Polymeric Membranes for H2S and CO2 Removal from Natural Gas for Hydrogen Production: A Review
Abstract
:1. Introduction
2. Solution–Diffusion Theory
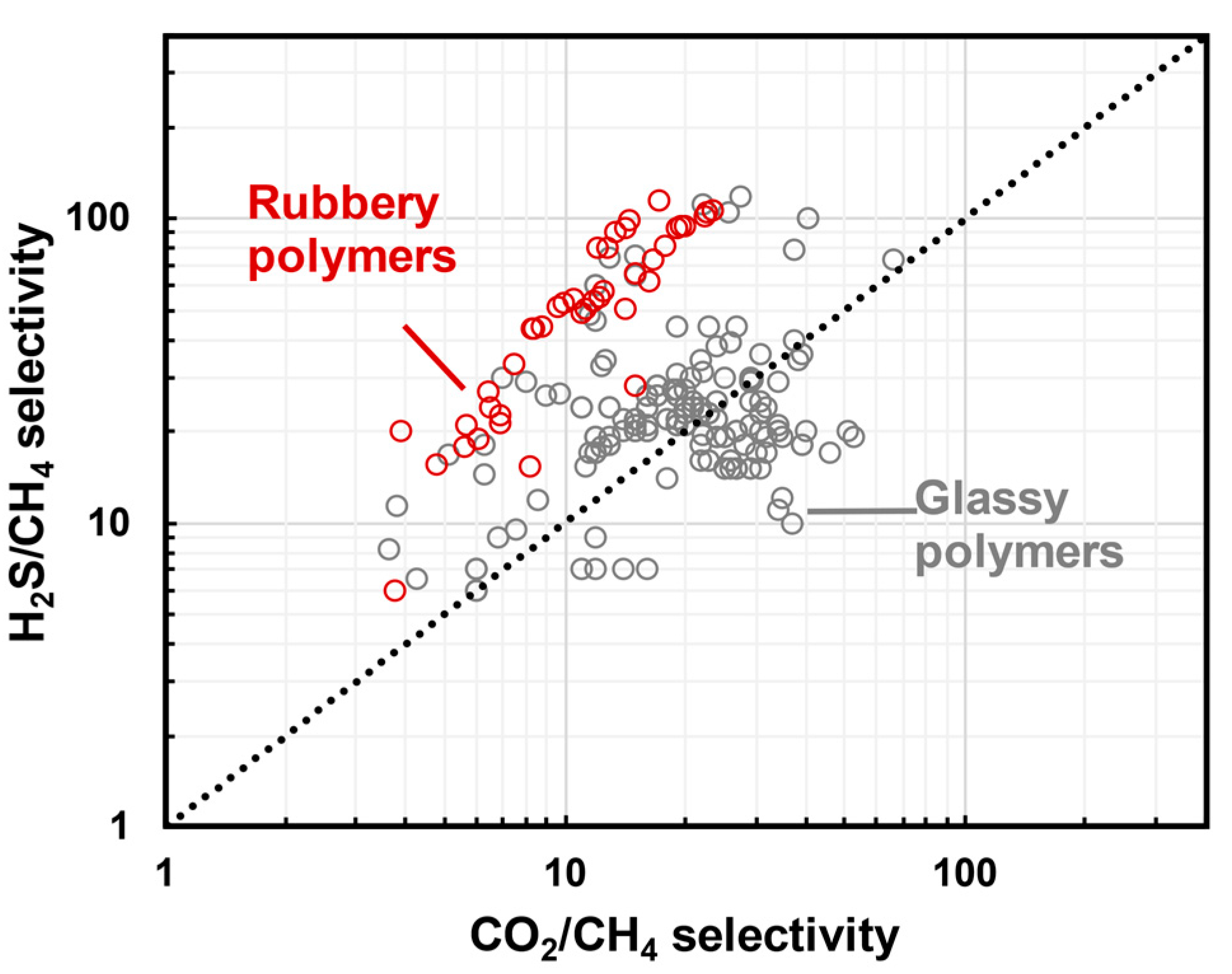
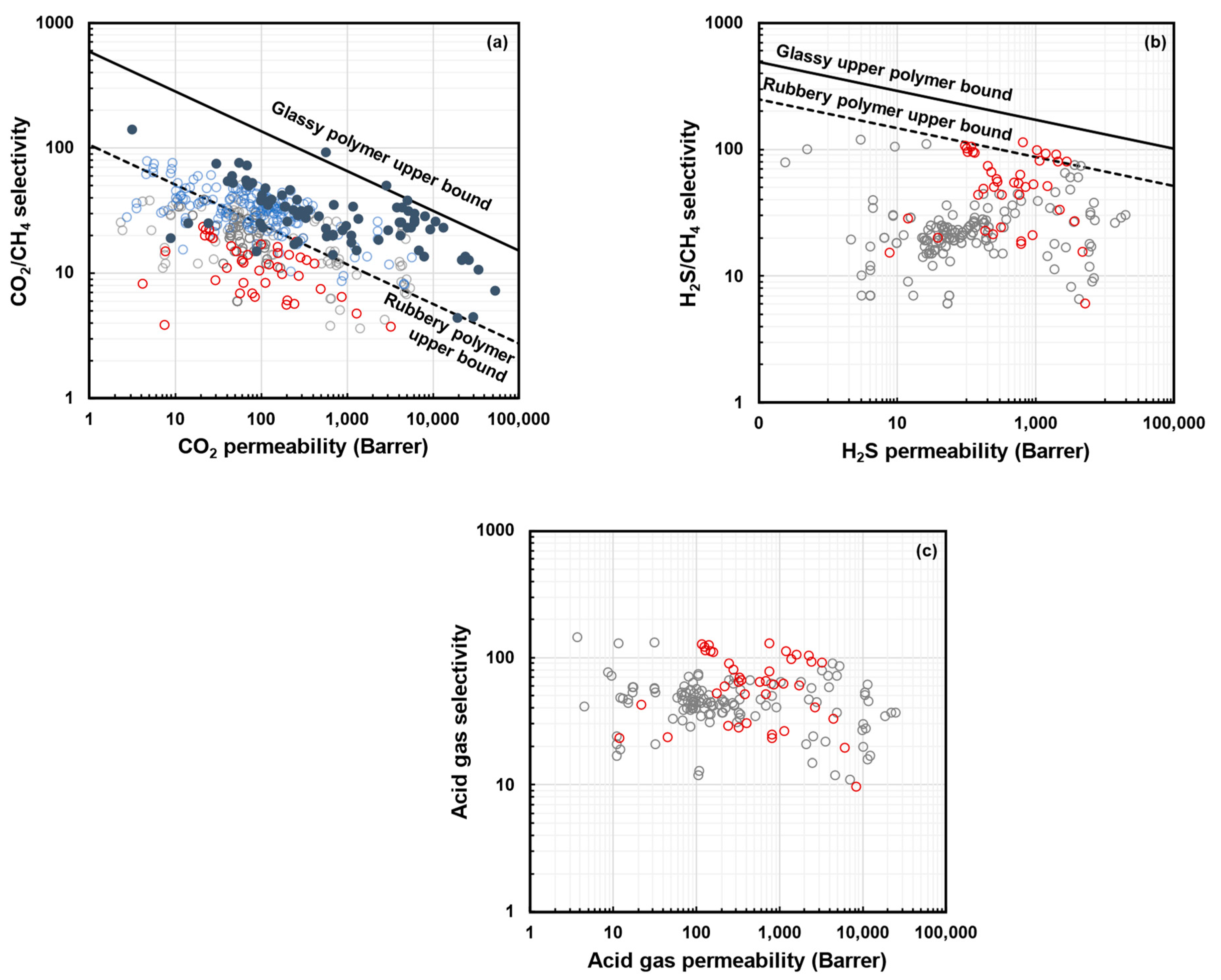
3. Upper Bounds for H2S/CH4 and CO2/CH4 Gas Pairs
4. Mixed-Gas CO2/CH4 Upper Bounds and Free Volume Model
- (1)
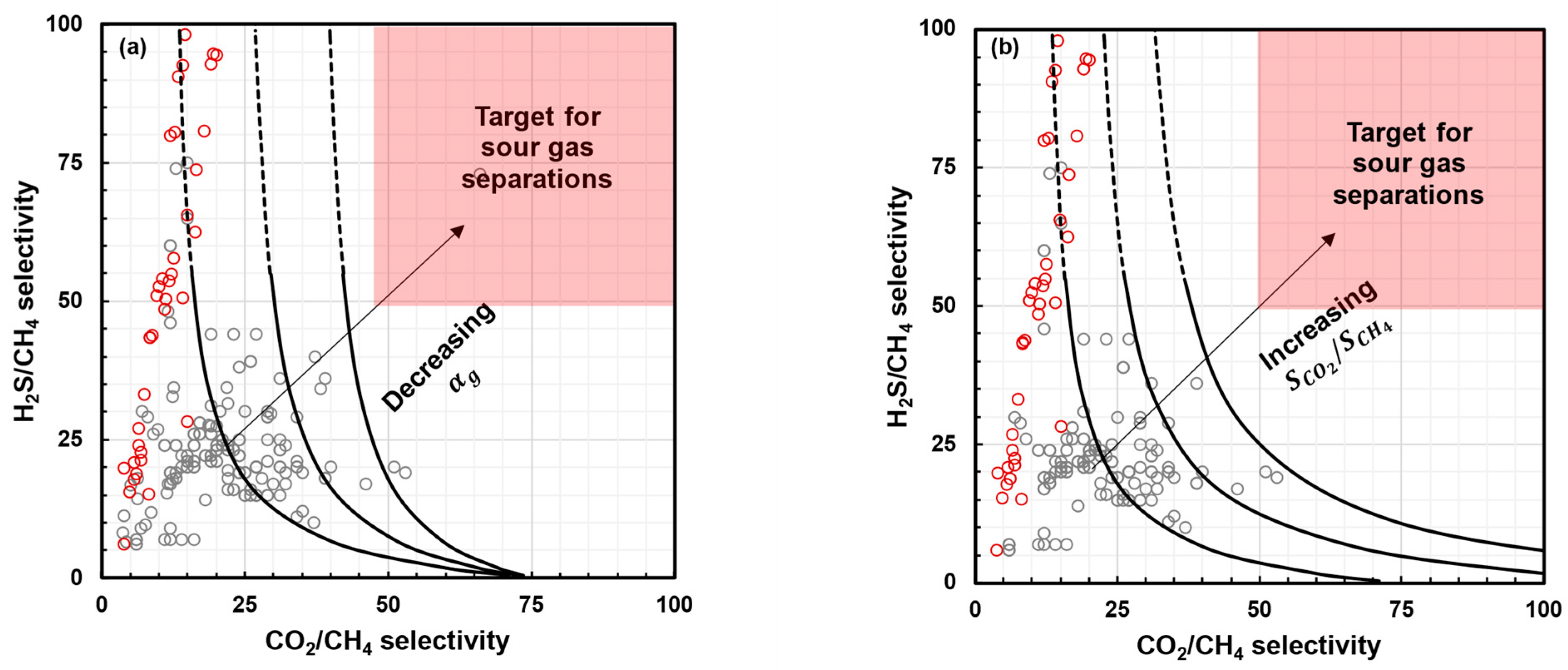
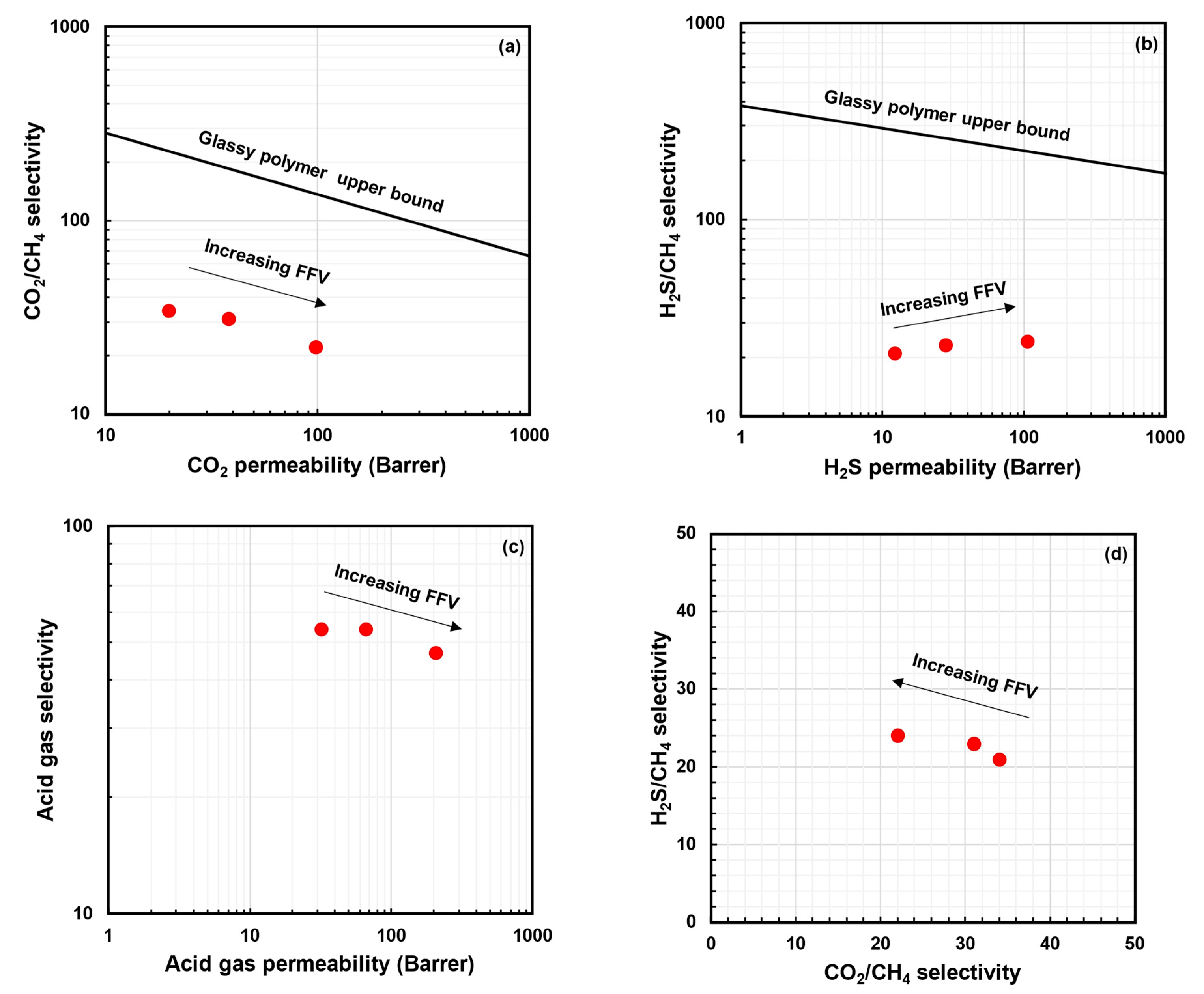
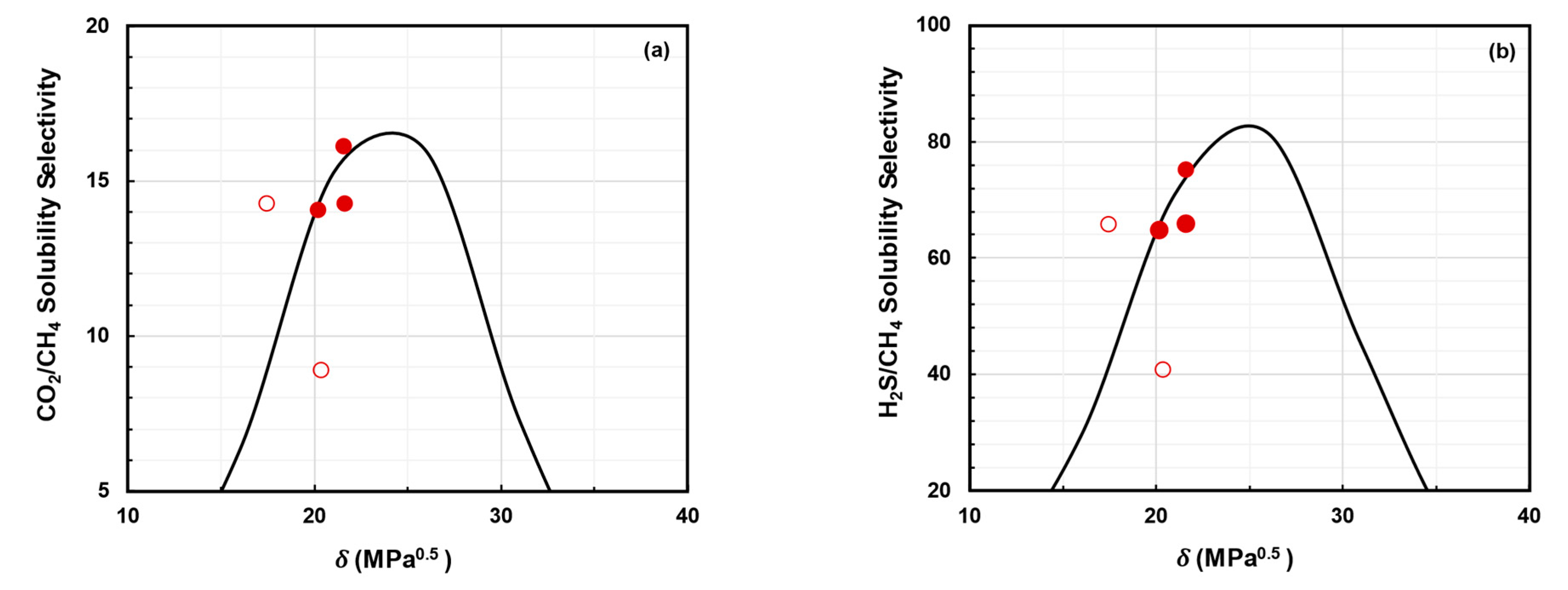
- Decreasing : As shown in Figure 4a, increasing the chain rigidity and improving plasticization resistance can allow for improvements in both H2S/CH4 selectivity and CO2/CH4 selectivity. This can be conducted through methods such as crosslinking, annealing, etc. [52,61,69]. Both these approaches generally result in decreased acid gas permeability.
- (2)
- Increasing : As shown in Figure 4b, increasing the solubility selectivity for CO2 over CH4 can mitigate the selectivity loss due to plasticization and improve both H2S/CH4 selectivity and CO2/CH4 selectivity. The most straightforward route to this is by incorporating functional groups that provide favorable sorption interactions for the acid gases [64,70,71]. It should be noted that, for such polymers, uncontrolled swelling and plasticization may occur [72]. While some degree of plasticization is acceptable and can even be beneficial, severe plasticization can cause deterioration in mechanical strength and should be avoided [69].
5. Polymeric Membranes for Natural Gas Purification
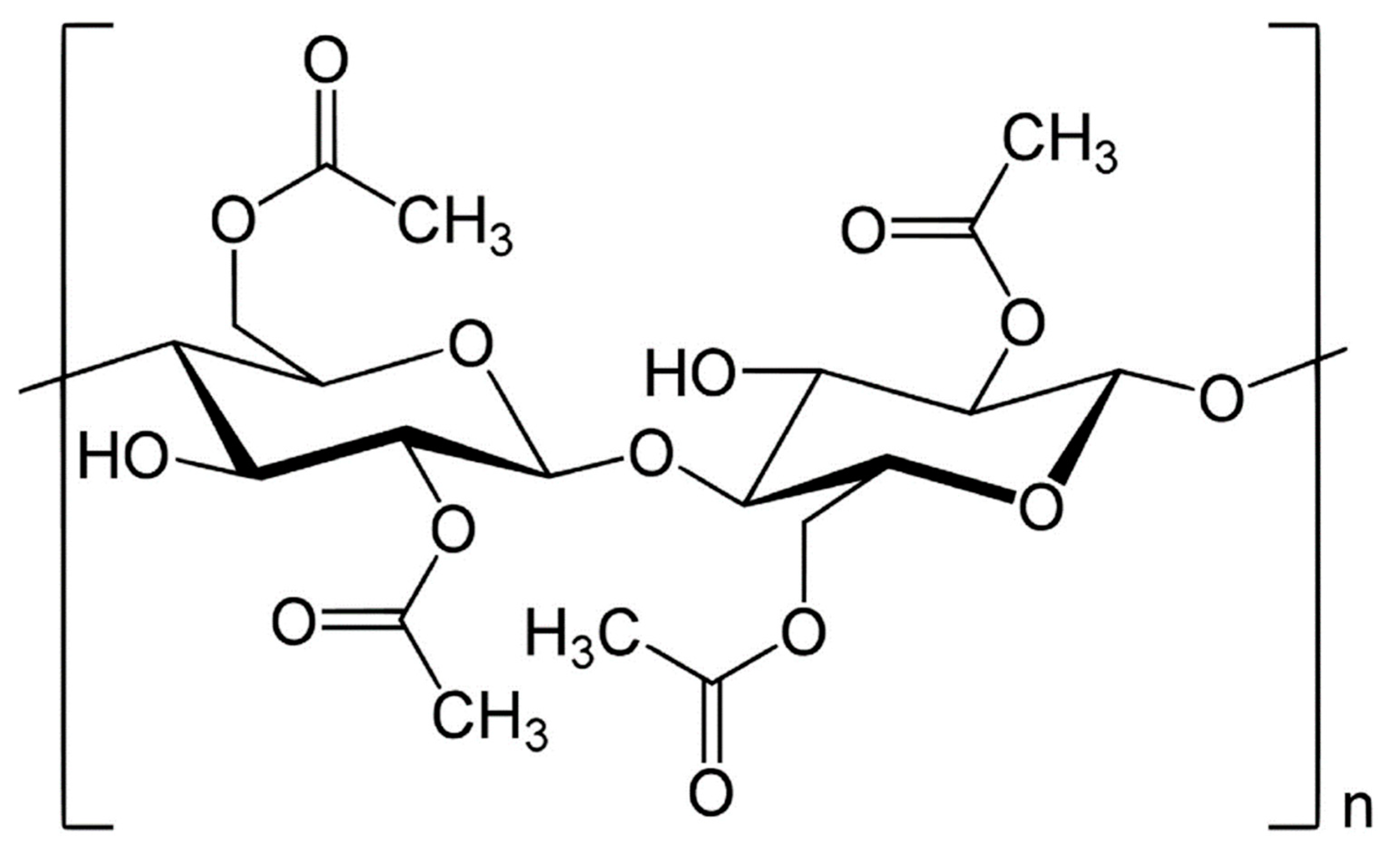
5.1. Cellulose Acetate (CA)
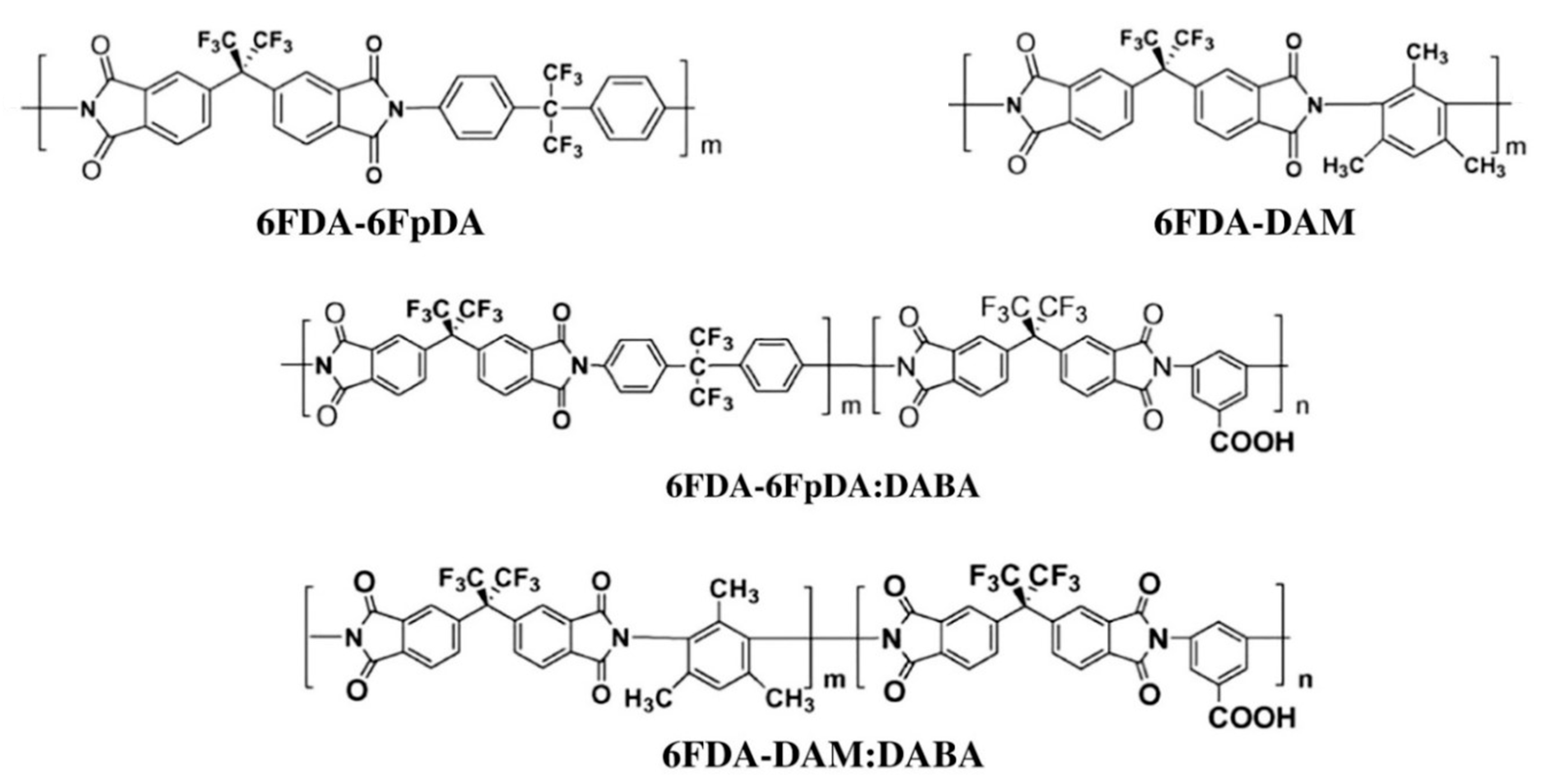
5.2. Polyimides
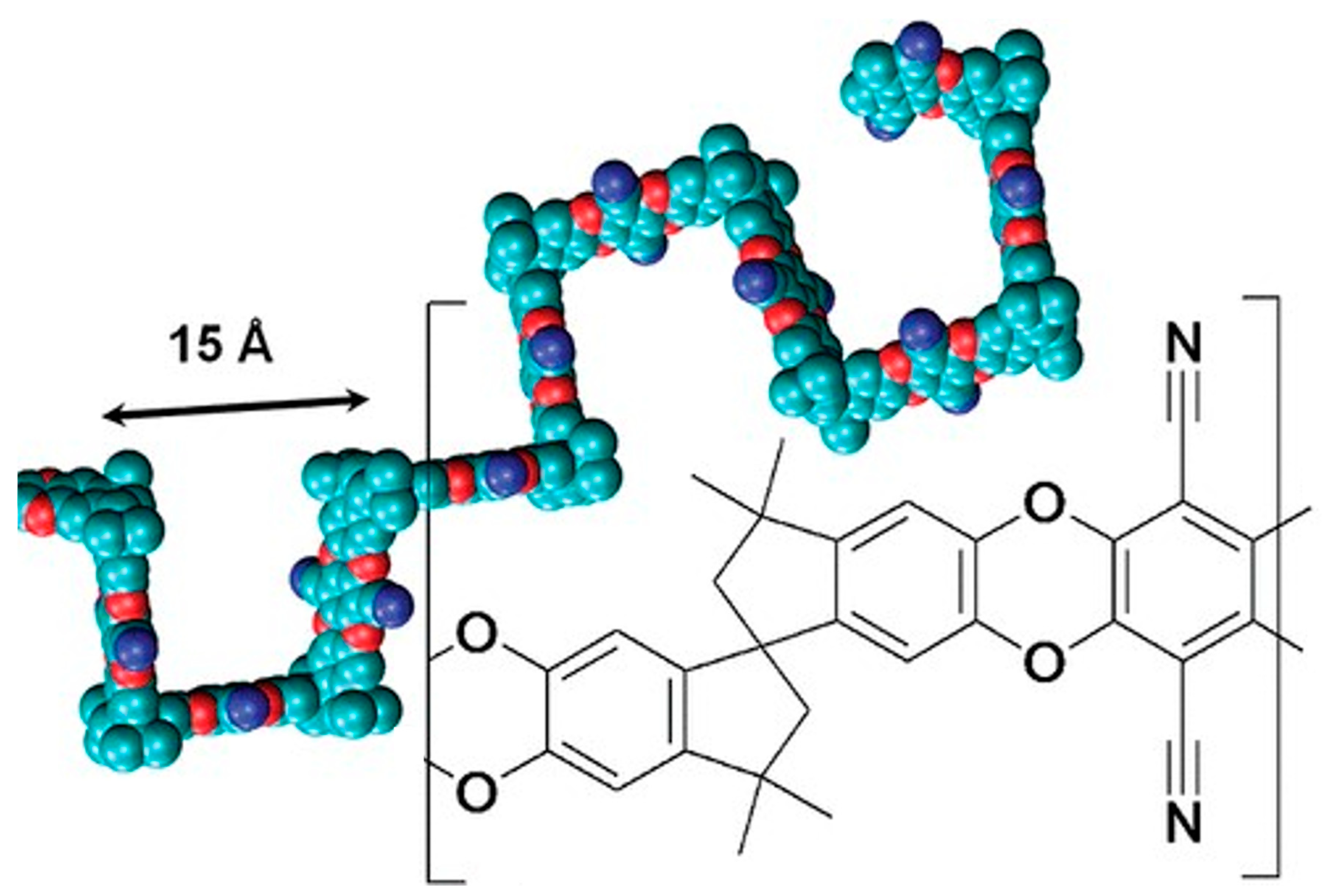
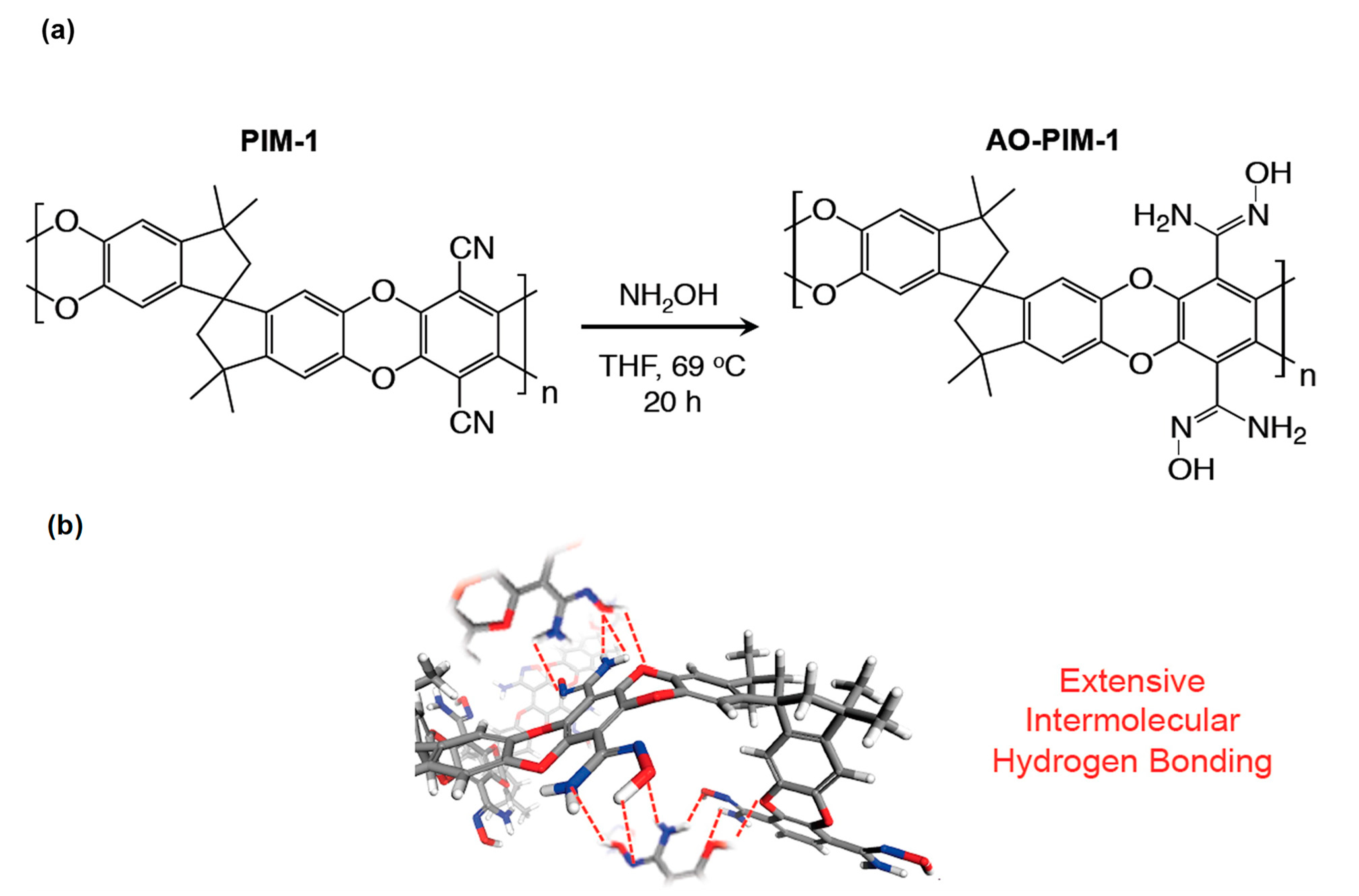
5.3. PIMs and Other Microporous Polymers
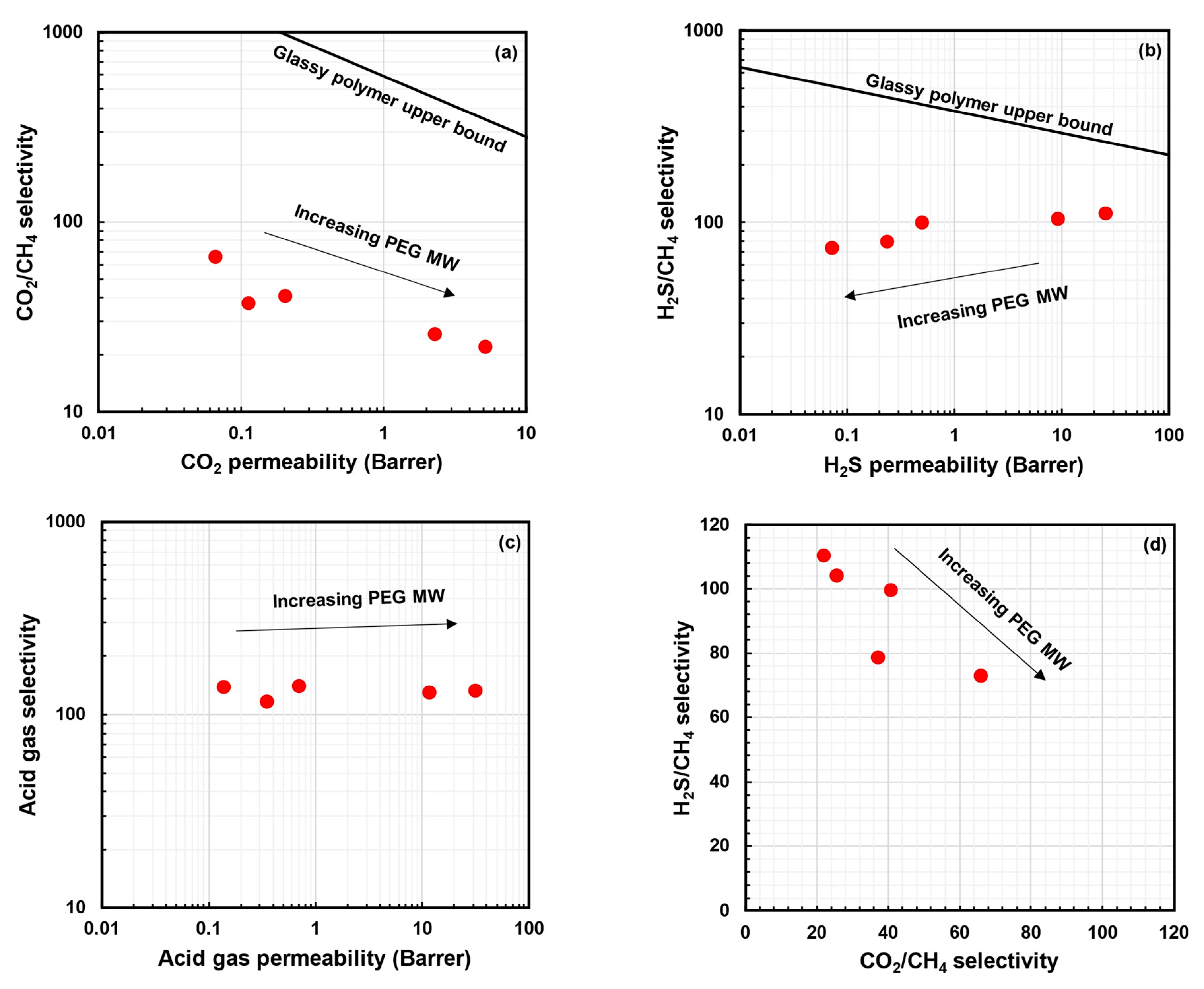
5.4. PEO-Based Polymers
5.5. H2 Production from H2S and Prospects for Polymeric Membranes
6. Concluding Remarks
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the green hydrogen market—Current state and outlook on green hydrogen demand and electrolyzer manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- Hunt, J.D.; Nascimento, A.; Nascimento, N.; Vieira, L.W.; Romero, O.J. Possible pathways for oil and gas companies in a sustainable future: From the perspective of a hydrogen economy. Renew. Sustain. Energy Rev. 2022, 160, 112291. [Google Scholar] [CrossRef]
- Espegren, K.; Damman, S.; Pisciella, P.; Graabak, I.; Tomasgard, A. The role of hydrogen in the transition from a petroleum economy to a low-carbon society. Int. J. Hydrogen Energy 2021, 46, 23125–23138. [Google Scholar] [CrossRef]
- Aguilera, R.F. The role of natural gas in a low carbon Asia Pacific. Appl. Energy 2014, 113, 1795–1800. [Google Scholar] [CrossRef] [Green Version]
- Amo, K.; Baker, R.W.; Helm, V.D.; Hofmann, T.; Lokhandwala, K.A.; Pinnau, I.; Ringer, M.B.; Su, T.T.; Toy, L.; Wijmans, J.G. Low-Quality Natural Gas Sulfur Removal/Recovery; Technical Report DE-AC21-92MC28133-01; Federal Energy Technology Center Pittsburgh: Pittsburgh, PA, USA, 1998; OSTI Identifier 775213. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Fayazi, A.; Arabloo, M.; Mohammadi, A.H. Efficient estimation of natural gas compressibility factor using a rigorous method. J. Nat. Gas Sci. Eng. 2014, 16, 8–17. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal–organic framework adsorbents and membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef]
- Sadooghi, P.; Rauch, R. Experimental and modeling study of hydrogen production from catalytic steam reforming of methane mixture with hydrogen sulfide. Int. J. Hydrogen Energy 2015, 40, 10418–10426. [Google Scholar] [CrossRef]
- Wachter, P.; Gaber, C.; Raic, J.; Demuth, M.; Hochenauer, C. Experimental investigation on H2S and SO2 sulphur poisoning and regeneration of a commercially available Ni-catalyst during methane tri-reforming. Int. J. Hydrogen Energy 2021, 46, 3437–3452. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Kurdymov, S.S.; Konstantinov, G.I.; Murzin, V.Y.; Bukhtenko, O.V.; Maksimov, Y.V. Core-shell bifunctional catalyst for steam methane reforming resistant to H2S: Activity and structure evolution. Int. J. Hydrogen Energy 2015, 40, 2963–2970. [Google Scholar] [CrossRef]
- Raju, A.S.K.; Park, C.S.; Norbeck, J.M. Synthesis gas production using steam hydrogasification and steam reforming. Fuel Process. Technol. 2009, 90, 330–336. [Google Scholar] [CrossRef]
- Huang, C.; T-Raissi, A. Thermodynamic analyses of hydrogen production from sub-quality natural gas. J. Power Sources 2007, 163, 645–652. [Google Scholar] [CrossRef]
- Burgers, W.F.J.; Northrop, P.S.; Kheshgi, H.S.; Valencia, J.A. Worldwide development potential for sour gas. Energy Procedia 2011, 4, 2178–2184. [Google Scholar] [CrossRef] [Green Version]
- Henni, A. Extracting profits from Abu Dhabi’s sour gas. J. Pet. Technol. 2013, 65, 76–79. [Google Scholar] [CrossRef]
- Boschee, P. Taking on the technical challenges of sour gas processing. Oil Gas Facil. 2014, 3, 21–25. [Google Scholar] [CrossRef]
- Hafezi, R.; Akhavan, A.; Pakseresht, S.; Wood, D.A. Global natural gas demand to 2025: A learning scenario development model. Energy 2021, 224, 120167. [Google Scholar] [CrossRef]
- Hafeznia, H.; Pourfayaz, F.; Maleki, A. An assessment of Iran’s natural gas potential for transition toward low-carbon economy. Renew. Sustain. Energy Rev. 2017, 79, 71–81. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Duan, Y.-Y.; Zhang, Y.-K.; Cui, Y.; Huang, Y.; Gao, D.; Shi, L.-J.; Wang, J.-C.; Yi, Q. Hydrogen energy deployment in decarbonizing transportation sector using multi-supply-demand integrated scenario analysis with nonlinear programming—A Shanxi case study. Int. J. Hydrogen Energy 2022, 47, 19338–19352. [Google Scholar] [CrossRef]
- Raza, M.A.; Khatri, K.L.; Israr, A.; Haque, M.I.U.; Ahmed, M.; Rafique, K.; Saand, A.S. Energy demand and production forecasting in Pakistan. Energy Strategy Rev. 2022, 39, 100788. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, H.; Selyanchyn, R.; Wang, B.; Deng, L.; Dai, Z.; Jiang, X. Hydrogen sulfide removal from natural gas using membrane technology: A review. J. Mater. Chem. A 2021, 9, 20211–20240. [Google Scholar] [CrossRef]
- Gong, D.; Huang, S.; Wu, W.; Yu, C.; Fang, C.; Liu, D. Characteristics of gas compositions in giant gas fields of China. Energy Explor. Exploit. 2014, 32, 635–656. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, R.; Lewis, K.; McCullough, J.G.; Hansen, D. Controlling Corrosion in Amine Treating Plants. In Proceedings of the Laurance Reid Gas Conditioning Conference, Norman, OK, USA, 26 February 1995. [Google Scholar]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Ghanem, B.S.; Alghunaimi, F.; Pinnau, I.; Han, Y. Polymers of intrinsic microporosity for energy-intensive membrane-based gas separations. Mater. Today Nano 2018, 3, 69–95. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Spatolisano, E.; De Angelis, A.R.; Pellegrini, L.A. Middle scale Hydrogen Sulfide conversion and valorization technologies: A review. ChemBioEng Rev. 2022, 9, 370–392. [Google Scholar] [CrossRef]
- Wagenfeld, J.-G.; Al-Ali, K.; Almheiri, S.; Slavens, A.F.; Calvet, N. Sustainable applications utilizing sulfur, a by-product from oil and gas industry: A state-of-the-art review. Waste Manag. 2019, 95, 78–89. [Google Scholar] [CrossRef]
- USGS. Mineral Commodities Summaries 2022; USGS: Reston, VA, USA, 2022. [Google Scholar]
- Jariwala, A. High H2S Gas Field Monetization: A Novel Approach. In Proceedings of the Offshore Technology Conference, OTC, Houston, TX, USA, 6–9 May 2019; p. D031S032R004. [Google Scholar] [CrossRef]
- Spatolisano, E.; De Guido, G.; Sangiorgio, V.A.; Pellegrini, L.A.; Mezzetta, A.; Guazzelli, L.; de Angelis, A.R.; Bonoldi, L. On the effect of the reaction medium on the HydroClaus Process: A novel sustainable H2S valorization strategy. ACS Sustain. Chem. Eng. 2023, 11, 6081–6089. [Google Scholar] [CrossRef]
- De Crisci, A.G.; Moniri, A.; Xu, Y. Hydrogen from hydrogen sulfide: Towards a more sustainable hydrogen economy. Int. J. Hydrogen Energy 2019, 44, 1299–1327. [Google Scholar] [CrossRef]
- Ozturk, M.; Midilli, A.; Dincer, I. Effective use of hydrogen sulfide and natural gas resources available in the Black Sea for hydrogen economy. Int. J. Hydrogen Energy 2021, 46, 10697–10707. [Google Scholar] [CrossRef]
- Ouali, S.; Chader, S.; Belhamel, M.; Benziada, M. The exploitation of hydrogen sulfide for hydrogen production in geothermal areas. Int. J. Hydrogen Energy 2011, 36, 4103–4109. [Google Scholar] [CrossRef]
- Joshi, A.S.; Jangam, K.V.; Mohammad, Z.; Fan, L.-S. Novel sulfur looping scheme to convert H2S to H2 using Ni3S2 supported over ZrO2: Thermodynamic, kinetic, and comparative system analyses. Fuel Process. Technol 2022, 237, 107443. [Google Scholar] [CrossRef]
- Jangam, K.V.; Joshi, A.S.; Chen, Y.-Y.; Mahalingam, S.; Sunny, A.A.; Fan, L.-S. Synergistic decomposition of H2S into H2 by Ni3S2 over ZrO2 support via a sulfur looping scheme with CO2 enabled carrier regeneration. Chem. Eng. J. 2021, 426, 131815. [Google Scholar] [CrossRef]
- Guldal, N.O.; Figen, H.E.; Baykara, S.Z. New catalysts for hydrogen production from H2S: Preliminary results. Int. J. Hydrogen Energy 2015, 40, 7452–7458. [Google Scholar] [CrossRef]
- Mostafaeipour, A.; Dehshiri, S.J.H.; Dehshiri, S.S.H. Ranking locations for producing hydrogen using geothermal energy in Afghanistan. Int. J. Hydrogen Energy 2020, 45, 15924–15940. [Google Scholar] [CrossRef]
- Reddy, S.; Nadgouda, S.G.; Tong, A.; Fan, L.-S. Metal sulfide-based process analysis for hydrogen generation from hydrogen sulfide conversion. Int. J. Hydrogen Energy 2019, 44, 21336–21350. [Google Scholar] [CrossRef]
- Seker, S.; Aydin, N. Hydrogen production facility location selection for Black Sea using entropy based TOPSIS under IVPF environment. Int. J. Hydrogen Energy 2020, 45, 15855–15868. [Google Scholar] [CrossRef]
- Fukuda, K.; Dokiya, M.; Kameyama, T.; Kotera, Y. Catalytic decomposition of hydrogen sulfide. Ind. Eng. Chem. Fundam. 1978, 17, 243–248. [Google Scholar] [CrossRef]
- León, N.E.; Liu, Z.; Irani, M.; Koros, W.J. How to get the best gas separation membranes from state-of-the-art glassy polymers. Macromolecules 2022, 55, 1457–1473. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Sirkar, K.K. (Eds.) Membrane Handbook; Chapman & Hall: New York, NY, USA; Kluwer Academic Publishers: Boston, MA, USA, 1992. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M.; Smith, Z.P.; Freeman, B.D.; Paul, D.R. Contributions of diffusion and solubility selectivity to the upper bound analysis for glassy gas separation membranes. J. Membr. Sci. 2014, 453, 71–83. [Google Scholar] [CrossRef]
- Robeson, L.M.; Liu, Q.; Freeman, B.D.; Paul, D.R. Comparison of transport properties of rubbery and glassy polymers and the relevance to the upper bound relationship. J. Membr. Sci. 2015, 476, 421–431. [Google Scholar] [CrossRef]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- Hayek, A.; Shalabi, Y.A.; Alsamah, A. Sour mixed-gas upper bounds of glassy polymeric membranes. Purif. Technol. 2021, 277, 119535. [Google Scholar] [CrossRef]
- Harrigan, D.J.; Yang, J.; Sundell, B.J.; Lawrence, J.A.; O’Brien, J.T.; Ostraat, M.L. Sour gas transport in poly(ether-b-amide) membranes for natural gas separations. J. Membr. Sci. 2020, 595, 117497. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Alaslai, N.; Maab, H.; Qasem, E.A.; Alhajry, R.H.; Alyami, N.M. Unprecedented sour mixed-gas permeation properties of fluorinated polyazole-based membranes. ACS Appl. Polym. Mater. 2020, 2, 2199–2210. [Google Scholar] [CrossRef]
- Harrigan, D.J.; Lawrence, J.A.; Reid, H.W.; Rivers, J.B.; O'Brien, J.T.; Sharber, S.A.; Sundell, B.J. Tunable sour gas separations: Simultaneous H2S and CO2 removal from natural gas via crosslinked telechelic poly(ethylene glycol) membranes. J. Membr. Sci. 2020, 602, 117947. [Google Scholar] [CrossRef]
- Chatterjee, G.; Houde, A.A.; Stern, S.A. Poly(ether urethane) and poly(ether urethane urea) membranes with high H2S/CH4 selectivity. J. Membr. Sci. 1997, 135, 99–106. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Kanehashi, S.; Nagai, K. Analysis of dual-mode model parameters for gas sorption in glassy polymers. J. Membr. Sci. 2005, 253, 117–138. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Starannikova, L.; Belov, N.; Bermeshev, M.; Gringolts, M.; Finkelshtein, E. Solubility controlled permeation of hydrocarbons: New membrane materials and results. J. Membr. Sci. 2014, 453, 532–545. [Google Scholar] [CrossRef]
- Merkel, T.C.; Toy, L.G. Comparison of hydrogen sulfide transport properties in fluorinated and nonfluorinated polymers. Macromolecules 2006, 39, 7591–7600. [Google Scholar] [CrossRef]
- Signorini, V.; Baschetti, M.G.; Pizzi, D.; Merlo, L. Hydrogen sulfide mix gas permeation in Aquivion® perfluorosulfonic acid (PFSA) ionomer membranes for natural gas sweetening. J. Membr. Sci. 2021, 640, 119809. [Google Scholar] [CrossRef]
- Achoundong, C.S.K.; Bhuwania, N.; Burgess, S.K.; Karvan, O.; Johnson, J.R.; Koros, W.J. Silane modification of cellulose acetate dense films as materials for acid gas removal. Macromolecules 2013, 46, 5584–5594. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.-C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]
- Kraftschik, B.; Koros, W.J.; Johnson, J.R.; Karvan, O. Dense film polyimide membranes for aggressive sour gas feed separations. J. Membr. Sci. 2013, 428, 608–619. [Google Scholar] [CrossRef]
- Lin, H.; Yavari, M. Upper bound of polymeric membranes for mixed-gas CO2/CH4separations. J. Membr. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Robeson, L.M.; Freeman, B.D.; Paul, D.R.; Rowe, B.W. An empirical correlation of gas permeability and permselectivity in polymers and its theoretical basis. J. Membr. Sci. 2009, 341, 178–185. [Google Scholar] [CrossRef]
- Yi, S.; Ghanem, B.; Liu, Y.; Pinnau, I.; Koros, W.J. Ultraselective glassy polymer membranes with unprecedented performance for energy-efficient sour gas separation. Sci. Adv. 2019, 5, eaaw5459. [Google Scholar] [CrossRef] [Green Version]
- Simha, R.; Boyer, R.F. On a general relation involving the glass temperature and coefficients of expansion of polymers. J. Chem. Phys. 1962, 37, 1003–1007. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Polymer free volume and its connection to the glass transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Qiu, W.; Koros, W.J. Molecularly engineered 6FDA-based polyimide membranes for sour natural gas separation. Angew. Chem. Int. Ed. 2020, 59, 14877–14883. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Tang, C.; Zhang, J.; Hu, D. Effects of hydrogen sulfide on the mechanical and thermal properties of cellulose insulation paper: A molecular dynamics simulation. Mater. Chem. Phys. 2020, 240, 122153. [Google Scholar] [CrossRef]
- Kraftschik, B.; Koros, W.J. Cross-Linkable polyimide membranes for improved plasticization resistance and permselectivity in sour gas separations. Macromolecules 2013, 46, 6908–6921. [Google Scholar] [CrossRef]
- Vaughn, J.; Koros, W.J. Effect of the amide bond diamine structure on the CO2, H2S, and CH4 transport properties of a series of novel 6FDA-based polyamide–imides for natural gas purification. Macromolecules 2012, 45, 7036–7049. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.S.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM-1. J. Membr. Sci. 2014, 457, 95–102. [Google Scholar] [CrossRef]
- Liu, Y.; Kraftschik, B.E.; Babu, V.P.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using TEGMC polyimide hollow fiber membranes. J. Membr. Sci. 2021, 632, 119361. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Heydari, I.; Zhang, Z. Hybrid systems: Combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J. CO2 Util. 2017, 18, 362–369. [Google Scholar] [CrossRef]
- Alcheikhhamdon, Y.; Hoorfar, M. Natural gas purification from acid gases using membranes: A review of the history, features, techno-commercial challenges, and process intensification of commercial membranes. Chem. Eng. Process. Process Intensif. 2017, 120, 105–113. [Google Scholar] [CrossRef]
- Torres, F.B.; Gutierrez, J.P.; Ruiz, L.A.; Bertuzzi, M.A.; Erdmann, E. Comparative analysis of absorption, membrane, and hybrid technologies for CO2 recovery. J. Nat. Gas Sci. Eng. 2021, 94, 104082. [Google Scholar] [CrossRef]
- Parekh, S. Hybrid System—An Emerging Solution to Sour Gas Treatment. In Proceedings of the International Petroleum Technology Conference, IPTC, Dhahran, Saudi Arabia, 13–15 January 2020; p. D022S143R001. [Google Scholar] [CrossRef]
- Alkatheri, M.; Grandas, R.; Betancourt-Torcat, A.; Almansoori, A. Ultra-Sour Natural Gas Sweetening Using Membranes/Amines Hybrid Systems for Recurrent Middle East Conditions. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, SPE, Abu Dhabi, United Arab Emirates, 31 October–3 November 2016; p. D011S003R002. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Kane, R.D. Selection of materials for sour service in petroleum production. J. Pet. Technol. 1986, 38, 1051–1061. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Musa, O.M.; Steed, J.W. Problems associated with sour gas in the oilfield industry and their solutions. Energy Fuels 2015, 29, 4667–4682. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Sliem, M.H.; Abdullah, A.M.; Youssef, K.M.; Al-Qahtani, N.H. Impact of Prolonged Exposure to Sour Service on the Mechanical Properties and Corrosion Mechanism of NACE Carbon Steel Material Used in Wet Sour Gas Multiphase Pipeline. Sustainability 2022, 14, 8015. [Google Scholar] [CrossRef]
- Agbroko, O.W.; Piler, K.; Benson, T.J. A comprehensive review of H2S scavenger technologies from oil and gas streams. ChemBioEng Rev. 2017, 4, 339–359. [Google Scholar] [CrossRef]
- Hao, J.; Rice, P.A.; Stern, S.A. Upgrading low-quality natural gas with H2S- and CO2-selective polymer membranes. J. Membr. Sci. 2002, 209, 177–206. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 74. [Google Scholar] [CrossRef]
- Stannett, V.T.; Koros, W.J.; Paul, D.R.; Lonsdale, H.K.; Baker, R.W. Recent Advances in Membrane Science and Technology. In Chemistry; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1979; Volume 32, pp. 69–121. [Google Scholar] [CrossRef]
- Schell, W.J.; Wensley, C.G.; Chen, M.S.K.; Venugopal, K.G.; Miller, B.D.; Stuart, J.A. Recent advances in cellulosic membranes for gas separation and pervaporation. Gas Sep. Purif. 1989, 3, 162–169. [Google Scholar] [CrossRef]
- Chen, G.Q.; Kanehashi, S.; Doherty, C.M.; Hill, A.J.; Kentish, S.E. Water vapor permeation through cellulose acetate membranes and its impact upon membrane separation performance for natural gas purification. J. Membr. Sci. 2015, 487, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Koros, W.J. Simplified analysis of gas/polymer selective solubility behavior. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1611–1628. [Google Scholar] [CrossRef]
- White, L.S.; Blinka, T.A.; Kloczewski, H.A.; Wang, I. Properties of a polyimide gas separation membrane in natural gas streams. J. Membr. Sci. 1995, 103, 73–82. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Donohue, M.D.; Minhas, B.S.; Lee, S.Y. Permeation behavior of carbon dioxide-methane mixtures in cellulose acetate membranes. J. Membr. Sci. 1989, 42, 197–214. [Google Scholar] [CrossRef]
- Ali, Z.; Pacheco, F.; Litwiller, E.; Wang, Y.; Han, Y.; Pinnau, I. Ultra-selective defect-free interfacially polymerized molecular sieve thin-film composite membranes for H2 purification. J. Mater. Chem. A 2018, 6, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Liu, L.; Kanehashi, S.; Scholes, C.A.; Kentish, S.E. The impact of toluene and xylene on the performance of cellulose triacetate membranes for natural gas sweetening. J. Membr. Sci. 2018, 555, 362–368. [Google Scholar] [CrossRef]
- Liu, L.; Doherty, C.M.; Ricci, E.; Chen, G.Q.; De Angelis, M.G.; Kentish, S.E. The influence of propane and n-butane on the structure and separation performance of cellulose acetate membranes. J. Membr. Sci. 2021, 638, 119677. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Omole, I.C.; Bhandari, D.A.; Miller, S.J.; Koros, W.J. Toluene impurity effects on CO2 separation using a hollow fiber membrane for natural gas. J. Membr. Sci. 2011, 369, 490–498. [Google Scholar] [CrossRef]
- Liu, Q.; Galizia, M.; Gleason, K.L.; Scholes, C.A.; Paul, D.R.; Freeman, B.D. Influence of toluene on CO2 and CH4 gas transport properties in thermally rearranged (TR) polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2016, 514, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Nemestóthy, N.; Bakonyi, P.; Lajtai-Szabó, P.; Bélafi-Bakó, K. The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane. Membranes 2020, 10, 324. [Google Scholar] [CrossRef]
- Hasan, R.; Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Effect of Hydrocarbons on the Separation of Carbon Dioxide from Methane through a Polyimide Gas Separation Membrane. Ind. Eng. Chem. Res. 2009, 48, 5415–5419. [Google Scholar] [CrossRef]
- Kawakami, M.; Iwanaga, H.; Hara, Y.; Iwamoto, M.; Kagawa, S. Gas permeabilities of cellulose nitrate/poly(ethylene glycol) blend membranes. J. Appl. Polym. Sci. 1982, 27, 2387–2393. [Google Scholar] [CrossRef]
- Li, J.; Nagai, K.; Nakagawa, T.; Wang, S. Preparation of polyethyleneglycol (PEG) and cellulose acetate (CA) blend membranes and their gas permeabilities. J. Appl. Polym. Sci. 1995, 58, 1455–1463. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Hsiao, M.-Y.; Nagai, K.; Lin, H. Suppressed crystallization and enhanced gas permeability in thin films of cellulose acetate blends. Polymer 2020, 205, 122790. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Nagai, K.; Nakagawa, T.; Mau, A.W.-H. Effect of polyethyleneglycol (PEG) on gas permeabilities and permselectivities in its cellulose acetate (CA) blend membranes. J. Membr. Sci. 1998, 138, 143–152. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- McCandless, F.P. Separation of binary mixtures of CO and H2 by permeation through polymeric films. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 470–478. [Google Scholar] [CrossRef]
- Stern, S.A.; Mi, Y.; Yamamoto, H.; Clair, A.K.S. Structure/permeability relationships of polyimide membranes. Applications to the separation of gas mixtures. J. Polym. Sci. Part. B Polym. Phys. 1989, 27, 1887–1909. [Google Scholar] [CrossRef]
- Kim, T.-H.; Koros, W.J.; Husk, G.R. Advanced gas separation membrane materials: Rigid aromatic polyimides. Sci. Technol. 1988, 23, 1611–1626. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Chern, R.T.; Koros, W.J.; Yui, B.; Hopfenberg, H.B.; Stannett, V.T. Selective permeation of CO2 and CH4 through Kapton polyimide: Effects of penetrant competition and gas-phase nonideality. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1061–1084. [Google Scholar] [CrossRef]
- Bos, A.; Pünt, I.G.M.; Wessling, M.; Strathmann, H. Plasticization-resistant glassy polyimide membranes for CO2/CO4 separations. Purif. Technol. 1998, 14, 27–39. [Google Scholar] [CrossRef]
- Kim, T.H.; Koros, W.J.; Husk, G.R.; O’Brien, K.C. Relationship between gas separation properties and chemical structure in a series of aromatic polyimides. J. Membr. Sci. 1988, 37, 45–62. [Google Scholar] [CrossRef]
- Coleman, M.R.; Koros, W.J. Isomeric polyimides based on fluorinated dianhydrides and diamines for gas separation applications. J. Membr. Sci. 1990, 50, 285–297. [Google Scholar] [CrossRef]
- Hirayama, Y.; Yoshinaga, T.; Kusuki, Y.; Ninomiya, K.; Sakakibara, T.; Tamari, T. Relation of gas permeability with structure of aromatic polyimides I. J. Membr. Sci. 1996, 111, 169–182. [Google Scholar] [CrossRef]
- Hirayama, Y.; Yoshinaga, T.; Kusuki, Y.; Ninomiya, K.; Sakakibara, T.; Tamari, T. Relation of gas permeability with structure of aromatic polyimides II. J. Membr. Sci. 1996, 111, 183–192. [Google Scholar] [CrossRef]
- Matsumoto, K.; Xu, P. Gas permeation properties of hexafluoro aromatic polyimides. J. Appl. Polym. Sci. 1993, 11, 1961–1972. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising plasticization benefits in natural gas upgrading using polyimide membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Kharul, U.K. Investigation of gas permeation properties of systematically modified polybenzimidazoles by N-substitution. J. Membr. Sci. 2010, 357, 134–142. [Google Scholar] [CrossRef]
- Kosuri, M.R.; Koros, W.J. Defect-free asymmetric hollow fiber membranes from Torlon®, a polyamide–imide polymer, for high-pressure CO2 separations. J. Membr. Sci. 2008, 320, 65–72. [Google Scholar] [CrossRef]
- Japip, S.; Liao, K.-S.; Chung, T.-S. Molecularly tuned free volume of vapor cross-linked 6FDA-Durene/ZIF-71 MMMs for H2/CO2 separation at 150 °C. Adv. Mater. 2017, 29, 1603833. [Google Scholar] [CrossRef]
- Yahaya, G.O.; Mokhtari, I.; Alghannam, A.A.; Choi, S.-H.; Maab, H.; Bahamdan, A.A. Cardo-type random co-polyimide membranes for high pressure pure and mixed sour gas feed separations. J. Membr. Sci. 2018, 550, 526–535. [Google Scholar] [CrossRef]
- Tanaka, K.; Okano, M.; Toshino, H.; Kita, H.; Okamoto, K.-I. Effect of methyl substituents on permeability and permselectivity of gases in polyimides prepared from methyl-substituted phenylenediamines. J. Polym. Sci. Part. B Polym. Phys. 1992, 30, 907–914. [Google Scholar] [CrossRef]
- Yahaya, G.O.; Qahtani, M.S.; Ammar, A.Y.; Bahamdan, A.A.; Ameen, A.W.; Alhajry, R.H.; Ben Sultan, M.M.; Hamad, F. Aromatic block co-polyimide membranes for sour gas feed separations. Chem. Eng. J. 2016, 304, 1020–1030. [Google Scholar] [CrossRef]
- Hayek, A.; Yahaya, G.O.; Alsamah, A.; Panda, S.K. Fluorinated copolyimide membranes for sour mixed-gas upgrading. J. Appl. Polym. Sci. 2020, 137, 48336. [Google Scholar] [CrossRef]
- Alghannam, A.A.; Yahaya, G.O.; Hayek, A.; Mokhtari, I.; Saleem, Q.; Sewdan, D.A.; Bahamdan, A.A. High pressure pure- and mixed sour gas transport properties of Cardo-type block co-polyimide membranes. J. Membr. Sci. 2018, 553, 32–42. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Yahaya, G.O.; Qasem, E.A.; Alhajry, R.H. Post-synthetic modification of CARDO-based materials: Application in sour natural gas separation. J. Mater. Chem. A 2020, 8, 23354–23367. [Google Scholar] [CrossRef]
- Lee, J.S.; Adams, R.T.; Madden, W.; Koros, W.J. Toluene and n-heptane sorption in Matrimid® asymmetric hollow fiber membranes. Polymer 2009, 50, 6049–6056. [Google Scholar] [CrossRef]
- Lee, J.S.; Madden, W.; Koros, W.J. Antiplasticization and plasticization of Matrimid® asymmetric hollow fiber membranes—Part A. Experimental. J. Membr. Sci. 2010, 350, 232–241. [Google Scholar] [CrossRef]
- Vaughn, J.T.; Koros, W.J.; Johnson, J.R.; Karvan, O. Effect of thermal annealing on a novel polyamide-imide polymer membrane for aggressive acid gas separations. J. Membr. Sci. 2012, 401–402, 163–174. [Google Scholar] [CrossRef]
- Babu, V.P.; Kraftschik, B.E.; Koros, W.J. Crosslinkable TEGMC asymmetric hollow fiber membranes for aggressive sour gas separations. J. Membr. Sci. 2018, 558, 94–105. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 2, 230. [Google Scholar] [CrossRef]
- Budd, P.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- McDermott, A.G.; Larsen, G.S.; Budd, P.M.; Colina, C.M.; Runt, J. Structural characterization of a polymer of intrinsic microporosity: X-ray scattering with interpretation enhanced by molecular dynamics simulations. Macromolecules 2011, 44, 14–16. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Litwiller, E.; Pinnau, I. Physical Aging, Plasticization and Their Effects on Gas Permeation in ‘Rigid’ Polymers of Intrinsic Microporosity. Macromolecules 2015, 48, 6553–6561. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Swaidan, R.; Belmabkhout, Y.; Zhu, Y.; Litwiller, E.; Jouiad, M.; Pinnau, I.; Han, Y. Synthesis and gas transport properties of hydroxyl-functionalized polyimides with intrinsic microporosity. Macromolecules 2012, 45, 3841–3849. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Al-Saeedi, M.; Litwiller, E.; Pinnau, I. Role of intrachain rigidity in the plasticization of intrinsically microporous triptycene-based polyimide membranes in mixed-gas CO2/CH4 separations. Macromolecules 2014, 47, 7453–7462. [Google Scholar] [CrossRef]
- Luo, S.; Liu, J.; Lin, H.; Kazanowska, B.A.; Hunckler, M.D.; Roeder, R.K.; Guo, R. Preparation and gas transport properties of triptycene-containing polybenzoxazole (PBO)-based polymers derived from thermal rearrangement (TR) and thermal cyclodehydration (TC) processes. J. Mater. Chem. A 2016, 4, 17050–17062. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Membr. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Corrado, T.J.; Huang, Z.; Huang, D.; Wamble, N.; Luo, T.; Guo, R. Pentiptycene-based ladder polymers with configurational free volume for enhanced gas separation performance and physical aging resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2022204118. [Google Scholar] [CrossRef]
- Yi, S.; Ma, X.; Pinnau, I.; Koros, W.J. A high-performance hydroxyl-functionalized polymer of intrinsic microporosity for an environmentally attractive membrane-based approach to decontamination of sour natural gas. J. Mater. Chem. A 2015, 3, 22794–22806. [Google Scholar] [CrossRef] [Green Version]
- Maroon, C.R.; Townsend, J.; Gmernicki, K.R.; Harrigan, D.J.; Sundell, B.J.; Lawrence, I.J.A.; Mahurin, S.M.; Vogiatzis, K.D.; Long, B.K. Elimination of CO2/N2 Langmuir sorption and promotion of ‘N2-Phobicity’ within high-Tg glassy membranes. Macromolecules 2019, 52, 1589–1600. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Harrigan, D.J.; Maroon, C.R.; Sharber, S.A.; Long, B.K.; Sundell, B.J. Promoting acid gas separations via strategic alkoxysilyl substitution of vinyl-added poly(norbornene)s. J. Membr. Sci. 2020, 616, 118569. [Google Scholar] [CrossRef]
- Staiger, C.L.; Pas, S.J.; Hill, A.J.; Cornelius, C.J. Gas separation, free volume distribution, and physical aging of a highly microporous spirobisindane polymer. Chem. Mater. 2008, 20, 2606–2608. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Jin, J.; Freeman, B.D.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371. [Google Scholar] [CrossRef]
- Pinnau, I.; Casillas, C.G.; Morisato, A.; Freeman, B.D. Long-term permeation properties of poly(1-trimethylsilyl-1-propyne) membranes in hydrocarbon/Vapor environment. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1483–1490. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Ringer, M.B.; Su, T.T.; He, Z.; Pinnau, I.; Wijmans, J.G.; Morisato, A.; Amo, K.; DaCosta, A.; Baker, R.W.; et al. Nitrogen Removal from Natural Gas; Technical Report DE--AC21-95MC32199—02; OSTI: Oak Ridge, TN, USA, 1999; p. 78 0455. [CrossRef] [Green Version]
- Dorkenoo, K.D.; Pfromm, P.H. Accelerated physical aging of thin poly[1-(trimethylsilyl)-1-propyne] films. Macromolecules 2000, 33, 3747–3751. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, H.B. High performance polyimide with high internal free volume elements. Macromol. Rapid Commun. 2011, 32, 579–586. [Google Scholar] [CrossRef]
- Luo, S.; Wiegand, J.R.; Gao, P.; Doherty, C.M.; Hill, A.J.; Guo, R. Molecular origins of fast and selective gas transport in pentiptycene-containing polyimide membranes and their physical aging behavior. J. Membr. Sci. 2016, 518, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Fu, L.; Tian, Z.; Cao, B.; Li, P. Post-crosslinking of triptycene-based Tröger’s base polymers with enhanced natural gas separation performance. J. Membr. Sci. 2018, 556, 277–284. [Google Scholar] [CrossRef]
- Tamaddondar, M.; Foster, A.B.; Carta, M.; Gorgojo, P.; McKeown, N.B.; Budd, P.M. Mitigation of physical aging with mixed matrix membranes based on cross-linked PIM-1 fillers and PIM-1. ACS Appl. Mater. Interfaces 2020, 12, 46756–46766. [Google Scholar] [CrossRef]
- Weng, Y.; Li, N.; Xu, Z.; Huang, J.; Huang, L.; Wang, H.; Li, J.; Wang, Y.; Ma, X. Super high gas separation performance membranes derived from a brominated alternative PIM by thermal induced crosslinking and carbonization at low temperature. Purif. Technol. 2023, 314, 123548. [Google Scholar] [CrossRef]
- Yong, W.F.; Kwek, K.H.A.; Liao, K.-S.; Chung, T.-S. Suppression of aging and plasticization in highly permeable polymers. Polymer 2015, 77, 377–386. [Google Scholar] [CrossRef]
- Michaeli, W.; Stein, W.H.; Unland, H. Selexol process: A contribution to selective treatment of sour gas. Erdoel-Erdgas Z 1975, 91, 341–347. [Google Scholar]
- Parker, P.E.M.E.; Northrop, S.; Valencia, J.A.; Foglesong, R.E.; Duncan, W.T. CO2 management at ExxonMobil’s LaBarge field, Wyoming, USA. Energy Procedia 2011, 4, 5455–5470. [Google Scholar] [CrossRef] [Green Version]
- Woelfer, W. Ruetenbrock natural gas purification plant. Modifications of the Selexol regeneration. Erdoel-Erdgas Z 1976, 92, 183–187. [Google Scholar]
- Woelfer, W. Construction and Start-up of the SELEXOL Natural Gas Purifying Plant Dueste II of Wintershall AG. In Proceedings of the Canadian Chemical Engineering Conference, Calgary, AB, Canada, 23–26 October 1977. [Google Scholar]
- Kuehne, D.L.; Friedlander, S.K. Selective transport of sulfur dioxide through polymer membranes. 1. polyacrylate and cellulose triacetate single-layer membranes. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 609–616. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 selective poly(ethylene oxide)-based membranes: From laboratory to pilot plant scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Flesher, J.R. Pebax® Polyether Block Amide—A New Family of Engineering Thermoplastic Elastomers. In Dordrecht: High Performance Polymers: Their Origin and Development; Seymour, R.B., Kirshenbaum, G.S., Eds.; Springer: Amsterdam, Netherlands, 1986; pp. 401–408. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Bengtson, G.; Bode, E. Pervaporation of low volatility aromatics from water. J. Membr. Sci. 1990, 53, 143–158. [Google Scholar] [CrossRef]
- Baker, R.W. Separation of Organic Azeotropic Mixtures by Pervaporation. Technical Report DOE/ER/14067-T2; OSTI: Oak Ridge, TN, USA, 1991; p. 5926894. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J. Separation of dichloroethane-trichloroethylene mixtures by means of a membrane pervaporation process. Desalination 1991, 80, 85–95. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Amo, K.A.; Baker, R.W.; Pinnau, I.; Toy, L.; Wijmans, J.G. Low-Quality Natural Gas Sulfur Removal/Recovery with Membranes; Technical Report DOE/MC/28133--94/C0307; OSTI: Oak Ridge, TN, USA, 1993. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Membrane Process for Separating H2S from Natural Gas. In Proceedings of the Natural Gas Research, Development and Demonstration Contractors Review Meeting, Baton Rouge, LA, USA, 4–6 April 1995. [Google Scholar]
- Okamoto, K.; Fuji, M.; Okamyo, S.; Suzuki, H.; Tanaka, K.; Kita, H. Gas permeation properties of poly(ether imide) segmented copolymers. Macromolecules 1995, 28, 6950–6956. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas transport properties of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part. B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Vu, J.; Ng, A.; Mohammed, M.; Kniep, J.; Merkel, T.C.; Wu, T.; Lambrecht, R.C. CO2-selective membranes for hydrogen production and CO2 capture—Part I: Membrane development. J. Membr. Sci. 2014, 457, 149–161. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-selective membranes for hydrogen production and CO2 capture—Part II: Techno-economic analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef] [Green Version]
- Munoz, D.; Maya, E.; Deabajo, J.; Delacampa, J.; Lozano, A. Thermal treatment of poly(ethylene oxide)-segmented copolyimide based membranes: An effective way to improve the gas separation properties. J. Membr. Sci. 2008, 323, 53–59. [Google Scholar] [CrossRef]
- Bucklin, R.W.; Schendel, R.L. Comparison of Fluor Solvent and Selexol Processes. Energy Prog. USA. 1984; 4, p. 3. Available online: https://www.osti.gov/biblio/6126903 (accessed on 23 July 2023).
- Kapetaki, Z.; Brandani, P.; Brandani, S.; Ahn, H. Process simulation of a dual-stage Selexol process for 95% carbon capture efficiency at an integrated gasification combined cycle power plant. Int. J. Greenh. Gas. Control 2015, 39, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Tanthana, J.; Rayer, A.V.; Gupta, V.; Mobley, P.D.; Soukri, M.; Zhou, J.; Lail, M. Experimental study of a hydrophobic solvent for natural gas sweetening based on the solubility and selectivity for light hydrocarbons (CH4, C2H6) and acid gases (CO2 and H2S) at 298–353 K. J. Chem. Eng. Data 2019, 64, 545–556. [Google Scholar] [CrossRef]
- Vaughn, J.T.; Koros, W.J. Analysis of feed stream acid gas concentration effects on the transport properties and separation performance of polymeric membranes for natural gas sweetening: A comparison between a glassy and rubbery polymer. J. Membr. Sci. 2014, 465, 107–116. [Google Scholar] [CrossRef]
- Mohammadi, T.; Moghadam, M.T.; Saeidi, M.; Mahdyarfar, M. Acid Gas Permeation Behavior Through Poly(Ester Urethane Urea) Membrane. Ind. Eng. Chem. Res. 2008, 47, 7361–7367. [Google Scholar] [CrossRef]
- Sadeghi, M.; Talakesh, M.M.; Arabi, A.; Soroush, M. Novel application of a polyurethane membrane for efficient separation of hydrogen sulfide from binary and ternary gas mixtures. Chem. Sel. 2018, 3, 3302–3308. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Yave, W.; Car, A.; Peinemann, K.-V.; Shaikh, M.Q.; Rätzke, K.; Faupel, F. Gas permeability and free volume in poly(amide-b-ethylene oxide)/polyethylene glycol blend membranes. J. Membr. Sci. 2009, 339, 177–183. [Google Scholar] [CrossRef]
- Alkatheri, M.; Grandas, R.; Betancourt-Torcat, A.; Almansoori, A. Tapping singular Middle Eastern ultrasour gas resources combining membrane and absorption systems: Potential for energy intensity reduction. Ind. Eng. Chem. Res. 2018, 57, 5748–5763. [Google Scholar] [CrossRef]
- Jirsáková, K.; Stanovský, P.; Dytrych, P.; Morávková, L.; Přibylová, K.; Petrusová, Z.; Jansen, J.C.; Izák, P. Organic vapour permeation in amorphous and semi-crystalline rubbery membranes: Experimental data versus prediction by solubility parameters. J. Membr. Sci. 2021, 627, 119211. [Google Scholar] [CrossRef]
- Lin, H.; Wagner, E.; Swinnea, J.S.; Freeman, B.D.; Pas, S.J.; Hill, A.J.; Kalakkunnath, S.; Kalika, D.S. Transport and structural characteristics of crosslinked poly(ethylene oxide) rubbers. J. Membr. Sci. 2006, 276, 145–161. [Google Scholar] [CrossRef]
- Lin, H.; Van Wagner, E.; Freeman, B.D.; Toy, L.G.; Gupta, R.P. Plasticization-enhanced hydrogen purification using polymeric membranes. Science 2006, 311, 639–642. [Google Scholar] [CrossRef]
- Callison, A.; Davidson, G. Offshore processing plant uses membranes for CO2 removal. Oil Gas J. 2007, 105, 20. [Google Scholar]
- Lokhandwala, K.A.; Pinnau, I.; He, Z.; Amo, K.D.; DaCosta, A.R.; Wijmans, J.G.; Baker, R.W. Membrane separation of nitrogen from natural gas: A case study from membrane synthesis to commercial deployment. J. Membr. Sci. 2010, 346, 270–279. [Google Scholar] [CrossRef]
- Hasse, D.; Kulkarni, S.; Sanders, E.; Corson, E.; Tranier, J.-P. CO2 capture by sub-ambient membrane operation. Energy Procedia 2013, 37, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Alders, M.; Von Bargen, C.; König, A.; Wessling, M. Chilled membranes—Efficient gas permeation at sub-ambient temperatures. J. Membr. Sci. 2019, 576, 171–181. [Google Scholar] [CrossRef]
- Beuscher, U.; Kappert, E.J.; Wijmans, J.G. Membrane research beyond materials science. J. Membr. Sci. 2022, 643, 119902. [Google Scholar] [CrossRef]
- Chan, Y.H.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Ngu, L.H.; How, B.S.; Li, C.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; et al. Hydrogen sulfide (H2S) conversion to hydrogen (H2) and value-added chemicals: Progress, challenges and outlook. Chem. Eng. J. 2023, 458, 141398. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Klemeš, J.J.; Varbanov, P.S.; Fabiano, B. A review on hydrogen production from hydrogen sulphide by chemical and photochemical methods. J. Clean. Prod. 2016, 136, 72–80. [Google Scholar] [CrossRef]
- Slimane, R.B.; Lau, F.S.; Abbasian, J. Hydrogen Production by Superadiabatic Combustion of Hydrogen Sulfide. In Proceedings of the 2000 US DOE Hydrogen Program Review, San Ramon, CA, USA, 9–11 May 2000. [Google Scholar]
- Edlund, D. A Membrane Reactor for H2S Decomposition. In Advanced Coal-Fired Power Systems Review Meeting; USDOE Morgantown Energy Technology Center (METC): Morgantown, WV, USA, 1996. [Google Scholar]
- Doong, S.-J.; Jadhav, R.A.; Lau, F. Membrane Reactor for H2S, CO2 and H2 Separation. US7938893B2, 10 May 2011. [Google Scholar]
- Lin, Y.S. Microporous and Dense Inorganic Membranes: Current Status and Prospective. Purif. Technol. 2001, 25, 39–55. [Google Scholar] [CrossRef]
- Mujeebu, M.A. Hydrogen and syngas production by superadiabatic combustion—A review. Appl. Energy 2016, 173, 210–224. [Google Scholar] [CrossRef]
- Cox, B. Economics of thermal dissociation of H2S to produce hydrogen. Int. J. Hydrogen Energy 1998, 23, 531–544. [Google Scholar] [CrossRef]
- Huang, C.; T-Raissi, A. Liquid hydrogen production via hydrogen sulfide methane reformation. J. Power Sources 2008, 175, 464–472. [Google Scholar] [CrossRef]
- Merkel, T. Mixed-gas permeation of syngas components in poly(dimethylsiloxane) and poly(1-trimethylsilyl-1-propyne) at elevated temperatures. J. Membr. Sci. 2001, 191, 85–94. [Google Scholar] [CrossRef]
- Li, N.; Funk, E.; Chang, Y.; Kulkarni, S.; Swamikannu, A.; White, L. Membrane Separation Processes in the Petrochemical Industry: Part 1: Phase 2, Final Summary Report, October 31, 1985-September 30, 1987; Technical Report DOE/ID/12422-2; Allied-Signal, Inc.: Des Plaines, IL, USA, 1987; p. 6290984. [Google Scholar] [CrossRef]
- Cardoso, A.R.T.; Ambrosi, A.; Di Luccio, M.; Hotza, D. Membranes for separation of CO2/CH4 at harsh conditions. J. Nat. Gas. Sci. Eng. 2022, 98, 104388. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Qiu, W.; Liu, G.; Eddaoudi, M.; Koros, W. Penetrant competition and plasticization in membranes: How negatives can be positives in natural gas sweetening. J. Membr. Sci. 2021, 627, 119201. [Google Scholar] [CrossRef]
- Orme, C.; Stewart, F. Mixed gas hydrogen sulfide permeability and separation using supported polyphosphazene membranes. J. Membr. Sci. 2005, 253, 243–249. [Google Scholar] [CrossRef]




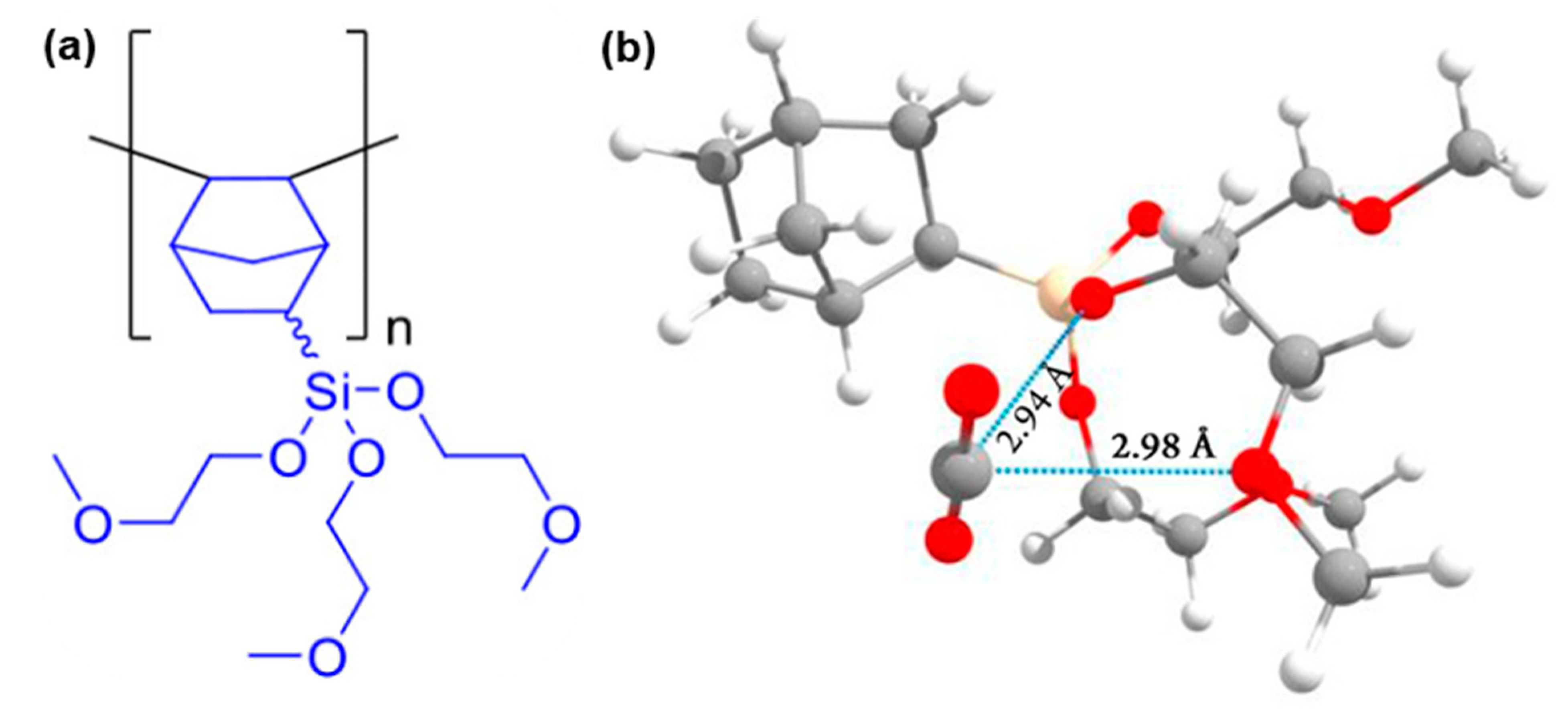
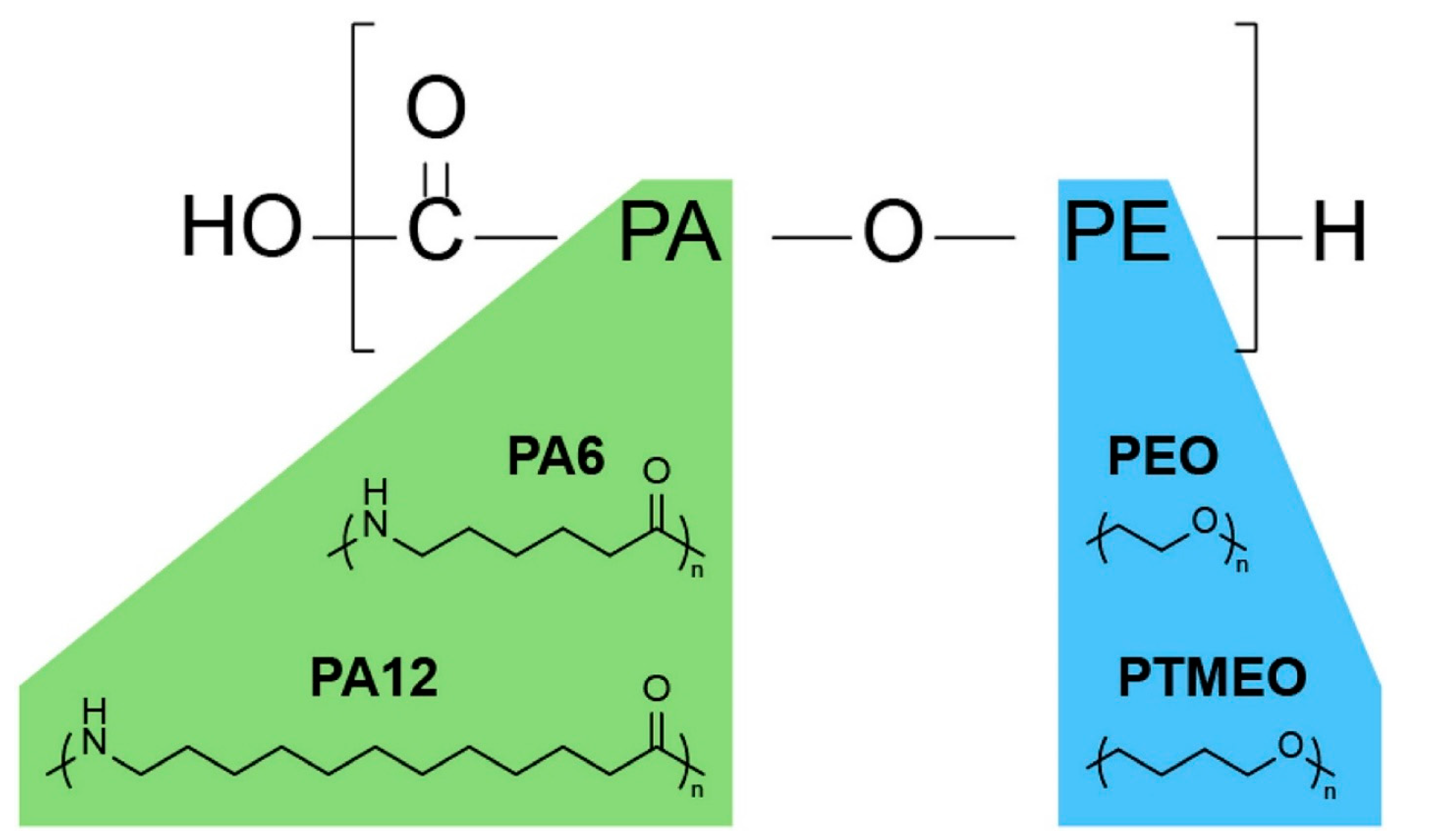
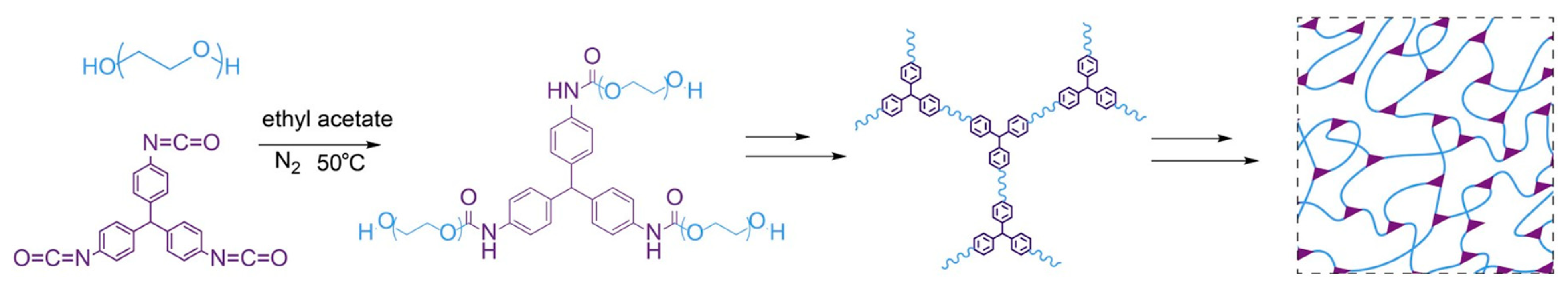



| Gas | Kinetic Diameter (Å) | Lennard–Jones Temperature (K) |
|---|---|---|
| H2S | 3.62 | 301 |
| CO2 | 3.32 | 195 |
| CH4 | 3.82 | 149 |
| Polymer | H2S/CH4 Selectivity | CO2/CH4 Selectivity | Strategies for Improving Sour Gas Performance |
|---|---|---|---|
| CA | 20–25 | 15–20 | Limited scope for improvement. Best suited to sweet natural gases. |
| Polyimide | 20–25 | ~30 | Crosslinking to improve resistance to H2S plasticization. |
| Microporous polymers | >50 | ~10 | Incorporation of functional groups to improve CO2 and H2S solubility. Limited scope for improving CO2/CH4 selectivity. |
| PEO | >50 | ~10 | Crosslinking, sub-ambient operation may improve CO2/CH4 selectivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Prasad, B.; Han, Y.; Ho, W.S.W. Polymeric Membranes for H2S and CO2 Removal from Natural Gas for Hydrogen Production: A Review. Energies 2023, 16, 5713. https://doi.org/10.3390/en16155713
Rao S, Prasad B, Han Y, Ho WSW. Polymeric Membranes for H2S and CO2 Removal from Natural Gas for Hydrogen Production: A Review. Energies. 2023; 16(15):5713. https://doi.org/10.3390/en16155713
Chicago/Turabian StyleRao, Shraavya, Babul Prasad, Yang Han, and W.S. Winston Ho. 2023. "Polymeric Membranes for H2S and CO2 Removal from Natural Gas for Hydrogen Production: A Review" Energies 16, no. 15: 5713. https://doi.org/10.3390/en16155713
APA StyleRao, S., Prasad, B., Han, Y., & Ho, W. S. W. (2023). Polymeric Membranes for H2S and CO2 Removal from Natural Gas for Hydrogen Production: A Review. Energies, 16(15), 5713. https://doi.org/10.3390/en16155713










