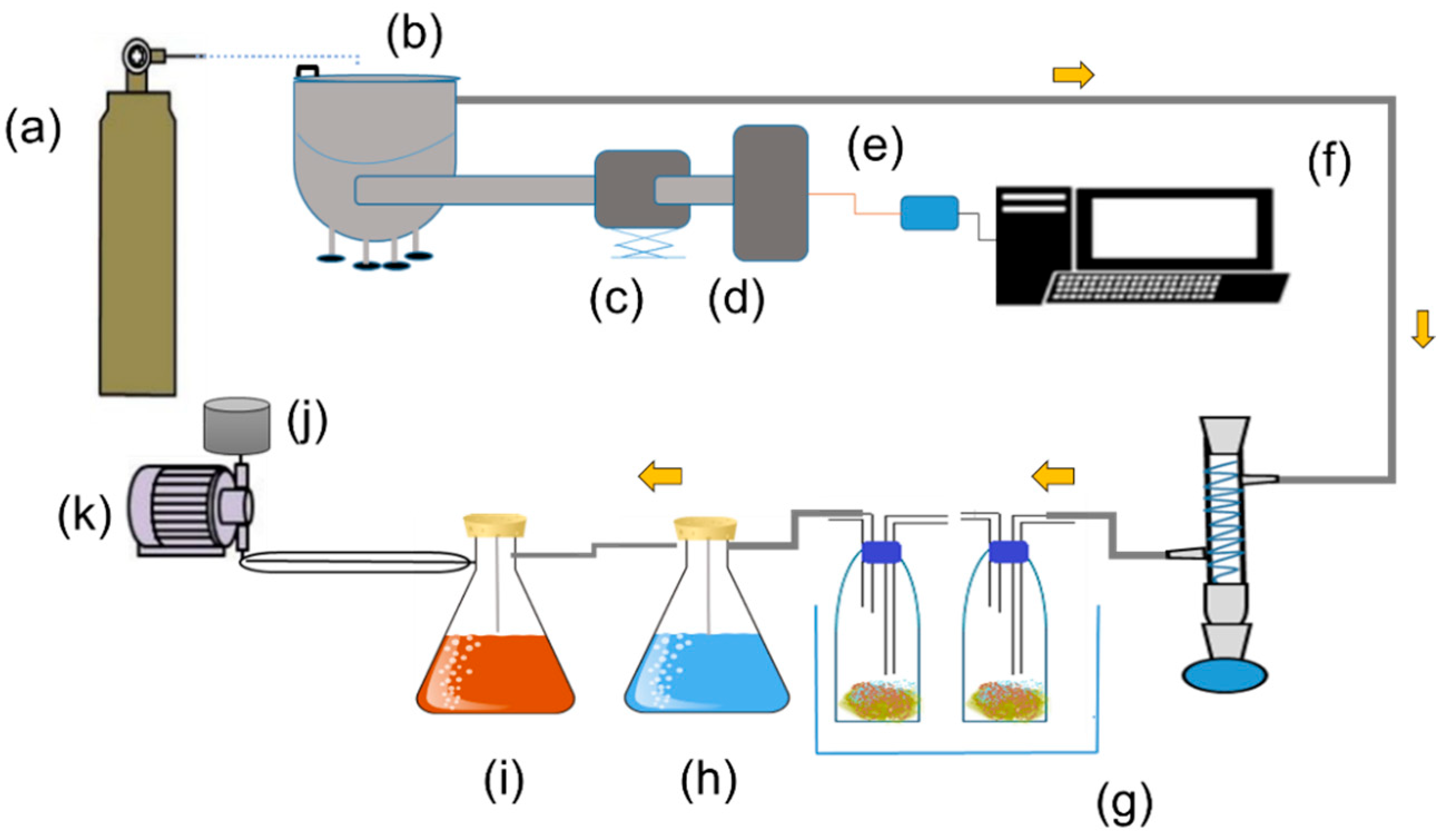
1. Introduction
One third of the world’s food is wasted, with Australian households generating 2.5 million tonnes of food waste annually, equivalent to around 4 kg per household per week [
1]. The disposal of food waste has a significant environmental impact and economic cost, and contributes to water source depletion, with 25% of agricultural water consumption dedicated to food growth, amounting to 2600 gigalitres per year in Australia [
1]. The economic loss due to food waste is substantial, estimated at AUD 36.6 billion annually [
1,
2,
3,
4]. Additionally, food waste disposal accounts for 8% of global greenhouse gas emissions, predominantly through the release of methane during decomposition in landfills [
1,
5]. The disposal of fruits, organic waste, and vegetables obstruct sustainable development in household, commercial, and agricultural sectors [
6,
7]. Vegetables and fruits constitute a significant portion of food waste, representing 23–65% [
6]. In Australia, the household sector alone produces between 150,695 and 461,721 tonnes of fruit and vegetable waste per year [
1]. To address these challenges, utilizing food waste as a fuel feedstock is a viable option to reduce the environmental impact and develop clean energy sources.
Common destinations for food waste include food recovery, composting, landfill, incineration, and animal feed [
1,
8,
9,
10]. However, these treatment methods have limitations in terms of scale, efficiency, pre-treatment requirements, management complexity, and output product selectivity [
9,
10,
11,
12]. Waste-to-energy (bioenergy) processes offer a green alternative, including biochemical methods such as anaerobic digestion and fermentation, as well as thermochemical methods like conventional and microwave pyrolysis [
7,
10,
11,
12,
13]. The biochemical approach utilises organic biomass for biogas and alcohol fuel production [
10,
13]. Conventional and microwave pyrolysis can be applied to any biomass type to generate biochar, bio-oil, and biogas [
11,
14]. Microwave pyrolysis holds an advantage over conventional pyrolysis due to its rapid and efficient heating mechanism. In microwave pyrolysis, electromagnetic waves directly target the material, distinguished by volumetric heating (electromagnetic energy), hence the better heat distribution, versatility of biomass uses, and high energy conversion efficiency through rapid and controlled heating. Unlike conventional pyrolysis, microwave-assisted pyrolysis transfers the heat energy through the interaction of the molecules inside the biomass rather than by heat transfer from external sources [
11,
15,
16,
17]. Overall, it results in faster and more uniform heating, leading to reduced processing times and higher energy yields. This technology also allows for precise control over temperature gradients, minimizing the formation of undesirable byproducts and enhancing the overall product quality. Additionally, microwave pyrolysis reduces energy consumption and emissions, making it a greener and more sustainable option for waste treatment and resource recovery [
14,
18].
Not all materials are natural microwave absorbers, and hence a microwave susceptor (MS) is essential in microwave pyrolysis to initiate and enhance the heating efficiency. MS absorbs microwave energy and initiates biomass heating, promoting uniform and rapid heating of the material being processed, leading to faster reactions, improved yields, and enhanced overall efficiency [
14]. The three by-products obtained from microwave pyrolysis, namely biochar, bio-oil, and biogas, have diverse applications, including power generation, heat production, chemical recovery, soil conditioning, fuel production, and electrochemical sensors [
14,
19]. Most of the agricultural waste is formed by lignocellulosic compounds, which have a range content of three fibres: lignin, cellulose, and hemicellulose. Biomass nature (fibre composition) is a relevant factor in terms of the yield and characterisation of the by-products; for example, biomass with a high cellulose content is favourable for bio-oil production, while biochar is derived from lignin [
7,
10,
11,
20,
21].
The high energy conversion efficiency of microwave pyrolysis reduces biomass treatment procedures, lowers processing costs, mitigates GHG (greenhouse gas) emissions from food waste decomposition, and facilitates clean energy recovery from the three by-products [
6,
7,
11]. Previous work has reported food waste processing using highly time-consuming technologies and expensive technologies, such as fermentation methods and biomass pre-treatment [
8]. While several studies focus on the conversion of food waste into valuable applications or energy analysis [
15,
22], the evaluation of yield, quality, and energy value of the by-products is often overlooked. This research aims to study the energy recovery of by-products from the microwave pyrolysis of pumpkin peel biomass under varied operating conditions, specifically focusing on biochar and bio-oil yield and quality. The study also includes economic and environmental analyses of the custom-made microwave pyrolysis system.
4. Discussion
This study assessed the conversion of food waste biomass into energy using microwave-assisted pyrolysis under different operating conditions. The analysis focused on the yield, CHN (carbon, hydrogen, nitrogen) elemental properties, functional group quality, and energy output of biochar and bio-oil. Pumpkin peel was utilized as the feedstock, and three operating conditions were employed (0.9 kW, 1.2 kW, and 1.5 kW). The biomass exhibited varying behaviours at different microwave power levels. Lower power (0.9 kW) resulted in a higher biochar yield (11 wt%), while the same power level led to the lowest bio-oil generation (20.3 wt%) due to intrinsic biomass characteristics. Biochar and bio-oil characterization revealed that 1.2 kW produced by-products with a high carbon content and low oxygen concentration, yielding high-energy heating value. At 1.2 kW for 40 min, the biochar exhibited well-defined pores, while bio-oil exhibited high aromatic functional groups and low oxygen content, indicating superior quality.
The energy balance analysis demonstrated that the food waste feedstock generated an output energy of 0.07 kWh, primarily attributed to the high energy generation from bio-oil. The energy conversion efficiency of the microwave pyrolysis system using food waste reached 6.9%. An economic feasibility analysis using the same scenario yielded a cost balance of AUD 0.01. The potential for the carbon dioxide sequestration of pumpkin biochar was found to be 14.29 g CO2 eq/kg.
This work also provides relevant findings related to the future application of biochar in advanced carbon nanomaterials due to its notable characteristics and quality generated from microwave pyrolysis—with promising uses in the electrochemistry sector and supercapacitor fabrication. It is recommended for future research to study the biogas composition from pumpkin peel and evaluate its energy potential in global energy recovery using microwave-assisted pyrolysis. Moreover, the optimisation of bio-oil quality is suggested, through esterification techniques (post-treatment) or by changing the microwave operating conditions (input power, microwave susceptor, reaction time). At the same time, this work provides the initial point to explore the processing of different types of agricultural wastes.