Pack-Level Modeling and Thermal Analysis of a Battery Thermal Management System with Phase Change Materials and Liquid Cooling
Abstract
:1. Introduction
2. Models and Methodologies
2.1. Physical Model Description
- (1)
- The heat generation part of the battery cell is retained, with the shell and positive/negative tabs being ignored. Additionally, the heat generation of each battery cell is uniform [25].
- (2)
- The thermal conductivity of the battery material is anisotropic, meaning that it varies in different directions. Additionally, the density, thermal conductivity, and specific heat of all materials are independent of temperature.
- (3)
- Based on the simplification of the battery model, it is considered that the filling height of the PCM is as high as the heat-generating part of the battery cell.
- (4)
- The liquid PCM is an incompressible Newtonian fluid and its natural convection is ignored because heat conduction plays an absolute role in the heat transfer process [26].
- (5)
- The radiation in the heat transfer process is negligible.
| Mass Fraction of EG (%) | Density (kg·m−3) | Specific Heat (J·kg−1·k−1) | Thermal Conductivity (W·m−1·k−1) | Latent Heat (kJ·kg−1) | Melting Temperature (°C) |
|---|---|---|---|---|---|
| 0 | 900 | 2560 | 0.3 | 226 | 32–36 |
| 5 | 928 | 2477 | 1.54 | 215 | 32–36 |
| 10 | 958 | 2394 | 2.97 | 203 | 32–36 |
| 15 | 990 | 2311 | 4.48 | 192 | 32–36 |
| 20 | 1025 | 2228 | 6.24 | 181 | 32–36 |
| The Parameter | Value | Units |
|---|---|---|
| Battery Cell [28] | ||
| Size | 148.3 × 26.7 × 98 | mm |
| The distance between the adjacent battery cell | 4 | mm |
| Density | 2519 | Kg·m−3 |
| Specific heat | 1022.8 | J·kg−1·k−1· |
| Thermal conductivity in the y-direction | 1.062 | W·m−1·k−1 |
| Thermal conductivity in the x/z-direction | 22.4459 | W·m−1·k−1 |
| Electrolyte material | LiPF6 | - |
| Cathode material | Graphite | - |
| Nominal capacity | 50 | Ah |
| Nominal voltage | 3.65 | V |
| PCM | ||
| Density | 928 | kg·m−3 |
| Specific heat | 2477 | J·kg−1·k−1· |
| Thermal conductivity | 1.54 | W·m−1·k−1 |
| Latent heat | 215,000 | J·kg−1 |
| Phase change temperature | 32 | °C |
| Liquid Cooling Equipment | ||
| Size of LCP | 1426 × 366 × 10 | mm |
| Density of aluminum | 2700 | kg·m−3 |
| Specific heat of aluminum | 903 | J·kg−1·k−1 |
| Thermal conductivity of aluminum | 238 | W·m−1·k−1 |
| Density of water | 998.2 | kg·m−3 |
| Specific heat of water | 4182 | J·kg−1·k−1· |
| Thermal conductivity of water | 0.6 | W·m−1·k−1 |
| Kinematic viscosity of water | 0.001 | kg·m−1·s−1 |
| Thermal Pad | ||
| Size | 1374 × 366 × 2 | mm |
| Density | 1130 | kg·m−3 |
| Specific heat | 1320 | J·kg−1·k−1· |
| Thermal conductivity | 2 | W·m−1·k−1 |
2.2. Numerical Methods
2.2.1. Battery Thermal Model
2.2.2. PCM Model
2.2.3. Liquid Cooling Model
2.2.4. Initial and Boundary Conditions
2.2.5. Mesh Independence Verification
3. Results and Discussions
3.1. Performance Comparison of Different BTM Patterns
3.2. Thermal Effects of the Mass Fraction of EG in PCM
3.3. Thermal Performance of the Battery Pack with PCM and Liquid Cooling
3.3.1. Influences of the Inlet Mass Flow Rate and Temperature of Coolant
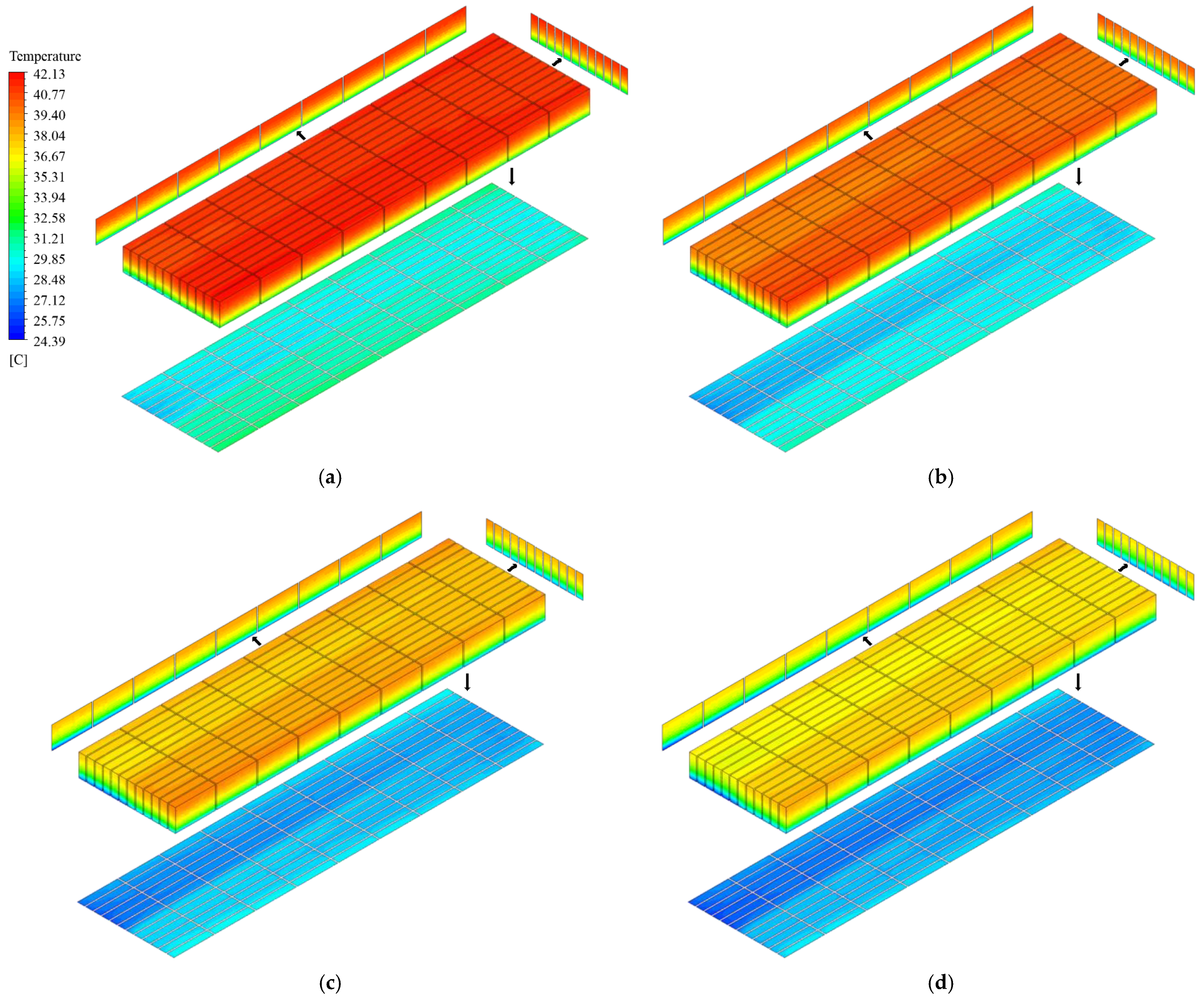
3.3.2. Temperature Distribution of the Battery Pack
4. Conclusions
- (1)
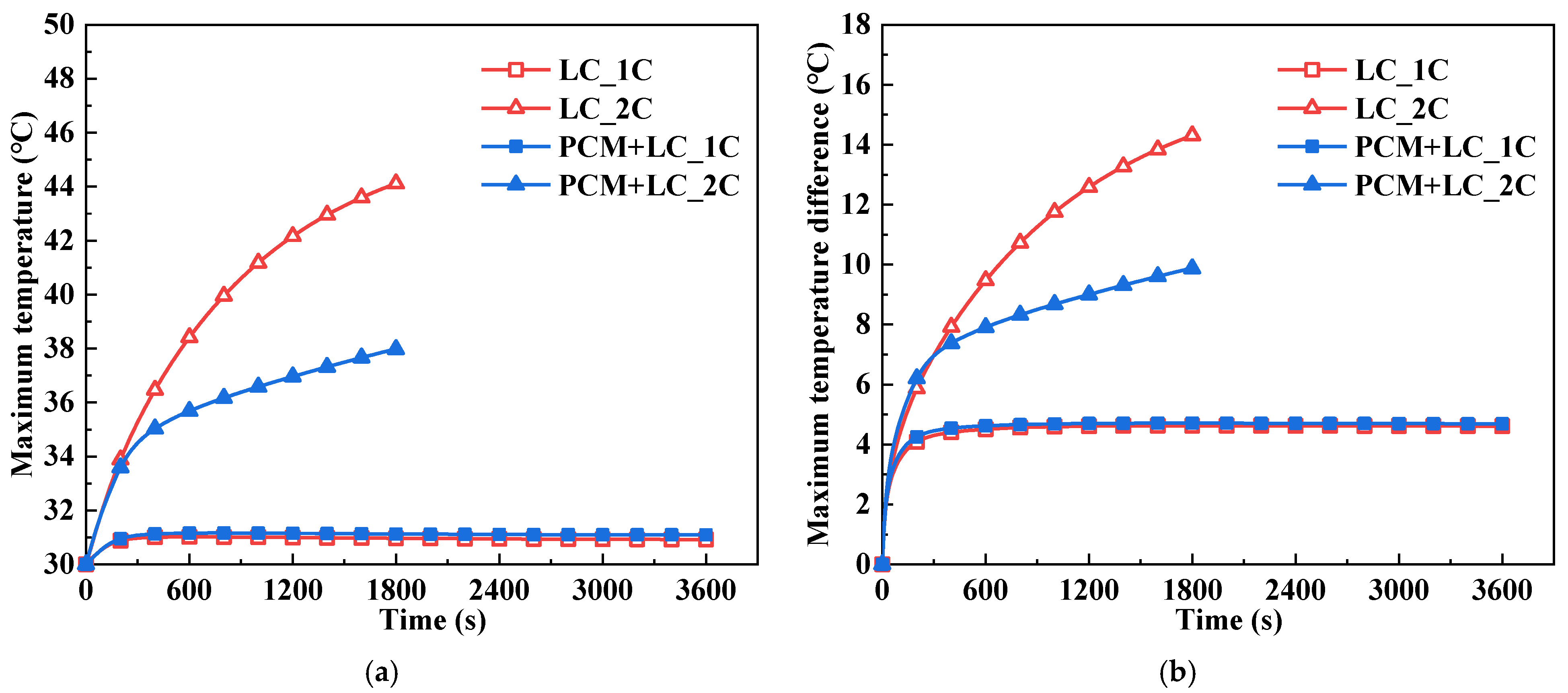
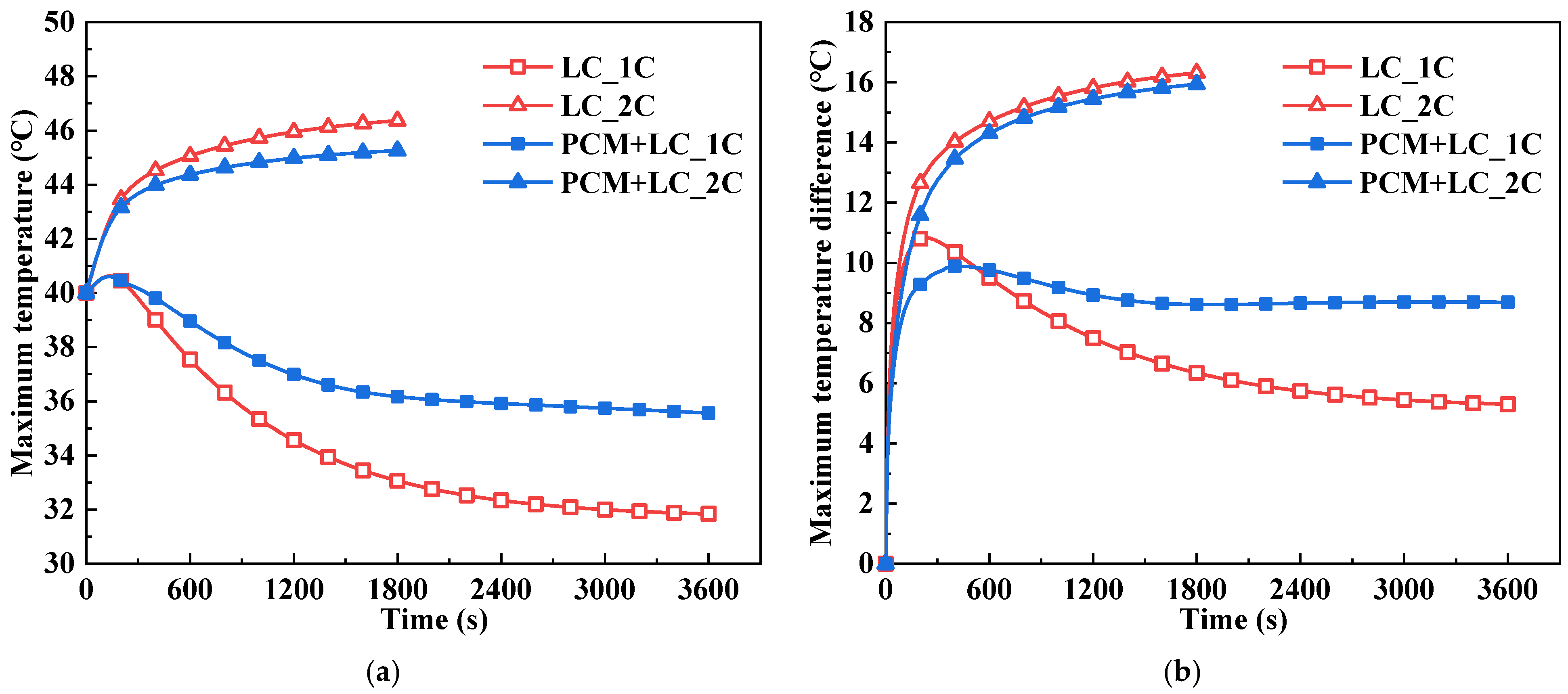
- Compared with the traditional BTM system using LC, the proposed composite BTM system with PCM and LC can reduce the battery pack’s maximum temperature and temperature difference after full 2C discharge at the ambient temperatures of both 30 °C and 40 °C. For the discharge process of 1C, the BTM performance of the two systems is similar at a 30 °C ambient temperature. This is because the battery heat production is relatively low and the PCM’s melting temperature of 32–36 °C is not reached. When the ambient temperature is 40 °C, the PCM cannot absorb heat and thus utilize the phase change process because the ambient temperature is higher than the melting temperature of the PCM. Besides, the heat load on the system has increased due to the PCM, resulting in poorer temperature characteristics for the composite system than the traditional system during the 1C discharge process.
- (2)
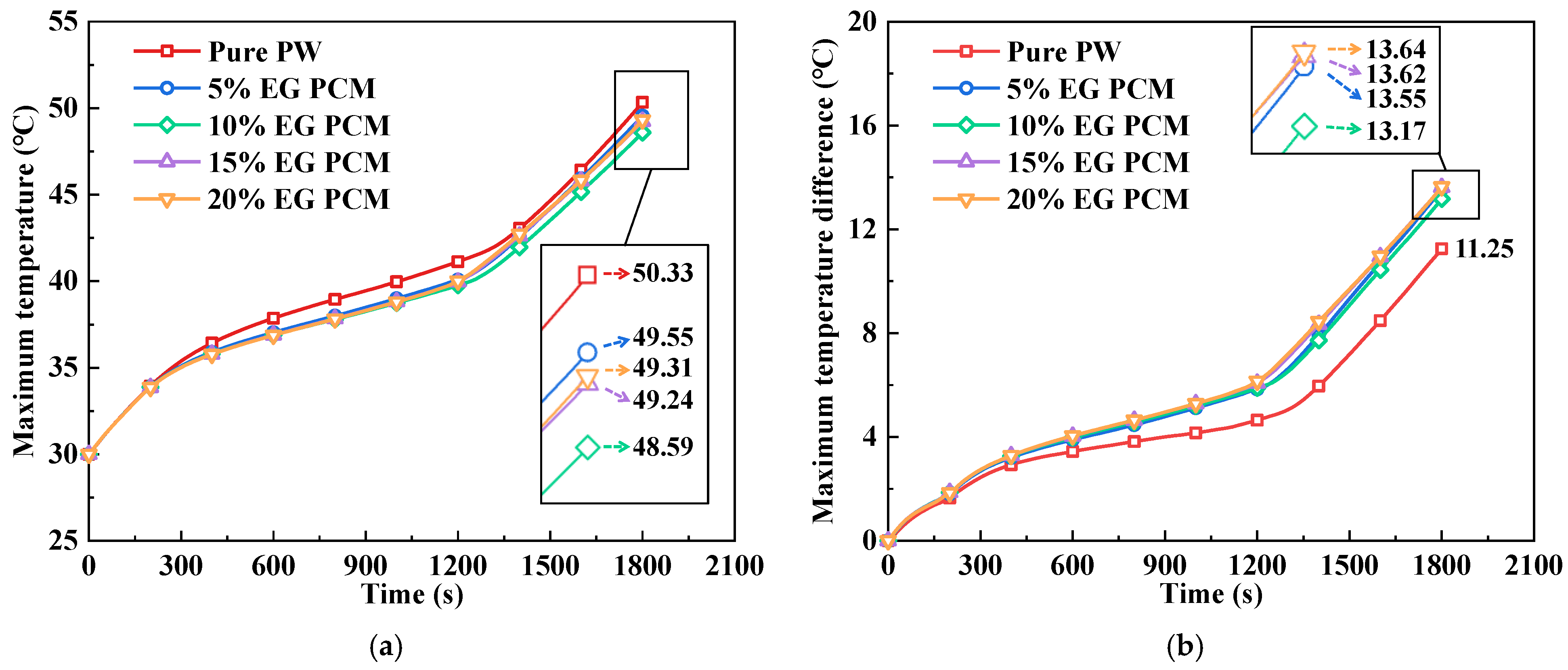
- Mixing EG with various mass fractions in PW can clearly enhance the PCM’s thermal performance. Compared with pure PW, the use of 10% EG in PCM reduced the battery pack’s maximum temperature at the terminal of 2C discharge by 1.74 °C. Additionally, compared to the 20% EG PCM, it resulted in a 0.47 °C decrease in the temperature difference. Furthermore, as determined through discussing the average temperature and liquid fraction of PCM, the addition of 10% EG in PCM was able to sustain a sufficient heat storage capacity, making this the ideal mass fraction ratio.
- (3)
- The maximum temperature of the battery pack during the 2C discharge process at a temperature of 40 °C can be decreased by increasing the mass flow rate and decreasing the flow temperature of the coolant. Additionally, the former can also reduce the maximum temperature difference of the battery pack. However, the latter will deeply reduce the temperature of the battery bottom and deteriorate the battery temperature difference. Hence, an ideal LC control logic for this model was proposed, which would be to increase the mass flow rate while maintaining the appropriate inlet temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Amush | Mushy zone constant [-] | Subscripts | |
| c | Specific heat [J·kg−1·K−1] | a | Ambient |
| d | Constant coefficient [-] | ave | Average value |
| H | Total enthalpy [J·kg−1] | b | Battery cell |
| h | Sensible enthalpy [J·kg−1] | f | Final state |
| hnat | Natural convective heat transfer | i | Initial state |
| coefficient [W·m−2·K−1] | l | Liquid cooling plate | |
| hl | Latent heat of PCM [J·kg−1] | m | Melting state |
| ΔH | Latent heat capacity [J·kg−1] | max | Maximum value |
| I | Current [A] | p | Phase change material |
| k | Thermal conductivity [W·m−1·k−1] | p,l | Liquid phase change material |
| Lp | Liquid fraction of PCM [%] | p,s | Solid phase change material |
| m | Mass [kg] | ref | Values in reference condition |
| n | Wall-normal direction [-] | s | Shell |
| Q | Total energy absorbed by PCM [J] | t | Thermal pad |
| q | Heat generation rate [J·s−1] | w | Water |
| qm | Mass flow rate of coolant [kg·s−1] | ||
| Re | Reynolds number | ||
| Rohm | Ohmic resistance [Ω] | Abbreviation | |
| Rpolar | Polarization resistance [Ω] | BTM | Battery thermal management |
| S | Momentum sink [kg·m·s−1] | CFD | Computational fluid dynamics |
| T | Temperature [K] | EG | Expanded graphite |
| t | Time [s] | LC | Liquid cooling |
| Uocv | Open-circuit voltage [V] | LCP | Liquid cooling plate |
| V | Volume [m3] | PCM | Phase change material |
| Velocity of the liquid PCM [m·s−1] | PW | Paraffin wax | |
| SOC | State of charge | ||
| Greek symbols | |||
| ρ | Density [kg·m−3] | ||
| ε | Constant [-] | ||
References
- Zhao, Y.; Dan, D.; Zheng, S.; Wei, M.; Xie, Y. A two-stage eco-cooling control strategy for electric vehicle thermal management system considering multi-source information fusion. Energy 2023, 267, 126606. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Dan, D.; Zhao, Y.; Wei, M.; Wang, X. Review of Thermal Management Technology for Electric Vehicles. Energies 2023, 16, 4693. [Google Scholar] [CrossRef]
- Li, M.; Zheng, S.; Wei, M. Flow Loss Analysis and Structural Optimization of Multiway Valves for Integrated Thermal Management Systems in Electric Vehicles. Energies 2023, 16, 5040. [Google Scholar] [CrossRef]
- Karimi, G.; Li, X. Thermal management of lithium-ion batteries for electric vehicles. Int. J. Energy Res. 2013, 37, 13–24. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Zhao, J.; Wei, Z. Thermal investigation of lithium-ion battery module with different cell arrangement structures and forced air-cooling strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M.; Li, C. An up-to-date review on the design improvement and optimization of the liquid-cooling battery thermal management system for electric vehicles. Appl. Therm. Eng. 2023, 219, 119626. [Google Scholar] [CrossRef]
- Zhao, J.; Rao, Z.; Li, Y. Thermal performance of mini-channel liquid cooled cylinder based battery thermal management for cylindrical lithium-ion power battery. Energy Convers. Manag. 2015, 103, 157–165. [Google Scholar] [CrossRef]
- Sheng, L.; Su, L.; Zhang, H.; Li, K.; Fang, Y.; Ye, W.; Fang, Y. Numerical investigation on a lithium ion battery thermal management utilizing a serpentine-channel liquid cooling plate exchanger. Int. J. Heat Mass Transf. 2019, 141, 658–668. [Google Scholar] [CrossRef]
- Weragoda, D.M.; Tian, G.; Burkitbayev, A.; Lo, K.-H.; Zhang, T. A comprehensive review on heat pipe based battery thermal management systems. Appl. Therm. Eng. 2023, 224, 120070. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Georgoulas, A.; Miché, N.; Rouaud, C.; Marengo, M. Novel battery thermal management system for electric vehicles with a loop heat pipe and graphite sheet inserts. Appl. Therm. Eng. 2021, 194, 117061. [Google Scholar] [CrossRef]
- Budiman, A.C.; Azzopardi, B.; Sudirja; Perdana, M.A.P.; Kaleg, S.; Hadiastuti, F.S.; Hasyim, B.A.; Amin; Ristiana, R.; Muharam, A. Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation. Energies 2023, 16, 3896. [Google Scholar] [CrossRef]
- Zheng, S.; Li, S.; Li, M.; Dai, R.; Wei, M.; Tian, R. Experimental and numerical investigation of a rectangular finned-tube latent heat storage unit for Carnot battery. J. Energy Storage 2023, 71, 108092. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, Q.; Huang, C. Investigation of the thermal performance of phase change material/mini-channel coupled battery thermal management system. Appl. Energy 2016, 164, 659–669. [Google Scholar] [CrossRef]
- Alrashdan, A.; Mayyas, A.T.; Al-Hallaj, S. Thermo-mechanical behaviors of the expanded graphite-phase change material matrix used for thermal management of Li-ion battery packs. J. Mater. Process. Technol. 2010, 210, 174–179. [Google Scholar] [CrossRef]
- Alipanah, M.; Li, X. Numerical studies of lithium-ion battery thermal management systems using phase change materials and metal foams. Int. J. Heat Mass Transf. 2016, 102, 1159–1168. [Google Scholar] [CrossRef]
- Samimi, F.; Babapoor, A.; Azizi, M.; Karimi, G. Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 2016, 96, 355–371. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, F.; Fang, X.; Gao, X.; Zhang, Z. A hybrid thermal management system for lithium ion batteries combining phase change materials with forced-air cooling. Appl. Energy 2015, 148, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Lebrouhi, B.E.; Lamrani, B.; Ouassaid, M.; Abd-Lefdil, M.; Maaroufi, M.; Kousksou, T. Low-cost numerical lumped modelling of lithium-ion battery pack with phase change material and liquid cooling thermal management system. J. Energy Storage 2022, 54, 105293. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Yang, C. Thermal analysis of conjugated cooling configurations using phase change material and liquid cooling techniques for a battery module. Int. J. Heat Mass Transf. 2019, 133, 827–841. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Kalogiannis, T.; Jin, L.; Karimi, D.; Van Mierlo, J.; Berecibar, M. Experimental and numerical thermal analysis of a lithium-ion battery module based on a novel liquid cooling plate embedded with phase change material. J. Energy Storage 2022, 50, 104673. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, M.; Zhang, Y.; Li, J.; Jiang, G.; Shi, H. Thermal management of cylindrical battery pack based on a combination of silica gel composite phase change material and copper tube liquid cooling. J. Energy Storage 2023, 71, 108205. [Google Scholar] [CrossRef]
- Senlin, C.; Mingshan, W.; Panpan, S.; Hongge, W. Cooling performance simulation of the power battery pack based on straight liquid cooling plate. J. Automot. Saf. Energy 2021, 12, 380. [Google Scholar]
- Wang, Y.; Dan, D.; Zhang, Y.; Qian, Y.; Panchal, S.; Fowler, M.; Li, W.; Tran, M.K.; Xie, Y. A novel heat dissipation structure based on flat heat pipe for battery thermal management system. Int. J. Energy Res. 2022, 46, 15961–15980. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Wang, S. Thermal optimization of composite PCM based large-format lithium-ion battery modules under extreme operating conditions. Energy Convers. Manag. 2017, 153, 22–33. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Zhang, Y.; Lee, K.; Liu, J.; Mou, L.; Chen, B.; Li, Y. A dynamic electro-thermal coupled model for temperature prediction of a prismatic battery considering multiple variables. Int. J. Energy Res. 2021, 45, 4239–4264. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, H.; Li, W.; Zhang, Y.; Chen, B.; Zhang, K. Improving battery thermal behavior and consistency by optimizing structure and working parameter. Appl. Therm. Eng. 2021, 196, 117281. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, R.; Li, J. High latent heat phase change materials (PCMs) with low melting temperature for thermal management and storage of electronic devices and power batteries: Critical review. Renew. Sustain. Energy Rev. 2022, 168, 112783. [Google Scholar] [CrossRef]
- Ogoh, W.; Groulx, D. Effects of the heat transfer fluid velocity on the storage characteristics of a cylindrical latent heat energy storage system: A numerical study. Heat Mass Transf. 2012, 48, 439–449. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Jones, B.J.; Sun, D.; Krishnan, S.; Garimella, S.V. Experimental and numerical study of melting in a cylinder. Int. J. Heat Mass Transf. 2006, 49, 2724–2738. [Google Scholar] [CrossRef]
- Panchal, S.; Khasow, R.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Thermal design and simulation of mini-channel cold plate for water cooled large sized prismatic lithium-ion battery. Appl. Therm. Eng. 2017, 122, 80–90. [Google Scholar] [CrossRef]
- Xia, B.; Liu, Y.; Huang, R.; Yang, Y.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. Thermal analysis and improvements of the power battery pack with liquid cooling for electric vehicles. Energies 2019, 12, 3045. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Li, X.; Song, L.; Xiang, L. Development of a theoretically based thermal model for lithium ion battery pack. J. Power Sources 2013, 223, 155–164. [Google Scholar] [CrossRef]
- Zhao, J.; Rao, Z.; Huo, Y.; Liu, X.; Li, Y. Thermal management of cylindrical power battery module for extending the life of new energy electric vehicles. Appl. Therm. Eng. 2015, 85, 33–43. [Google Scholar] [CrossRef]







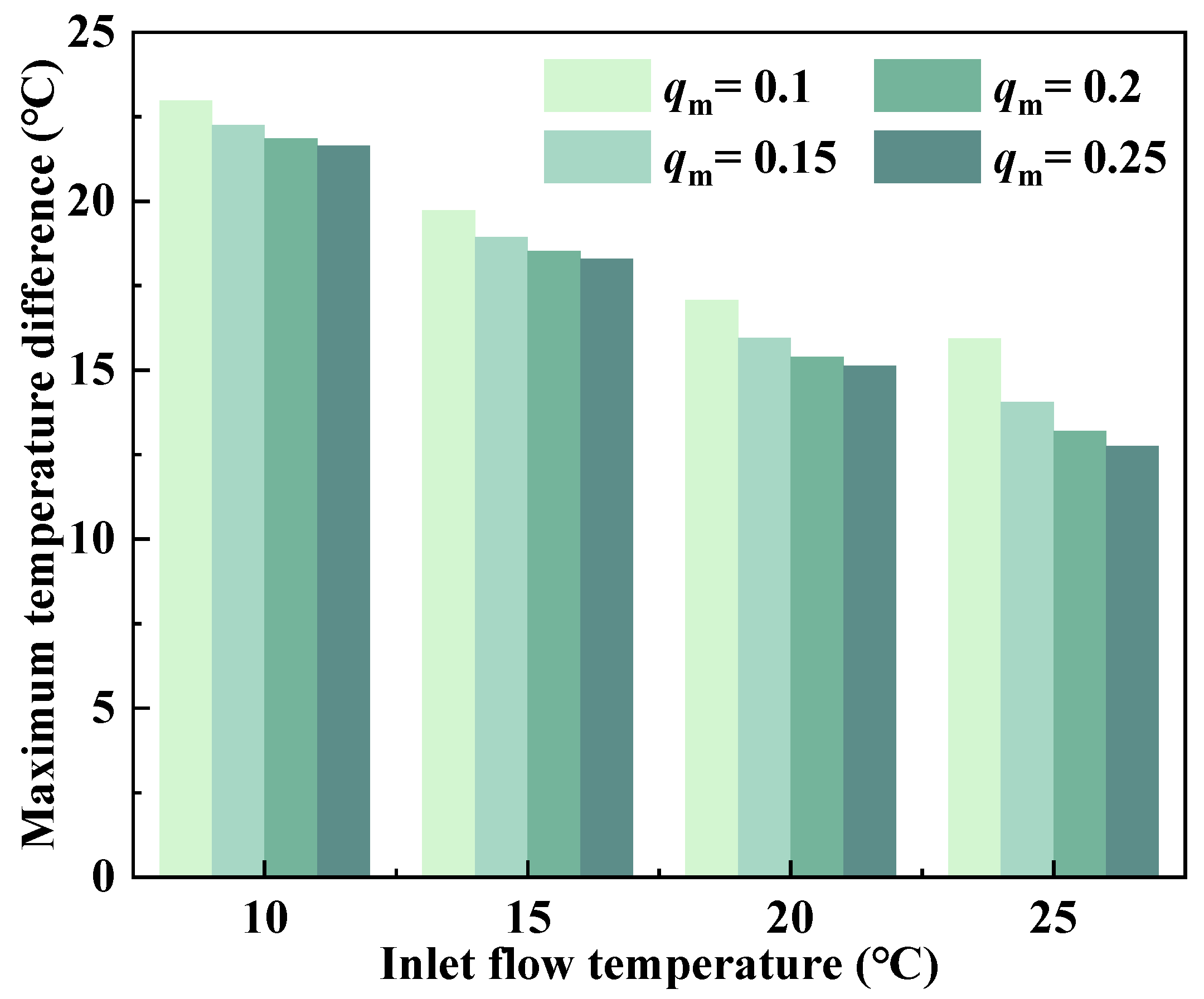
| Flow Temperature (°C) | Mass Flow Rate (kg·s−1) | |||
|---|---|---|---|---|
| 0.1 | 0.15 | 0.2 | 0.25 | |
| 10 | −3.16 | −4.04 | −4.28 | −4.46 |
| 15 | −1.20 | −2.70 | −3.36 | −3.71 |
| 20 | +1.56 | −0.30 | −1.16 | −1.68 |
| 25 | +5.26 | +3.18 | +2.19 | +1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Dan, D.; Wei, M.; Cai, S.; Zhao, Y.; Wright, E. Pack-Level Modeling and Thermal Analysis of a Battery Thermal Management System with Phase Change Materials and Liquid Cooling. Energies 2023, 16, 5815. https://doi.org/10.3390/en16155815
Sun J, Dan D, Wei M, Cai S, Zhao Y, Wright E. Pack-Level Modeling and Thermal Analysis of a Battery Thermal Management System with Phase Change Materials and Liquid Cooling. Energies. 2023; 16(15):5815. https://doi.org/10.3390/en16155815
Chicago/Turabian StyleSun, Jixian, Dan Dan, Mingshan Wei, Senlin Cai, Yihang Zhao, and Edward Wright. 2023. "Pack-Level Modeling and Thermal Analysis of a Battery Thermal Management System with Phase Change Materials and Liquid Cooling" Energies 16, no. 15: 5815. https://doi.org/10.3390/en16155815






