Numerical Study on Heat and Mass Transfer of Evaporated Binary Zeotropic Mixtures in Porous Structure
Abstract
:1. Introduction
2. Governing Equation
3. Numerical Model
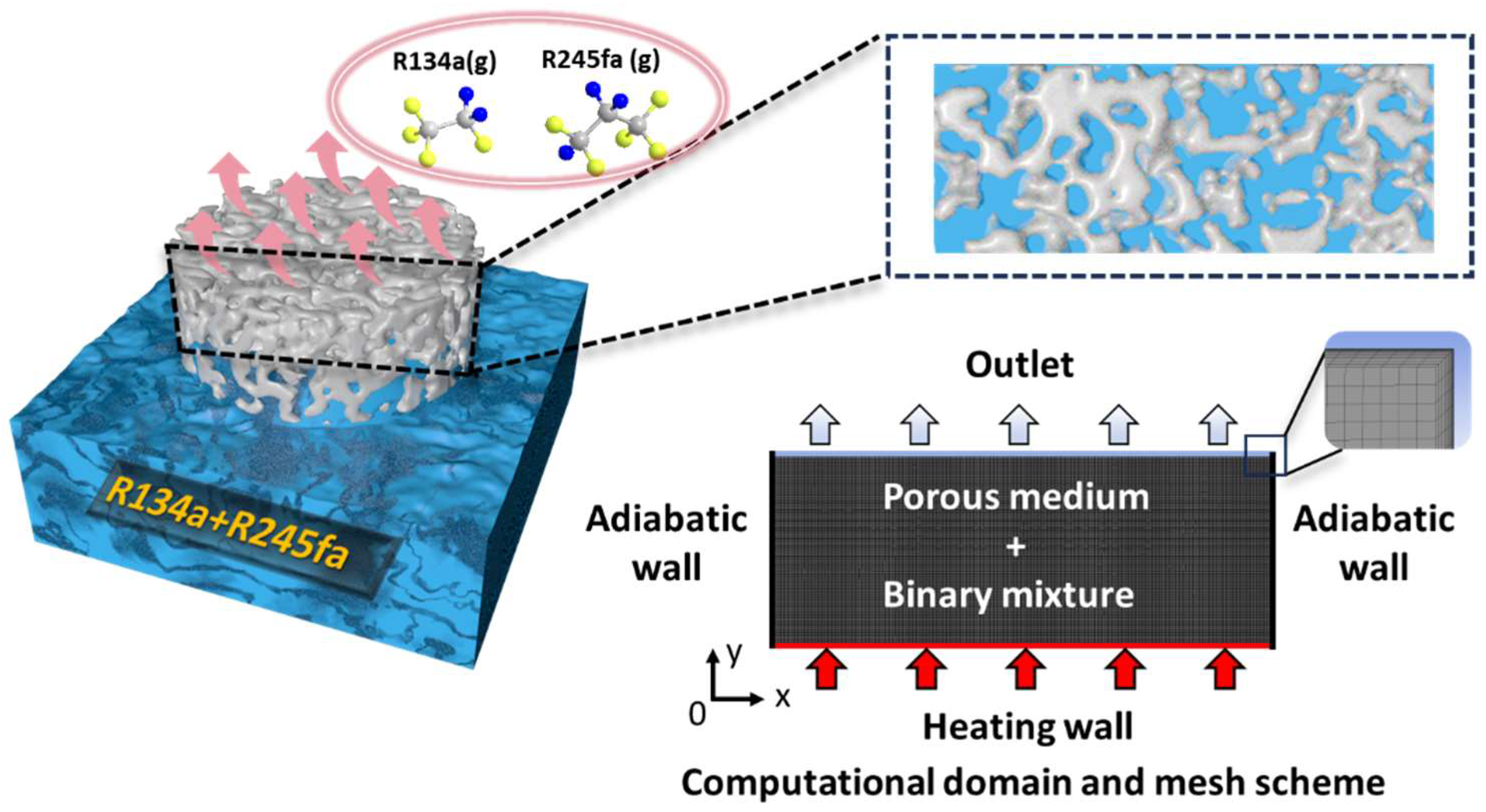
3.1. Physical Model and Mesh Scheme
3.2. Boundary Conditions and Initial Conditions
- (1)
- At the initial time, the porous medium is fully saturated with the liquid phase. The composition ratio of the solution is set to 3:7, 5:5, and 7:3 for R134a and R245fa, respectively.
- (2)
- The gauge pressure inside the porous medium is initially set to 0 Pa.
- (3)
- The initial temperature inside the porous medium is set to 296 K, resulting in a subcooling of 3 K.
- (4)
- The initial velocity of the computational cells inside the porous medium is set to 0 m/s.
- (1)
- The outlet boundary condition is set as a pressure outlet, and the backflow temperature at the outlet is set to the saturation temperature.
- (2)
- The side walls are considered adiabatic ones, and the velocity is set to a no-slip boundary condition.
- (3)
- The heating wall is specified as a constant heat flux boundary condition, ranging from 100 kW/m2 to 500 kW/m2.
3.3. Case Setup
3.4. Grid Independence Analysis
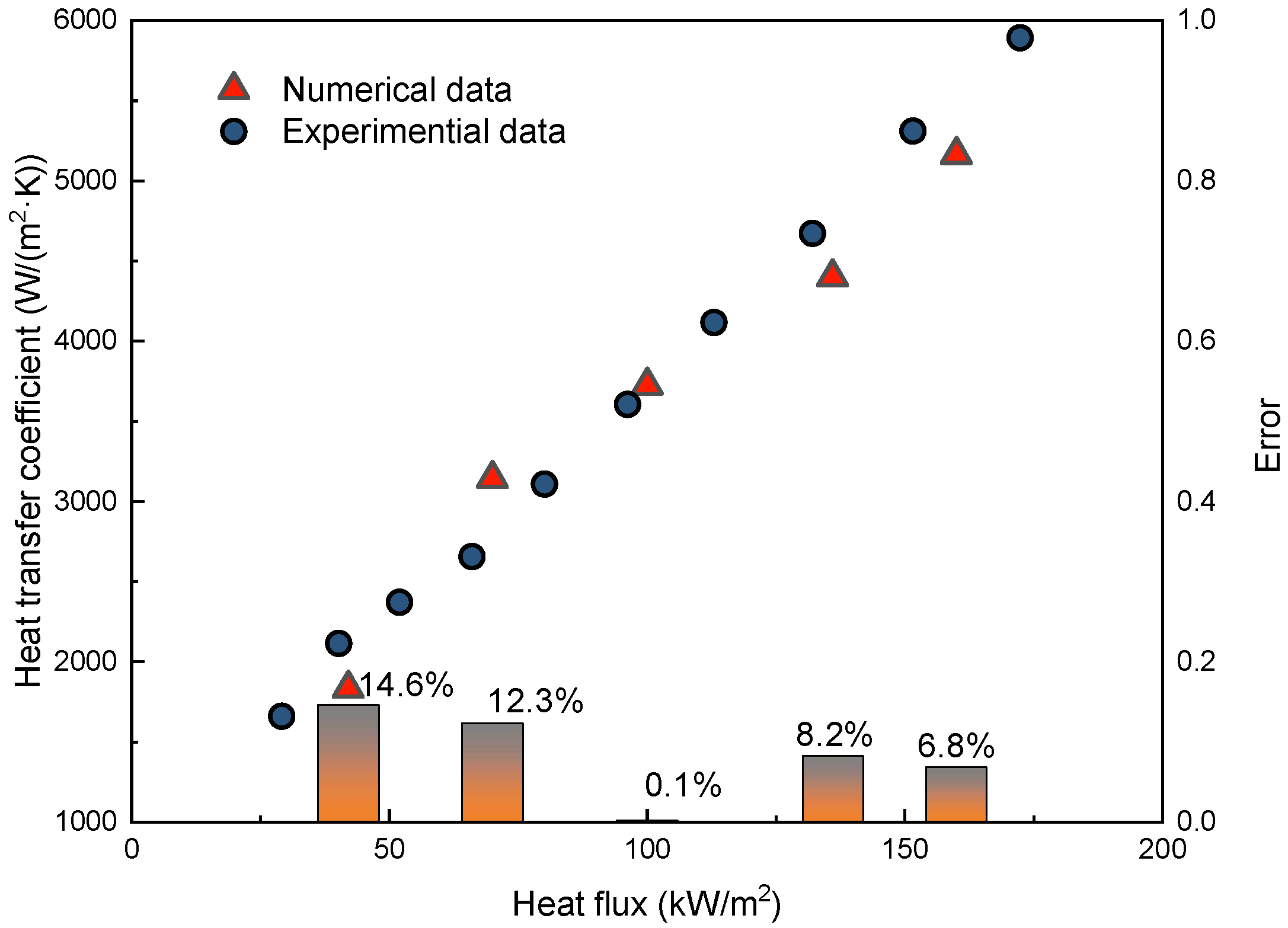
3.5. Validation
4. Results and Discussion
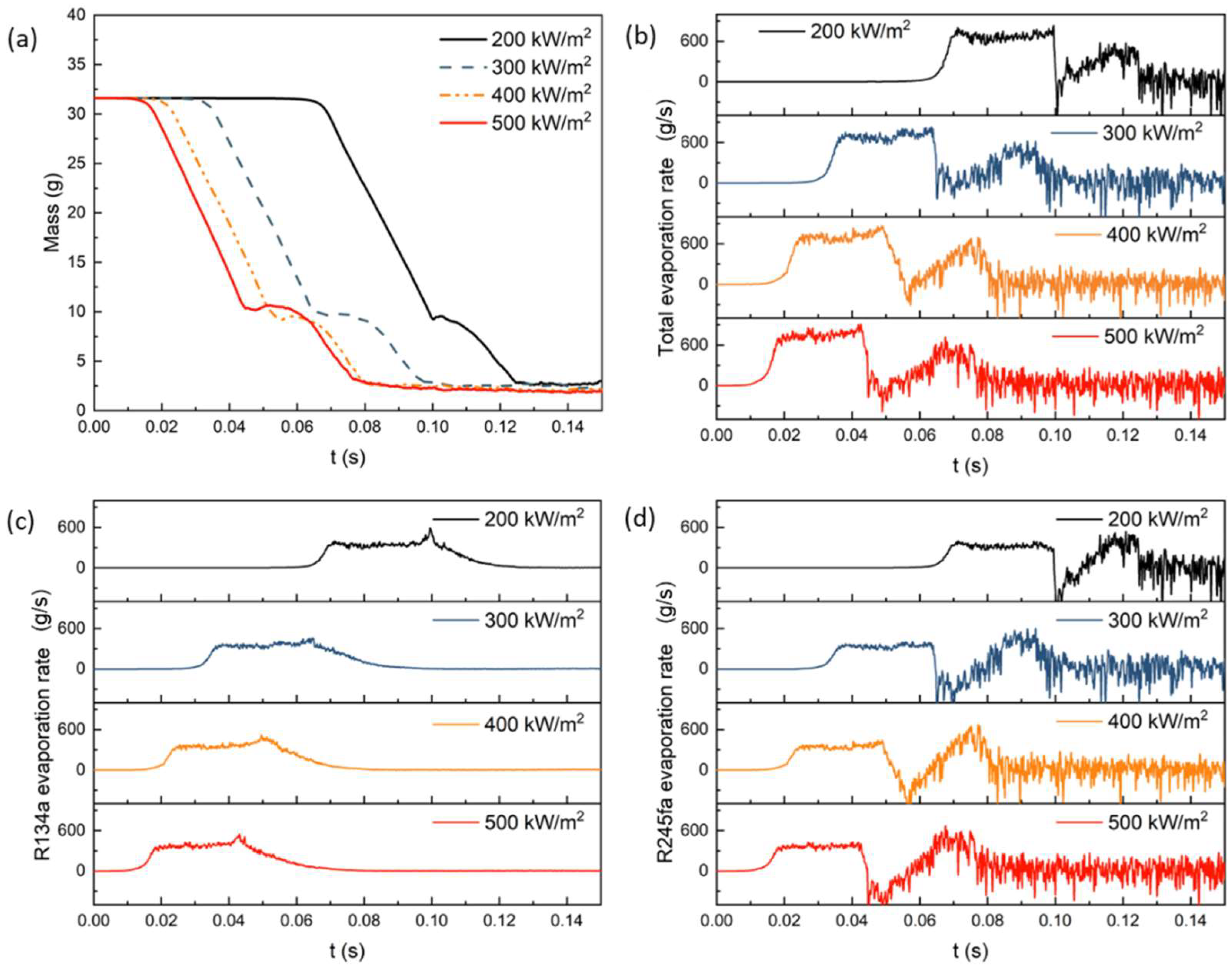
4.1. Effect of Heat Flux on Evaporation Heat Transfer
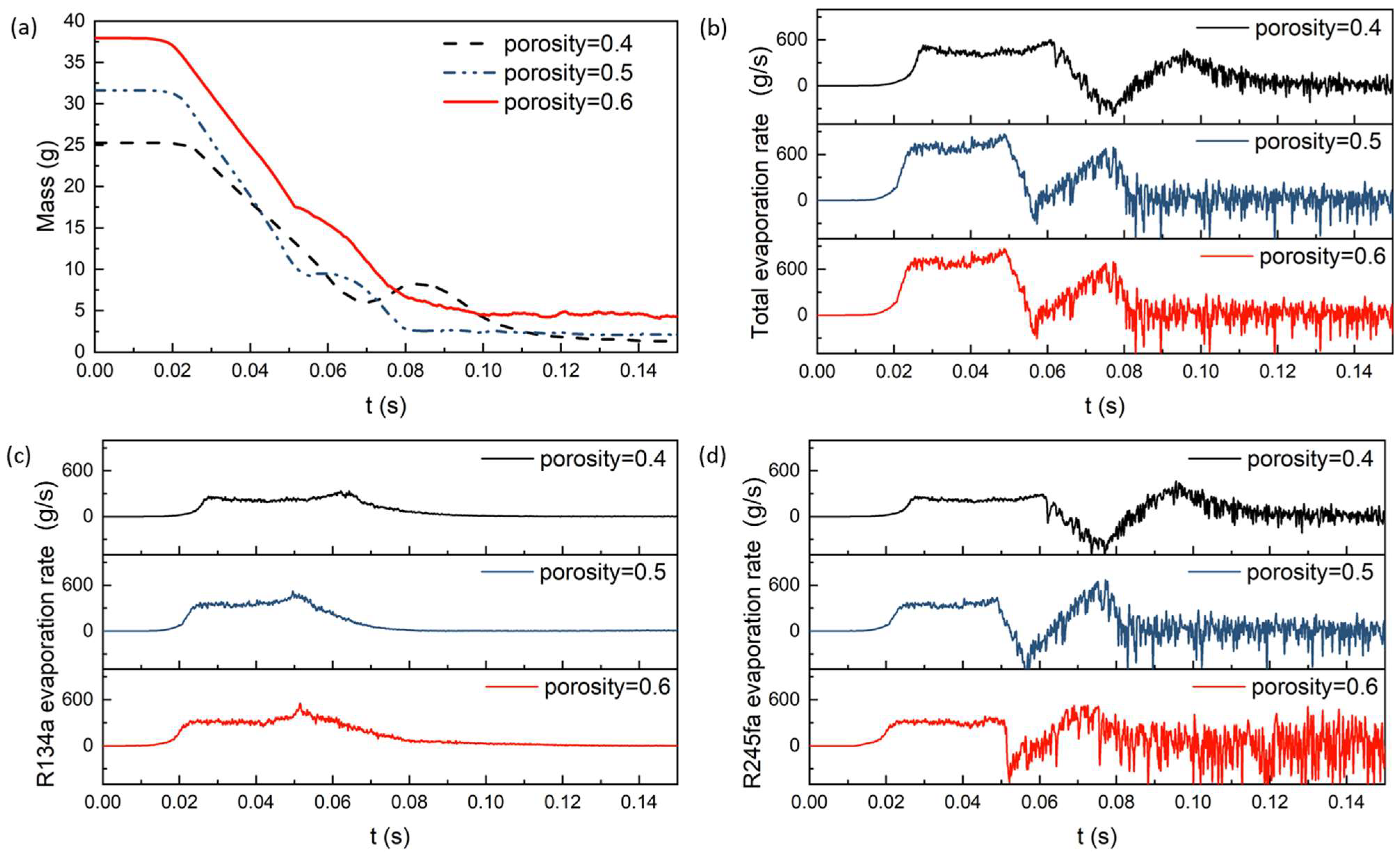
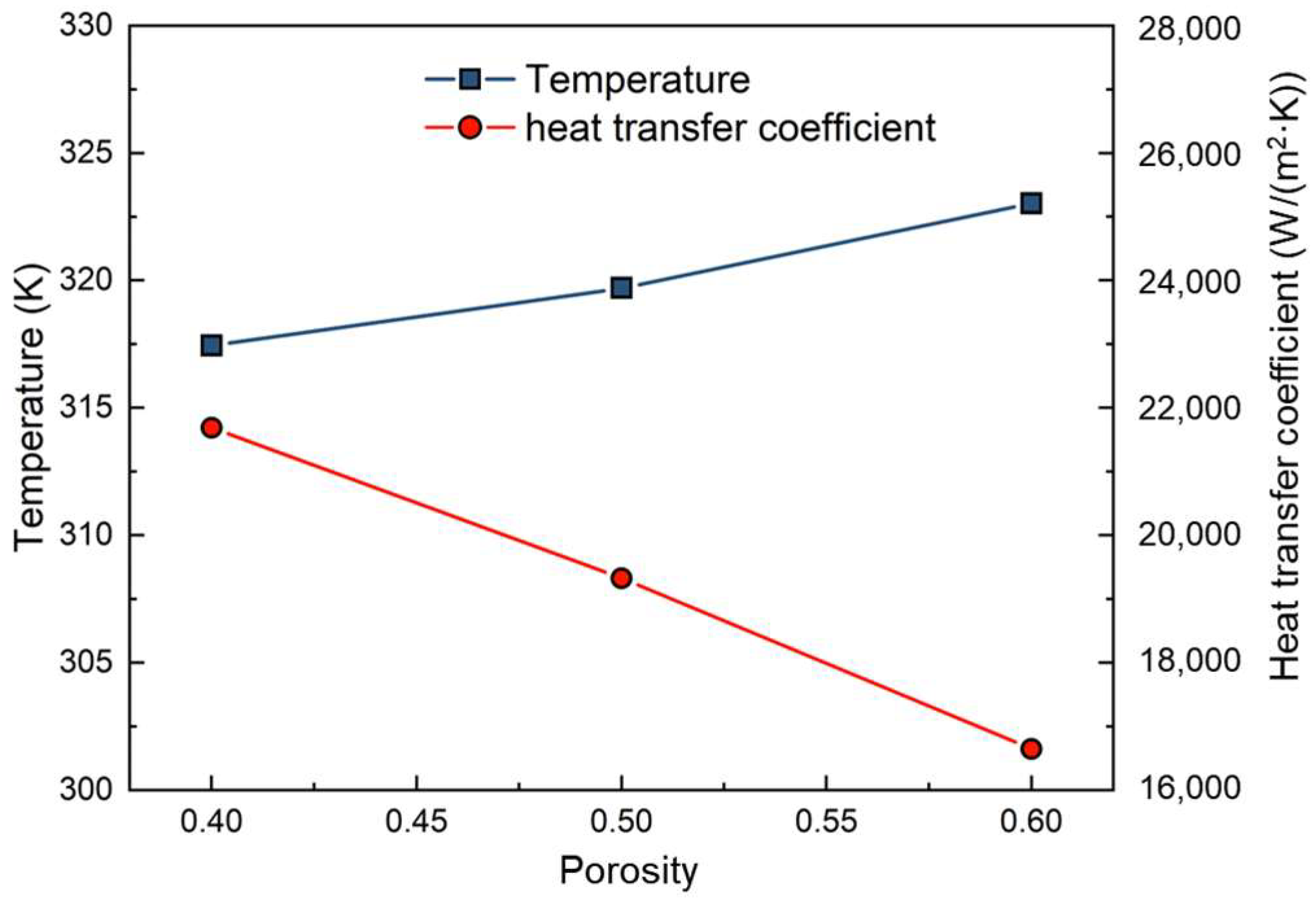
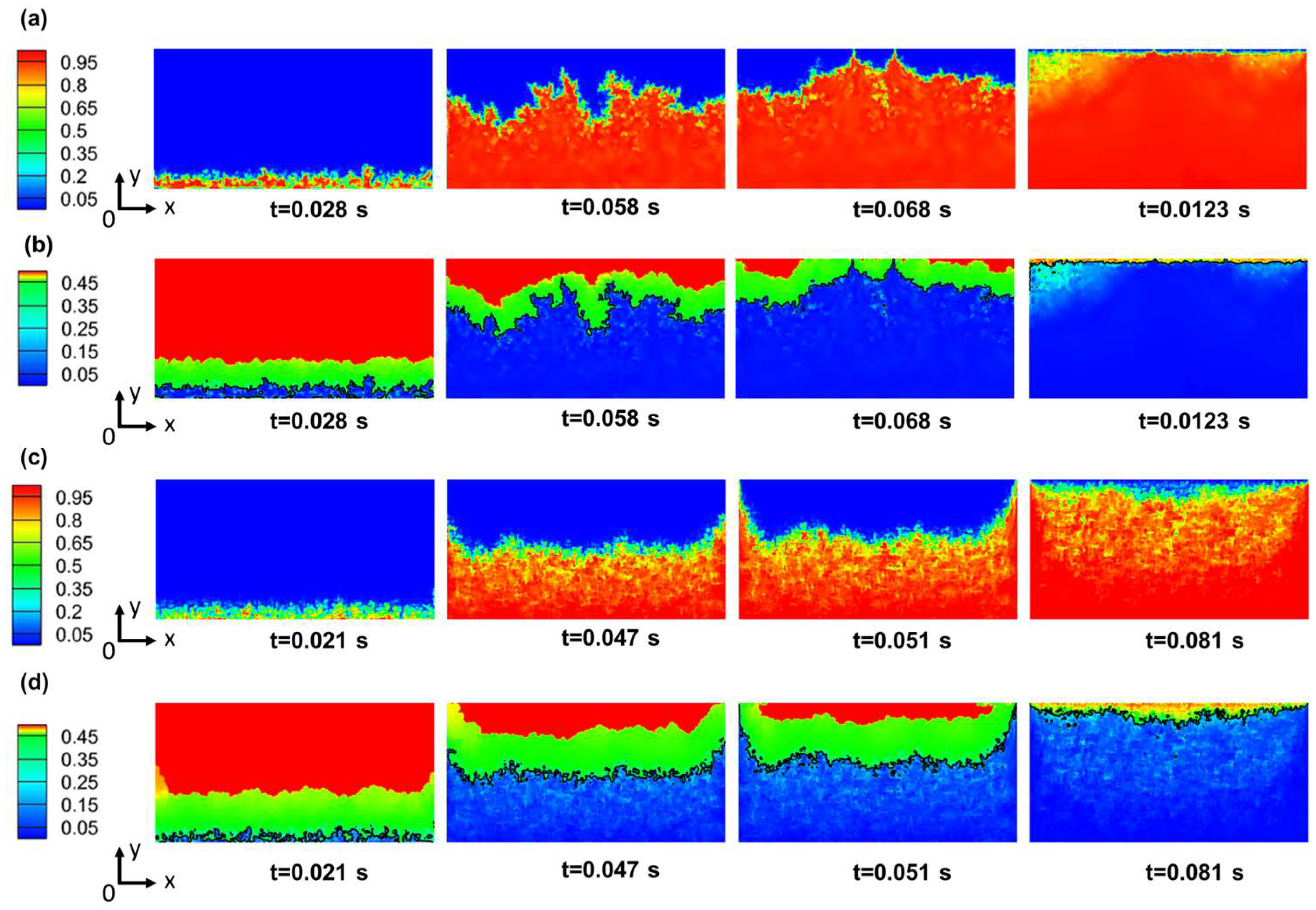
4.2. The Effect of Porosity on Evaporation Heat Transfer
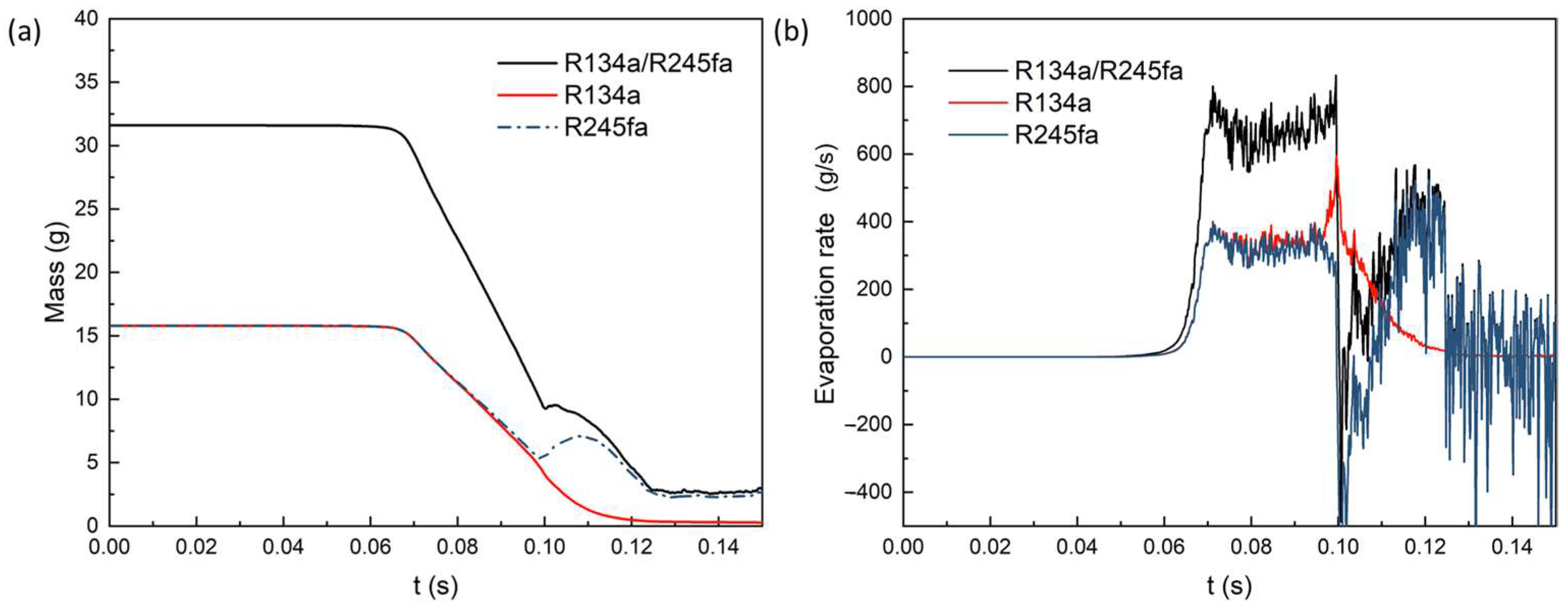
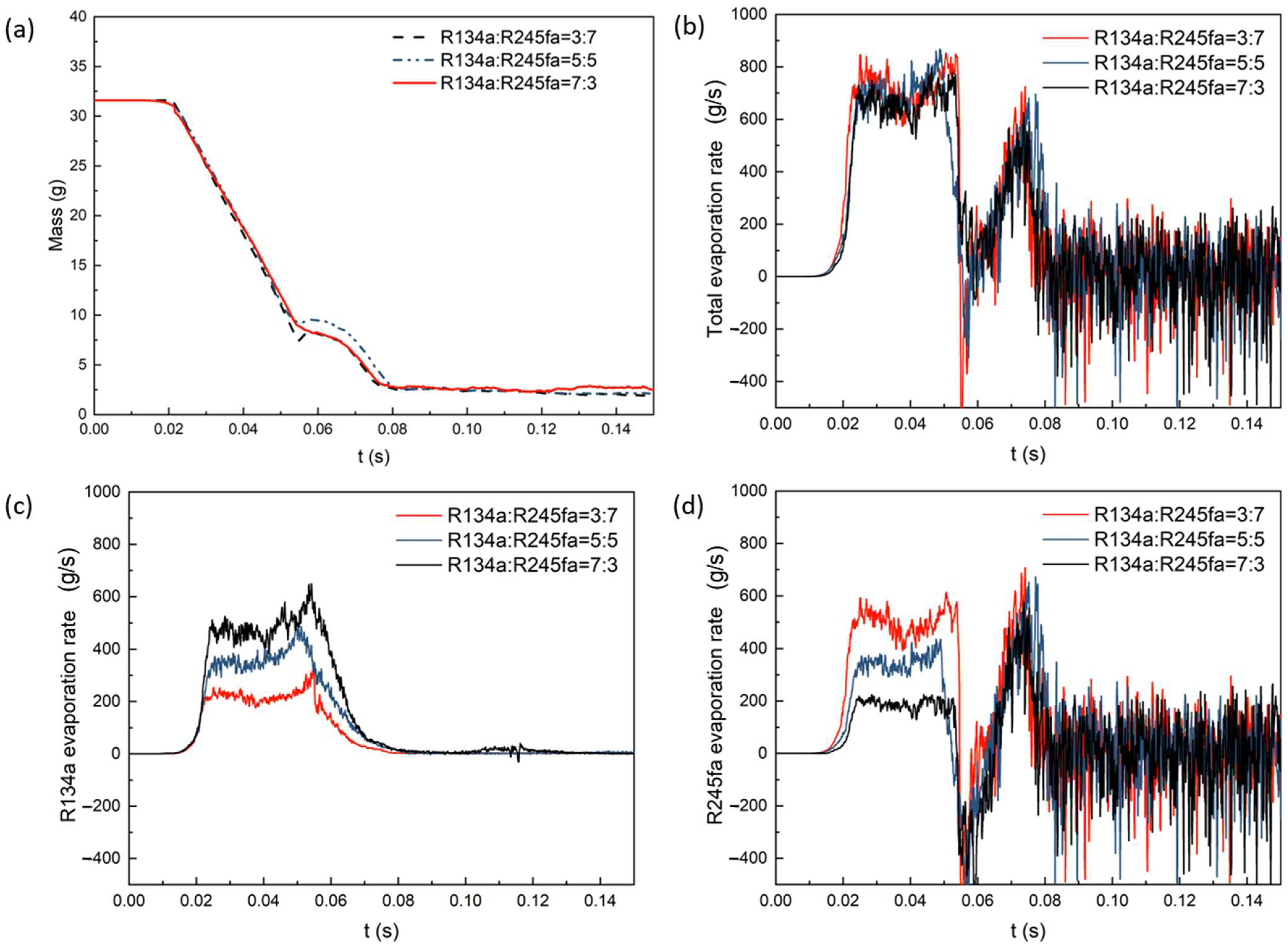
4.3. The Effect of Mass Fraction on Evaporation Heat Transfer
5. Conclusions
- (1)
- With the increase in heat flux, the overall evaporation rate, R134a evaporation rate, and R245fa evaporation rate show certain improvements, with the increases of 11.3%, 6.9%, and 16.3%, respectively. The evaporation rate of R245fa is more sensitive to changes in heat flux. As the heat flux increases, the heat transfer coefficient slightly increases, with a maximum improvement of 1.4%.
- (2)
- Taking into account factors such as inertia resistance coefficient, viscosity resistance coefficient, flow space, and effective thermal conductivity, the overall evaporation rate and R134a (R245fa) evaporation rates achieve their maximum values at a porosity of 0.5 in the porous medium. Additionally, the maximum heat transfer coefficient is observed at a porosity of 0.4.
- (3)
- In a binary mixture, as the mass fraction of the component increases, the corresponding evaporation rate also increases. When the mass fraction ratio (R134a/R245fa) is 5:5, the evaporation rate of R134a is slightly higher than that of R245fa. However, for three different mass fractions, the overall evaporation rate and heat transfer coefficient remain nearly constant.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.; Vazquez, I.R.; Jackson, N. Atomization of High Viscous Liquids Using a MEMS Vibrating Mesh with Integrated Microheater. J. Microelectromechanical Syst. 2023, 32, 362–370. [Google Scholar] [CrossRef]
- Aceti, P.; Rosso, M.; Ardito, R.; Pienazza, N.; Nastro, A.; Baù, M.; Ferrari, M.; Rouvala, M.; Ferrari, V.; Corigliano, A. Optimization of an Impact-Based Frequency up-Converted Piezoelectric Vibration Energy Harvester for Wearable Devices. Sensors 2023, 23, 1391. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Phan, H.-P.; Kashaninejad, N.; Nguyen, T.-K.; Dao, D.V.; Nguyen, N.-T. An On-Chip SiC MEMS Device with Integrated Heating, Sensing, and Microfluidic Cooling Systems. Adv. Mater. Interfaces 2018, 5, 1800764. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, S.; Chen, X.; Chen, D.; Wang, F. PDMS-PDMS Micro Channels Filled with Phase-Change Material for Chip Cooling. Micromachines 2018, 9, 165. [Google Scholar] [CrossRef]
- Clark, M.D.; Weibel, J.A.; Garimella, S.V. Impact of pressure drop oscillations and parallel channel instabilities on microchannel flow boiling and critical heat flux. Int. J. Multiph. Flow 2023, 161, 104380. [Google Scholar] [CrossRef]
- Ren, C.; Li, W.; Ma, J.; Huang, G.; Li, C. Flow boiling in microchannels enhanced by parallel microgrooves fabricated on the bottom surfaces. Int. J. Heat Mass Transf. 2021, 166, 120756. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, J.; Qu, J.; Lei, Q.; Li, C.; Chen, Y. Characterizing effect of particle size on flow boiling in sintered porous-microchannels. Appl. Therm. Eng. 2023, 229, 120571. [Google Scholar] [CrossRef]
- Azzolin, M.; Bortolin, S.; Del Col, D. Flow boiling heat transfer of a zeotropic binary mixture of new refrigerants inside a single microchannel. Int. J. Therm. Sci. 2016, 110, 83–95. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Yang, R.; Liu, W.; Wu, H.; Ding, Y.; Li, Y. A review of boiling heat transfer characteristics in binary mixtures. Int. J. Heat Mass Transf. 2021, 164, 120570. [Google Scholar] [CrossRef]
- Li, J.; Lin, L.; Li, S.; Yang, Z.; Duan, Y. Experimental study on nucleate pool boiling heat transfer characteristics of R32 + R1234yf binary mixtures. Appl. Therm. Eng. 2022, 205, 118047. [Google Scholar] [CrossRef]
- Dang, C.; Jia, L.; Peng, Q.; Huang, Q.; Zhang, X. Experimental and analytical study on nucleate pool boiling heat transfer of R134a/R245fa zeotropic mixtures. Int. J. Heat Mass Transf. 2018, 119, 508–522. [Google Scholar] [CrossRef]
- Diddens, C.; Tan, H.; Lv, P.; Versluis, M.; Kuerten, J.G.M.; Zhang, X.; Lohse, D. Evaporating pure, binary and ternary droplets: Thermal effects and axial symmetry breaking. J. Fluid Mech. 2017, 823, 470–497. [Google Scholar] [CrossRef]
- Gurrala, P.; Katre, P.; Balusamy, S.; Banerjee, S.; Sahu, K.C. Evaporation of ethanol-water sessile droplet of different compositions at an elevated substrate temperature. Int. J. Heat Mass Transf. 2019, 145, 118770. [Google Scholar] [CrossRef]
- Duursma, G.; Sefiane, K.; Clarke, J. Diffusion-Evaporation Studies of Binary Mixtures in Capillary Tubes. Defect Diffus. Forum 2008, 273–276, 577–582. [Google Scholar] [CrossRef]
- Lv, C.; Varanakkottu, S.N.; Hardt, S. Liquid plug formation from heated binary mixtures in capillary tubes. J. Fluid Mech. 2020, 889, A15. [Google Scholar] [CrossRef]
- Dang, C.; Jia, L.; Peng, Q.; Yin, L.; Qi, Z. Experimental study on flow boiling heat transfer for pure and zeotropic refrigerants in multi-microchannels with segmented configurations. Int. J. Heat Mass Transf. 2018, 127, 758–768. [Google Scholar] [CrossRef]
- Vajc, V.; Moe, M.; Hadi, A.; Ŝulc, R.; Golobi, I. Saturated and subcooled pool boiling heat transfer in mixtures of water and glycerin. Exp. Heat Transf. 2023, 36, 283–311. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, S.; Huang, J.; Song, M. Marangoni effect on pool boiling heat transfer enhancement of self-rewetting fluid. Int. J. Heat Mass Transf. 2018, 127, 1263–1270. [Google Scholar] [CrossRef]
- Canbazoglu, F.M.; Fan, B.; Vemuri, K.; Bandaru, P.R. Enhanced Solar Thermal Evaporation of Ethanol-Water Mixtures, through the Use of Porous Media. Langmuir 2018, 34, 10523–10528. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Duan, Y. Numerical simulation of single bubble growth and heat transfer of R32 + R1234yf binary mixtures during saturated pool boiling. Int. J. Therm. Sci. 2023, 191, 108380. [Google Scholar] [CrossRef]
- Yang, R.; Wang, Y.; Li, Y. Numerical investigations on flow boiling heat transfer of ammonia water binary solution (NH3/H2O) in a horizontal microchannel. Int. J. Heat Mass Transf. 2021, 171, 121091. [Google Scholar] [CrossRef]
- Lee, H.; Kharangate, C.R.; Mascarenhas, N.; Park, I.; Mudawar, I. Experimental and computational investigation of vertical downflow condensation. Int. J. Heat Mass Transf. 2015, 85, 865–879. [Google Scholar] [CrossRef]
- Lee, H.; Kharangate, C.R.; Mascarenhas, N.; Park, I.; Mudawar, I. Selective evaporation rate modeling of volatile binary mixture droplets. Int. J. Heat Mass Transf. 2021, 178, 121584. [Google Scholar] [CrossRef]



| No. | Grid Number | (K) | e% | m (g) | e% |
|---|---|---|---|---|---|
| 1 | 10,000 | 300.281 | 0.014% | 31.28 | 0.477% |
| 2 | 20,000 | 300.324 | 0.007% | 31.43 | 0.412% |
| 3 | 80,000 | 300.345 | 0.006% | 31.56 | 0.032% |
| 4 | 125,000 | 300.328 | - | 31.55 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Cui, P.; Wang, Z.; Sun, Z.; Kong, B.; Wang, W.; Du, W.; Huang, P.; Pan, Z.; Liu, Z. Numerical Study on Heat and Mass Transfer of Evaporated Binary Zeotropic Mixtures in Porous Structure. Energies 2023, 16, 6526. https://doi.org/10.3390/en16186526
Zhang B, Cui P, Wang Z, Sun Z, Kong B, Wang W, Du W, Huang P, Pan Z, Liu Z. Numerical Study on Heat and Mass Transfer of Evaporated Binary Zeotropic Mixtures in Porous Structure. Energies. 2023; 16(18):6526. https://doi.org/10.3390/en16186526
Chicago/Turabian StyleZhang, Bo, Peilin Cui, Zhiguo Wang, Zhiwei Sun, Bo Kong, Wei Wang, Wen Du, Ping Huang, Zhenhai Pan, and Zhenyu Liu. 2023. "Numerical Study on Heat and Mass Transfer of Evaporated Binary Zeotropic Mixtures in Porous Structure" Energies 16, no. 18: 6526. https://doi.org/10.3390/en16186526
APA StyleZhang, B., Cui, P., Wang, Z., Sun, Z., Kong, B., Wang, W., Du, W., Huang, P., Pan, Z., & Liu, Z. (2023). Numerical Study on Heat and Mass Transfer of Evaporated Binary Zeotropic Mixtures in Porous Structure. Energies, 16(18), 6526. https://doi.org/10.3390/en16186526







