Some Perspectives for the Gasification Process in the Energy Transition World Scenario
Abstract
1. Introduction
2. Conventional Gasification Process
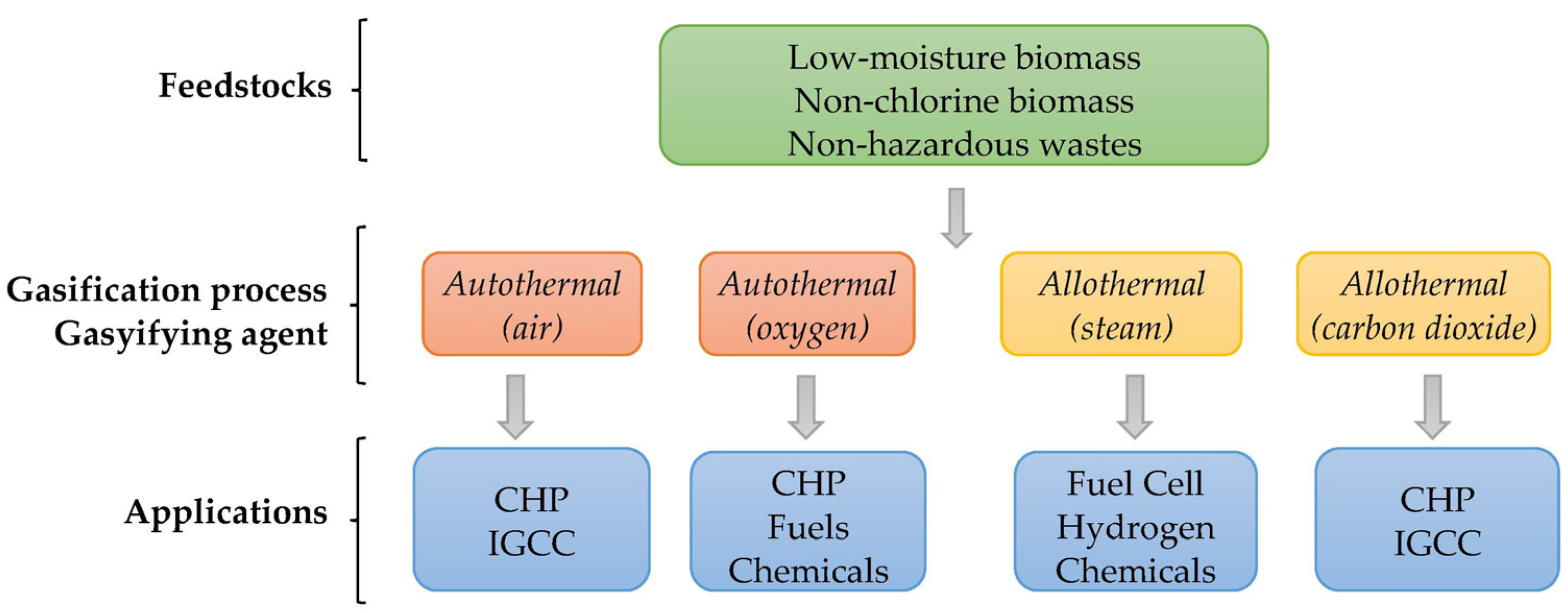
2.1. Effect of Feedstock Characteristics
- Biomass particle dimensions, which are reactor-dependent (5–100 mm for fixed bed reactors, 6–50 mm for fluidized bed reactors, and <1 mm for entrained flow reactors [18]) and have a great influence on the gasification rate. Smaller particles can mix with each other and to willingly fit into any remaining space, boosting the overall energy efficiency of the gasification process. However, their size reduction incurs an additional expense due to the necessary milling/grinding pretreatment. Moreover, smaller particles increase the yields of hydrogen and syngas as well as the carbon conversion [19]. However, they should respect the gasifier and feeding system limitations to prevent agglomeration. The fibrous biomass frequently becomes stuck in the feeding system. These problems can be overcome by densifying the biomass, which reduces the spaces between particles and thus increases the bulk density.
- Low moisture, low sulfur, and low chlorine contents. Feedstocks with higher moisture necessitate drying prior to the gasification stage. Increased moisture content increases hydrogen while decreasing carbon monoxide, owing to the consumption of this latter component in the water–gas shift reaction. Yet, because carbon monoxide has a higher heating value than H2, the drop in carbon monoxide yield has a greater impact than the increase in hydrogen production, resulting in a negative balance in syngas quality. Typical moisture requirements for downdraft reactors are below 12%, <40% for updraft reactors, and between 10% and 60% for fluidized bed reactors [18]. Sulfur content is usually residual (<0.1%) in lignocellulosic biomasses. However, the presence of this compound gives rise to SO2 formation, enhancing the corrosion potential of the process [20].
- Chlorine can also be present in some lignocellulosic biomasses but in residual amounts (<0.1%) and must the submitted to dichlorination prior to the gasification process [18]. This chlorine would lead to the formation of chlorine acid (Cl2), enhancing the corrosion potential of the process.
- Ash content and melting temperature are also a fundamental biomass characteristics from thermal conversion. However, the ash fusion temperatures of the biomass ash oscillate from 1100 °C to 1300 °C [21], which is generally above the temperature range of the conventional gasification processes, thus preventing the melting of the inorganics and allowing some of them to act as catalysts in fluidized bed reactors such as potassium and sodium.
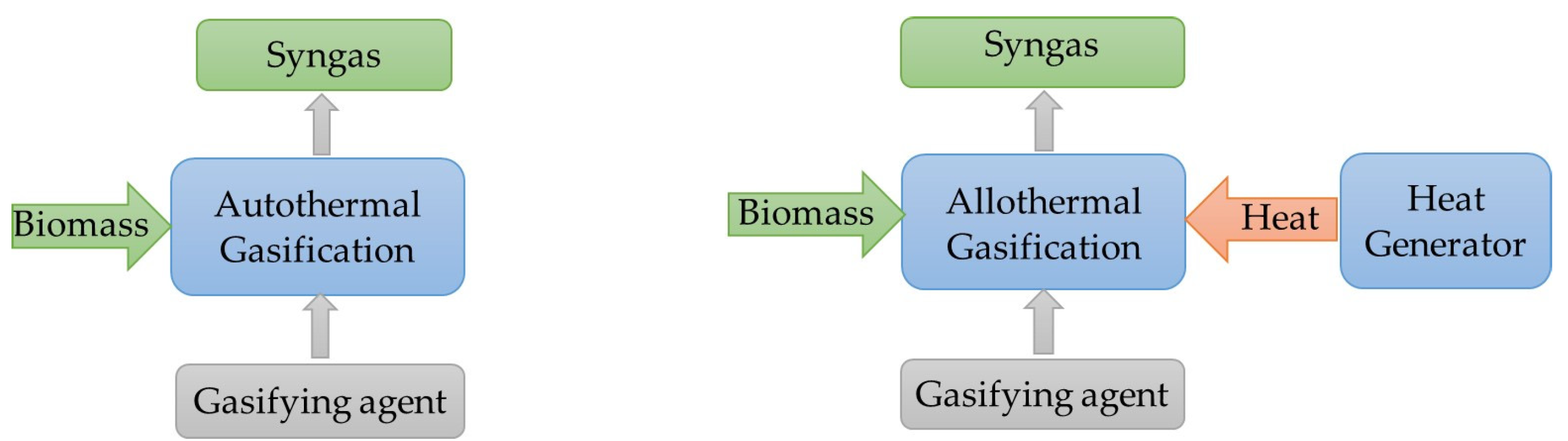
2.2. Effect of Gasifying Agent
- Due to its low cost, air is the main gasifying agent, with the resulting syngas being heavily diluted by nitrogen (about 50%) and having a lower heating value (typically 4–6 MJ/m3) and hydrogen concentration below 20% [22]. To improve the syngas quality, air has been used combined with steam and pure oxygen (oxygen-enriched air). Hernandez et al. [23] demonstrated that the use of a mixture of air and steam as the gasifying agent enhances the quality of the syngas, increasing its hydrogen and methane contents. Ismail et al. [7] showed that the use of oxygen-enriched air allows the production of a syngas with a reasonable heating value (around 9–15 MJ/m3); however, this has the shortcoming of being more costly once oxygen separators from air are needed.
- Steam use for biomass gasification allows obtaining a syngas with a higher heating value (typically 10–18 MJ/m3) and hydrogen content [24], although an external source of heat is required to increase the temperature of the process (allothermal gasification). Steam gasification also has the advantage of operating with both wet and dry biomass [19]. The review of Ahmad et al. [19] also presents the use of a mixture of steam and pure oxygen to promote biomass conversion, accompanied by a carbon dioxide increase and the decrease of carbon monoxide and hydrogen.
- Pure oxygen usage as a gasifying agent in biomass gasification systems was reviewed by Mishra and Upadhyay [25]. They conclude that an improved gasification as well as enhanced carbon conversion, and a heating value between 10 and 18 MJ/m3 was obtained, mainly because of the absence of nitrogen in the gasifying medium. However, due to the separation of oxygen from the air, oxygen-blown gasification is an energy-intensive and expensive process.
- Carbon dioxide is the least studied gasifying agent. A recent review by Chan et al. [26] concluded that the use of carbon dioxide as the gasifying agent provides a syngas rich in carbon monoxide (40–80 vol. %), essentially because of the dominant Boudouard reaction that is only thermodynamically active over 700 °C. Carbon dioxide gasification is a very promising conversion method that takes advantage of the abundance of carbon dioxide and biomass to generate value-added carbon monoxide. The successful commercialization of this method would have a significant impact on achieving the UN Sustainable Development Goals and the Paris Climate Agreement.
2.3. Syngas Applications
2.3.1. Heat and Power
2.3.2. Fuels
2.3.3. Chemicals
3. Some Future Perspectives for the Gasification Process
- Optimizing and comprehending the behavior of the reactor, because it is the least efficient piece of a gasification plant. Its design and operation require a thorough understanding of the gasification process and how the design, feedstock, and key operating settings have an impact on entire performance.
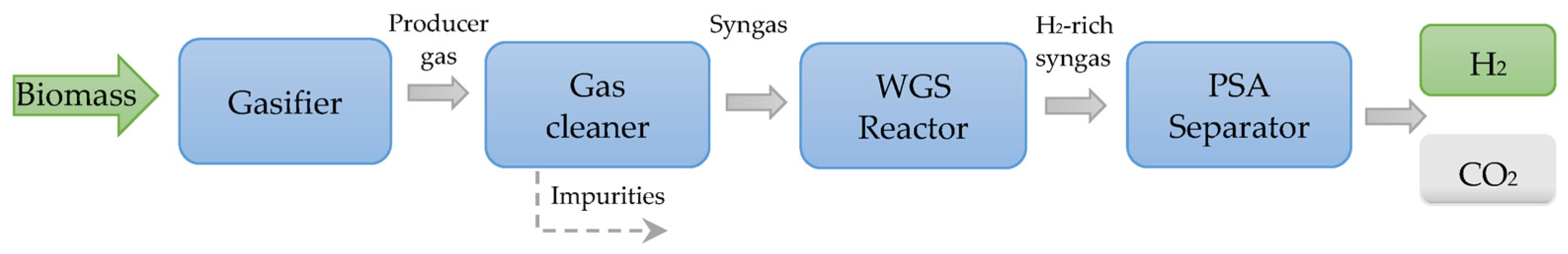
- Efficient and cost-effective gas cleaning systems for the downstream application of syngas. Gas cleaning is required to avoid erosion, corrosion, and environmental issues in downstream applications. Tars are generally heavy hydrocarbons produced by the biomass gasification process, which can clog engine valves or cause turbine fouling, resulting in greater maintenance costs and reduced performance. Tars also interfere with the production of fuels and chemicals.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, E.; Ferreira, S. Biomass Waste for Energy Production. Energies 2022, 15, 5943. [Google Scholar] [CrossRef]
- Danish, R.; Ozcan, U.B. An empirical investigation of nuclear energy consumption and carbon dioxide (CO2) emission in India: Bridging IPAT and EKC hypotheses. Nucl. Eng. Technol. 2021, 53, 2056–2065. [Google Scholar] [CrossRef]
- Gielen, D.; Francisco, B.; Deger, S.; Morgan, D.B.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- IEA, Bioenergy, IEA, Paris. License: CC BY 4.0. 2022. Available online: https://www.iea.org/reports/bioenergy (accessed on 5 May 2023).
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. CBC Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renew. Sustain. Energy Rev. 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Ismail, T.; Ramos, A.; Monteiro, E.; El-Salam, M.; Rouboa, A. Parametric studies in the gasification agent and fluidization velocity during oxygen-enriched gasification of biomass in a pilot-scale fluidized bed: Experimental and numerical assessment. Renew. Energy 2020, 147, 2429–2439. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; Yoshida, A.; Abudula, A.; Guan, G. Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev. 2020, 117, 109486. [Google Scholar]
- Lanjekar, P.R.; Panwar, N.L.; Agrawal, C. A comprehensive review on hydrogen production through thermochemical conversion of biomass for energy security. Bioresour. Technol. Rep. 2023, 21, 101293. [Google Scholar] [CrossRef]
- MMR, Maximize Market Research, Biomass Gasification Market—Global Industry Analysis and Forecast (2022–2029) by Fuel Type, Applications, and by Region, MMR, India. 2022. Available online: https://www.maximizemarketresearch.com/market-report/biomass-gasification-market/66858/ (accessed on 30 May 2023).
- Ferreira, S.; Monteiro, E.; Brito, P.; Costa, C.; Calado, L.; Vilarinho, C. Experimental analysis of brewers´ spent grains steam gasification in an allothermal batch reactor. Energies 2019, 12, 912. [Google Scholar] [CrossRef]
- Bourguig, O.; Ramos, A.; Bouziane, K.; Asbik, M.; Monteiro, E.; Rouboa, A. Performance assessment of the co-gasification for sustainable management of municipal solid waste: Moroccan Case. Energy Rep. 2022, 8, 1530–1540. [Google Scholar] [CrossRef]
- Witold, M.L.; Ryms, M.; Kosakowski, W. Thermal Biomass Conversion: A Review. Processes 2020, 8, 516. [Google Scholar]
- Alves, O.; Monteiro, E.; Brito, P.; Gonçalves, M. Clean-up treatments for syngas obtained by gasification of coal, lignocellulosic biomass or municipal residues. Prog. Ind. Ecol. 2022, 15, 128–161. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Khosravani, H.; Rahimpour, H.R.; Rahimpour, M.R. Characteristics of syngas impurities; Physical and chemical properties. In Advances in Synthesis Gas: Methods, Technologies and Applications; Rahimpour, M.R., Makarem, M.A., Meshksar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 3–25. [Google Scholar]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass pre-treatment techniques for the production of biofuels using thermal conversion methods—A review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Dai, J.; Cui, H.; Grace, J.R. Biomass feeding for thermochemical reactors. Prog. Energy Combust. Sci. 2012, 38, 716–736. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Elbersen, W.; Lammens, T.M.; Alakangas, E.A.; Annevelink, B.; Harmsen, P.; Elbersen, B. Chapter 3—Lignocellulosic Biomass Quality: Matching Characteristics with Biomass Conversion Requirements. In Modeling and Optimization of Biomass Supply Chains; Panoutsou, C., Ed.; Academic Press: London, UK, 2017; pp. 55–78. [Google Scholar]
- Zhai, M.; Li, X.; Yang, D.; Ma, Z.; Dong, P. Ash fusion characteristics of biomass pellets during combustion. J. Clean. Prod. 2022, 336, 130361. [Google Scholar] [CrossRef]
- Sansaniwal, S.; Pal, K.; Rosen, M.; Tyagi, S. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Hernandez, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air-steam flow on biomass entrained flow gasification. Fuel Process. Technol. 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Kuo, P.C.; Wu, W. Design, optimization and energetic efficiency of producing hydrogen-rich gas from biomass steam gasification. Energies 2015, 8, 94–110. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman, S.F.S.A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278, 116843. [Google Scholar] [CrossRef] [PubMed]
- Ahrenfeldt, J.; Thomsen, T.P.; Henriksen, U.; Clausen, L.R. Biomass gasification cogeneration- A review of state of the art technology and near future perspectives. Appl. Therm. Eng. 2013, 50, 1407–1417. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, H.; Guo, W.; Song, T.; Shen, L. System simulation and experimental verification: Biomass-based integrated gasification combined cycle (BIGCC) coupling with chemical looping gasification (CLG) for power generation. Fuel 2019, 241, 118–128. [Google Scholar] [CrossRef]
- Escudero, M.; Gonzalez, C.; Lopez, I. Quantitative analysis of potential power production and environmental benefits of biomass integrated gasification combined cycles in the European Union. Energy Policy 2013, 53, 63–75. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Lora, E.S.; Venturini, O.J.; Maya, D.M.Y.; Garcia-Pérez, M. Gas cleaning systems for integrating biomass gasification with Fischer-Tropsch synthesis—A review of impurity removal processes and their sequences. Renew. Sustain. Energy Rev. 2023, 172, 113047. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Arsalanfar, M.; Bozorgzadeh, H.R.; Samimi, A. A review of Fischer-Tropsch synthesis on the cobalt based catalysts. Phys. Chem. Res. 2014, 2, 179–201. [Google Scholar]
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Kanwal, S.; Mehran, M.T.; Hassan, M.; Anwar, M.; Naqvi, S.R.; Khoja, A.H. An integrated future approach for the energy security of Pakistan: Replacement of fossil fuels with syngas for better environment and socio-economic development. Renew. Sustain. Energy Rev 2022, 156, 111978. [Google Scholar] [CrossRef]
- Monteiro, E.; Brito, P. Hydrogen supply chain: Current status and prospects. Energy Storage 2023, e466. [Google Scholar] [CrossRef]
- Malik, F.R.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar]
- Martins, A.H.; Rouboa, A.; Monteiro, E. On the green hydrogen production through gasification processes: A techno-economic approach. J. Clean. Prod. 2023, 383, 135476. [Google Scholar] [CrossRef]
- Zhao, F.; Fan, Y.; Zhang, S.; Eichhammer, W.; Haendel, M.; Yu, S. Exploring pathways to deep de-carbonization and the associated environmental impact in China’s ammonia industry. Environ. Res. Lett. 2022, 17, 045029. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Oliveira, M.; Ramos, A.; Ismail, T.; Monteiro, E.; Rouboa, A. A Review on Plasma Gasification of Solid Residues: Recent Advances and Developments. Energies 2022, 15, 1475. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 109529. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. Advances in the thermo-chemical production of hydrogen from biomass and residual wastes: Summary of recent techno-economic analyses. Bioresour. Technol. 2020, 299, 122557. [Google Scholar] [CrossRef]
| Impurity | Examples | Issues | Cleanup Method |
|---|---|---|---|
| Particulates | Ash, char, bed material | Erosion | Filtration, scrubbing |
| Alkali metals | Sodium and potassium compounds | Corrosion | Cooling, condensation, filtration, adsorption |
| Nitrogen components | NH3 e HCN | NOx formation | Scrubbing, selective catalytic reduction |
| Tars | Aromatics | Clog filters, difficult to burn, deposit internally | Tar cracking; tar removal |
| Sulfur, Chloride | H2S, HCl | Corrosion, emissions | Lime or dolomite scrubbing or absorption |
| Product/Property | Fischer–Tropsch Synthesis | Methanol | Hydrogen | Heat and Power | |
|---|---|---|---|---|---|
| Boiler | Turbine | ||||
| H2/CO | 0.6 (a) | ~2.0 | High | Irrelevant | Irrelevant |
| CO2 | Low | Low (c) | Unimportant (b) | Not critical | Not critical |
| Hydrocarbons | Low (d) | Low (d) | Low (d) | High | High |
| N2 | Low | Low | Low | (e) | (e) |
| H2O | Low | Low | High (f) | Low | (g) |
| Contaminants | <1 ppm sulfur Low particulates | <1 ppm sulfur Low particulates | <<1 ppm sulfur Low particulates | (k) | Low particulates and metals |
| Heating value | Irrelevant (h) | Irrelevant (h) | Irrelevant (h) | High (i) | High (i) |
| Pressure (bar) | ~20–30 | ~50 (liquid phase) ~140 (vapor phase) | ~28 | Low | ~400 |
| Temperature (°C) | 200–300 (j) 300–400 | 100–200 | 100–200 | 250 | 500–600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, E.; Ferreira, S. Some Perspectives for the Gasification Process in the Energy Transition World Scenario. Energies 2023, 16, 5543. https://doi.org/10.3390/en16145543
Monteiro E, Ferreira S. Some Perspectives for the Gasification Process in the Energy Transition World Scenario. Energies. 2023; 16(14):5543. https://doi.org/10.3390/en16145543
Chicago/Turabian StyleMonteiro, Eliseu, and Sérgio Ferreira. 2023. "Some Perspectives for the Gasification Process in the Energy Transition World Scenario" Energies 16, no. 14: 5543. https://doi.org/10.3390/en16145543
APA StyleMonteiro, E., & Ferreira, S. (2023). Some Perspectives for the Gasification Process in the Energy Transition World Scenario. Energies, 16(14), 5543. https://doi.org/10.3390/en16145543







