Numerical Analysis of Tar and Syngas Formation during the Steam Gasification of Biomass in a Fluidized Bed
Abstract
:1. Introduction
2. Materials and Methods
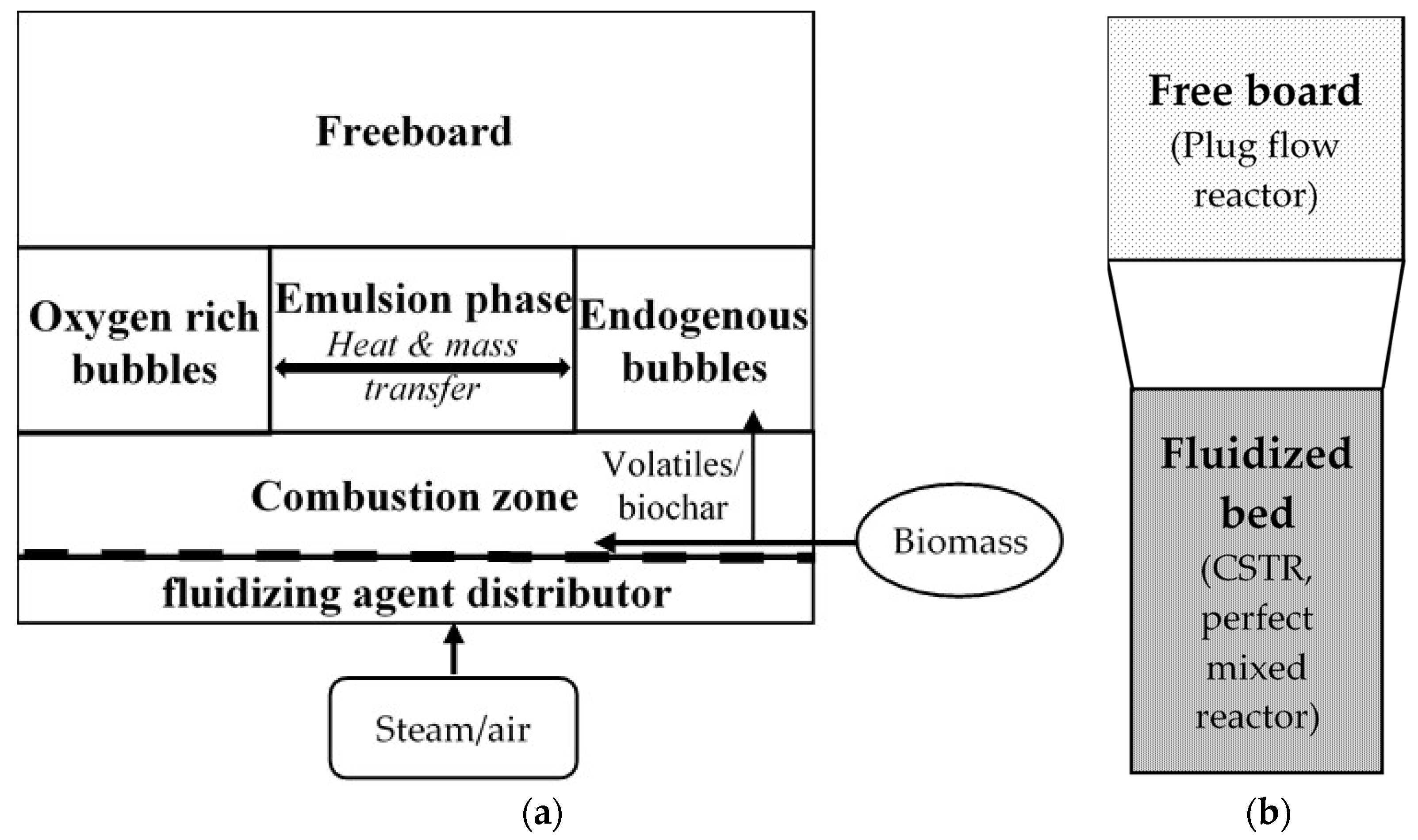
2.1. Sequential Modular Modeling of Hydrodynamics in a Fluidized Bed
2.2. Heat Transfer in a Biomass Particle
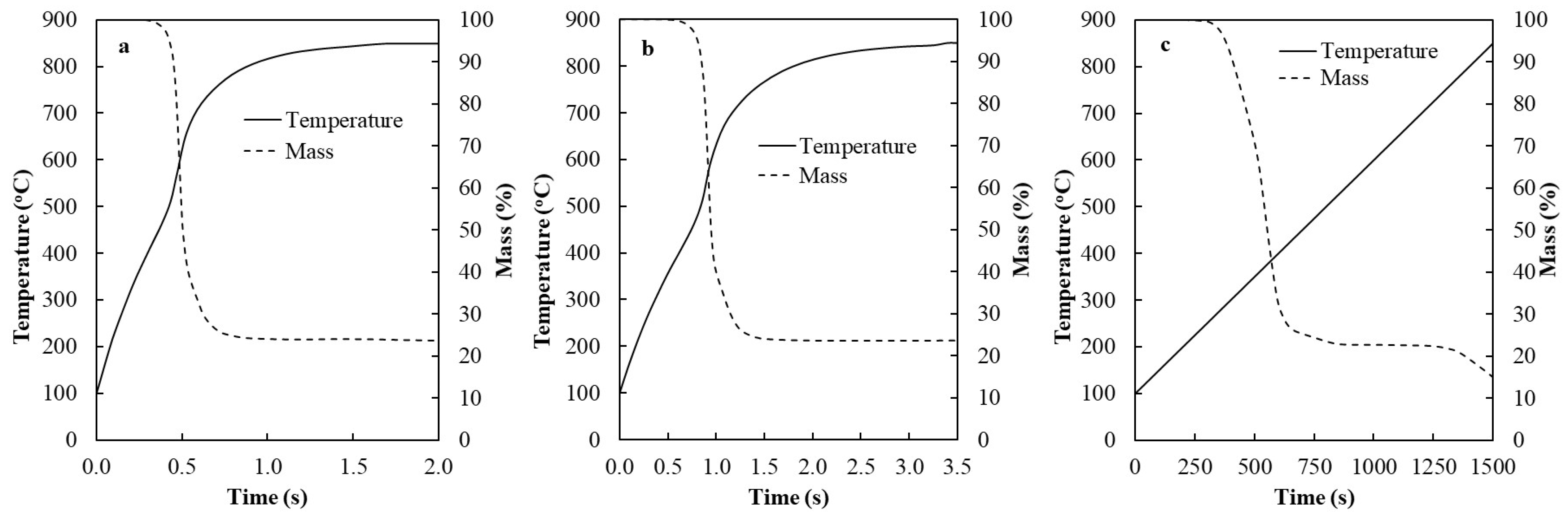
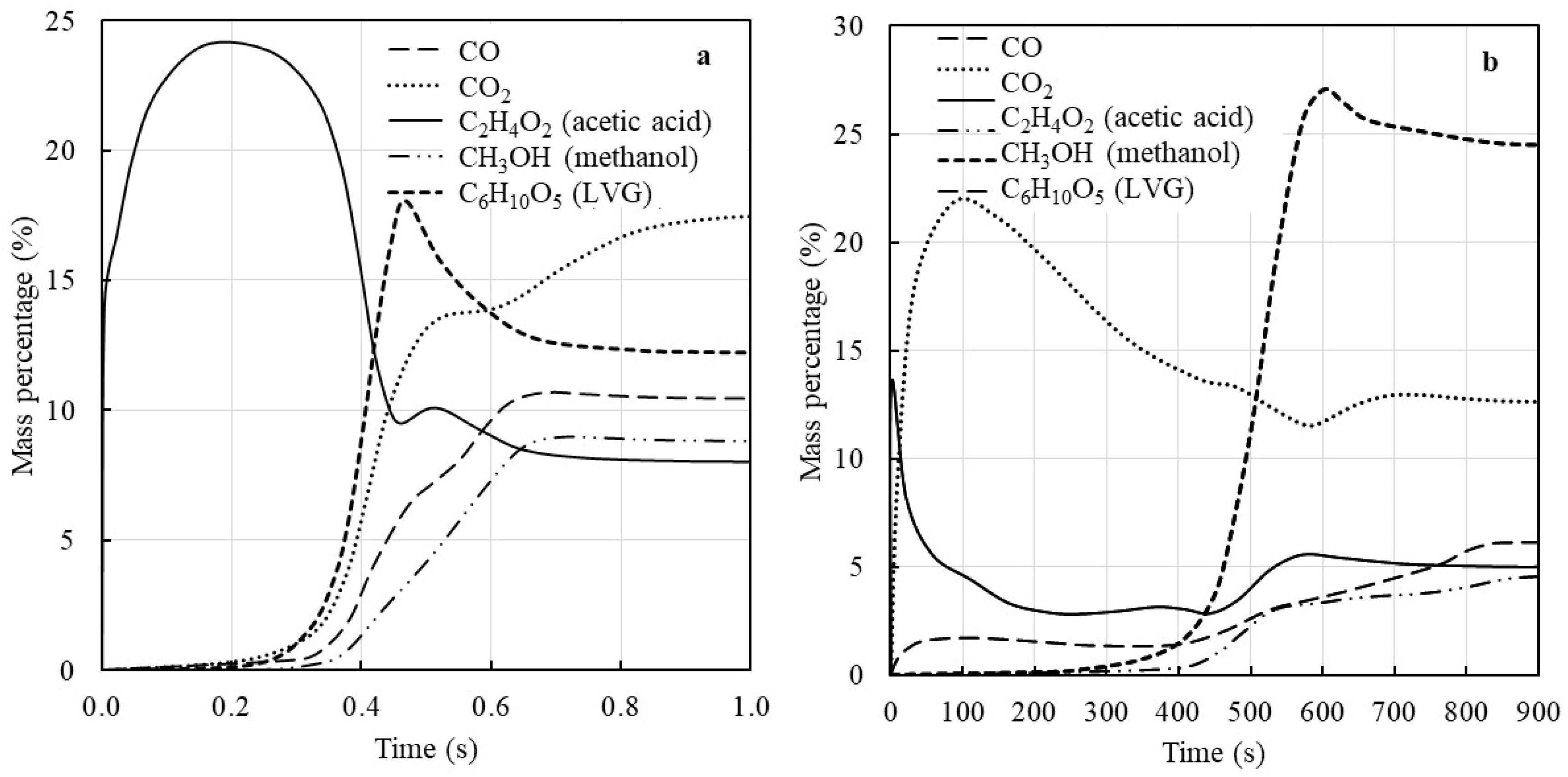
2.3. Biomass Devolatilization Kinetics
2.4. Solution and Validation
3. Results and Discussion
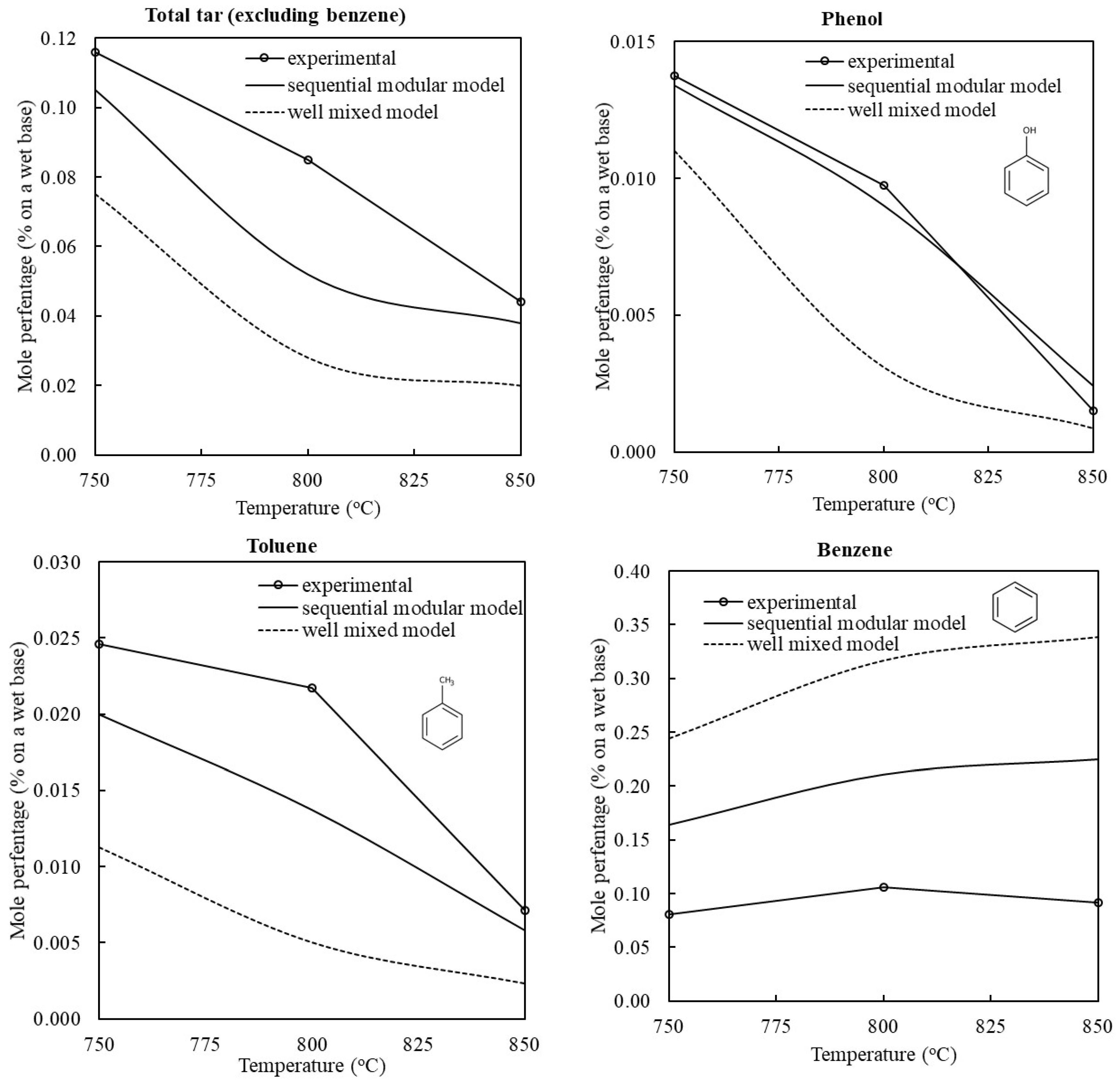
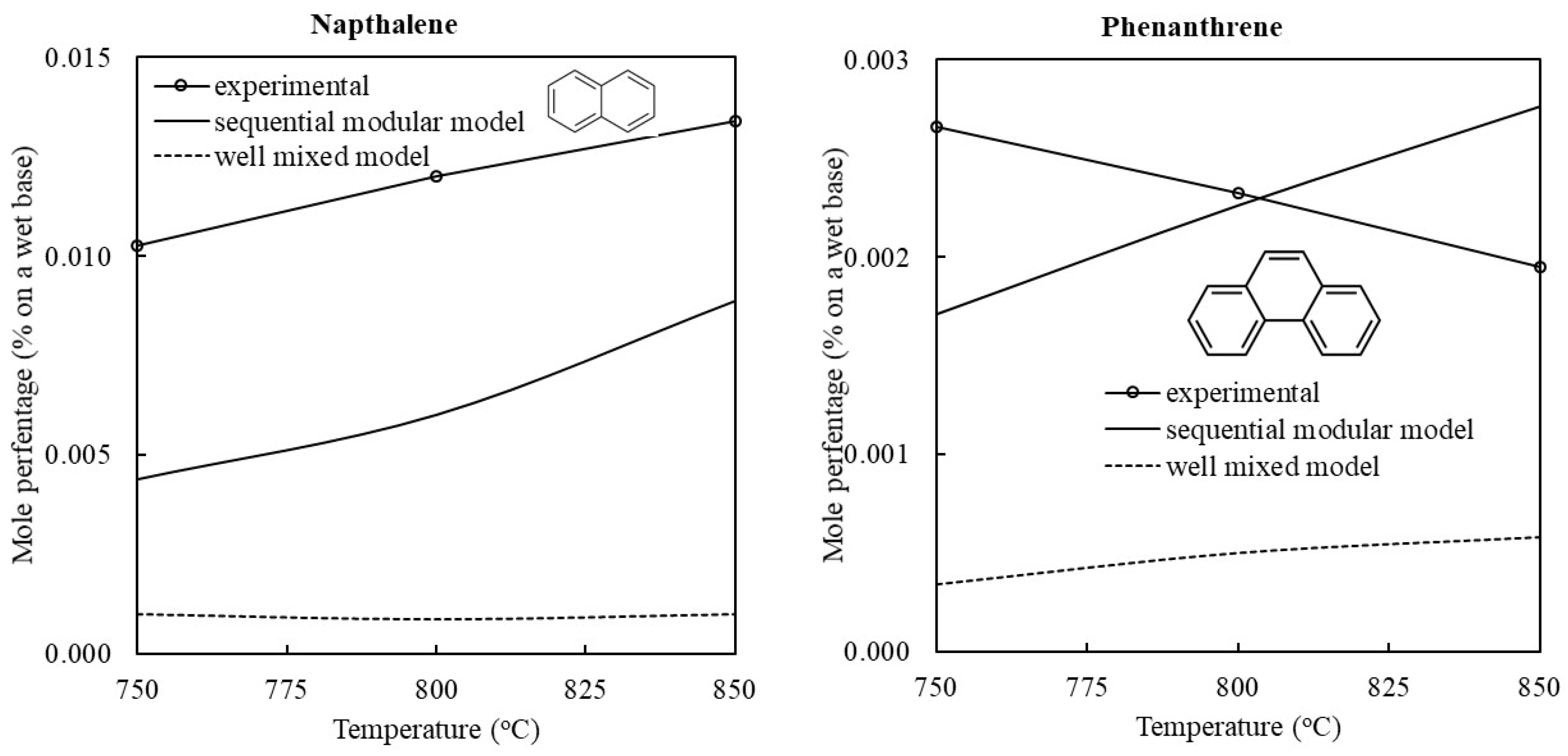
3.1. Validation of the Model
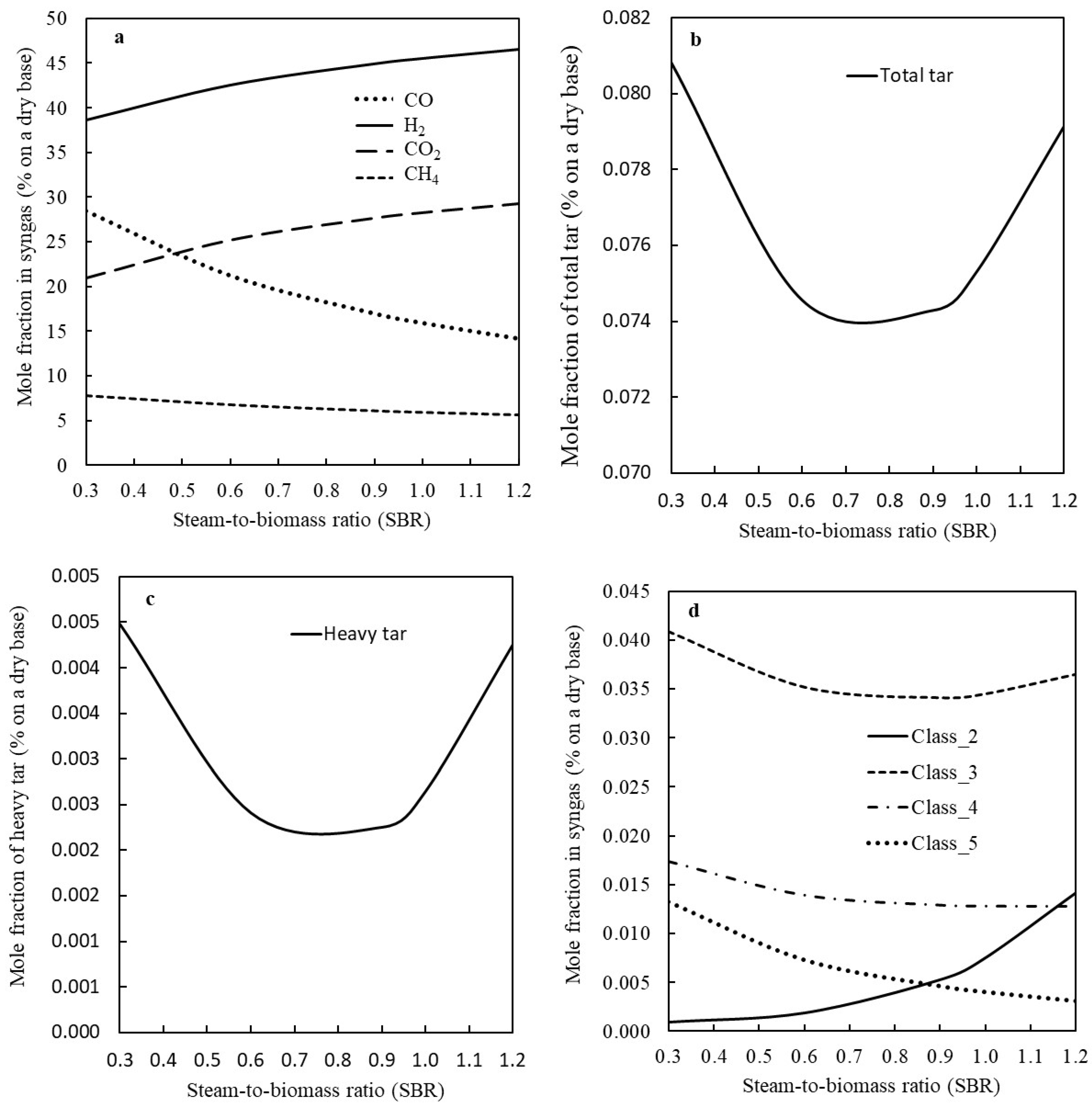
3.2. The Effect of Steam-to-Biomass Ratio on the Syngas Quality
3.3. The Effect of Oxygen Injection in Steam Gasification on the Syngas Quality
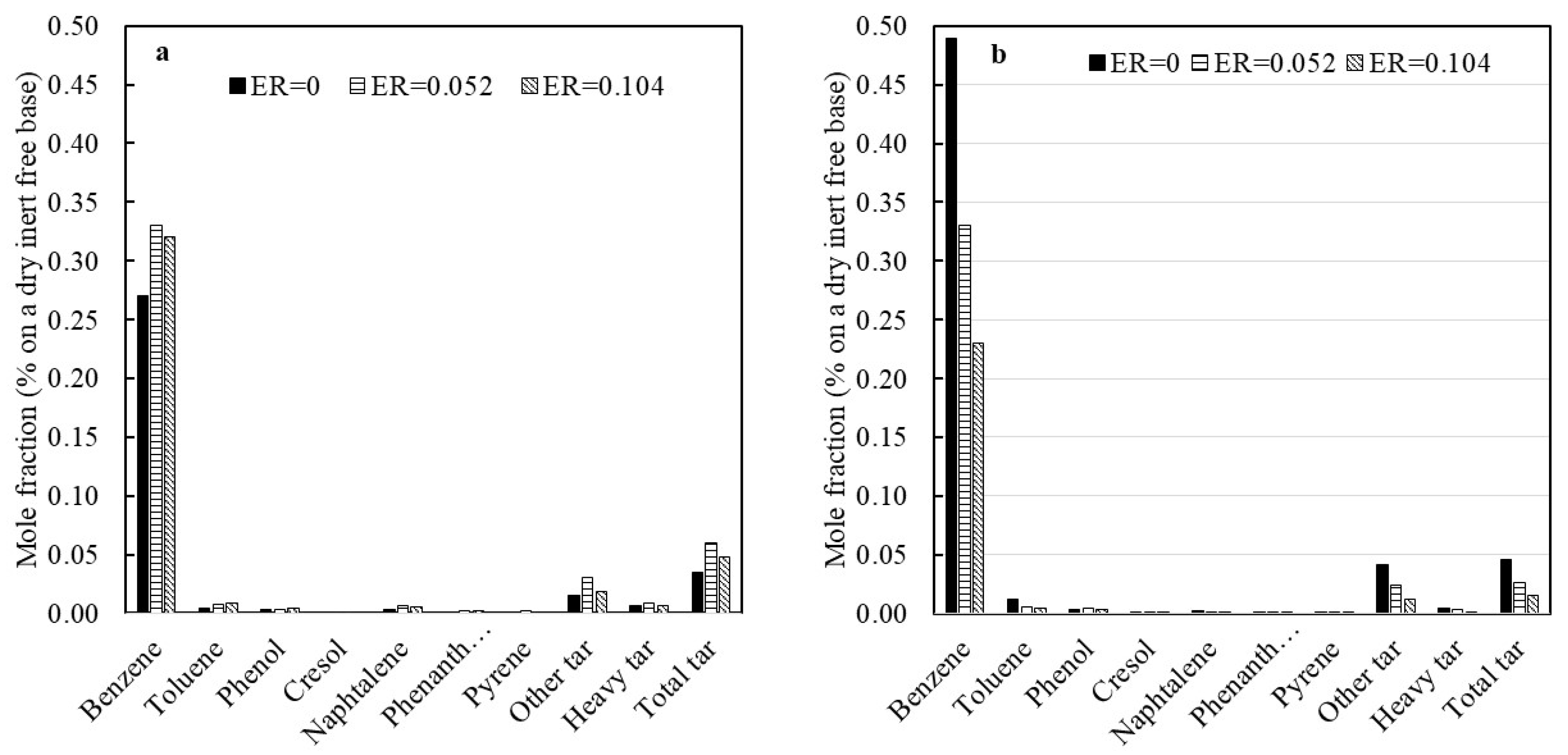
3.4. The Effect of Devolatilization Rate on Tar Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Dayton, D. Review of the Literature on Catalytic Biomass tar Destruction: Milestone Completion Report; Department of Energy’s Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2002. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.J.; Hanna, M.A.; Weller, C.L.; Jones, D.D. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenerg. 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.J.; Zhang, B.; Khajeh, A.; Shahbazi, A. Iron oxide supported on silicalite-1 as a multifunctional material for biomass chemical looping gasification and syngas upgrading. Chem. Eng. J. 2020, 401, 125943. [Google Scholar] [CrossRef]
- Wang, L.J.; Agyemang, S.A.; Amini, H.; Shahbazi, A. Mathematical modeling of production and biorefinery of energy crops. Renew. Sustain. Energy Rev. 2015, 43, 530–544. [Google Scholar] [CrossRef] [Green Version]
- Gerber, S.; Behrendt, F.; Oevermann, M. An Eulerian modeling approach of wood gasification in a bubbling fluidized bed reactor using char as bed material. Fuel 2010, 89, 2903–2917. [Google Scholar] [CrossRef]
- Oevermann, M.; Gerber, S.; Behrendt, F. Euler-Lagrange/DEM simulation of wood gasification in a bubbling fluidized bed reactor. Particuology 2009, 7, 307–316. [Google Scholar] [CrossRef]
- Debiagi, P.; Gentile, G.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A predictive model of biochar formation and characterization. J. Anal. Appl. Pyrolysis 2018, 134, 326–335. [Google Scholar] [CrossRef]
- Ranzi, E.; Debiagi, P.E.A.; Frassoldati, A. Mathematical modeling of fast biomass pyrolysis and bio-oil formation. Note I: Kinetic mechanism of biomass pyrolysis. ACS Sustain. Chem. Eng. 2017, 5, 2867–2881. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Frassoldati, A.; Bennadji, H.; Smith, K.; Serapiglia, M.J.; Gauthier, G.; Melkior, T.; Ranzi, E.; Fisher, E.M. Pyrolysis of centimeter-scale woody biomass particles: Kinetic modeling and experimental validation. Energy Fuels 2014, 28, 3884–3898. [Google Scholar] [CrossRef]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical kinetics of biomass pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F.; Jablonski, W.S.; Carpenter, D.L.; Altantzis, C.; Garg, A.; Barton, J.L.; Chen, R.; Field, R.P. Steam-air blown bubbling fluidized bed biomass gasification (BFBBG): Multi-scale models and experimental validation. AIChE J. 2017, 63, 1543–1565. [Google Scholar] [CrossRef]
- Rafati, M.; Hashemisohi, A.; Wang, L.J.; Shahbazi, A. Sequential modular simulation of hydrodynamics and reaction kinetics in a biomass bubbling fluidized-bed gasifier using Aspen plus. Energy Fuels 2015, 29, 8261–8272. [Google Scholar] [CrossRef]
- Cui, H.; Mostoufi, N.; Chaouki, J. Characterization of dynamic gas-solid distribution in fluidized beds. Chem. Eng. J. 2000, 79, 133–143. [Google Scholar] [CrossRef]
- Davidson, J.F.; Harrison, D. (Eds.) Fludized Particles; Cambridge University Press: Cambridge, UK, 1964; p. 155. [Google Scholar]

| Parameters | Correlation | Reference |
|---|---|---|
| Voidage in bubble phase | [14] | |
| Voidage in emulsion phase | [14] | |
| Bubble fraction | [14] | |
| Mass transfer coefficient between bubble and cloud | [15] | |
| Mass transfer coefficient between cloud and emulsion | [15] | |
| Overall bubble–emulsion mass transfer coefficient | [15] |
| Parameter | Quantity | Reference |
|---|---|---|
| Biomass density (kg/m3) | 850 | [10] |
| Biomass-specific heat [J/(kg K)] | 1500 + T | [10] |
| Cellulose | 35% | [12] |
| Hemicellulose | 40% | [12] |
| Total lignin (LIG) | 25% | [12] |
| Lignin with rich C (LIGC) represented by C15H14O4 | 0.99% | [12] |
| Lignin with rich H (LIGH) represented by C16H10O6(OCH3)4 | 9.85% | [12] |
| Lignin with rich O (LIGO) represented by C17H13O4(OCH3)5 | 14.17% | [12] |
| Ultimate analysis (%wt dry basis) | ||
| Carbon | 49% | [12] |
| Hydrogen | 6% | [12] |
| Oxygen | 45% | [12] |
| Moisture content | 3.95% | [12] |
| Volatile matter | 84.79% | [12] |
| Fixed carbon | 10.9% | [12] |
| Component | Molar Percentage | |
|---|---|---|
| TGA at a Heating Rate of 30 °C/min to 850 °C | Fluidized Bed at 850 °C | |
| H2O | 26.59 | 17.69 |
| H2 | 1.07 | 0.59 |
| CO | 13.74 | 16.98 |
| CO2 | 19.89 | 17.73 |
| CH4 | 2.06 | 0.94 |
| CH2O (Formaldehyde) | 12.52 | 18.89 |
| CH3OH (Methanol) | 8.78 | 11.54 |
| C2H2O2 (Glyoxal) | 1.27 | 2.22 |
| C2H4 (Ethylene) | 2.39 | 3.80 |
| C2H4O (Acetaldehyde) | 1.57 | 1.70 |
| C2H4O2 (Acetic acid) | 5.83 | 7.32 |
| C2H5OH (Ethanol) | 1.23 | 2.13 |
| C5H8O4 (Glutaric acid) | 10.68 | 2.35 |
| C6H5OH (Phenol) | 0.03 | 0.03 |
| C6H6O3 (HMFU) | 1.58 | 2.77 |
| C6H10O5 (LVG) | 10.20 | 4.68 |
| C9H10O2 (Ethyl benzoate) | 0.04 | 0.03 |
| C11H12O4 (Ethyl acetylsalicylate) | 1.94 | 0.27 |
| HCOOH (Formic acid) | 1.06 | 1.00 |
| C2H5CHO (Propionaldehyde) | 3.28 | 4.92 |
| C6H5OCH3 (Methoxybenzene) | 0.83 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemisohi, A.; Wang, L.; Shahbazi, A. Numerical Analysis of Tar and Syngas Formation during the Steam Gasification of Biomass in a Fluidized Bed. Energies 2023, 16, 5283. https://doi.org/10.3390/en16145283
Hashemisohi A, Wang L, Shahbazi A. Numerical Analysis of Tar and Syngas Formation during the Steam Gasification of Biomass in a Fluidized Bed. Energies. 2023; 16(14):5283. https://doi.org/10.3390/en16145283
Chicago/Turabian StyleHashemisohi, Abolhasan, Lijun Wang, and Abolghasem Shahbazi. 2023. "Numerical Analysis of Tar and Syngas Formation during the Steam Gasification of Biomass in a Fluidized Bed" Energies 16, no. 14: 5283. https://doi.org/10.3390/en16145283




