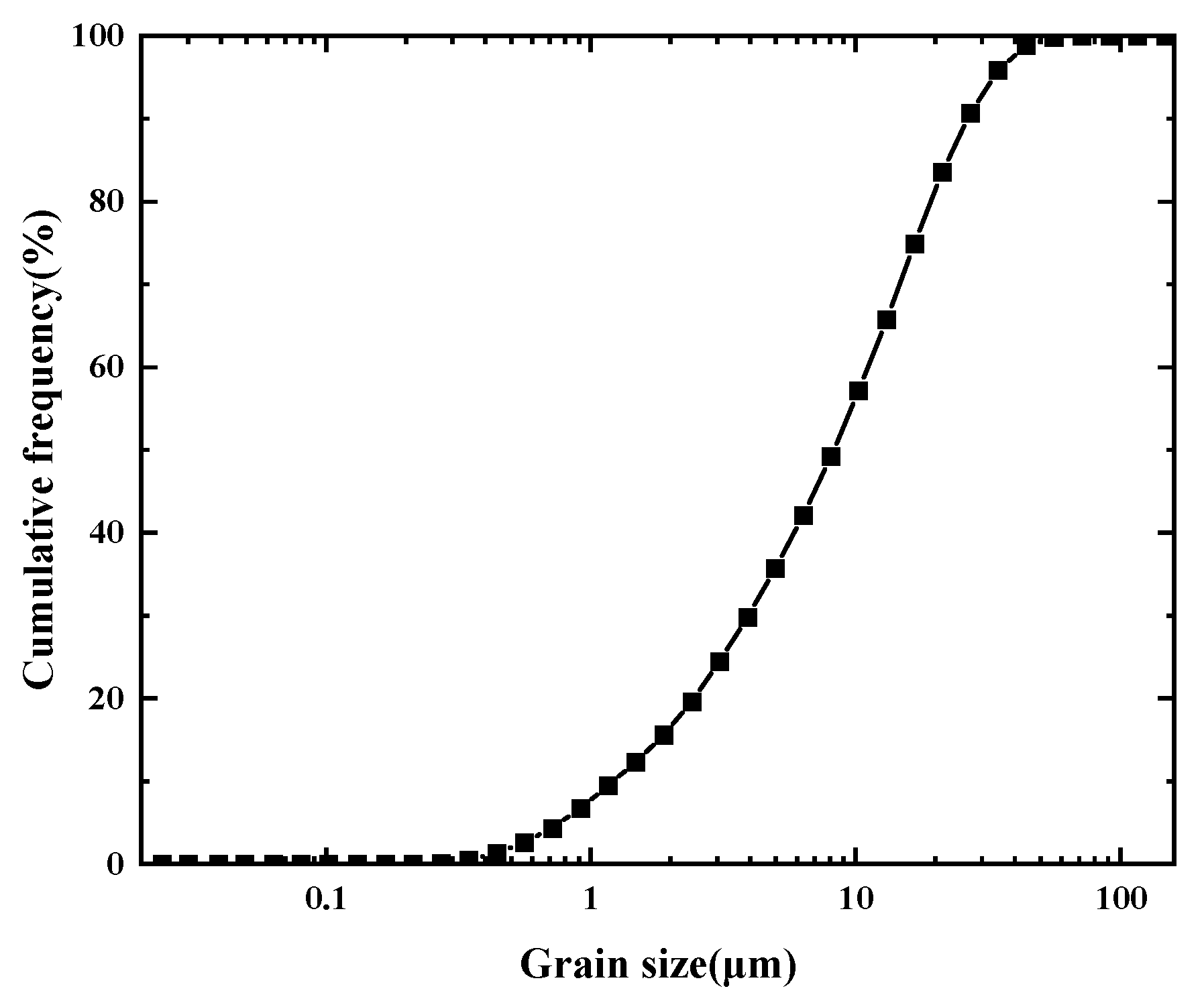


| Average Particle Size (μm) | Quartz Sand Content (%) | Clay Content (%) | Carbonate Content (%) | Density (g/cm3) | ||||
|---|---|---|---|---|---|---|---|---|
| Montmorillonite | Illite | Quartz Sand | Clay | Carbonate | ||||
| Sediments of this study | <11.44 | 54 | 10 | 20 | 16 | 2.65 | 2.75 | 2.7 |
| Sediments of South China Sea | 12 | 53 | 26~30 | 16 | 2.65 | 2.58 | 2.71 | |
| Main Parameters | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 | Run 8 |
|---|---|---|---|---|---|---|---|---|
| Quartz sand mass (g) | 182.54 | 168.50 | 154.46 | 168.50 | 168.50 | 168.50 | 182.54 | 182.54 |
| Montmorillonite mass (g) | 35.08 | 32.38 | 29.68 | 32.38 | 32.38 | 32.38 | 35.08 | 35.08 |
| Illite mass (g) | 70.16 | 64.76 | 59.37 | 64.76 | 64.76 | 64.76 | 70.16 | 70.16 |
| Calcite mass (g) | 55.11 | 50.87 | 46.63 | 50.87 | 50.87 | 50.87 | 55.11 | 55.11 |
| Water saturation | 0.60 | 0.60 | 0.60 | 0.30 | 0.70 | 0.60 | 0.60 | 0.60 |
| SH | 0.345 | 0.313 | 0.339 | 0.139 | 0.445 | 0.340 | 0.278 | 0.313 |
| n | 0.35 | 0.40 | 0.45 | 0.40 | 0.40 | 0.40 | 0.35 | 0.35 |
| Pe (MPa) | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 6 |
| Pp (MPa) | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 0.1 | 2.2 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, F.; Huang, F.; Zhang, G.; Li, B.; Lv, J. Experimental Investigation on Deformation and Permeability of Clayey–Silty Sediment during Hydrate Dissociation by Depressurization. Energies 2023, 16, 5005. https://doi.org/10.3390/en16135005
Jin F, Huang F, Zhang G, Li B, Lv J. Experimental Investigation on Deformation and Permeability of Clayey–Silty Sediment during Hydrate Dissociation by Depressurization. Energies. 2023; 16(13):5005. https://doi.org/10.3390/en16135005
Chicago/Turabian StyleJin, Fang, Feng Huang, Guobiao Zhang, Bing Li, and Jianguo Lv. 2023. "Experimental Investigation on Deformation and Permeability of Clayey–Silty Sediment during Hydrate Dissociation by Depressurization" Energies 16, no. 13: 5005. https://doi.org/10.3390/en16135005





