Pyrolysis of Energy Cane Bagasse: Investigating Kinetics, Thermodynamics, and Effect of Temperature on Volatile Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation and Characterization
2.2. Pyrolysis of Energy Cane Bagasse
2.3. Kinetic Modeling of Energy Cane Bagasse Pyrolysis
3. Results and Discussion
3.1. Energy Cane Bagasse Characterization
3.2. Products of Energy Cane Bagasse Pyrolysis
3.3. Multi-Component Kinetic Modeling
3.3.1. Results for the Four Deconvoluted Independent Devolatilization Reactions
3.3.2. Results for Activation Energy
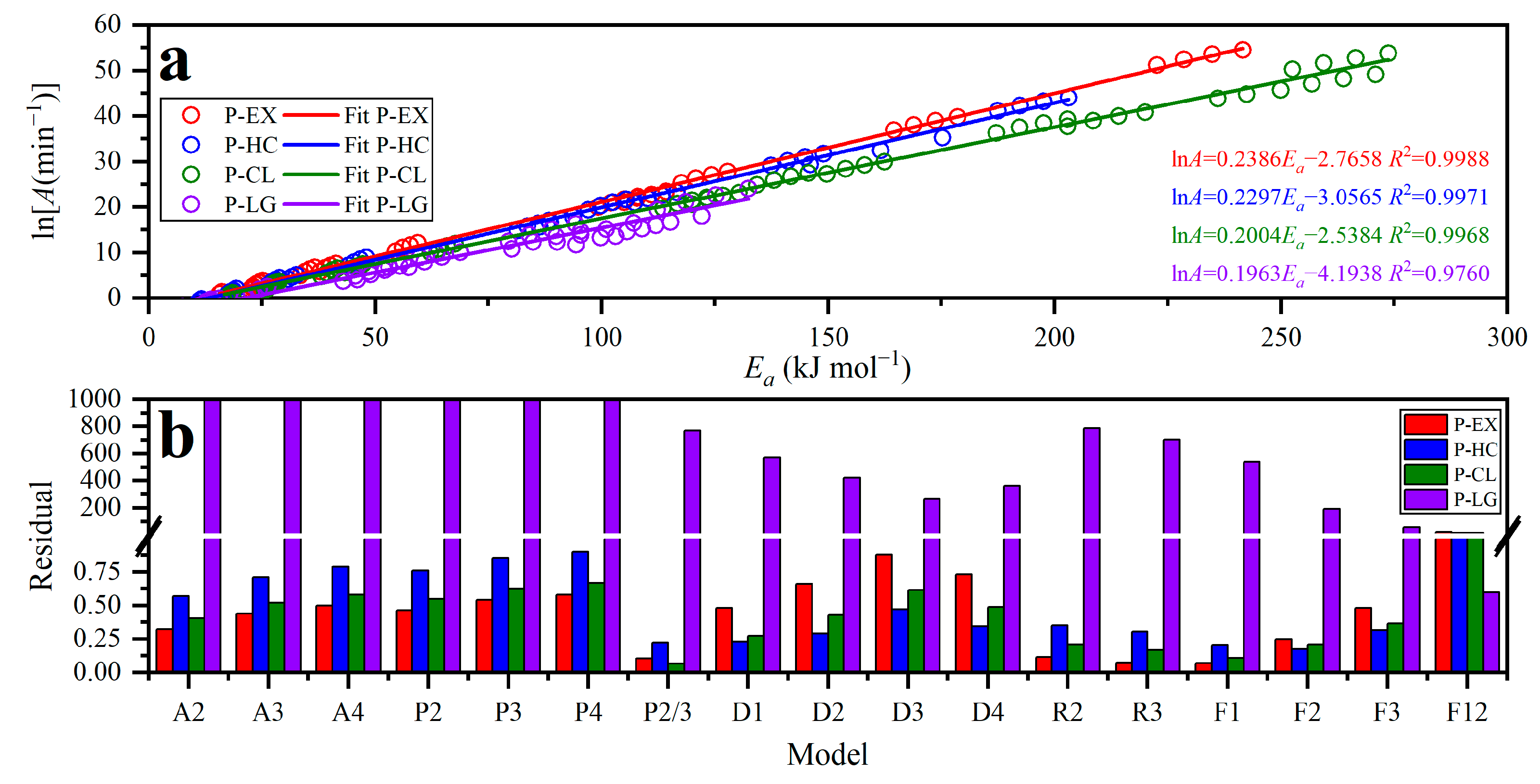
3.3.3. Results for Pre-Exponential Factor of Energy Cane Bagasse
3.3.4. Results for Reaction Model
3.3.5. Results for Verification Step of Summative Kinetic Expression
3.3.6. Results for Thermodynamic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Notation
| Notation | Definition |
| α | conversion |
| dα/dT | conversion rate |
| maximum amplitude of the curve | |
| T | temperature |
| Tp | peak temperature |
| w1 | curve width |
| w2 and w3 | shape parameters |
| M | total number of experimental data points |
| dα/dTexp | experimentally measured conversion rate |
| dα/dTdec | conversion rate deconvoluted |
| rate of change of temperature | |
| pre-exponential factor | |
| activation energy | |
| universal gas constant | |
| reaction model | |
| a and b | compensation coefficients |
| p(x)/p(x0.5) | represents the experimental master plot |
| ΔH | change in enthalpy |
| ΔG | change in Gibbs free energy |
| ΔS | change in entropy |
| kB | Boltzmann constant (1.381 × 10−23 J K−1) |
| h | Planck constant (6.626 × 10−34 J s) |
| Tm | DTG peak temperature |
References
- De Bhowmick, G.; Sarmaha, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.M.C.L.; Souza, T.P.C.; Elihimas, D.R.M.; Silva, J.P.; Albuquerque, A.A.; Pacheco, J.G.A.; Silva, J.M.F. Ethanol dehydration by absorption and biodiesel production by reactive distillation: An innovative integrated process. Biomass Bioenergy 2021, 154, 106263. [Google Scholar] [CrossRef]
- Souza, T.P.C.; Silva, R.J.M.C.L.; Melo, J.C.; Tschoeke, I.C.P.; Silva, J.P.; Pacheco, J.G.A.; Silva, J.M.F. Kinetic modeling of cottonseed oil transesterification with ethanol. React. Kinet. Mech. Catal. 2019, 128, 707–722. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Balances. Overview. Available online: https://www.iea.org/data-and-statistics/data-browser?country=WORLD&fuel=Energy%20supply&indicator=TESbySource (accessed on 30 June 2022).
- Das, P.; Gundimeda, H. Is biofuel expansion in developing countries reasonable? A review of empirical evidence of food and land use impacts. J. Clean. Prod. 2022, 372, 133501. [Google Scholar] [CrossRef]
- Caldeira, V.P.S.; Santos, A.G.D.; Oliveira, D.S.; Lima, R.B.; Souza, L.D. Polyethylene catalytic cracking by thermogravimetric analysis: Effects of zeolitic properties and homogenization process. J. Therm. Anal. Calorim. 2017, 130, 1939–1951. [Google Scholar] [CrossRef]
- Silva, J.B.; Cabral, G.G.; Araujo, M.D.S.; Caldeira, V.P.S.; Coriolano, A.C.F.; Fernandes, V.J., Jr. Catalytic pyrolysis of atmospheric residue of petroleum using pillared interlayed clay containing lanthanum and aluminum polyhydroxications (LaAl13-PILC). Pet. Sci. Technol. 2021, 39, 704–717. [Google Scholar] [CrossRef]
- Melo, J.A.; de Sá, M.S.; Moral, A.; Bimbela, F.; Gandía, L.M.; Wisniewski, A., Jr. Renewable Hydrocarbon Production from Waste Cottonseed Oil Pyrolysis and Catalytic Upgrading of Vapors with Mo-Co and Mo-Ni Catalysts Supported on γ-Al2O3. Nanomaterials 2021, 11, 1659. [Google Scholar] [CrossRef]
- Arias, S.; Vascocelos, D.P.; de Oliveira Libório, D.; Gonzalez, J.F.; Câmara, A.G.; Barbosa, C.M.; Pacheco, J.G.A. Hydrogen-free deoxygenation of industrial vegetable oil waste using Ce, Zr-NiAl catalysts for second-generation biofuels production. Mol. Catal. 2022, 529, 112554. [Google Scholar] [CrossRef]
- Wisniewski Jr, A.; Wosniak, L.; Scharf, D.R.; Wiggers, V.R.; Meier, H.F.; Simionatto, E.L. Upgrade of biofuels obtained from waste fish oil pyrolysis by reactive distillation. J. Braz. Chem. Soc. 2015, 26, 224–232. [Google Scholar] [CrossRef]
- Borel, L.D.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Santana, K.V.R.; Apolônio, F.C.S.O.; Wisniewski, A., Jr. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. Bioenergy Res. 2020, 13, 605–617. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Rossi, R.A.S.; Andrade, L.A.; Barrozo, M.A.S.; Vieira, L.G.M. A study of optimization of solar pyrolysis and catalyst recovery and reuse. Energy Convers. Manag. 2021, 237, 114094. [Google Scholar] [CrossRef]
- Santos, V.O.; Araujo, R.O.; Ribeiro, F.C.; Queiroz, L.S.; Guimarães, M.N.; Colpani, D.; de Souza, L.K. Non-isothermal kinetics evaluation of buriti and inaja seed biomass waste for pyrolysis thermochemical conversion technology. Biomass Convers. Biorefin. 2021, 1–17. [Google Scholar] [CrossRef]
- Padilha, J.F.; Frety, R.; Santos, A.P.; Pontes, L.A.M.; Santos, M.R.; Arias, S.; Pacheco, J.G.A. Deoxygenation of Oleic Acid Methyl Ester in FCC Process Conditions Over Protonated and Sodium Exchanged Y and ZSM-5 Zeolites. Waste Biomass Valorization 2022, 13, 185–194. [Google Scholar] [CrossRef]
- Amaral, A.R.; Bernar, L.P.; Ferreira, C.C.; de Oliveira, R.M.; Pereira, A.M.; Pereira, L.M.; Santos, M.C.; Assunção, F.P.d.C.; Bezerra, K.C.A.; Almeida, H.d.S.; et al. Economic Feasibility Assessment of the Thermal Catalytic Process of Wastes: Açaí Seeds (Euterpe oleracea) and Scum from Grease Traps. Energies 2022, 15, 7718. [Google Scholar] [CrossRef]
- De Rocha, C.D.A.; da Silva, R.H.J.; Hamoy, G.L.H.; Pinto, B.L.; Jonatan, B.S.; Costa, S.M.; Teixeira, M.N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- De Almeida, R.P.; Aciole, R.C.G.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Pacheco, J.G.A.; Barros, I.C.L. Residue-based activated carbon from passion fruit seed as support to H3PW12O40 for the esterification of oleic acid. J. Clean. Prod. 2021, 282, 124477. [Google Scholar] [CrossRef]
- do Nascimento, L.A.; Peçanha, S.R.S.; Arias, S.; Santos, B.S.; Pacheco, J.G.A.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Barros, I.D.C.L. NiAlCe mixed oxides obtained from layered double hydroxides applied to anisole hydrodeoxygenation. Catal. Today 2022, 394–396, 282–294. [Google Scholar] [CrossRef]
- CONAB. Companhia Nacional de Abastecimento. Boletin de Safra de Cana-de-açúCar, Published on 27 April 2022. Available online: https://www.conab.gov.br/info-agro/safras/cana (accessed on 30 June 2022).
- Schmitt, C.C.; Moreira, R.; Neves, R.C.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.; Dahmena, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199–106215. [Google Scholar] [CrossRef]
- Sohaib, Q.; Muhammad, A.; Younas, M. Fast pyrolysis of sugarcane bagasse: Effect of pyrolysis conditions on final product distribution and properties. Energ. Source Part A 2017, 39, 184–190. [Google Scholar] [CrossRef]
- Barros, J.A.S.; Krause, M.C.; Lazzari, E.; Bjerk, T.R.; do Amaral, A.L.; Caramão, E.B.; Krause, L.C. Chromatographic characterization of bio-oils from fast pyrolysis of sugar cane residues (straw and bagasse) from four genotypes of the Saccharum Complex. Microchem. J. 2017, 137, 30–36. [Google Scholar] [CrossRef]
- Durange, J.A.C.; Santos, M.R.L.; Pereira, M.M.; Fernandes, L.A.P., Jr.; Souza, M.N.; Mendes, A.N.; Mesa, L.M.; Sánchez, C.G.; Sanchez, E.M.S.; Pérez, J.M.M.; et al. Physicochemical Properties of Pyrolysis Bio-Oil from Sugarcane Straw and Sugarcane in Natura. J. Biomater. Nanobiotechnol. 2013, 4, 10–19. [Google Scholar] [CrossRef]
- De Carvalho, V.S.; Tannous, K. Thermal decomposition kinetics modeling of energy cane Saccharum robustum. Thermochim. Acta 2017, 657, 56–65. [Google Scholar] [CrossRef]
- Carvalho-Netto, O.V.; Bressiani, J.A.; Soriano, H.L.; Fiori, C.S.; Santos, J.M.; Barbosa, G.V.S.; Xavier, M.A.; Landell, M. GA and Pereira, G. AG. The potential of the energy cane as the main biomass crop for the cellulosic industry. Chem. Biol. Technol. Agric. 2014, 1, 20. [Google Scholar] [CrossRef]
- de Abreu, L.G.F.; Grassia, M.C.B.; de Carvalho, L.M.; da Silva, J.J.B.; Oliveira, J.V.C.; Bressiani, J.A.; Pereira, G.A.G. Energy cane Vs sugarcane: Watching the race in plant development. Ind. Crops Prod. 2020, 156, 112868. [Google Scholar] [CrossRef]
- Hoffstadt, K.; Pohen, G.D.; Dicke, M.D.; Paulsen, S.; Krafft, S.; Zang, J.W.; da Fonseca-Zang, W.A.; Leite, A.; Kuperjans, I. Challenges and Prospects of Biogas from Energy Cane as Supplement to Bioethanol Production. Agronomy 2020, 10, 821. [Google Scholar] [CrossRef]
- Pokhrel, P.; Rajan, N.; Jifon, J.; Roonoey, W.; Jessup, R.; da Silva, J.; Enciso, J.; Attia, A. Evaluation of the DSSAT-CANEGRO model for simulating the growth of energy cane (Saccharum spp.), a biofuel feedstock crop. Crop Sci. 2021, 62, 466–478. [Google Scholar] [CrossRef]
- Henkel, C.; Muley, P.D.; Abdollahi, K.K.; Marculescu, C.; Boldor, D. Pyrolysis of energy cane bagasse and invasive Chinese tallow tree (Triadica sebifera, L.) biomass in an inductively heated reactor. Energy Convers. Manag. 2016, 109, 175–183. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting pecan nutshell pyrolysis as a source of bioenergy and bio-based chemicals using multicomponent kinetic modeling, thermodynamic parameters estimation, and Py-GC/MS analysis. Renew. Sust. Energ. Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Bruce, E.D.; Frety, R.; Pacheco, J.G.A.; Teixeira, C.M.; Barbosa, C.B.M. Thermocatalytic cracking kinetics of myristic acid adsorbed on catalysts with different acidity. Catal. Today 2017, 289, 280–288. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Prospection of catole coconut (Syagrus cearensis) as a new bioenergy feedstock: Insights from physicochemical characterization, pyrolysis kinetics, and thermodynamics parameters. Renew. Energ 2022, 181, 207–218. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Di Domenico, M.; Bolzan, A.; Machado, R.A.F.; Marangoni, C. Evaluating the bioenergy potential of cupuassu shell through pyrolysis kinetics, thermodynamic parameters of activation, and evolved gas analysis with TG/FTIR technique. Thermochim. Acta 2022, 711, 179187. [Google Scholar] [CrossRef]
- Peres, C.B.; Rosa, A.H.; de Morais, L.C. Investigation of pyrolysis kinetics parameters and thermal behavior of thermochemically modified bagasse for bioenergy potential. SN Appl. Sci. 2021, 3, 337. [Google Scholar] [CrossRef]
- Van-Nam, H.; Tam, T.T.; Tho, V.D.S. Kinetic Modeling of thermal decomposition of sugarcane bagasse in the inert gas environment. Vietnam. J. Chem. 2019, 57, 574–580. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Guimarães, H.R.; Tannous, K. Influence of torrefaction on the pyrolysis of energy cane. J. Therm. Anal. Calorim. 2020, 139, 2221–2233. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Tannous, K.; de Lima, E.C.T. Pyrolysis of the hybrid energy cane: Thermal decomposition and kinetic modeling using non-isotermal thermogravimetric analysis. J. Therm. Anal. Calorim. 2022, 147, 7431–7448. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Investigation on prospective bioenergy from pyrolysis of butia seed waste using TGA-FTIR: Assessment of kinetic triplet, thermodynamic parameters and evolved volatiles. Renew. Energ. 2022, 191, 238–250. [Google Scholar] [CrossRef]
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- TAPPI T 204 cm-97; Solvent Extractives of Wood and Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 1997.
- TAPPI T222 om-02; Acid-Insoluble Lignin in Wood and Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 2002.
- TAPPI T 203 cm-99; Alpha-, Beta-and Gamma-Cellulose in Pulp. TAPPI Test Methods. Technical Association of the Pulp and Paper Industry TAPPI: Peachtree Corners, GA, USA, 2009.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Arias, S.; González, J.F.; Sousa, L.V.; Barbosa, C.B.M.; Silva, A.O.S.; Fréty, R.; Pacheco, J.G.A. Influence of Ni/Al ratio on the fast pyrolysis of myristic acid when adsorbed on unsupported mixed oxides derived from layered double hydroxides. Catal. Today 2021, 381, 181–191. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; de Albuquerque, J.G.; Galdino, W.V.d.A.; de Sena, R.F.; Andersen, S.L.F. Single-step and multi-step thermokinetic study—Deconvolution method as a simple pathway for describe properly the biomass pyrolysis for energy conversion. Energy Convers Manag. 2020, 209, 112653. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Potential of macauba endocarp (Acrocomia aculeate) for bioenergy production: Multi-component kinetic study and estimation of thermodynamic parameters of activation. Thermochim. Acta 2022, 708, 179134. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Liu, C.-G.; Nawaz, M.; Tawab, A.; Shen, X.; Shen, B.; Mehmood, M.A. Elucidating the pyrolysis reaction mechanism of Calotropis procera and analysis of pyrolysis products to evaluate its potential for bioenergy and chemicals. Bioresour. Technol. 2021, 322, 124545. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, G.K.; Mondal, M.K. Thermal degradation characteristics, kinetics, thermodynamic, and reaction mechanism analysis of pistachio shell pyrolysis for its bioenergy potential. Biomass Convers. Biorefinery 2020, 12, 4847–4861. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Lup, A.N.K.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on reaction mechanisms of metal-catalyzed deoxygenation process in bio-oil model compounds. Appl. Catal. A-Gen. 2017, 541, 87–106. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Moine, E.C.; Groune, K.; El Hamidi, A.; Khachani, M.; Halim, M.; Arsalane, S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy 2016, 115, 931–941. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic studies on the pyrolysis of pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Yao, Z.; Cai, D.; Chen, X.; Sun, Y.; Jin, M.; Qi, W.; Ding, J. Thermal behavior and kinetic study on the co-pyrolysis of biomass with polymer waste. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Sun, C.; Yang, Z.; Zheng, Z.; Li, W.; Tan, H.; Huang, Y.; Zhang, Y. Exploring how lignin promoting the co-pyrolysis with polylactic acid: Artificial neural network modeling, kinetic analysis and product distribution. Sustain. Mater. Technol. 2023, 35, e00549. [Google Scholar] [CrossRef]
- Jin, W.; Singh, K.; Zondlo, J. Pyrolysis kinetics of physical components of wood and wood-polymers using isoconversion method. Agriculture 2013, 3, 12–32. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martínez, J.D. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour. Technol. 2019, 288, 121485. [Google Scholar] [CrossRef]
- da Silva, J.C.G.; Andersen, S.L.F.; Costa, R.L.; Moreira, R.d.F.P.M.; José, H.J. Bioenergetic potential of Ponkan peel waste (Citrus reticulata) pyrolysis by kinetic modelling and product characterization. Biomass Bioenergy 2019, 131, 105401. [Google Scholar] [CrossRef]
- Sangaré, D.; Bostyn, S.; Moscosa-Santillan, M.; García-Alamilla, P.; Belandria, V.; Gökalp, I. Comparative pyrolysis studies of lignocellulosic biomasses: Online gas quantification, kinetics triplets, and thermodynamic parameters of the process. Bioresour. Technol. 2021, 346, 126598. [Google Scholar] [CrossRef] [PubMed]
- Alhumade, H.; da Silva, J.C.G.; Ahmad, M.S.; Çakman, G.; Yıldız, A.; Ceylan, S.; Elkamel, A. Investigation of pyrolysis kinetics and thermal behavior of Invasive Reed Canary (Phalaris arundinacea) for bioenergy potential. J. Anal. Appl. Pyrolysis 2019, 140, 385–392. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ahmad, M.S.; Liu, Q.; Liu, C.-G.; Tahir, M.H.; Aloqbi, A.A.; Tarbiah, N.I.; Alsufiani, H.M.; Gull, M. Helianthus tuberosus as a promising feedstock for bioenergy and chemicals appraised through pyrolysis, kinetics, and TG-FTIR-MS based study. Energy Convers. Manag. 2019, 194, 37–45. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Pinzi, S.; Buratti, C.; Bartocci, P.; Marseglia, G.; Fantozzi, F.; Barbanera, M. A simplified method for kinetic modeling of coffee silver skin pyrolysis by coupling pseudo-components peaks deconvolution analysis and model free-isoconversional methods. Fuel 2020, 278, 118260. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, O.P.; Diwan, P.K. Non-isothermal kinetics of pseudo-components of waste biomass. Fuel 2019, 253, 1149–1161. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Romero Millán, L.M.; Sierra Vargas, F.E.; Nzihou, A. Kinetic Analysis of Tropical Lignocellulosic Agrowaste Pyrolysis. BioEnergy Res. 2017, 10, 832–845. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Hu, W.; Chen, Z.; Liu, S.; Hu, Z.; Xiao, B. Thermogravimetric kinetic study of agricultural residue biomass pyrolysis based on combined kinetics. Bioresour. Technol. 2016, 219, 510–520. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chai, M.; Li, C.; Yellezuome, D.; Liu, R. Pyrolysis kinetics and thermodynamic parameters of bamboo residues and its three main components using thermogravimetric analysis. Biomass Bioenergy 2023, 170, 106705. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Kinetics and thermodynamic analysis of maize cob pyrolysis for its bioenergy potential using thermogravimetric analyzer. J. Therm. Anal. Calorim. 2019, 137, 1431–1441. [Google Scholar] [CrossRef]
- Tahir, M.H.; Çakman, G.; Goldfarb, J.L.; Topcu, Y.; Naqvi, S.R.; Ceylan, S. Demonstrating the suitability of canola residue biomass to biofuel conversion via pyrolysis through reaction kinetics, thermodynamics and evolved gas analyses. Bioresour. Technol. 2019, 279, 67–73. [Google Scholar] [CrossRef]
- Boonchom, B. Kinetics and Thermodynamic Properties of the Thermal Decomposition of Manganese Dihydrogenphosphate Dihydrate. J. Chem. Eng. Data 2008, 53, 1533–1538. [Google Scholar] [CrossRef]








| Analyses | Energy Cane Bagasse | ||
|---|---|---|---|
| This Work | [25] | [40] | |
| Proximate | |||
| Moisture (wt.%) | 4.8 | - | 5.7 |
| Volatile Matter (wt.%) | 74.0 | 83.4 | 78.4 |
| Fixed Carbon (wt.%) | 12.7 | 14.0 | 20.1 |
| Ash (wt.%) | 8.4 | 2.6 | 1.4 |
| Ultimate | |||
| C (wt.%) | 48.9 | 42.3 | 42.5 |
| H (wt.%) | 6.2 | 6.0 | 6.4 |
| O (wt.%) | 44.2 | 48.4 | 49.3 |
| N (wt.%) | 0.5 | 0.2 | 0.4 |
| S (wt.%) | 0.2 | - | - |
| Biochemical | |||
| Extratives (wt.%) | 11.2 | 23.2 | - |
| Holocellulose (wt.%) | 70.4 | 70.8 | 89.0 * |
| Lignin (wt.%) | 18.4 | 10.8 | 9.4 * |
| Heating Value | |||
| LHV (MJ·kg−1) | 18.5 | 15.5 | 15.4 |
| DSC (MJ·kg−1) | 12.5 | - | - |
| Peak | Retention Time (min) | Compound | Area (%) | |||
|---|---|---|---|---|---|---|
| 500 °C | 550 °C | 600 °C | 650 °C | |||
| 1 | 4.47 | Carbon dioxide | 27.53 | 46.42 | 41.55 | 53.32 |
| 2 | 4.67 | Acetaldehyde | 1.22 | 1.19 | 1.94 | - |
| 3 | 4.75 | Glyoxal | 2.74 | 1.66 | 2.48 | 6.68 |
| 4 | 5.23 | Acetone | 7.17 | 6.92 | 7.91 | 8.08 |
| 5 | 5.66 | 1,3-cyclopentadiene | 0.49 | 0.66 | 0.73 | 0.37 |
| 6 | 5.93 | 2,3-butanedione | 0.59 | - | - | - |
| 7 | 6.18 | Methyl vinyl ketone | 3.55 | 4.06 | 4.18 | 5.32 |
| 8 | 6.39 | Acetic acid | 6.58 | 2.96 | 2.22 | 0.50 |
| 9 | 7.38 | 2,3-dihydro-furan | 0.42 | 0.56 | 0.51 | 0.34 |
| 10 | 7.60 | Benzene | 0.69 | 1.18 | 1.29 | 0.52 |
| 11 | 8.13 | 3-methyl-1-pentanol | - | 0.63 | 0.68 | 0.59 |
| 12 | 10.33 | Toluene | 2.78 | 0.98 | 1.21 | 0.82 |
| 13 | 10.75 | Succinaldehyde | 1.23 | 1.10 | 0.64 | - |
| 14 | 11.03 | 2-oxo-,methyl ester propanoic acid | 1.33 | 0.36 | 0.68 | 0.45 |
| 15 | 12.71 | Furfural | 1.74 | 1.27 | 1.10 | 0.71 |
| 16 | 14.56 | Butyl-cyclopentane | - | 0.31 | 0.36 | 0.11 |
| 17 | 14.88 | Styrene | - | 0.31 | 0.26 | 0.23 |
| 18 | 15.68 | 2(5H)-furanone | 0.83 | 1.05 | 0.32 | 0.14 |
| 19 | 16.00 | 1,2-cyclopentanedione | 1.15 | 0.39 | 0.23 | - |
| 20 | 17.47 | 5-methyl-2-furancarboxaldehyde | - | 0.49 | 0.45 | 0.24 |
| 21 | 17.92 | Phenol | 1.25 | 1.74 | 1.66 | 1.01 |
| 22 | 18.24 | 1-decene | - | - | 0.17 | 0.20 |
| 23 | 18.76 | 3,4-dihydroxy-3-cyclobutene | 1.30 | - | - | - |
| 24 | 19.78 | 2-hydroxy-3-methyl-2-cyclopenten-1-one | 0.40 | - | - | - |
| 25 | 21.32 | 2-methyl-phenol | 0.44 | 0.49 | 0.49 | 0.45 |
| 26 | 21.77 | 1-undecene | - | - | 0.19 | 0.17 |
| 27 | 22.05 | 2-methyoxy-phenol | 0.59 | 0.42 | 0.41 | - |
| 28 | 25.34 | Heptanal | 1.11 | 0.72 | 0.40 | 0.14 |
| 29 | 25.52 | Creosol | 0.45 | 0.48 | - | - |
| 30 | 26.12 | 2,3-dihydro-benzofuran | 6.63 | 5.11 | 5.04 | 3.70 |
| 31 | 27.41 | Sucrose | 1.17 | 0.26 | 0.22 | - |
| 32 | 28.25 | 1-tridecene | - | - | 0.23 | 0.14 |
| 33 | 29.34 | 2-methoxy-4-vinylphenol | 2.98 | 1.53 | 1.24 | 0.28 |
| 34 | 30.39 | 2,6-dimethoxy-phenol | 0.56 | 0.53 | - | - |
| 35 | 31.19 | 1-tetradecene | - | - | 0.22 | 0.16 |
| 36 | 34.09 | D-allose | 2.78 | - | - | - |
| 37 | 36.12 | 4-ethenyl-2,6-dimethoxy-phenol | 0.55 | 0.47 | - | - |
| 38 | 39.06 | 2,6-dimethoxy-4-(2-propenyl)-phenol | 0.45 | 0.33 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liborio, D.O.; Gonzalez, J.F.; Arias, S.; Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Silva, J.M.F.; Barbosa, C.M.B.M.; Carvalho, F.R.; Soares, R.R.; et al. Pyrolysis of Energy Cane Bagasse: Investigating Kinetics, Thermodynamics, and Effect of Temperature on Volatile Products. Energies 2023, 16, 5669. https://doi.org/10.3390/en16155669
Liborio DO, Gonzalez JF, Arias S, Mumbach GD, Alves JLF, da Silva JCG, Silva JMF, Barbosa CMBM, Carvalho FR, Soares RR, et al. Pyrolysis of Energy Cane Bagasse: Investigating Kinetics, Thermodynamics, and Effect of Temperature on Volatile Products. Energies. 2023; 16(15):5669. https://doi.org/10.3390/en16155669
Chicago/Turabian StyleLiborio, Denisson O., Juan F. Gonzalez, Santiago Arias, Guilherme D. Mumbach, Jose Luiz F. Alves, Jean C. G. da Silva, Jose Marcos F. Silva, Celmy M. B. M. Barbosa, Florival R. Carvalho, Ricardo R. Soares, and et al. 2023. "Pyrolysis of Energy Cane Bagasse: Investigating Kinetics, Thermodynamics, and Effect of Temperature on Volatile Products" Energies 16, no. 15: 5669. https://doi.org/10.3390/en16155669
APA StyleLiborio, D. O., Gonzalez, J. F., Arias, S., Mumbach, G. D., Alves, J. L. F., da Silva, J. C. G., Silva, J. M. F., Barbosa, C. M. B. M., Carvalho, F. R., Soares, R. R., Simões, D. A., & Pacheco, J. G. A. (2023). Pyrolysis of Energy Cane Bagasse: Investigating Kinetics, Thermodynamics, and Effect of Temperature on Volatile Products. Energies, 16(15), 5669. https://doi.org/10.3390/en16155669








