Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects
Abstract
1. Introduction
2. Pretreatment of Woody Biomass
2.1. Thermomechanical Pretreatments
2.2. Chemical Pretreatments
2.3. Thermal/Thermochemical Pretreatments
3. Enzymatic Hydrolysis and Fermentation of Woody Biomass
3.1. Enzymatic Hydrolysis of Woody Biomass after Thermomechanical PT
3.2. Enzymatic Hydrolysis of Woody Biomass after Chemical PT
3.3. Enzymatic Hydrolysis of Woody Biomass after Thermal/Thermochemical PT
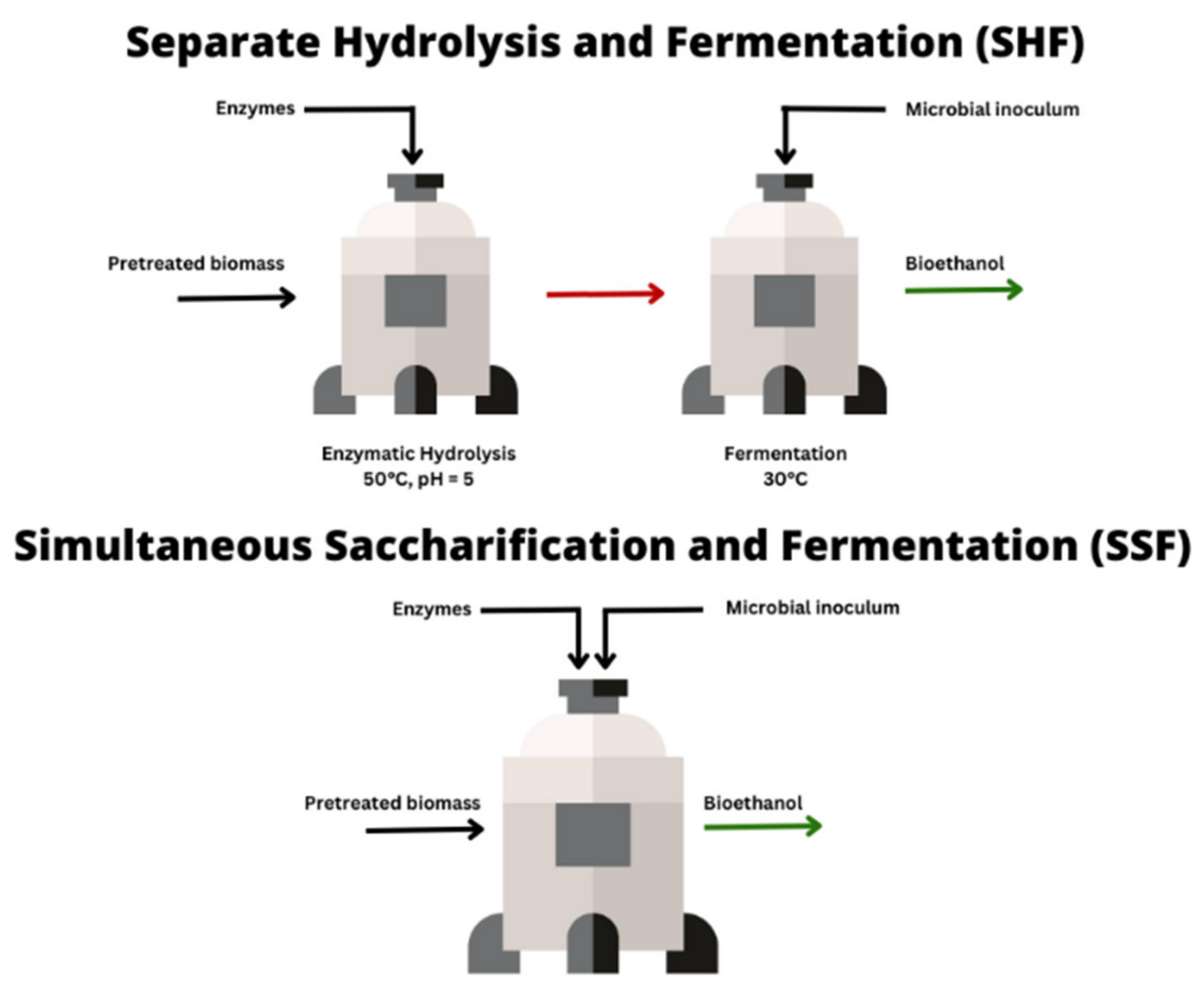
4. Simultaneous Saccharification and Fermentation of Pretreated Woody Biomass
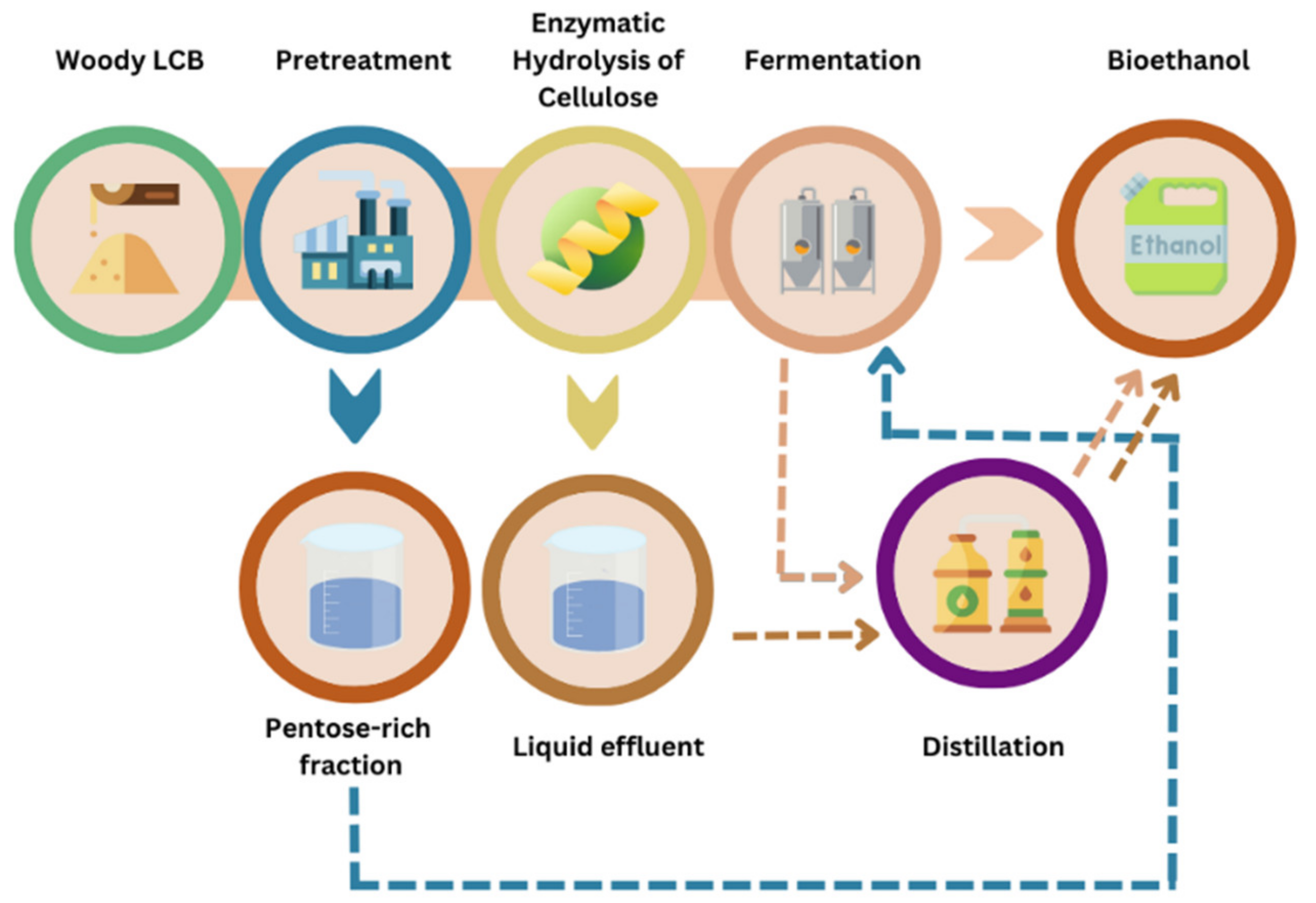
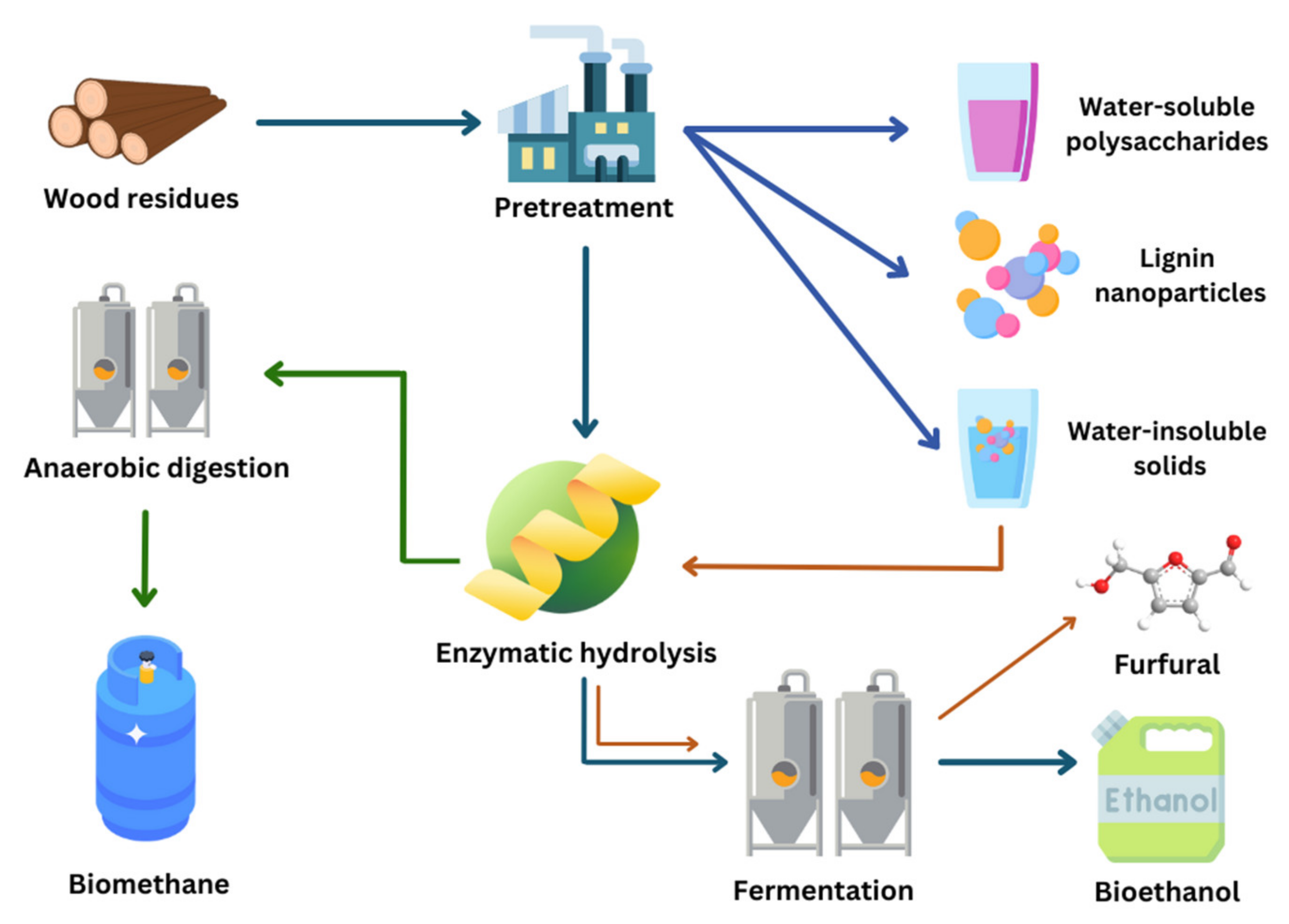
5. Overview of the Bioconversion Processes of Woody Biomass into Bioethanol and Other Products
6. Energy, Economic, and Environmental Aspects
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBP | Consolidated bioprocessing |
| CSF (or R0) | Combined severity factor |
| DB | Dry basis |
| E/W | Ethanol/water |
| EH | Enzymatic hydrolysis |
| IV-HMT | Intensive vacuum heat–moisture treatment |
| LCB | Lignocellulosic biomass |
| MT | Mannitol |
| 2N | 2-naphtol |
| NS | 2-naphtol-7-sulfonate |
| NaCS | Sodium cumene sulfonate solution |
| PHP | Phosphoric acid + hydrogen peroxide |
| PSSF | Pre-saccharification followed by simultaneous saccharification and fermentation |
| PT | Pretreatment |
| S/G Ratio | Ratio of S-to-G subunits |
| SE | Steam explosion |
| SHF | Separate hydrolysis and fermentation |
| SP | Steam pressure |
| SSCF | Simultaneous saccharification and co-fermentation |
| SSF | Simultaneous saccharification and fermentation |
| TsOH | p-toluenesulfonic acid |
| w/v | Weight on volume |
| w/w | Weight on weight |
References
- U.S. Energy Information Administration (EIA). U.S. Energy Information Administration—EIA—Independent Statistics and Analysis. Available online: https://www.eia.gov/todayinenergy/detail.php?id=56040 (accessed on 22 May 2023).
- ExxonMobil Energy Demand: Three Drivers. Available online: https://corporate.exxonmobil.com/what-we-do/energy-supply/outlook-for-energy/energy-demand#Residentialandcommercial (accessed on 22 May 2023).
- Morris, J. Renewable Energy. Available online: https://climate.mit.edu/explainers/renewable-energy (accessed on 22 May 2023).
- International Renewable Energy Agency (IRENA). Renewable Power Remains Cost-Competitive amid Fossil Fuel Crisis. Available online: https://www.irena.org/news/pressreleases/2022/Jul/Renewable-Power-Remains-Cost-Competitive-amid-Fossil-Fuel-Crisis (accessed on 22 May 2023).
- Rodrigue, J.-P. The Geography of Transport Systems. In The Geography of Transport Systems; Routledge: London, UK, 2020; ISBN 978-0-367-36463-2. [Google Scholar]
- International Energy Agency Renewables 2022—Analysis. Available online: https://www.iea.org/reports/renewables-2022/executive-summary (accessed on 28 April 2023).
- IEA. Transport Biofuels—Renewables 2022—Analysis. Available online: https://www.iea.org/reports/renewables-2022/transport-biofuels (accessed on 3 April 2023).
- International Energy Agency. Global Energy Review: CO2 Emissions in 2021; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Eurostat Renewable Energy Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics#Share_of_renewable_energy_more_than_doubled_between_2004_and_2021 (accessed on 10 March 2023).
- Papathanasiou, D. The Developing Countries Leading the Way on Renewable Energy. World Economic Forum. Available online: https://www.weforum.org/agenda/2022/07/renewables-are-the-key-to-green-secure-affordable-energy (accessed on 10 March 2023).
- International Energy Agency. World Energy Outlook 2022; International Energy Agency: Paris, France, 2022; p. 524. [Google Scholar]
- Xu, F.; Li, Y. Biomass Digestion. Encycl. Sustain. Technol. 2017, 3, 197–204. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Niju, S.; Swathika, M.; Balajii, M. Pretreatment of Lignocellulosic Sugarcane Leaves and Tops for Bioethanol Production. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–324. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of the World’s Forests 2022; FAO: Rome, Italy, 2022; ISBN 9789251359846. [Google Scholar]
- Merklein, K.; Fong, S.S.; Deng, Y. Biomass Utilization. Biotechnology for Biofuel Production and Optimization; Elsevier: Amsterdam, The Netherlands, 2016; pp. 291–324. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Yano, H.; Fu, W. Hemp: A Sustainable Plant with High Industrial Value in Food Processing. Foods 2023, 12, 651. [Google Scholar] [CrossRef]
- European Commission. Hemp Production in the EU. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/hemp_en (accessed on 12 March 2023).
- United Nations Conference on Trade and Development Hemp’s Versatility and Sustainability Offer Huge Opportunities for Developing Countries. UNCTAD. Available online: https://unctad.org/news/hemps-versatility-and-sustainability-offer-huge-opportunities-developing-countries (accessed on 12 March 2023).
- Vosper, J. The Role of Industrial Hemp in Carbon Farming; GoodEarth Resources PTY Ltd.: Newport, NSW, Australia, 2011; pp. 1–6. [Google Scholar]
- University of York. Hemp-30 Phase I Final Report; University of York: Heslington, UK, 2022. [Google Scholar]
- Tutek, K.; Masek, A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials 2022, 15, 2565. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a Potential Raw Material toward a Sustainable World: A Review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of Hemp in Textiles, Paper Industry, Insulation and Building Materials, Horticulture, Animal Nutrition, Food and Beverages, Nutraceuticals, Cosmetics and Hygiene, Medicine, Agrochemistry, Energy Production and Environment: A Review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Semhaoui, I. Etude de la Bioconversion de la Chènevotte (Cannabis Sativa) et de l’alfa (Stipa Tenacissima) par Prétraitement Thermomécanique en Présence d’un Catalyseur Acide ou Alcalin. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2019. [Google Scholar]
- Bruker Extracting High-Value Products from Hemp Waste. Available online: https://www.bruker.com/es/resources/library/application-notes-mr/extracting-high-value-products-from-hemp-waste.html (accessed on 13 March 2023).
- Naithani, V.; Tyagi, P.; Jameel, H.; Lucia, L.A.; Pal, L. Ecofriendly and Innovative Processing of Hemp Hurds Fibers for Tissue and Towel Paper. BioResources 2020, 15, 706–720. [Google Scholar] [CrossRef]
- Periyasamy, S.; Beula Isabel, J.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Sivashanmugam, P.; Aminabhavi, T.M. Recent Advances in Consolidated Bioprocessing for Conversion of Lignocellulosic Biomass into Bioethanol—A Review. Chem. Eng. J. 2023, 453, 139783. [Google Scholar] [CrossRef]
- Semhaoui, I.; Maugard, T.; Zarguili, I.; Rezzoug, S.A.; Zhao, J.M.Q.; Toyir, J.; Nawdali, M.; Maache-Rezzoug, Z. Eco-Friendly Process Combining Acid-Catalyst and Thermomechanical Pretreatment for Improving Enzymatic Hydrolysis of Hemp Hurds. Bioresour. Technol. 2018, 257, 192–200. [Google Scholar] [CrossRef]
- Paramasivan, S.; Sankar, S.; Velavan, R.S.; Krishnakumar, T.; Batcha, R.S.I.; Muthuvelu, K.S. Assessing the Potential of Lignocellulosic Energy Crops as an Alternative Resource for Bioethanol Production Using Ultrasound Assisted Dilute Acid Pretreatment. Mater. Today Proc. 2021, 45, 3279–3285. [Google Scholar] [CrossRef]
- Smullen, E.; Finnan, J.; Dowling, D.; Mulcahy, P. The Environmental Performance of Pretreatment Technologies for the Bioconversion of Lignocellulosic Biomass to Ethanol. Renew. Energy 2019, 142, 527–534. [Google Scholar] [CrossRef]
- Roni, K.A.; Hastarina, M.; Herawati, N. Effect of Time and Concentration of Sulfuric Acid on Yield Bioethanol Produced In Making Bioethanol from Peat Soil. J. Phys. Conf. Ser. 2019, 1167, 012056. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Consolidated Briefing of Biochemical Ethanol Production from Lignocellulosic Biomass. Electron. J. Biotechnol. 2016, 23, 44–53. [Google Scholar] [CrossRef]
- El Hage, M.; Rajha, N.H.; Maache-Rezzoug, Z.; Koubaa, M. Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies 2022, 15, 6912. [Google Scholar] [CrossRef]
- Demichelis, F.; Laghezza, M.; Chiappero, M.; Fiore, S. Technical, Economic and Environmental Assessement of Bioethanol Biorefinery from Waste Biomass. J. Clean. Prod. 2020, 277, 124111. [Google Scholar] [CrossRef]
- Silva Ortiz, P.A.; Maréchal, F.; de Oliveira Junior, S. Exergy Assessment and Techno-Economic Optimization of Bioethanol Production Routes. Fuel 2020, 279, 118327. [Google Scholar] [CrossRef]
- Araghi, M.K.; Barkhordari, S.; Hasannia, R. Economic Impacts of Producing Bioethanol in Iran: A CGE Approach. Energy 2023, 263, 125765. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Sganzerla, W.G.; Larnaudie, V.; Veersma, R.J.; van Erven, G.; Shiva; Ríos-González, L.J.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Ferrari, M.D.; et al. Advances in Process Design, Techno-Economic Assessment and Environmental Aspects for Hydrothermal Pretreatment in the Fractionation of Biomass under Biorefinery Concept. Bioresour. Technol. 2023, 369, 128469. [Google Scholar] [CrossRef] [PubMed]
- Gandam, P.K.; Chinta, M.L.; Pabbathi, N.P.P.; Baadhe, R.R.; Sharma, M.; Thakur, V.K.; Sharma, G.D.; Ranjitha, J.; Gupta, V.K. Second-Generation Bioethanol Production from Corncob—A Comprehensive Review on Pretreatment and Bioconversion Strategies, Including Techno-Economic and Lifecycle Perspective. Ind. Crops Prod. 2022, 186, 115245. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of Pretreatment of Feedstocks for Optimized Bioethanol Production: Distinct and Integrated Approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Liu, S.; Chang, H.; Christensen, T.H. Bioethanol from Corn Stover—Integrated Environmental Impacts of Alternative Biotechnologies. Resour. Conserv. Recycl. 2020, 155, 104652. [Google Scholar] [CrossRef]
- Lancha, J.P. A Multiscale Approach to Understand and Predict the Effects of Hydrothermal Treatment on Lignocellulosic Biomass; Université Paris-Saclay: Paris, France, 2020. [Google Scholar]
- Keskin, T.; Abubackar, H.N.; Arslan, K.; Azbar, N. Biohydrogen Production From Solid Wastes. Biohydrogen 2019, 321–346. [Google Scholar] [CrossRef]
- Tabil, L.; Adapa, P.; Kashaninejad, M. Biomass Feedstock Pre-Processing—Part 1: Pre-Treatment. In Biofuel’s Engineering Process Technolog; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. In Biotechnology Application Biomass; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Bajpai, P. Xylanases. Encycl. Microbiol. 2009, 600–612. [Google Scholar] [CrossRef]
- Simangunsong, E.; Ziegler-Devin, I.; Chrusciel, L.; Girods, P.; Wistara, N.; Brosse, N. Steam Explosion of Beech Wood-Effect of the Particle Size on the Xylans Recovery. HAL Id: Hal-02125966. Waste Biomass Valorization 2019, 11, 625. [Google Scholar] [CrossRef]
- Guo, M.; Littlewood, J.; Joyce, J.; Murphy, R. The Environmental Profile of Bioethanol Produced from Current and Potential Future Poplar Feedstocks in the EU. Green Chem. 2014, 16, 4680–4695. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Zhou, Y.; Wang, W.; Sun, Y.; Guo, Y.; Kang, X. Biorefining of Ethanol and Methane from NaOH Pretreated Poplar Residues: Mass Balance and Energy Flow Analyses. Fuel 2023, 333, 126293. [Google Scholar] [CrossRef]
- Kizhakkepurakkal, A.R. Opportunities and Challenges Associated with Development of Wood Biomass Energy Production in Louisiana; Kerala Agricultural University: Thrissur, India, 2008. [Google Scholar]
- Geng, W.; Venditti, R.A.; Pawlak, J.J.; Chang, H.M. Effect of Delignification on Hemicellulose Extraction from Switchgrass, Poplar, and Pine and Its Effect on Enzymatic Convertibility of Cellulose-Rich Residues. BioResources 2019, 13, 4946–4963. [Google Scholar] [CrossRef]
- Bay, M.S.; Eslami, F.; Karimi, K. The Relationship between Structural Features of Lignocellulosic Materials and Ethanol Production Yield. Designs 2022, 6, 119. [Google Scholar] [CrossRef]
- Houghton, R.A. Biomass. Biofuels Biorefining 2008, 1, 448–453. [Google Scholar]
- Paul, S.K.; Chakraborty, S. Microwave-Assisted Ionic Liquid-Mediated Rapid Catalytic Conversion of Non-Edible Lignocellulosic Sunn Hemp Fibres to Biofuels. Bioresour. Technol. 2018, 253, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.; Senn, T. Energy Self-Sufficient Production of Bioethanol from a Mixture of Hemp Straw and Triticale Seeds: Life-Cycle Analysis. Biomass Bioenergy 2016, 95, 99–108. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Jesús RANGEL, M.; Hornus, M.; Felissia, F.E.; Area, M.C. Hydrothermal Treatment of Eucalyptus Sawdust for a Forest Biorefinery. Cellul. Chem. Technol. 2016, 50, 521–528. [Google Scholar]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-Production of Bioethanol and Xylosaccharides from Steam-Exploded Eucalyptus Sawdust Using High Solid Loads in Enzymatic Hydrolysis: Effect of Alkaline Impregnation. Ind. Crops Prod. 2022, 175, 114253. [Google Scholar] [CrossRef]
- Bodîrlău, R.; Teacă, C.A.; Spiridon, I. Chemical Modification of Beech Wood: Effect on Thermal Stability. BioResources 2008, 3, 789–800. [Google Scholar] [CrossRef]
- Szczepkowski, A.; Nicewicz, D.; Koczoń, P. The Relationship Between Tree Health and Chemical Composition Of Beech (Fagus Sylvatica L.) and Oak (Quercus Robur L.) Wood of Polish Provenances. Acta Sci. Pol. Tecnol. Aliment. 2007, 6, 77–88. [Google Scholar]
- Bari, E.; Mohebby, B.; Naji, H.R.; Oladi, R.; Yilgor, N.; Nazarnezhad, N.; Ohno, K.M.; Nicholas, D.D. Monitoring the Cell Wall Characteristics of Degraded Beech Wood by White-Rot Fungi: Anatomical, Chemical, and Photochemical Study. Maderas Cienc. Tecnol. 2018, 20, 35–56. [Google Scholar] [CrossRef]
- Rowell, R.M.; Keany, F.M. Fiberboards Made From Acetylated Bagasse Fiber. Wood Fiber Sci. 1991, 23, 15–22. [Google Scholar]
- Wang, Z.; Winestrand, S.; Gillgren, T.; Jönsson, L.J. Chemical and Structural Factors Influencing Enzymatic Saccharification of Wood from Aspen, Birch and Spruce. Biomass Bioenergy 2018, 109, 125–134. [Google Scholar] [CrossRef]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Zimonin, D.V.; Skripnikov, A.M.; Miroshnikova, A.V.; Ionin, V.A.; Kazachenko, A.S.; Sychev, V.V.; et al. Composition and Structure of Aspen (Pópulus Trémula) Hemicelluloses Obtained by Oxidative Delignification. Polymers 2022, 14, 4521. [Google Scholar] [CrossRef]
- Buzala, K.P.; Kalinowska, H.; Malachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies 2019, 12, 2952. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Wan, X.; Liu, J.; Zhang, Y.; Tian, D.; Liu, Y.; Zhao, L.; Huang, M.; Hu, J.; Shen, F. Conversion of Agricultural and Forestry Biomass into Bioethanol, Water-Soluble Polysaccharides, and Lignin Nanoparticles by an Integrated Phosphoric Acid plus Hydrogen Peroxide Process. Ind. Crops Prod. 2023, 191, 115969. [Google Scholar] [CrossRef]
- Geffert, A.; Geffertova, J.; Dudiak, M. Direct Method of Measuring the PH Value of Wood. Forests 2019, 10, 852. [Google Scholar] [CrossRef]
- Kučerová, V.; Hrčka, R.; Hýrošová, T. Relation of Chemical Composition and Colour of Spruce Wood. Polymers 2022, 14, 5333. [Google Scholar] [CrossRef]
- Čabalová, I.; Bélik, M.; Kučerová, V.; Jurczyková, T. Chemical and Morphological Composition of Norway Spruce Wood (Picea Abies L.) in the Dependence of Its Storage. Polymers 2021, 13, 1619. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- University of Knoxville Tennessee. Typical H:G:S Ratio for Lignin from Biomass. Available online: https://static.igem.org/mediawiki/2018/c/c2/T--CCU_Taiwan--CCUlignin_HGS.pdf (accessed on 22 June 2023).
- Paliwal, R.; Giri, K.; Rai, J.P. Microbial Ligninolysis: Avenue for Natural Ecosystem Management; IGI Global: Hershey, PE, USA, 2015; pp. 120–144. ISBN 9781466686830. [Google Scholar]
- Papa, G.; Varanasi, P.; Sun, L.; Cheng, G.; Stavila, V.; Holmes, B.; Simmons, B.A.; Adani, F.; Singh, S. Exploring the Effect of Different Plant Lignin Content and Composition on Ionic Liquid Pretreatment Efficiency and Enzymatic Saccharification of Eucalyptus Globulus L. Mutants. Bioresour. Technol. 2012, 117, 352–359. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Phillips, L.; Flint, H.; Geddes, B.; Lu, F.; Ralph, J. Syringyl Lignin Production in Conifers: Proof of Concept in a Pine Tracheary Element System. Proc. Natl. Acad. Sci. USA 2015, 112, 6218–6223. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, C.S. Recent Efforts to Prevent Undesirable Reactions from Fractionation to Depolymerization of Lignin: Toward Maximizing the Value from Lignin. Front. Energy Res. 2018, 6, 92. [Google Scholar] [CrossRef]
- Eom, T.; Chaiprapat, S.; Charnnok, B. Enhanced Enzymatic Hydrolysis and Methane Production from Rubber Wood Waste Using Steam Explosion. J. Environ. Manag. 2019, 235, 231–239. [Google Scholar] [CrossRef]
- Li, W.C.; Han, L.J.; Peng, T.B.; Xie, Y.Y.; Zou, Y.; Li, L.Z.; Jia, S.R.; Zhong, C. Structural and Behavior Changes of Herbaceous and Hardwood Biomass during Steam Explosion Pretreatment and Enzymatic Hydrolysis. BioResources 2020, 15, 691–705. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented Digestion of Lignocellulose by Steam Explosion, Acid and Alkaline Pretreatment Methods: A Review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- He, Q.; Hou, Q.; Hong, L.; Lu, X.; Ziegler-Devin, I.; Chrusciel, L.; Besserer, A.; Brosse, N. Effect of Highly Efficient Steam Explosion Treatment on Beech, Poplar and Spruce Solid Wood Physicochemical and Permeable Performances. Ind. Crops Prod. 2022, 182, 114901. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Yang, Q.; Peng, H.; Li, Y.; Zhan, D.; Wei, H.; Lu, H.; Bakr, M.M.A.; EI-Sheekh, M.M.; et al. Combined Steam Explosion and Optimized Green-Liquor Pretreatments Are Effective for Complete Saccharification to Maximize Bioethanol Production by Reducing Lignocellulose Recalcitrance in One-Year-Old Bamboo. Renew. Energy 2021, 175, 1069–1079. [Google Scholar] [CrossRef]
- Adawiyah, D.R.; Akuzawa, S.; Sasaki, T.; Kohyama, K. A Comparison of the Effects of Heat Moisture Treatment (HMT) on Rheological Properties and Amylopectin Structure in Sago (Metroxylon Sago) and Arenga (Arenga Pinnata) Starches. J. Food Sci. Technol. 2017, 54, 3404. [Google Scholar] [CrossRef] [PubMed]
- Aklouche, L.; Monteau, J.-Y.; Rezzoug, S.-A.; Maugard, T.; Guihard, L.; Cohendoz, S.; Maache-Rezzoug, Z. Prediction of Thermal Conductivity and Specific Heat of Native Maize Starch and Comparison with Hmt Treated Starch. J. Renew. Mater. 2019, 7, 535–546. [Google Scholar] [CrossRef]
- Maruta, I.; Kurahashi, Y.; Takano, R.; Hayashi, K.; Yoshino, Z. Reduced-Pressurized Heat-Moisture Treatment: A New Method for Heat-Moisture Treatment of Starch. Available online: https://agris.fao.org/agris-search/search.do?recordID=DE94S0213 (accessed on 28 April 2023).
- Bahrani, S.A.; Monteau, J.Y.; Rezzoug, S.A.; Loisel, C.; Maache-Rezzoug, Z. Physics-Based Modeling of Simultaneous Heat and Mass Transfer Intensification during Vacuum Steaming Processes of Starchy Material. Chem. Eng. Process. Process Intensif. 2014, 85, 216–226. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Ballesteros, I.; Ballesteros, M. Second Generation Bioethanol from Steam Exploded Eucalyptus Globulus Wood. Fuel 2013, 111, 66–74. [Google Scholar] [CrossRef]
- Troncoso-Ortega, E.; Castillo, R.D.P.; Reyes-Contreras, P.; Castaño-Rivera, P.; Mendonça, R.T.; Schiappacasse, N.; Parra, C. Effects on Lignin Redistribution in Eucalyptus Globulus Fibres Pre-Treated by Steam Explosion: A Microscale Study to Cellulose Accessibility. Biomolecules 2021, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.D.H.; Fontana, R.C.; Baudel, H.M.; de Siqueira, F.G.; Rencoret, J.; Gutiérrez, A.; de Eugenio, L.I.; Prieto, A.; Martínez, M.J.; Martínez, Á.T.; et al. Lignin Degradation and Detoxification of Eucalyptus Wastes by On-Site Manufacturing Fungal Enzymes to Enhance Second-Generation Ethanol Yield. Appl. Energy 2020, 262, 114493. [Google Scholar] [CrossRef]
- Pažitný, A.; Russ, A.; Boháček, Š.; Stankovská, M.; Ihnát, V. Effect of Steam Explosion on Enzymatic Hydrolysis of Various Parts of Poplar Tree. Wood Res. 2020, 65, 579–590. [Google Scholar] [CrossRef]
- Barbanera, M.; Lascaro, E.; Foschini, D.; Cotana, F.; Buratti, C. Optimization of Bioethanol Production from Steam Exploded Hornbeam Wood (Ostrya Carpinifolia) by Enzymatic Hydrolysis. Renew. Energy 2018, 124, 136–143. [Google Scholar] [CrossRef]
- Besserer, A.; Obame, S.N.; Safou-Tchima, R.; Saker, S.; Ziegler-Devin, I.; Brosse, N. Biorefining of Aucoumea Klaineana Wood: Impact of Steam Explosion on the Composition and Ultrastructure the Cell Wall. Ind. Crops Prod. 2022, 177, 114432. [Google Scholar] [CrossRef]
- Mihiretu, G.T.; Chimphango, A.F.; Görgens, J.F. Steam Explosion Pre-Treatment of Alkali-Impregnated Lignocelluloses for Hemicelluloses Extraction and Improved Digestibility. Bioresour. Technol. 2019, 294, 122121. [Google Scholar] [CrossRef]
- Pielhop, T.; Amgarten, J.; Von Rohr, P.R.; Studer, M.H. Steam Explosion Pretreatment of Softwood: The Effect of the Explosive Decompression on Enzymatic Digestibility. Biotechnol. Biofuels 2016, 9, 152. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Catalytic Conversion of Structural Carbohydrates and Lignin to Chemicals. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2017; Volume 60, pp. 59–123. [Google Scholar]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam Explosion as Lignocellulosic Biomass Pretreatment; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128025611. [Google Scholar]
- Russ, A.; Fišerová, M.; Letko, M.; Opálen, E. Effect of Steam Explosion Temperature on Wheat Straw Enzymatic Hydrolysis. Wood Res. 2016, 61, 65–74. [Google Scholar]
- Jeong, S.Y.; Lee, J.W. Hydrothermal Treatment. Pretreat. Biomass Process. Technol. 2015, 61–74. [Google Scholar] [CrossRef]
- Cullis, I.F.; Saddler, J.N.; Mansfield, S.D. Effect of Initial Moisture Content and Chip Size on the Bioconversion Efficiency of Softwood Lignocellulosics. Biotechnol. Bioeng. 2004, 85, 413–421. [Google Scholar] [CrossRef]
- Meehnian, H.; Jana, A.K.; Jana, M.M. Effect of Particle Size, Moisture Content, and Supplements on Selective Pretreatment of Cotton Stalks by Daedalea Flavida and Enzymatic Saccharification. 3 Biotech 2016, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Monlau, F.; Solhy, A.; Carrere, H. Mechanical Dissociation and Fragmentation of Lignocellulosic Biomass: Effect of Initial Moisture, Biochemical and Structural Proprieties on Energy Requirement. Appl. Energy 2015, 142, 240–246. [Google Scholar] [CrossRef]
- Pawar, P.M.A.; Derba-Maceluch, M.; Chong, S.L.; Gandla, M.L.; Bashar, S.S.; Sparrman, T.; Ahvenainen, P.; Hedenström, M.; Özparpucu, M.; Rüggeberg, M.; et al. In Muro Deacetylation of Xylan Affects Lignin Properties and Improves Saccharification of Aspen Wood. Biotechnol. Biofuels 2017, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Peng, H.; Li, H.; Xu, J. Effect of Acetylation/Deacetylation on Enzymatic Hydrolysis of Corn Stalk. Biomass Bioenergy 2014, 71, 294–298. [Google Scholar] [CrossRef]
- Chen, X.; Shekiro, J.; Franden, M.A.; Wang, W.; Zhang, M.; Kuhn, E.; Johnson, D.K.; Tucker, M.P. The Impacts of Deacetylation Prior to Dilute Acid Pretreatment on the Bioethanol Process. Biotechnol. Biofuels 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A Review on Delignification of Lignocellulosic Biomass for Enhancement of Ethanol Production Potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Onyenwoke, C.; Tabil, L.G.; Dumonceaux, T.; Cree, D.; Mupondwa, E.; Adapa, P.; Karunakaran, C. Investigation of Steam Explosion Pretreatment of Sawdust and Oat Straw to Improve Their Quality as Biofuel Pellets. Energies 2022, 15, 7168. [Google Scholar] [CrossRef]
- Jacquet, N.; Vanderghem, C.; Danthine, S.; Quiévy, N.; Blecker, C.; Devaux, J.; Paquot, M. Influence of Steam Explosion on Physicochemical Properties and Hydrolysis Rate of Pure Cellulose Fibers. Bioresour. Technol. 2012, 121, 221–227. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021, 14, 588–605. [Google Scholar] [CrossRef]
- Jȩdrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and Chemical Pretreatment of Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128151624. [Google Scholar]
- Zhao, J.; Tian, D.; Shen, F.; Hu, J.; Zeng, Y.; Huang, C. Valorizing Waste Lignocellulose-Based Furniture Boards by Phosphoric Acid and Hydrogen Peroxide (Php) Pretreatment for Bioethanol Production and High-Value Lignin Recovery. Sustainability 2019, 11, 6175. [Google Scholar] [CrossRef]
- Ajayo, P.C.; Huang, M.; Zhao, L.; Tian, D.; He, J.; Zou, J.; Fang, D.; Zeng, Y.; Shen, F. High Yield of Fermentable Sugar from Paper Mulberry Woods Using Phosphoric Acid plus Hydrogen Peroxide Pretreatment: Multifactorial Investigation and Optimization. Ind. Crops Prod. 2022, 180, 114771. [Google Scholar] [CrossRef]
- Santos, R.B.; Jameel, H.; Chang, H.M.; Hart, P.W. Impact of Lignin and Carbohydrate Chemical Structures on Degradation Reactions during Hardwood Kraft Pulping Processes. BioResources 2013, 8, 158–171. [Google Scholar] [CrossRef]
- Normark, M.; Winestrand, S.; Lestander, T.A.; Jönsson, L.J. Analysis, Pretreatment and Enzymatic Saccharification of Different Fractions of Scots Pine. BMC Biotechnol. 2014, 14, 20. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Horváth, I.S. Improvement of Solid-State Biogas Production from Wood by Concentrated Phosphoric Acid Pretreatment. BioResources 2016, 11, 3230–3243. [Google Scholar] [CrossRef]
- Tong, D.; Zhan, P.; Zhang, W.; Zhou, Y.; Huang, Y.; Qing, Y.; Chen, J. A Novel Surfactant-Assisted Dilute Phosphoric Acid plus Steam Explosion Pretreatment of Poplar Wood for Fermentable Sugar Production. Res. Sq. 2021, 18. [Google Scholar] [CrossRef]
- Qin, W.; Wu, L.; Zheng, Z.; Dong, C.; Yang, Y. Lignin Hydrolysis and Phosphorylation Mechanism during Phosphoric Acid–Acetone Pretreatment: A DFT Study. Molecules 2014, 19, 21335. [Google Scholar] [CrossRef]
- Banerjee, G.; Car, S.; Liu, T.; Williams, D.L.; Meza, S.L.; Walton, J.D.; Hodge, D.B. Scale-up and Integration of Alkaline Hydrogen Peroxide Pretreatment, Enzymatic Hydrolysis, and Ethanolic Fermentation. Biotechnol. Bioeng. 2012, 109, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of Alkaline Hydrogen Peroxide Pretreatment and Tween 80 to Enhance Enzymatic Hydrolysis of Sugarcane Bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Investigation of Alkaline Hydrogen Peroxide Pretreatment to Enhance Enzymatic Hydrolysis and Phenolic Compounds of Oil Palm Trunk. 3 Biotech 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Novia, N.; Hasanudin, H.; Hermansyah, H.; Fudholi, A. Kinetics of Lignin Removal from Rice Husk Using Hydrogen Peroxide and Combined Hydrogen Peroxide–Aqueous Ammonia Pretreatments. Fermentation 2022, 8, 157. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Vega-Aguilar, C.A.; Rodrigues, A.E. Added-Value Chemicals from Lignin Oxidation. Molecules 2021, 26, 4602. [Google Scholar] [CrossRef]
- Abdou Alio, M.; Tugui, O.C.; Rusu, L.; Pons, A.; Vial, C. Hydrolysis and Fermentation Steps of a Pretreated Sawmill Mixed Feedstock for Bioethanol Production in a Wood Biorefinery. Bioresour. Technol. 2020, 310, 123412. [Google Scholar] [CrossRef]
- Chu, Q.; Tong, W.; Chen, J.; Wu, S.; Jin, Y.; Hu, J.; Song, K. Organosolv Pretreatment Assisted by Carbocation Scavenger to Mitigate Surface Barrier Effect of Lignin for Improving Biomass Saccharification and Utilization. Biotechnol. Biofuels 2021, 14, 136. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Pires, F.; Van-Dúnem, V.; Sanfins, L.; Roseiro, L.B.; Gírio, F. Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues. Energies 2022, 15, 5654. [Google Scholar] [CrossRef]
- Bhalla, A.; Cai, C.M.; Xu, F.; Singh, S.K.; Bansal, N.; Phongpreecha, T.; Dutta, T.; Foster, C.E.; Kumar, R.; Simmons, B.A.; et al. Performance of Three Delignifying Pretreatments on Hardwoods: Hydrolysis Yields, Comprehensive Mass Balances, and Lignin Properties. Biotechnol. Biofuels 2019, 12, 213. [Google Scholar] [CrossRef]
- Michelin, M.; Liebentritt, S.; Vicente, A.A.; Teixeira, J.A. Lignin from an Integrated Process Consisting of Liquid Hot Water and Ethanol Organosolv: Physicochemical and Antioxidant Properties. Int. J. Biol. Macromol. 2018, 120, 159–169. [Google Scholar] [CrossRef]
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for Olive Wastes Management and Efficient Bioenergy Production. Energy Convers. Manag. 2021, 244, 114467. [Google Scholar] [CrossRef]
- Rencoret, J.; Gutiérrez, A.; Castro, E.; Del Río, J.C. Structural Characteristics of Lignin in Pruning Residues of Olive Tree (Olea Europaea L.). Holzforschung 2019, 73, 25–34. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-First Biomass Fractionation Using a Hybrid Organosolv—Steam Explosion Pretreatment Technology Improves the Saccharification and Fermentability of Spruce Biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-Lignin Formation and Its Impact on Enzymatic Hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent Advances in Understanding the Effects of Lignin Structural Characteristics on Enzymatic Hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- He, J.; Huang, C.; Lai, C.; Huang, C.; Li, X.; Yong, Q. Elucidation of Structure-Inhibition Relationship of Monosaccharides Derived Pseudo-Lignin in Enzymatic Hydrolysis. Ind. Crops Prod. 2018, 113, 368–375. [Google Scholar] [CrossRef]
- Brunner, G. Processing of Biomass with Hydrothermal and Supercritical Water. Supercrit. Fluid Sci. Technol. 2014, 5, 395–509. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Fractional Study of Alkali-Soluble Hemicelluloses Obtained by Graded Ethanol Precipitation from Sugar Cane Bagasse. J. Agric. Food Chem. 2009, 58, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Wawro, A.; Batog, J.; Gieparda, W. Polish Varieties of Industrial Hemp and Their Utilisation in the Efficient Production of Lignocellulosic Ethanol. Molecules 2021, 26, 6467. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, I.J.; Kim, S.; Olawuyi, I.F.; Kim, K.M.; Kim, S.R. Deacetylation Kinetics of Promising Energy Crops, Hemp and Kenaf, for Cellulosic Ethanol Production. GCB Bioenergy 2022, 14, 1150–1161. [Google Scholar] [CrossRef]
- Yang, S.; Franden, M.A.; Yang, Q.; Chou, Y.C.; Zhang, M.; Pienkos, P.T. Identification of Inhibitors in Lignocellulosic Slurries and Determination of Their Effect on Hydrocarbon-Producing Microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 23. [Google Scholar] [CrossRef]
- Madadi, M.; Elsayed, M.; Sun, F.; Wang, J.; Karimi, K.; Song, G.; Tabatabaei, M.; Aghbashlo, M. Sustainable Lignocellulose Fractionation by Integrating P-Toluenesulfonic Acid/Pentanol Pretreatment with Mannitol for Efficient Production of Glucose, Native-like Lignin, and Furfural. Bioresour. Technol. 2023, 371, 128591. [Google Scholar] [CrossRef]
- Pielhop, T.; Reinhard, C.; Hecht, C.; Del Bene, L.; Studer, M.H.; Rudolf von Rohr, P. Application Potential of a Carbocation Scavenger in Autohydrolysis and Dilute Acid Pretreatment to Overcome High Softwood Recalcitrance. Biomass Bioenergy 2017, 105, 164–173. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Bian, H.; Yelle, D.J.; Vuorinen, T.; Fu, S.; Pan, X.; et al. Rapid and Near-Complete Dissolution of Wood Lignin at ≤80 °C by a Recyclable Acid Hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Tang, J.; Wang, M.; Luo, X.; Liu, X.; Qin, Z. One-Pot Conversion of Industrial Hemp Residue into Fermentable Feedstocks Using Green Catalyst and Enzyme Cocktails Generated by Solid-State Fermentation. Ind. Crops Prod. 2022, 182, 114885. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. High-Pressure Microwave-Assisted Pretreatment of Softwood, Hardwood and Non-Wood Biomass Using Different Solvents in the Production of Cellulosic Ethanol. Biotechnol. Biofuels Bioprod. 2023, 16, 19. [Google Scholar] [CrossRef]
- Madadi, M.; Bakr, M.M.A.; Abdulkhani, A.; Zahoor; Asadollahi, M.A.; Sun, C.; Sun, F.; Abomohra, A.E.F. Alleviating Lignin Repolymerization by Carbocation Scavenger for Effective Production of Fermentable Sugars from Combined Liquid Hot Water and Green-Liquor Pretreated Softwood Biomass. Energy Convers. Manag. 2022, 251, 114956. [Google Scholar] [CrossRef]
- Sierra-Ibarra, E.; Alcaraz-Cienfuegos, J.; Vargas-Tah, A.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martinez, A. Ethanol Production by Escherichia Coli from Detoxified Lignocellulosic Teak Wood Hydrolysates with High Concentration of Phenolic Compounds. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab077. [Google Scholar] [CrossRef]
- Raina, N.; Slathia, P.S.; Sharma, P. Experimental Optimization of Thermochemical Pretreatment of Sal (Shorea Robusta) Sawdust by Central Composite Design Study for Bioethanol Production by Co-Fermentation Using Saccharomyces Cerevisiae (MTCC-36) and Pichia Stipitis (NCIM-3498). Biomass Bioenergy 2020, 143, 105819. [Google Scholar] [CrossRef]
- Lee, I.; Yu, J.H. Design of Hydrothermal and Subsequent Lime Pretreatment for Fermentable Sugar and Bioethanol Production from Acacia Wood. Renew. Energy 2021, 174, 170–177. [Google Scholar] [CrossRef]
- Lee, I.; Yu, J.H. The Production of Fermentable Sugar and Bioethanol from Acacia Wood by Optimizing Dilute Sulfuric Acid Pretreatment and Post Treatment. Fuel 2020, 275, 117943. [Google Scholar] [CrossRef]
- Augustyn, A. Surfactant. Definition, Properties, Examples, & Facts. Britannica. Available online: https://www.britannica.com/science/surfactant (accessed on 3 April 2023).
- Domínguez, E.; Nóvoa, T.; del Río, P.G.; Garrote, G.; Romaní, A. Sequential Two-Stage Autohydrolysis Biorefinery for the Production of Bioethanol from Fast-Growing Paulownia Biomass. Energy Convers. Manag. 2020, 226, 113517. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose Biorefinery Feedstock Engineering. Lignocellul. Biorefinery Eng. 2015, 37–86. [Google Scholar] [CrossRef]
- Fan, Z. Consolidated Bioprocessing for Ethanol Production. In Biorefineries Integrated Biochemical Processes for Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2014; pp. 141–160. [Google Scholar] [CrossRef]
- Stickel, J.J.; Elander, R.T.; MCmillan, J.D.; Brunecky, R. Enzymatic Hydrolysis of Lignocellulosic Biomass. In Bioprocessing of Renewable Resources to Commodity Bioproducts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 77–103. ISBN 9781118175835. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B.; Alhussaini, M.S.; Ramteke, P.W. Current Applications and Future Trends of Extremozymes in Detergent Industries. Microb. Extrem. 2022, 223–230. [Google Scholar] [CrossRef]
- Yi, Y. Tiny Bugs Play Big Role: Microorganisms’ Contribution to Biofuel Production. In Advances in 2nd Generation of Bioethanol Production; Elsevier: Amsterdam, The Netherlands, 2021; pp. 113–136. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Penicillium Enzymes for the Food Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–186. [Google Scholar] [CrossRef]
- Purkait, M.K.; Haldar, D. Enzymatic Hydrolysis of Lignocellulosic Biomass: Mechanistic Insight and Advancement. In Lignocellulosic Biomass to Value-Added Products: Fundamental Strategies and Technological Advancements; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–94. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A Review of Lignocellulose Bioconversion Using Enzymatic Hydrolysis and Synergistic Cooperation between Enzymes--Factors Affecting Enzymes, Conversion and Synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding Factors That Limit Enzymatic Hydrolysis of Biomass. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2009; Volume 121, pp. 1081–1099. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. The Effect of Agitation Speed, Enzyme Loading and Substrate Concentration on Enzymatic Hydrolysis of Cellulose from Brewer’s Spent Grain. Cellulose 2008, 15, 711–721. [Google Scholar] [CrossRef]
- Shiva; Climent Barba, F.; Rodríguez-Jasso, R.M.; Sukumaran, R.K.; Ruiz, H.A. High-Solids Loading Processing for an Integrated Lignocellulosic Biorefinery: Effects of Transport Phenomena and Rheology—A Review. Bioresour. Technol. 2022, 351, 127044. [Google Scholar] [CrossRef] [PubMed]
- Zanuso, E.; Ruiz, H.A.; Domingues, L.; Teixeira, J.A. Oscillatory Flow Bioreactor Operating at High Solids Loading for Enzymatic Hydrolysis of Lignocellulosic Biomass. Biochem. Eng. J. 2022, 187, 108632. [Google Scholar] [CrossRef]
- Lu, M.; He, D.; Li, J.; Han, L.; Xiao, W. Rheological Characterization of Ball-Milled Corn Stover with Different Fragmentation Scales at High-Solids Loading. Ind. Crops Prod. 2021, 167, 113517. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A Short Review on SSF—An Interesting Process Option for Ethanol Production from Lignocellulosic Feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Rana, V.; Eckard, A.D.; Ahring, B.K. Comparison of SHF and SSF of Wet Exploded Corn Stover and Loblolly Pine Using In-House Enzymes Produced from T. Reesei RUT C30 and A. Saccharolyticus. Springerplus 2014, 3, 516. [Google Scholar] [CrossRef] [PubMed]
- Dahnum, D.; Tasum, S.O.; Triwahyuni, E.; Nurdin, M.; Abimanyu, H. Comparison of SHF and SSF Processes Using Enzyme and Dry Yeast for Optimization of Bioethanol Production from Empty Fruit Bunch. Energy Procedia 2015, 68, 107–116. [Google Scholar] [CrossRef]
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces Cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7, 464. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Wan, C.; Li, D.; Mao, Z. Effect of Hot Water Pretreatment Severity on the Degradation and Enzymatic Hydrolysis of Corn Stover. Trans. ASABE 2010, 53, 1929–1934. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Hardwood and Softwood Lignocellulosic Residues for Selective Hemicellulose Recovery and Improved Cellulose Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Bardak, S.; Nemli, G.; Bardak, T.; Bardak, S.; Nemli, G.; Bardak, T. The Quality Comparison of Particleboards Produced from Heartwood and Sapwood of European Larch. Maderas. Cienc. Tecnol. 2019, 21, 511–520. [Google Scholar] [CrossRef]
- Benouadah, N.; Aliouche, D.; Pranovich, A.; Willför, S. Chemical Characterization of Pinus Halepensis Sapwood and Heartwood. Wood Mater. Sci. Eng. 2018, 14, 157–164. [Google Scholar] [CrossRef]
- Chitambar, J. Marasmiellus Palmivorus. Pest Rating Proposals and Final Ratings. Available online: https://blogs.cdfa.ca.gov/Section3162/?p=4525 (accessed on 4 April 2023).
- Cavka, A.; Jönsson, L.J. Detoxification of Lignocellulosic Hydrolysates Using Sodium Borohydride. Bioresour. Technol. 2013, 136, 368–376. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial Detoxification of Lignocellulosic Biomass Hydrolysates: Biochemical and Molecular Aspects, Challenges, Exploits and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Wang, X.; Wang, N.; Wang, W.; Bao, J. Biodetoxification of Toxins Generated from Lignocellulose Pretreatment Using a Newly Isolated Fungus, Amorphotheca Resinae ZN1, and the Consequent Ethanol Fermentation. Biotechnol. Biofuels 2010, 3, 26. [Google Scholar] [CrossRef]
- Cheng, Y.; Mondal, A.K.; Wu, S.; Xu, D.; Ning, D.; Ni, Y.; Huang, F. Study on the Anti-Biodegradation Property of Tunicate Cellulose. Polymers 2020, 12, 3071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass Recalcitrance. Part II: Fundamentals of Different Pre-Treatments to Increase the Enzymatic Digestibility of Lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Singh, N.; Devi, A.; Bishnoi, M.B.; Jaryal, R.; Dahiya, A.; Tashyrev, O.; Hovorukha, V.; Singh, N.; Devi, A.; Bishnoi, M.B.; et al. Overview of the Process of Enzymatic Transformation of Biomass. In Elements of Bioeconomy; Biernat, K., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-862-4. [Google Scholar]
- Kumar, D.; Murthy, G.S. Stochastic Molecular Model of Enzymatic Hydrolysis of Cellulose for Ethanol Production. Biotechnol. Biofuels 2013, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A. The Crystallinity of Nanocellulose: Dispersion-Induced Disordering of the Grain Boundary in Biologically Structured Cellulose. ACS Appl. Nano Mater. 2018, 1, 5774–5785. [Google Scholar] [CrossRef]
- Xu, H.; Che, X.; Ding, Y.; Kong, Y.; Li, B.; Tian, W. Effect of Crystallinity on Pretreatment and Enzymatic Hydrolysis of Lignocellulosic Biomass Based on Multivariate Analysis. Bioresour. Technol. 2019, 279, 271–280. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Han, L.; Feng, J.; Zhang, S.; Ma, Z.; Wang, Y.; Zhang, X. Alkali Pretreated of Wheat Straw and Its Enzymatic Hydrolysis. Braz. J. Microbiol. 2012, 43, 53–61. [Google Scholar] [CrossRef]
- Eriksson, T.; Börjesson, J.; Tjerneld, F. Mechanism of Surfactant Effect in Enzymatic Hydrolysis of Lignocellulose. Enzyme Microb. Technol. 2002, 31, 353–364. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a Nonionic Surfactant on Enzymatic Hydrolysis of Lignocellulose Based on Lignocellulosic Features and Enzyme Adsorption. ACS Omega 2020, 5, 15812–15820. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the Mechanism of Surfactant-Promoted Enzymatic Hydrolysis of Dilute Acid Pretreated Bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef]
- Oliva-Taravilla, A.; Carrasco, C.; Jönsson, L.J.; Martín, C. Effects of Biosurfactants on Enzymatic Saccharification and Fermentation of Pretreated Softwood. Molecules 2020, 25, 3559. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.S.; Balbino, T.R.; Alba, E.M.; Barbosa, F.C.; de Pier, F.T.; de Almeida, A.L.M.; Zilla, A.H.B.; Antunes, F.A.F.; Hilares, R.T.; Balagurusamy, N.; et al. Surfactants in Biorefineries: Role, Challenges & Perspectives. Bioresour. Technol. 2022, 345, 126477. [Google Scholar] [CrossRef]
- Singh, S. Carbohydrates 10. Available online: https://www.uou.ac.in/lecturenotes/science/MSCCH-17/CHEMISTRY%20LN%204%20CARBOHYDRATES-converted%20(1).pdf (accessed on 22 June 2023).
- Frankó, B.; Carlqvist, K.; Galbe, M.; Lidén, G.; Wallberg, O. Removal of Water-Soluble Extractives Improves the Enzymatic Digestibility of Steam-Pretreated Softwood Barks. Appl. Biochem. Biotechnol. 2018, 184, 599. [Google Scholar] [CrossRef]
- Shi, J.; Gladden, J.M.; Sathitsuksanoh, N.; Kambam, P.; Sandoval, L.; Mitra, D.; Zhang, S.; George, A.; Singer, S.W.; Simmons, B.A.; et al. One-Pot Ionic Liquid Pretreatment and Saccharification of Switchgrass. Green Chem. 2013, 15, 2579–2589. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Kitiborwornkul, N.; Tantayotai, P.; Rattanaporn, K.; Show, P.L. One-Pot Ionic Liquid-Mediated Bioprocess for Pretreatment and Enzymatic Hydrolysis of Rice Straw with Ionic Liquid-Tolerance Bacterial Cellulase. Bioengineering 2022, 9, 17. [Google Scholar] [CrossRef]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean Protein as a Cost-Effective Lignin-Blocking Additive for the Saccharification of Sugarcane Bagasse. Bioresour. Technol. 2016, 221, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Simões, I.R.; Brondi, M.G.; Farinas, C.S. In-House Extracted Soybean Protein Can Reduce the Enzyme Dosage in Biomass Saccharification. Fermentation 2023, 9, 142. [Google Scholar] [CrossRef]
- Manzanares, P. Integrated Hydrolysis, Fermentation and Co-Fermentation of Lignocellulosic Biomass. In Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 205–223. ISBN 9781845695101. [Google Scholar]
- Joshi, A.; Kanthaliya, B.; Meena, S.; Khan, F.; Arora, J. Process Consolidation Approaches for Cellulosic Ethanol Production. Sustain. Biofuels Oppor. Challenges 2021, 43–72. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, Y.C.; Ciou, Y.L.; Chu, I.M.; Tsai, S.L.; Lan, J.C.W.; Chang, Y.K.; Wei, Y.H. Producing Bioethanol from Pretreated-Wood Dust by Simultaneous Saccharification and Co-Fermentation Process. J. Taiwan Inst. Chem. Eng. 2017, 79, 43–48. [Google Scholar] [CrossRef]
- Nurdin, M.; Abimanyu, H.; Cahyaningrum, R.; Arham, Z.; Natsir, M.; Maulidiyah, M. Bioethanol Production Based on OPEFB Biomass by Sulfuric Acid Followed by Saccharification and Co-Fermentation Simultaneously. AIP Conf. Proc. 2023, 2719, 030016. [Google Scholar] [CrossRef]
- Nait M’Barek, H.; Arif, S.; Taidi, B.; Hajjaj, H. Consolidated Bioethanol Production from Olive Mill Waste: Wood-Decay Fungi from Central Morocco as Promising Decomposition and Fermentation Biocatalysts. Biotechnol. Rep. 2020, 28, e00541. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Recent Insights into Consolidated Bioprocessing for Lignocellulosic Biohydrogen Production. Int. J. Hydrog. Energy 2019, 44, 14362–14379. [Google Scholar] [CrossRef]
- Singhvi, M.; Kim, B.S. Green Hydrogen Production through Consolidated Bioprocessing of Lignocellulosic Biomass Using Nanobiotechnology Approach. Bioresour. Technol. 2022, 365, 128108. [Google Scholar] [CrossRef]
- Shahab, R.L.; Luterbacher, J.S.; Brethauer, S.; Studer, M.H. Consolidated Bioprocessing of Lignocellulosic Biomass to Lactic Acid by a Synthetic Fungal-Bacterial Consortium. Biotechnol. Bioeng. 2018, 115, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Devos, R.J.B.; Colla, L.M. Simultaneous Saccharification and Fermentation to Obtain Bioethanol: A Bibliometric and Systematic Study. Bioresour. Technol. Rep. 2022, 17, 100924. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Y.; Zhang, Q.; Wang, X.; Wu, D.; Kong, H. Optimisation of Simultaneous Saccharification and Fermentation of Wheat Straw for Ethanol Production. Fuel 2013, 112, 331–337. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Rudolf, A.; Lidén, G.; Zacchi, G. Influence of Strain and Cultivation Procedure on the Performance of Simultaneous Saccharification and Fermentation of Steam Pretreated Spruce. Enzym. Microb. Technol. 2006, 38, 279–286. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of Simultaneous Saccharification and Fermentation (SSF) from Viscosity Reducing of Raw Sweet Potato for Bioethanol Production at Laboratory, Pilot and Industrial Scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef]
- Palniandy, T.; Nafsun, A.I.; Mohd Jamil, N.; Herz, F.; Azmi, N.A.A.; Muhammad Zaki, M.H. Influence of Stirring Speed on Glucose and Ethanol Production in Simultaneous Saccharification and Fermentation Process. J. Chem. Eng. Ind. Biotechnol. 2022, 8, 20–25. [Google Scholar] [CrossRef]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-Step Pretreatment of Oil Palm Trunk for Ethanol Production by Thermotolerent Saccharomyces Cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef]
- Kooprasertying, P.; Vanichsriratana, W.; Sirisansaneeyakul, S.; Laemsak, N.; Tareen, A.K.; Ullah, Z.; Parakulsuksatid, P.; Sultan, I.N. Ethanol Production through Optimized Alkaline Pretreated Elaeis Guineensis Frond Waste from Krabi Province, Thailand. Fermentation 2022, 8, 648. [Google Scholar] [CrossRef]
- Ko, J.K.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S.M. Improved Bioconversion of Lignocellulosic Biomass by Saccharomyces Cerevisiae Engineered for Tolerance to Acetic Acid. GCB Bioenergy 2020, 12, 90–100. [Google Scholar] [CrossRef]
- Hashemi, S.; Joseph, P.; Mialon, A.; Moe, S.; Lamb, J.J.; Lien, K.M. Enzymatic Pretreatment of Steam-Exploded Birch Wood for Increased Biogas Production and Lignin Degradation. Bioresour. Technol. Rep. 2021, 16, 100874. [Google Scholar] [CrossRef]
- Zhu, J.; Jiao, N.; Cheng, J.; Zhang, H.; Xu, G.; Xu, Y.; Zhu, J.Y. Integrated Process for the Co-Production of Bioethanol, Furfural, and Lignin Nanoparticles from Birch Wood via Acid Hydrotropic Fractionation. Renew. Energy 2023, 204, 176–184. [Google Scholar] [CrossRef]
- Wagner, E.; Sierra-Ibarra, E.; Rojas, N.L.; Martinez, A. One-Pot Bioethanol Production from Brewery Spent Grain Using the Ethanologenic Escherichia Coli MS04. Renew. Energy 2022, 189, 717–725. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Magaña, G.; Rodriguez, F.; De Leon-Rodriguez, A.; Sanchez, A. Co-Production of Ethanol-Hydrogen by Genetically Engineered Escherichia Coli in Sustainable Biorefineries for Lignocellulosic Ethanol Production. Chem. Eng. J. 2021, 406, 126829. [Google Scholar] [CrossRef]
- Díaz, M.J.; Moya, M.; Castro, E. Bioethanol Production from Steam-Exploded Barley Straw by Co-Fermentation with Escherichia Coli SL100. Agronomy 2022, 12, 874. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent Advances in Green Pre-Treatment Methods of Lignocellulosic Biomass for Enhanced Biofuel Production. J. Clean. Prod. 2021, 321, 129038. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Comparative Techno-Economic Analysis of Steam Explosion, Dilute Sulfuric Acid, Ammonia Fiber Explosion and Biological Pretreatments of Corn Stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Malik, K.; Sharma, P.; Yang, Y.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Lignocellulosic Biomass for Bioethanol: Insight into the Advanced Pretreatment and Fermentation Approaches. Ind. Crops Prod. 2022, 188, 115569. [Google Scholar] [CrossRef]
- Kuittinen, S.; Hietaharju, J.; Bhattarai, I.; Hassan, M.K.; Kupiainen, L.; Kangas, J.; Tanskanen, J.; Pappinen, A. Technoeconomic Analysis and Environmental Sustainability Estimation of Bioalcohol Production from Barley Straw. Biocatal. Agric. Biotechnol. 2022, 43, 102427. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chandra, R.P.; Tang, Y.; Huang, X.Y.; Bai, F.W.; Liu, C.G. Instant Catapult Steam Explosion: An Efficient Preprocessing Step for the Robust and Cost-Effective Chemical Pretreatment of Lignocellulosic Biomass. Ind. Crops Prod. 2022, 188, 115664. [Google Scholar] [CrossRef]
- Sui, W.; Chen, H. Multi-Stage Energy Analysis of Steam Explosion Process. Chem. Eng. Sci. 2014, 116, 254–262. [Google Scholar] [CrossRef]
- Quinn, T.J.; Martin, J.E. Radiometric Measurements of the Stefan-Boltzmann Constant and Thermodynamic Temperature between −40 °C and +100 °C. Metrologia 1984, 20, 163. [Google Scholar] [CrossRef]
- Quintero, J.A.; Moncada, J.; Cardona, C.A. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Residues in Colombia: A Process Simulation Approach. Bioresour. Technol. 2013, 139, 300–307. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y. Second-Generation Bioenergy from Oilseed Crop Residues: Recent Technologies, Techno-Economic Assessments and Policies. Energy Convers. Manag. 2022, 267, 115869. [Google Scholar] [CrossRef]
- Aghaei, S.; Karimi Alavijeh, M.; Shafiei, M.; Karimi, K. A Comprehensive Review on Bioethanol Production from Corn Stover: Worldwide Potential, Environmental Importance, and Perspectives. Biomass Bioenergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Kuglarz, M.; Gunnarsson, I.B.; Svensson, S.E.; Prade, T.; Johansson, E.; Angelidaki, I. Ethanol Production from Industrial Hemp: Effect of Combined Dilute Acid/Steam Pretreatment and Economic Aspects. Bioresour. Technol. 2014, 163, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.; Silva, C.C.; Mussatto, S.I.; Osseweijer, P.; van der Wielen, L.A.M.; Posada, J.A. Integrated 1st and 2nd Generation Sugarcane Bio-Refinery for Jet Fuel Production in Brazil: Techno-Economic and Greenhouse Gas Emissions Assessment. Renew. Energy 2018, 129, 733–747. [Google Scholar] [CrossRef]
- Bello, S.; Galán-Martín, Á.; Feijoo, G.; Moreira, M.T.; Guillén-Gosálbez, G. BECCS Based on Bioethanol from Wood Residues: Potential towards a Carbon-Negative Transport and Side-Effects. Appl. Energy 2020, 279, 115884. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Gelosia, M.; Giannoni, T.; Barros Lovate Temporim, R.; Nicolini, A.; Cotana, F.; Bertini, A. Acid-Catalyzed Steam Explosion for High Enzymatic Saccharification and Low Inhibitor Release from Lignocellulosic Cardoon Stalks. Biochem. Eng. J. 2021, 174, 108121. [Google Scholar] [CrossRef]

| Substrate | Cellulose (% DB) | Hemicellulose (% DB) | Lignin (% DB) | Reference |
|---|---|---|---|---|
| Poplar | 35–52 | 12–33 | 21–28 | [49,50,51,52,53] |
| Hemp | 40–76 | 10–18 | 10–21 | [26,54,55,56] |
| Eucalyptus | 53–54 | 11–19 | 21–34 | [57,58,59] |
| Beech | 38–58 | 8–36 | 20–26 | [48,60,61,62] |
| Aspen | 44–48 | 18–23 | 19–24 | [63,64,65] |
| Birch | 39–46 | 25–27 | 26.5–28.5 | [64,66] |
| Pine | 37–48 | 13–26 | 23–30 | [52,53,67,68,69] |
| Spruce | 39–44 | 20.5–35.5 | 24–35 | [64,70,71] |
| Substrate | Pretreatment and Conditions | SSF Results | Reference |
|---|---|---|---|
| Eucalyptus sawdust | Thermomechanical; steam explosion (Optimal: 200 °C, 10 min, no catalyst) | [Ethanol] = 75.6 g/L | [59] |
| Eucalyptus wood | Thermomechanical; steam explosion (195 °C, 5.87 min) | [Ethanol] = 51 g/L | [88] |
| Aspen wood | Thermomechanical; steam explosion (204 °C, 10 min, 5% NaOH w/w (NaOH/H2O) | Yethanol = 79.4% | [94] |
| Poplar wood | Chemical; NaOH (2% NaOH w/w (NaOH/H2O), 80 °C, 2 h) | [Ethanol] = 12.2–15.8 g/L | [50] |
| Poplar and pine wood | Chemical; autohydrolysis, dilute H2SO4 (0.5% w/w H2SO4 (H2SO4/H2O), 180 °C), cold NaOH (NaOH 2 M, 4 °C), hot NaOH (NaOH 2M, 100 °C), Na2CO3 (Na2CO3 0.5 M, 100 °C). | [Ethanol] = 221.2 g ethanol/g of poplar wood [Ethanol] = 177.6 g ethanol/g pine wood | [53] |
| Poplar sawdust and pine sawdust | Chemical; PHP (Solid–liquid ratio 1:10 m substrate/v PHP mixture; 50 °C, 3 h) | Yethanol (poplar) = 18.4% Yethanol (pine) = 12.3% | [68] |
| Furniture boards | Chemical; PHP (Solid–liquid ratio 1:10 m substrate/v PHP mixture, 40.2 °C, 2.9 h) | [Ethanol] = 8.1–10.4 g/L | [114] |
| Hemp hurds from 4 varieties | Chemical; NaOH (NaOH 2% w/w (NaOH/H2O), 90 °C, 5 h, solid–liquid ratio 1:10 m substrate/v water) | [Ethanol] = 7.5 g/L for Rajan variety | [139] |
| Hemp | Chemical; dilute H2SO4 (1% H2SO4 v/v (H2SO4/H2O), 121 °C, 30 min, sterilization 121 °C, 15 min) | [Ethanol] = 18.9 g/L | [140] |
| Oil Palm Trunk | Thermomechanical; SE + chemical; NaOH (SE: 210 °C, 6 min; NaOH: 15% w/v (NaOH/H2O), 90 °C) | [Ethanol] = 44.25 g/L | [212] |
| Oil Palm Frond Fibers | Thermomechanical + Thermal + Optimized chemical (SE + autohydrolysis + NaOH) (SE: 210 °C, 4 min; autohydrolysis: 80 °C, 30 min; NaOH: 15% w/v (NaOH/H2O), 90 °C, 60 min) | [Ethanol] = 33.15 g/L | [213] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hage, M.; Louka, N.; Rezzoug, S.-A.; Maugard, T.; Sablé, S.; Koubaa, M.; Debs, E.; Maache-Rezzoug, Z. Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects. Energies 2023, 16, 5052. https://doi.org/10.3390/en16135052
El Hage M, Louka N, Rezzoug S-A, Maugard T, Sablé S, Koubaa M, Debs E, Maache-Rezzoug Z. Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects. Energies. 2023; 16(13):5052. https://doi.org/10.3390/en16135052
Chicago/Turabian StyleEl Hage, Maria, Nicolas Louka, Sid-Ahmed Rezzoug, Thierry Maugard, Sophie Sablé, Mohamed Koubaa, Espérance Debs, and Zoulikha Maache-Rezzoug. 2023. "Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects" Energies 16, no. 13: 5052. https://doi.org/10.3390/en16135052
APA StyleEl Hage, M., Louka, N., Rezzoug, S.-A., Maugard, T., Sablé, S., Koubaa, M., Debs, E., & Maache-Rezzoug, Z. (2023). Bioethanol Production from Woody Biomass: Recent Advances on the Effect of Pretreatments on the Bioconversion Process and Energy Yield Aspects. Energies, 16(13), 5052. https://doi.org/10.3390/en16135052









