Numerical Study of a High-Temperature Latent Heat Thermal Energy Storage Device with AlSi12 Alloy
Abstract
:1. Introduction
2. Numerical Simulation
2.1. Physical Model
2.1.1. Dimensions of the Physical Model
2.1.2. Physical Properties
2.2. Numerical Modeling
2.2.1. Numerical Model of the Heat Exchanger
2.2.2. Numerical Model of the HTF
2.2.3. Numerical Model of PCM
2.2.4. Computational Setup
2.3. Governing Equation
2.4. Validation of Flow Models
2.5. Validation of the LTNE Model
3. Results and Discussion
3.1. Study of Heat Discharge through a Round Tube Heat Exchanger
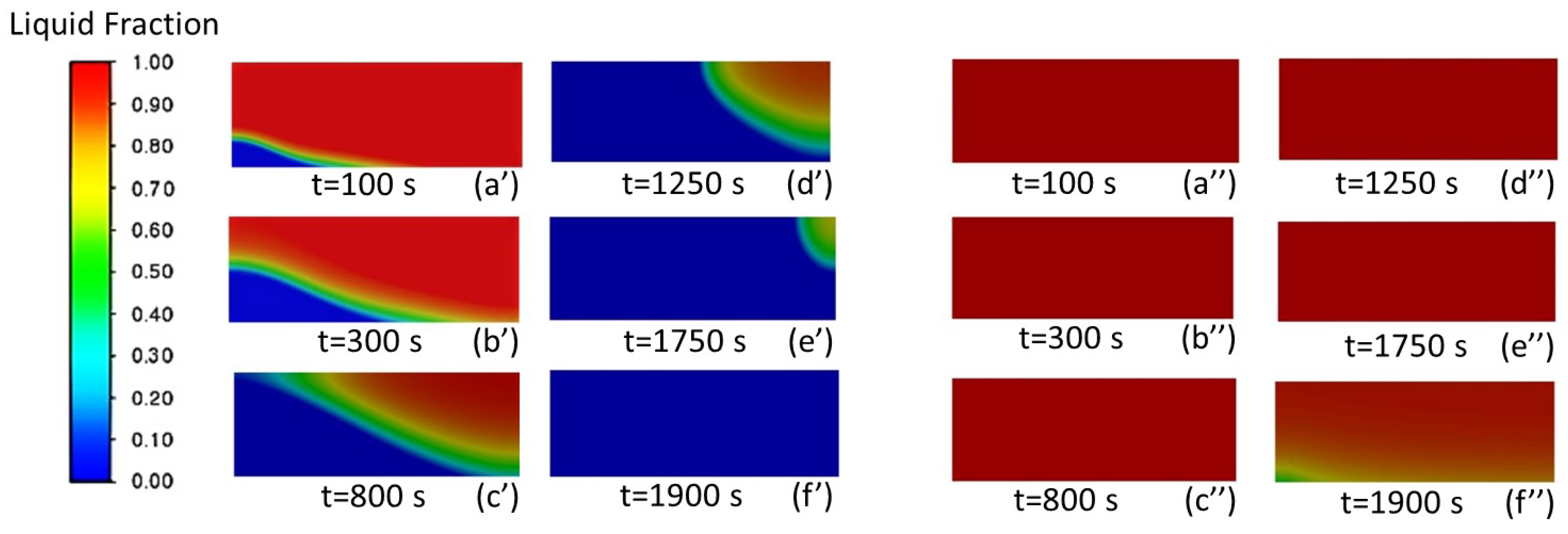
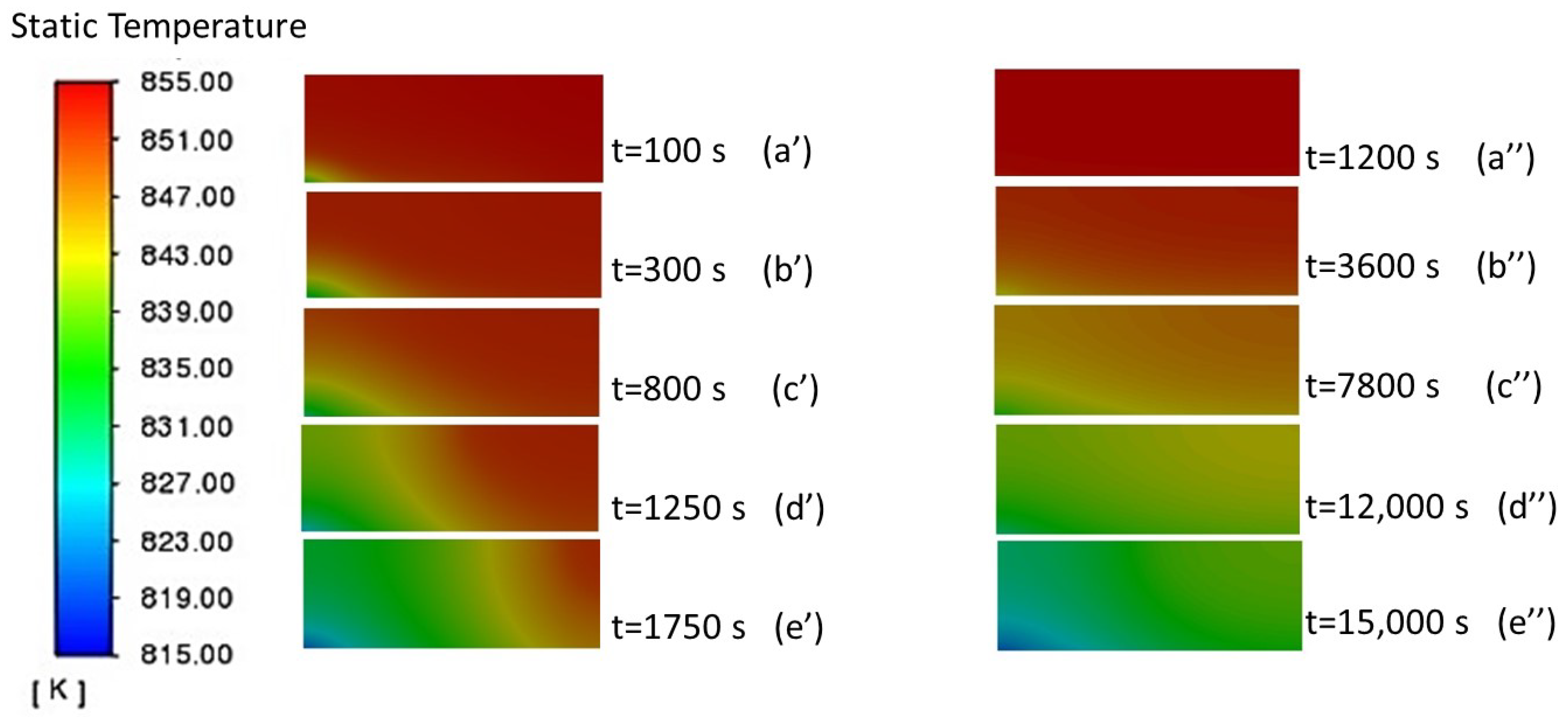
3.1.1. Analysis of the Liquid-Phase Fraction and Temperature Distribution of PCM
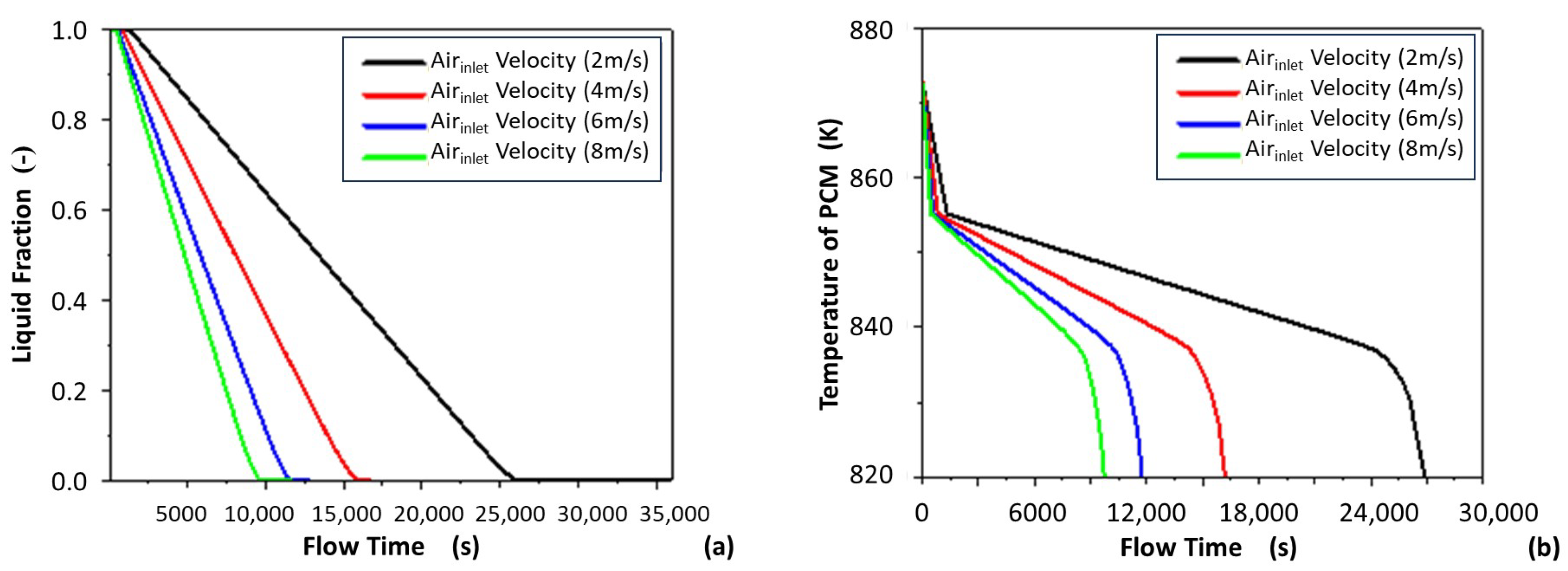
3.1.2. Effect of Air Inlet Velocity on the Solidification Speed of PCM
3.1.3. Average Temperature of PCM
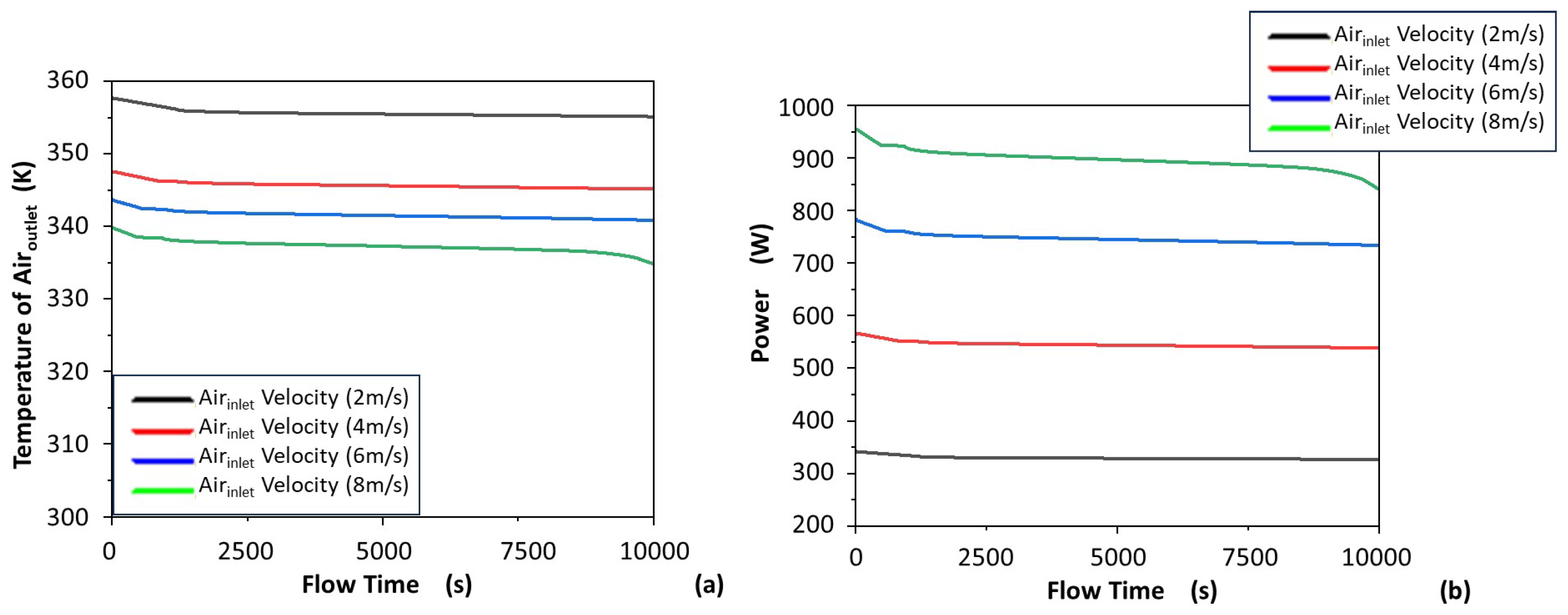
3.1.4. Air Outlet Temperature and Heat Discharge Power
3.2. Analysis of the Addition of Nickel Foam to HTF Tubes
3.2.1. Analysis of the Liquid-Phase Fraction and Temperature Distribution of PCM
3.2.2. Liquid Fraction of PCM
3.2.3. Average Temperature of PCM
3.3. Air Outlet Temperature and Discharge Power

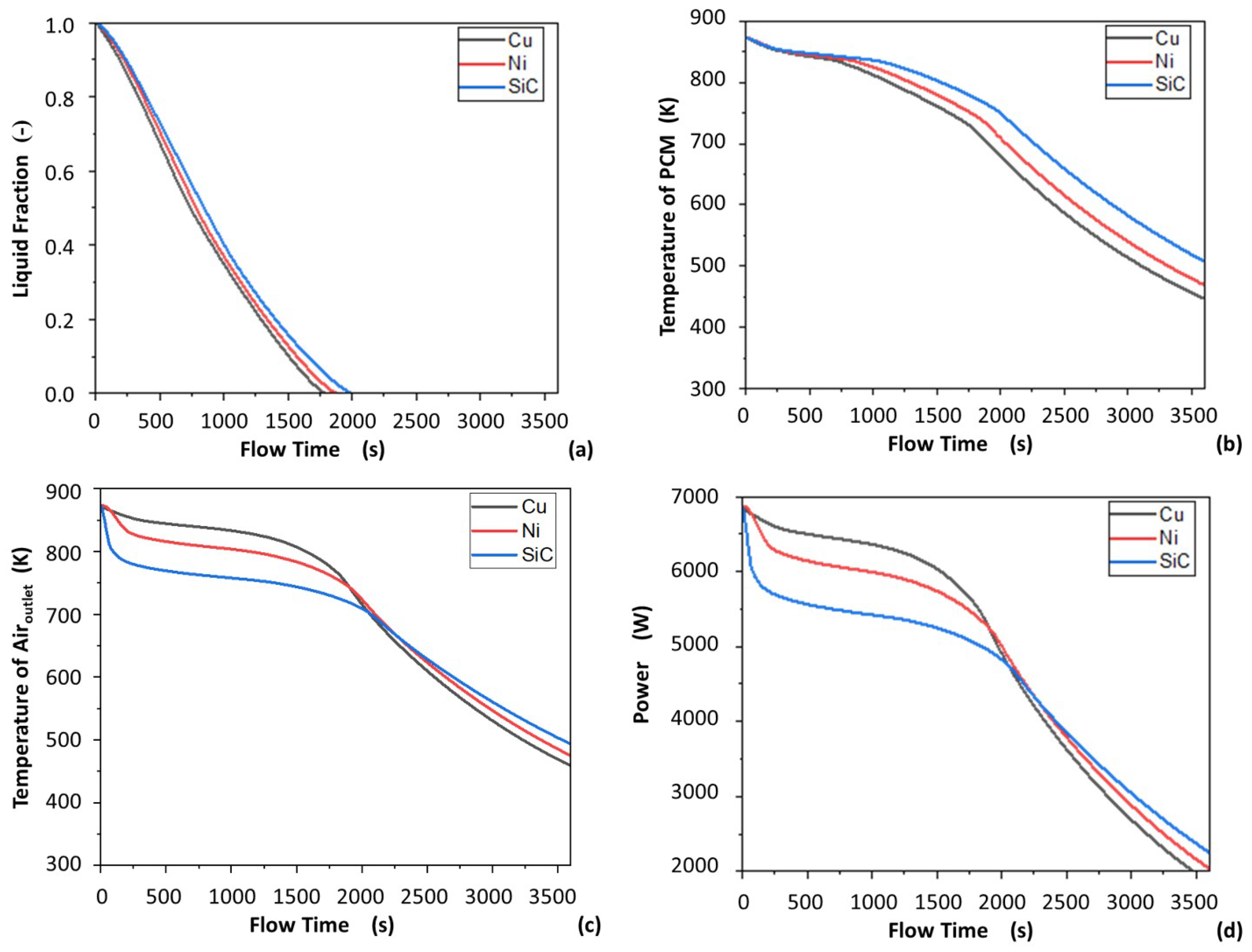
3.4. Comparison of Added Nickel Foam, Copper Foam, and Silicon Carbide Foam
4. Conclusions
- Validation of the flow model and LTNE model to ensure simulation accuracy.
- Investigation of the effects of air inlet velocity and the addition of porous materials on an LHS device.
- Increasing the air inlet velocity, which accelerates the solidification and temperature drop of the AlSi alloy.
- Solidification of PCM occurring in axial and radial directions without porous material in HTF tube.
- Increasing the air inlet velocity, which was shown to not change direction of solidification and temperature drop.
- Increasing the air inlet velocity, which was shown to lead to decreased air outlet temperature and increased heat discharge power.
- Addition of porous material, which accelerates the solidification process and temperature drop of PCM, reducing the solidification time by more than 85%.
- Increasing outlet temperature of air and heat discharge power of LHS with the addition of porous material.
- Addition of porous material, which increases the temperature difference of PCM.
- Addition of porous material, causing solidification and temperature drop of PCM to occur in the axial direction.
- Copper foam, nickel foam, and silicon carbide foam were shown to have similar effects on the LHS device, while minimal variation was shown in PCM temperature, solidification time, air exit temperature, and discharge power between different porous materials in the HTF tube.
- The segmented calculation approach was shown to reduce the computing time.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CFD | computational fluid dynamics | |
| CSP | concentrating solar power | |
| HTF | heat transfer fluid | |
| LHS | constant current constant voltage | |
| LNTE | local non-thermal equilibrium | |
| LTE | local thermal equilibrium | |
| mPCM | metallic phase-change material | |
| PCM | phase-change material | |
| SIMPLE | semi-implicit method for pressure linked equations | |
| SHS | sensible heat storage | |
| TES | thermal energy storage | |
| THS | thermochemical heat storage | |
| The following symbols are used in this manuscript: | ||
| Symbol | Meaning | Unit |
| A | heat transfer area | m |
| additional term in momentum equation | - | |
| interfacial area density | m | |
| Biot number | - | |
| C | mushy zone constant | - |
| drag coefficient | ||
| specific heat capacity of porous materials | J·kg·K | |
| specific heat capacity of HTF | J·kg·K | |
| specific heat capacity of PCM | J·kg·K | |
| the average specific heat of the liquid phase | J·kg·K | |
| the average specific heat of the solid phase | J·kg·K | |
| Darcy number | - | |
| pore diameter | m | |
| d | diameter of air tube | m |
| f | melt fraction | - |
| g | gravitational acceleration | m·s |
| H | enthalpy of PCM | J/kg |
| h | specific sensible heat of the PCM | J·kg |
| convective heat transfer coefficient of HTF | W·m·K | |
| reference enthalpy | J·kg | |
| fluid-to-solid heat transfer coefficient | W·m·K | |
| K | permeability | m |
| heat transfer coefficient between cold and heat objects | W·m·K | |
| L | latent heat of the PCM | J·kg |
| length of the air tube in LHS | m | |
| length of the LHS tube | m | |
| l | air tube length | m |
| m | the mass of PCM | kg |
| Nusselt number | - | |
| pressure of HTF | Pa | |
| Peclet number | - | |
| pressure of PCM | Pa | |
| Prandtl Number | - | |
| heat capacity of the LHS | J | |
| R | radius of the LHS tube | m |
| Reynolds number | - | |
| r | radius of the air tube in LHS | m |
| , S | source term | - |
| temperature of HTF | K | |
| liquid-phase temperature of PCM | K | |
| fluid inlet temperature | K | |
| temperature of PCM | K | |
| solid phase temperature of PCM | K | |
| temperature of porous materials | K | |
| temperature of wall | K | |
| the final temperature | K | |
| the initial temperature | K | |
| the melting temperature | K | |
| velocity of HTF | m·s | |
| velocity of PCM | m·s | |
| velocity vector of HTF | m·s | |
| velocity vector of PCM | m·s | |
| liquid fraction of PCM | - | |
| porosity | - | |
| small constant in additional term of momentum equation | - | |
| density of HTF | kg·m | |
| density of PCM | kg·m | |
| dynamic viscosity of HTF | kg·m·s | |
| dynamic viscosity of PCM | kg·m·s | |
| thermal conductivity of HTF | W·m·K | |
| thermal conductivity of PCM | W·m·K | |
| effective thermal conductivity of fluid | W·m·K | |
| effective thermal conductivity of solid | W·m·K | |
| heat transfer rate | W | |
| change in latent heat during the phase change of the PCM | J·kg | |
| temperature difference | K | |
References
- Inglesi-Lotz, R. The impact of renewable energy consumption to economic growth: A panel data application. Energy Econ. 2016, 53, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Gowrisankaran, G.; Reynolds, S.S.; Samano, M. Intermittency and the value of renewable energy. J. Political Econ. 2016, 124, 1187–1234. [Google Scholar] [CrossRef] [Green Version]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Tao, Y.B.; He, Y.L. A review of phase change material and performance enhancement method for latent heat storage system. Renew. Sustain. Energy Rev. 2018, 93, 245–259. [Google Scholar] [CrossRef]
- Fan, L.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Saari, J. Heat Exchanger Dimensioning; Lappeenranta University of Technology: Lappeenranta, Finland, 2010. [Google Scholar]
- Tao, Y.B.; Liu, Y.K.; He, Y.L. Effects of PCM arrangement and natural convection on charging and discharging performance of shell-and-tube LHS unit. Int. J. Heat Mass Transf. 2017, 115, 99–107. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Barreneche, C.; Belusko, M.; Bruno, J.C.; Bristow, D.; Tadeo, F. Considerations for the use of metal alloys as phase change materials for high temperature applications. Sol. Energy Mater. Sol. Cells 2017, 171, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and numerical analysis of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage. J. Energy Storage 2022, 56, 105975. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2021, 33, 101913. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite. Sol. Energy Mater. Sol. Cells 2011, 95, 636–643. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, G.; Sun, H.; Chen, P.; Zhu, Z.; Li, A. Graphene–nickel/n-carboxylic acids composites as form-stable phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 425–430. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, F.; Xiao, X. Thermal energy storage and retrieval characteristics of a molten-salt latent heat thermal energy storage system. Appl. Energy 2016, 173, 255–271. [Google Scholar] [CrossRef]
- Siahpush, A.; O’brien, J.; Crepeau, J. Phase change heat transfer enhancement using copper porous foam. In Proceedings of the ASME Summer Heat Transfer Conference, Jacksonville, FL, USA, 10–14 August 2008; American Society of Mechanical Engineers: New York, NY, USA, 2008. [Google Scholar]
- Meng, Z.N.; Zhang, P. Experimental and numerical investigation of a tube-in-tank latent thermal energy storage unit using composite PCM. Appl. Energy 2017, 190, 524–539. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Jia, L.; Yang, L. Paraffin and paraffin/aluminum foam composite phase change material heat storage experimental study based on thermal management of Li-ion battery. Appl. Therm. Eng. 2015, 78, 428–436. [Google Scholar] [CrossRef]
- Mat, S.; Al-Abidi, A.A.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Enhance heat transfer for PCM melting in triplex tube with internal-external fins. Energy Convers. Manag. 2013, 74, 223–236. [Google Scholar] [CrossRef]
- Stritih, U. An experimental study of enhanced heat transfer in rectangular PCM thermal storage. Int. J. Heat Mass Transf. 2004, 47, 2841–2847. [Google Scholar] [CrossRef]
- Nithyanandam, K.; Pitchumani, R. Analysis and optimization of a latent thermal energy storage system with embedded heat pipes. Int. J. Heat Mass Transf. 2011, 54, 4596–4610. [Google Scholar] [CrossRef]
- Hemati, R.; Veysi, F.; Qaderi, A. Experimental investigation of the simultaneous effect of using phase change material/plate-fin heat sink on thermal performance of a power supply unit. Sustain. Energy Technol. Assess. 2022, 52, 102009. [Google Scholar] [CrossRef]
- Tao, Y.B.; He, Y.L.; Liu, Y.K.; Tao, W.Q. Performance optimization of two-stage latent heat storage unit based on entransy theory. Int. J. Heat Mass Transf. 2014, 77, 695–703. [Google Scholar] [CrossRef]
- Kolsi, L.; Hussein, A.K.; Hassen, W.; Ben Said, L.; Ayadi, B.; Rajhi, W.; Labidi, T.; Shawabkeh, A.; Ramesh, K. Numerical Study of a Phase Change Material Energy Storage Tank Working with Carbon Nanotube-Water Nanofluid under Ha’il City Climatic Conditions. Mathematics 2023, 11, 1057. [Google Scholar] [CrossRef]
- Ouri, H.; Selimefendigil, F.; Bouterra, M.; Omri, M.; Alshammari, B.M.; Kolsi, L. MHD hybrid nanofluid convection and phase change process in an L-shaped vented cavity equipped with an inner rotating cylinder and PCM-packed bed system. Alex. Eng. J. 2023, 63, 563–582. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, G.; Mura, E.; Li, C.; Fischer, L.; Ding, Y. Experimental and numerical studies of a fatty acid based phase change dispersion for enhancing cooling of high voltage electrical devices. Energy 2020, 198, 117280. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Y. Experimental and Numerical Studies on Phase Change Materials. In Phase Change Materials and Their Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- Nikanorov, S.P.; Volkov, M.P.; Gurin, V.N.; Burenkov, Y.A.; Derkachenko, L.I.; Kardashev, B.K.; Regel, L.L.; Wilcox, W.R. Structural and Mechanical Properties of Al–Si Alloys Obtained by Fast Cooling of a Levitated Melt. Mater. Sci. Eng. A 2005, 390, 63–69. [Google Scholar] [CrossRef]
- Schmitz, J.; Hallstedt, B.; Brillo, J.; Egry, I.; Schick, M. Density and Thermal Expansion of Liquid Al–Si Alloys. J. Mater. Sci. 2012, 47, 3706–3712. [Google Scholar] [CrossRef]
- Assael, M.J.; Mihailidou, E.K.; Brillo, J.; Stankus, S.V.; Wu, J.T.; Wakeham, W.A. Reference correlation for the density and viscosity of eutectic liquid alloys Al+ Si, Pb+ Bi, and Pb+ Sn. J. Phys. Chem. Ref. Data 2012, 41, 033103. [Google Scholar] [CrossRef] [Green Version]
- Dry Air—Thermodynamic and Physical Properties. Available online: https://www.engineeringtoolbox.com/dry-air-properties-d_973.html (accessed on 21 January 2023).
- Chen, X. Investigation on Forced Convection and Coupled Heat Transfer with High Temperature Radiation in Cellular Porous Material. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2016. [Google Scholar]
- Yang, Q.; Tao, W. Heat Transfer, 3rd ed.; Higher Education Press: Beijing, China, 1998. [Google Scholar]
- Khashan, S.A.; Al-Amiri, A.M.; Al-Nimr, M.A. Assessment of the local thermal non-equilibrium condition in developing forced convection flows through fluid-saturated porous tubes. Appl. Therm. Eng. 2005, 25, 1429–1445. [Google Scholar] [CrossRef]

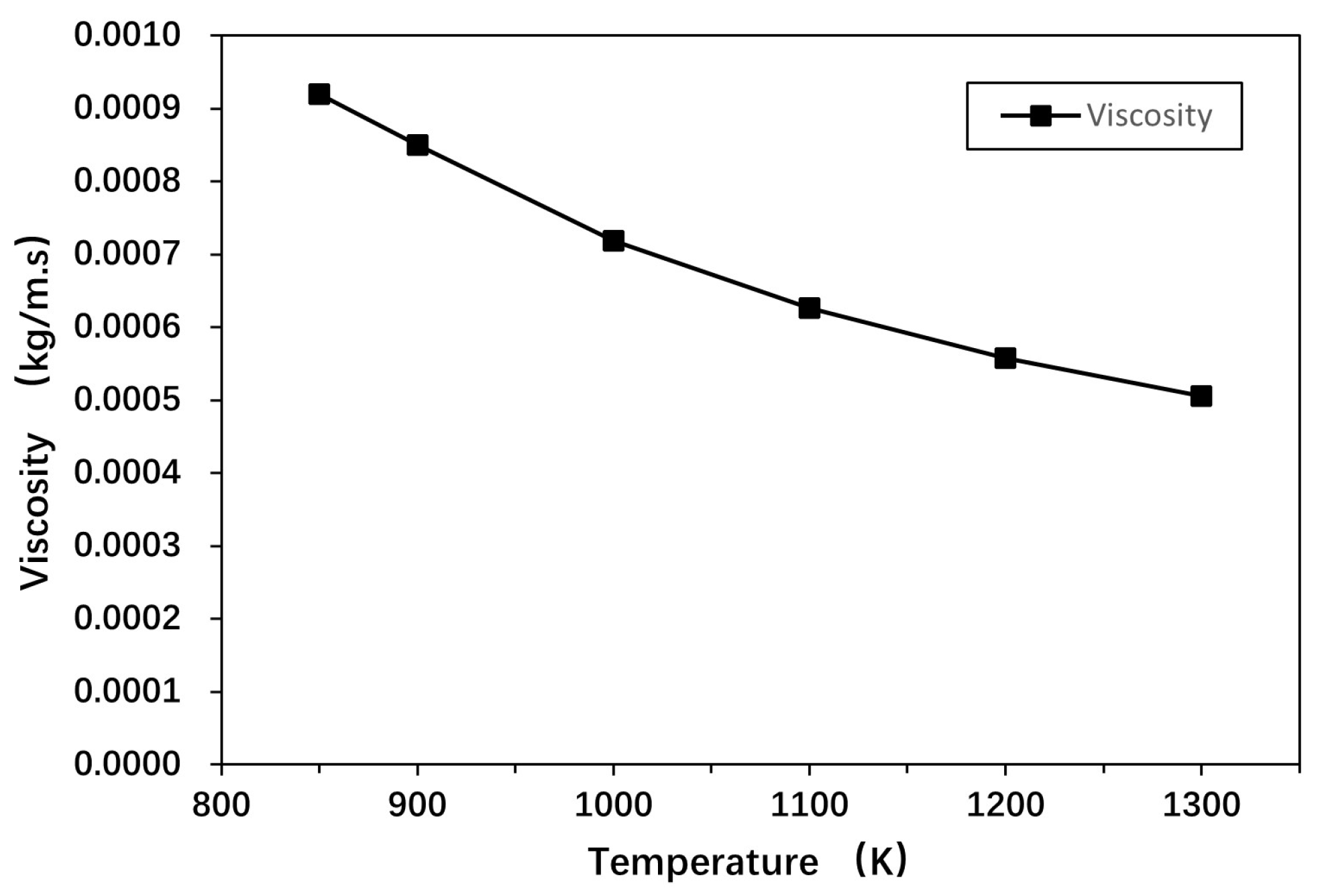

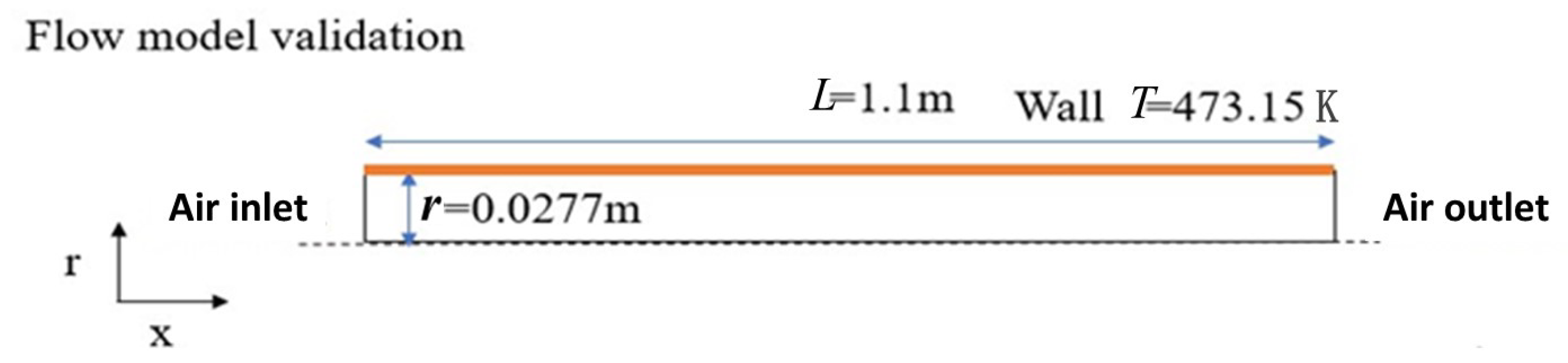


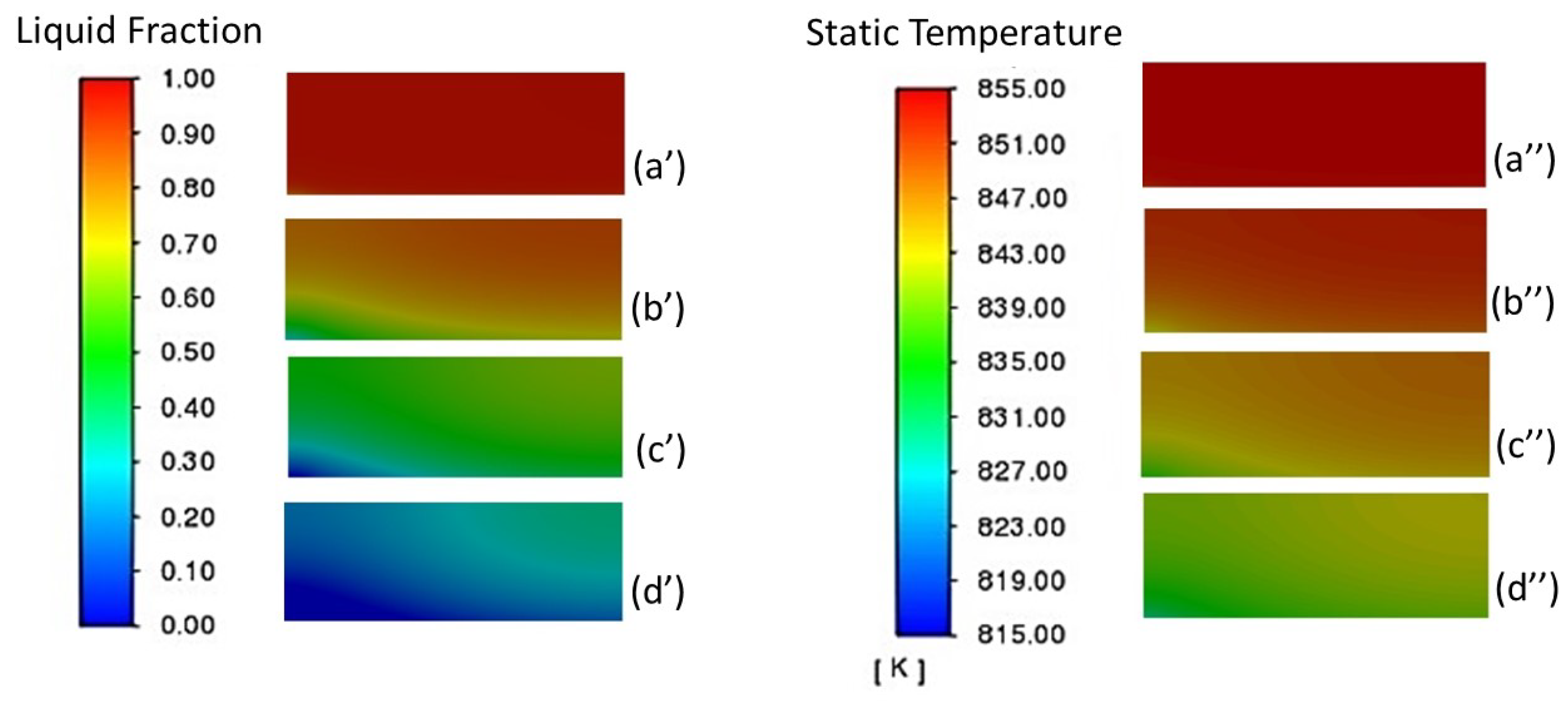
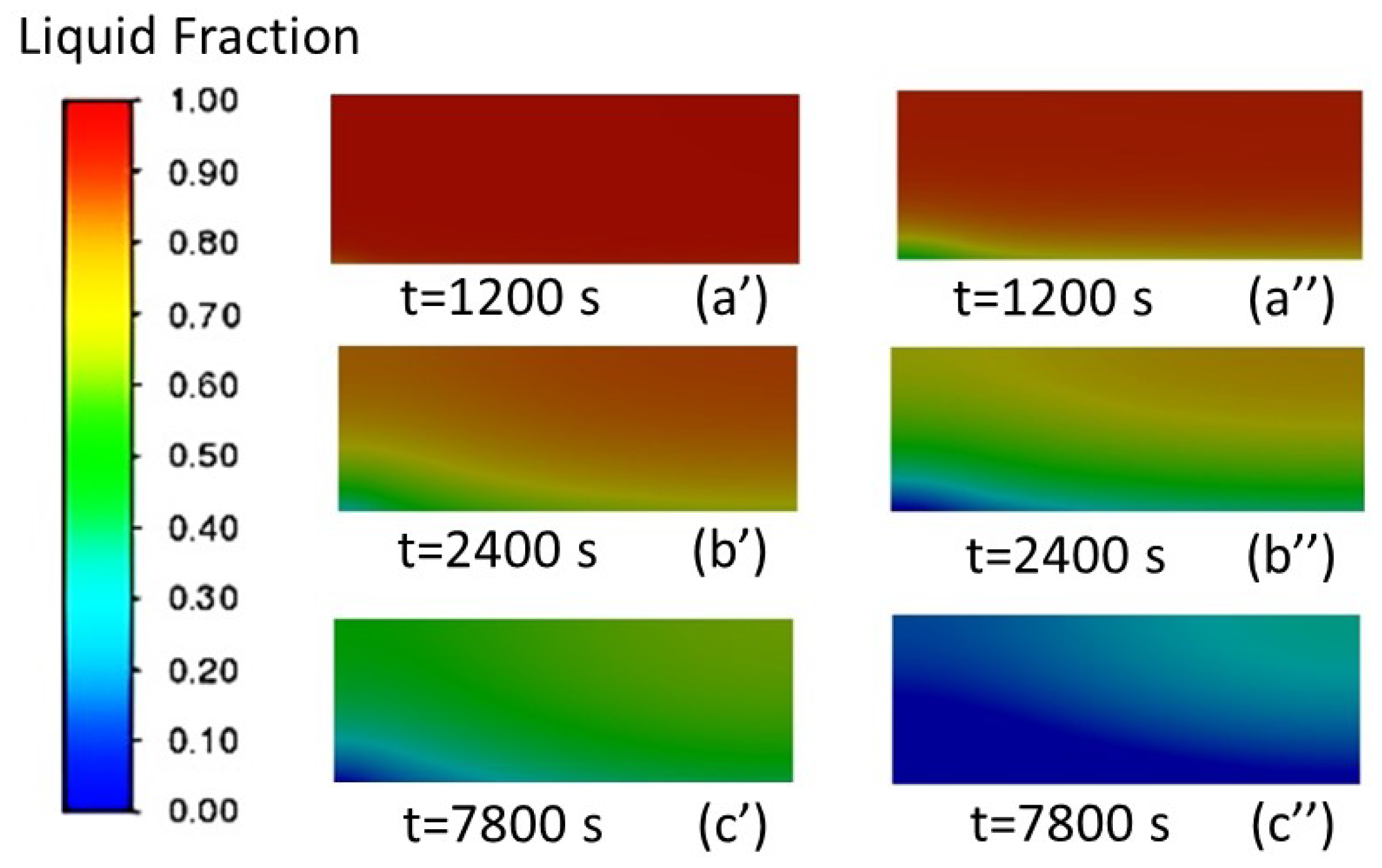

| Properties | Unit | Value |
|---|---|---|
| Density at 298.15 K (25 C) | kg·m | 2642 |
| Density at Liquidus Temperature | kg·m | 2460 |
| Cp (Specific Heat) at Solid 298.15 K (25 C) | J·kg·K | 1038 |
| Cp (Specific Heat) at Liquid | J·kg·K | 1741 |
| Thermal Conductivity, solid | W·m·K | 180 |
| Thermal Conductivity, liquid | W·m·K | 70 |
| Thermal Expansion Coefficient | K | 0.000131 |
| Pure Solvent Melting Heat | J·kg | 470,000 |
| Melting Temperature | C | 576 |
| T (K) | (kg·m) | (J·kg·K) | (W·m·K) |
|---|---|---|---|
| 275 | 1.284 | 1003.8 | 0.02428 |
| 300 | 1.177 | 1004.9 | 0.02624 |
| 325 | 1.086 | 1006.3 | 0.02816 |
| 350 | 1.009 | 1008.2 | 0.03003 |
| 375 | 0.9413 | 1010.6 | 0.03186 |
| 400 | 0.8824 | 1013.5 | 0.03365 |
| 450 | 0.7844 | 1020.6 | 0.0371 |
| 500 | 0.706 | 1029.5 | 0.04041 |
| 550 | 0.6418 | 1039.8 | 0.04357 |
| 600 | 0.5883 | 1051.1 | 0.04661 |
| 650 | 0.543 | 1062.9 | 0.04954 |
| 700 | 0.5043 | 1075 | 0.05236 |
| 750 | 0.4706 | 1087 | 0.05509 |
| 800 | 0.4412 | 1098.7 | 0.05774 |
| 850 | 0.4153 | 1110.1 | 0.0603 |
| 900 | 0.3922 | 1120.9 | 0.06276 |
| Raw Materials for Porous Materials | Density (kg·m) | Specific Heat Capacity (J·kg·K) | Thermal Conductivity (W·m·K) |
|---|---|---|---|
| Copper | 8930 | 386 | 398 |
| Nickel | 8900 | 444 | 91.4 |
| Silicon carbide | 3210 | 1244 | 80 |
| Porous Materials | Permeability (m) | Drag Coefficient (m) | Pore Diameter (m) | Porosity |
|---|---|---|---|---|
| Copper foam | 1.092 × 10 | 451.88 | 0.001047 | 0.9 |
| Nickel foam | 7.345 × 10 | 659.2 | 0.001007 | 0.87 |
| Silicon carbide foam | 2.369 × 10 | 515.2 | 0.001851 | 0.88 |
| Physical Properties | Unit | Value |
|---|---|---|
| W·m·K | 10 | |
| W·m·K | 99 | |
| J·kg·K | 1000 | |
| kg·m | 1 | |
| kg·m·K | ||
| - | 0.99 | |
| m·s | 1 | |
| D | m | 0.1 |
| K | m | |
| m | ||
| W·m·K | 99 | |
| m | 5 | |
| W·m·K | 9.9 | |
| W·m·K | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaomurilige; Qiao, G.; Zhao, P.; Li, Y.; Li, Y. Numerical Study of a High-Temperature Latent Heat Thermal Energy Storage Device with AlSi12 Alloy. Energies 2023, 16, 5729. https://doi.org/10.3390/en16155729
Chaomurilige, Qiao G, Zhao P, Li Y, Li Y. Numerical Study of a High-Temperature Latent Heat Thermal Energy Storage Device with AlSi12 Alloy. Energies. 2023; 16(15):5729. https://doi.org/10.3390/en16155729
Chicago/Turabian StyleChaomurilige, Geng Qiao, Peng Zhao, Yang Li, and Yongliang Li. 2023. "Numerical Study of a High-Temperature Latent Heat Thermal Energy Storage Device with AlSi12 Alloy" Energies 16, no. 15: 5729. https://doi.org/10.3390/en16155729






