Research on Thermal Management Coupling by CPCM and Liquid Cooling for Vehicle Lithium-Ion Batteries
Abstract
:1. Introduction
2. Battery Module Modeling
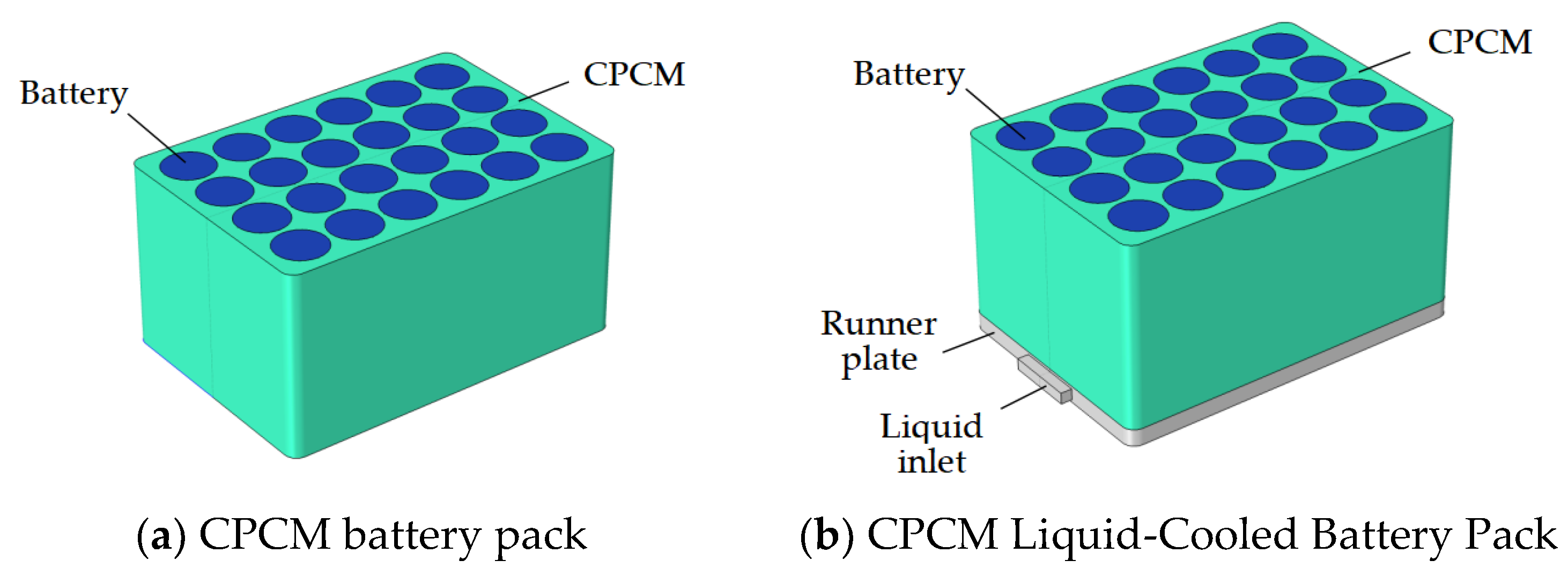
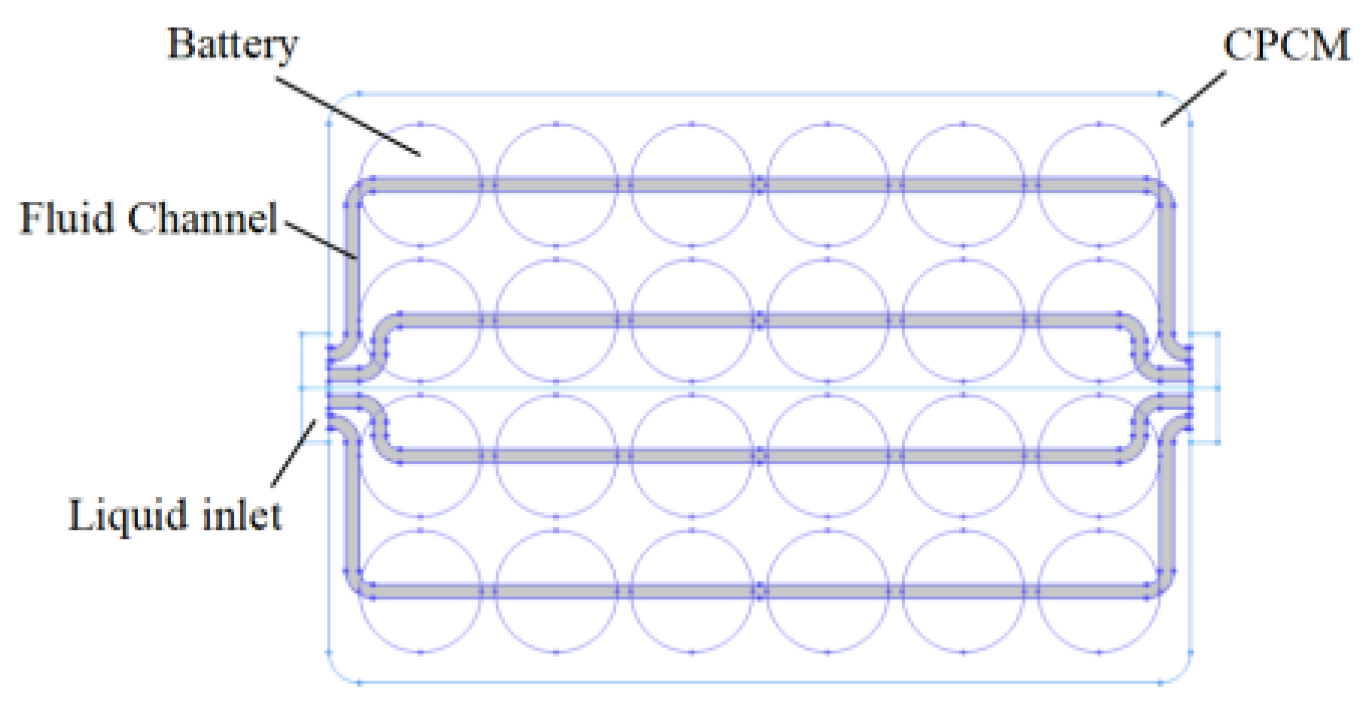
2.1. Geometric Modeling
2.2. Battery Heat Generation Model
2.3. CPCM Model
2.4. Initial and Boundary Conditions
2.5. Battery Pack Layout Model
2.6. Mesh Partitioning
3. Results and Discussion
3.1. Lithium-Ion Battery Pack with Natural Convection Air Heat Dissipation
3.2. Lithium Ion Battery Pack with CPCM Heat Dissipation
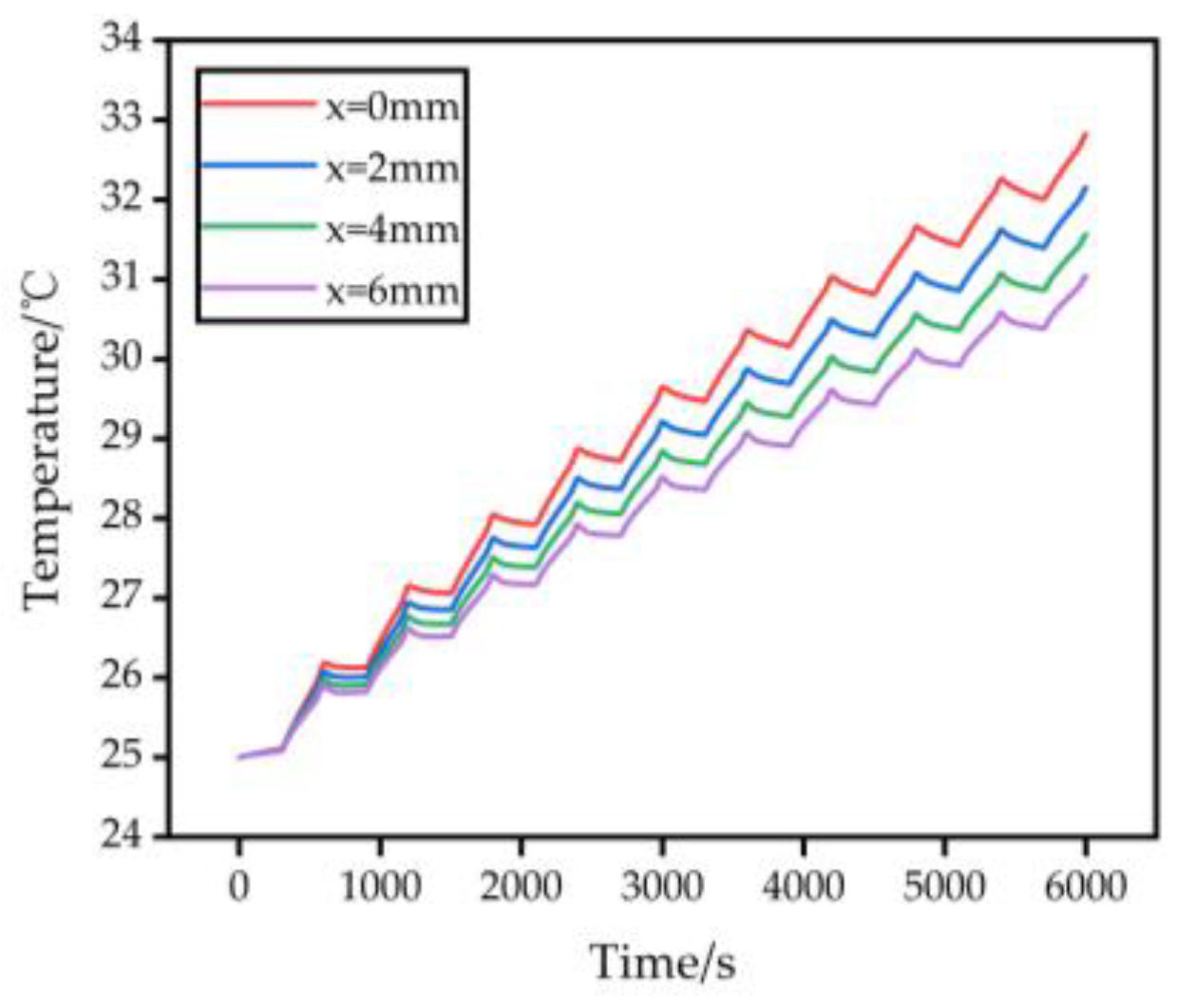
3.3. Effect of Varying Battery Spacing on Temperature Rise of Battery Pack Models
3.4. Lithium-Ion Battery Pack with CPCM Liquid Cooling Heat Dissipation
4. Conclusions
- (1)
- The addition of expanded graphite to paraffin in the CPCM enhances its thermal conductivity, resulting in improved heat absorption during the sensible heat stage. Under the 1–5 C charge-discharge rate, the maximum surface temperature of the battery pack using the paraffin expanded graphite CPCM heat dissipation model is lower by 2.22, 4.56, 14.00, 15.84, and 12.36 °C compared to the air cooling heat dissipation model.
- (2)
- Increasing the spacing between individual batteries in the battery pack can provide a certain cooling effect. However, the cooling efficiency improvement diminishes as the spacing increases. Therefore, it is crucial to consider cooling requirements, battery pack size, and cost in order to determine the optimal spacing.
- (3)
- CPCM liquid cooling coupled with heat dissipation effectively reduces the maximum surface temperature of the battery pack at a charging and discharging rate of 1–5 C. The temperature decrease is 2.51, 5.28, 0.57, 5.92, and 10.13 °C. This study attempts to reduce the battery spacing to 0 mm. When the charging and discharging rate is between 1–3 C, the maximum temperature of the battery can be maintained below 45 °C. Thus, CPCM liquid cooling can reduce battery size while ensuring heat dissipation efficiency.
- (4)
- By coupling CPCM with liquid cooling, the cooling effect can be improved on the basis of CPCM heat dissipation, while also improving the temperature uniformity of the battery on the basis of liquid cooling heat dissipation. Moreover, this structure places the flow channel plate below the CPCM battery pack, which is simpler than the structure of inserting pipes or liquid cooling plates in the middle of the battery, making it easy to install, repair, and replace, and reducing costs. This study optimizes the cooling effect, temperature uniformity, structural size, and cost of the lithium-ion battery pack thermal management system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Sovacool, B.K. Valuing the greenhouse gas emissions from nuclear power: A critical survey. Energy Policy 2008, 36, 2950–2963. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Gong, H.; Wang, M.Q.; Wang, H. New energy vehicles in China: Policies, demonstration, and progress. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 207–228. [Google Scholar] [CrossRef]
- Li, L.Y. Current status and development trend of Li-ion batteries for new energy vehicles in China. Chin. J. Power Sources 2020, 44, 628–630. [Google Scholar]
- German, R.; Delarue, P.; Bouscayrol, A. Battery pack self-heating during the charging process. In Proceedings of the 2018 IEEE International Conference on Industrial Technology (ICIT), Lyon, France, 20–22 February 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Zhang, Y.; Guo, X.; Xue, W.; Zhang, Z.; Liang, J.; Wang, H. Research Progress on multi-scale thermal safety of lithium-ion power battery system. Chin. J. Beijing Univ. Aeronaut. Astronaut. 2021, 49, 31–44. [Google Scholar] [CrossRef]
- Chung, Y.H.; Jhang, W.C.; Chen, W.C.; Wang, Y.W.; Shu, C.M. Thermal hazard assessment for three C rates for a Li-polymer battery by using vent sizing package 2. J. Therm. Anal. Calorim. 2016, 127, 809–817. [Google Scholar] [CrossRef]
- Pesaran, A.A. Battery Thermal Models for Hybrid Vehicle Simulations. J. Power Sources 2002, 110, 377–382. [Google Scholar] [CrossRef]
- Zichen, W.; Changqing, D. A comprehensive review on thermal management systems for power lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 139, 110685. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.-Q.; Guan, C. Progress in Application of Phase Change Materials in Thermal Management Systems for Power Battery. Chin. Bull. Chin. Ceram. Soc. 2016, 35, 150–153. [Google Scholar] [CrossRef]
- Ma, X.; Zou, D.; Liu, X.; Li, L. Research progress on phase change material for power battery thermal management. Chin. New Chem. Mater. 2017, 45, 23–25. [Google Scholar]
- Zhang, J. Research on Power Batteries Thermal Management Technology Based on PCM. Master’s Thesis, Chinese Guangdong University of Technology, Guangzhou, China, 2013. [Google Scholar]
- Rami Sabbah, R.; Kizilel, R.; Selman, J.R.; Al-Hallaj, S. Active (air-cooled) vs. passive (phase change material) thermal management of high power lithium-ion packs: Limitation of temperature rise and uniformity of temperature distribution. J. Power Sources 2008, 182, 630–638. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Peng, J.; Fang, X.; Gao, X.; Fang, Y. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material. Appl. Energy 2012, 91, 426–431. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Fu, Y.; Cao, M.; Liu, M. Thermal optimization of composite phase change material/ expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 2016, 108, 1119–1125. [Google Scholar] [CrossRef]
- Rao, Z.; Huo, Y.; Liu, X.; Zhang, G. Experimental investigation of battery thermal management system for electric vehicle based on paraffin/copper foam. J. Energy Inst. 2015, 88, 241–246. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Xu, S.-C.; Li, Z.; Lin, C.; Chang, G. Research on active-passive thermal management system of LiFePO4 battery pack. Chin. J. Power Sources 2016, 40, 4. [Google Scholar]
- Huang, J.H.; Liu, Z.Q.; Cao, M.; Jiang, G.; Yan, Q. Thermal management ofbattery pack based on phase change material and liquid cooling coupling. Chin. J. Power Sources 2019, 43, 4. [Google Scholar]
- Liu, Z.; Huang, J.; Cao, M.; Jiang, G.; Yan, Q.; Hu, J. Experimental study on the thermal management of batteries based on the coupling of composite phase change materials and liquid cooling. Appl. Therm. Eng. Des. Process. Equip. Econ. 2021, 185, 116415. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5. [Google Scholar] [CrossRef] [Green Version]








| Parameter | Value |
|---|---|
| Capacity/Ah | 3.4 |
| Diameter/mm | 18 |
| Height/mm | 65 |
| Weight/g | 46 |
| Nominal Voltage/V | 3.7 |
| Discharge Cut-off Voltage/V | 2.5 |
| Internal Resistance/mΩ | 35 |
| Radial Thermal Conductivity/W·(m·K)−1 | 0.897 |
| Axial Thermal Conductivity/W·(m·K)−1 | 29.557 |
| Density/kg·m−3 | 2055.2 |
| Specific heat capacity/J·(kg·K)−1 | 1399.1 |
| Parameter | Value |
|---|---|
| Melting temperature/°C | 42.98 |
| melting range/°C | 2 |
| thermal conductivity/W·(m·K)−1 | 7.85 |
| Density/kg·m−3 | 714 |
| Specific heat capacity/J·(kg·K)−1 | 2500 |
| latent heat/kJ·kg−1 | 181 |
| Rate of Charge and Discharge/C | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Maximum temperature of air-cooled battery pack/°C | 34.36 | 47.40 | 58.27 | 67.46 | 75.42 |
| Maximum temperature of CPCM battery pack/°C | 32.15 | 42.84 | 44.27 | 51.61 | 63.06 |
| Maximum temperature drop/°C | 2.22 | 4.56 | 14.00 | 15.84 | 12.36 |
| Battery Spacing/mm | 0 | 2 | 4 | 6 |
|---|---|---|---|---|
| Maximum surface temperature of battery pack/°C | 32.82 | 32.15 | 31.56 | 31.04 |
| Rate of Charge and Discharge/C | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Maximum temperature of battery pack/°C | CPCM | 32.15 | 42.84 | 44.27 | 51.61 | 63.06 |
| CPCM-Liquid (x = 2 mm) | 29.64 | 37.56 | 43.70 | 45.69 | 52.93 | |
| CPCM-Liquid (x = 0 mm) | 30.41 | 39.16 | 44.22 | 48.84 | 57.71 | |
| Maximum temperature drop | x = 2 mm | 2.51 | 5.28 | 0.57 | 5.92 | 10.13 |
| x = 0 mm | 1.74 | 3.68 | 0.05 | 2.77 | 5.35 | |
| Rate of Charge and Discharge/C | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Temperature difference of liquid cooled battery pack/°C | 1.05 | 3.07 | 4.89 | 6.57 | 8.35 |
| Temperature difference of CPCM and liquid cooled battery pack/°C | 0.79 | 2.12 | 2.94 | 5.65 | 5.32 |
| Temperature difference drop/°C | 0.26 | 0.95 | 1.95 | 0.92 | 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Du, C.; Wang, Z. Research on Thermal Management Coupling by CPCM and Liquid Cooling for Vehicle Lithium-Ion Batteries. Energies 2023, 16, 5260. https://doi.org/10.3390/en16145260
Wang Y, Du C, Wang Z. Research on Thermal Management Coupling by CPCM and Liquid Cooling for Vehicle Lithium-Ion Batteries. Energies. 2023; 16(14):5260. https://doi.org/10.3390/en16145260
Chicago/Turabian StyleWang, Yijin, Changqing Du, and Zichen Wang. 2023. "Research on Thermal Management Coupling by CPCM and Liquid Cooling for Vehicle Lithium-Ion Batteries" Energies 16, no. 14: 5260. https://doi.org/10.3390/en16145260





