Experimental Investigation of Compression Ignition Engine Combustion, Performance, and Emission Characteristics of Ternary Blends with Higher Alcohols (1-Heptanol and n-Octanol)
Abstract
:1. Introduction
Motivation, Novelty, and Objective of the Study
2. Materials and Methods
2.1. Preparations of the Fuels
2.2. Testing of the Fuels
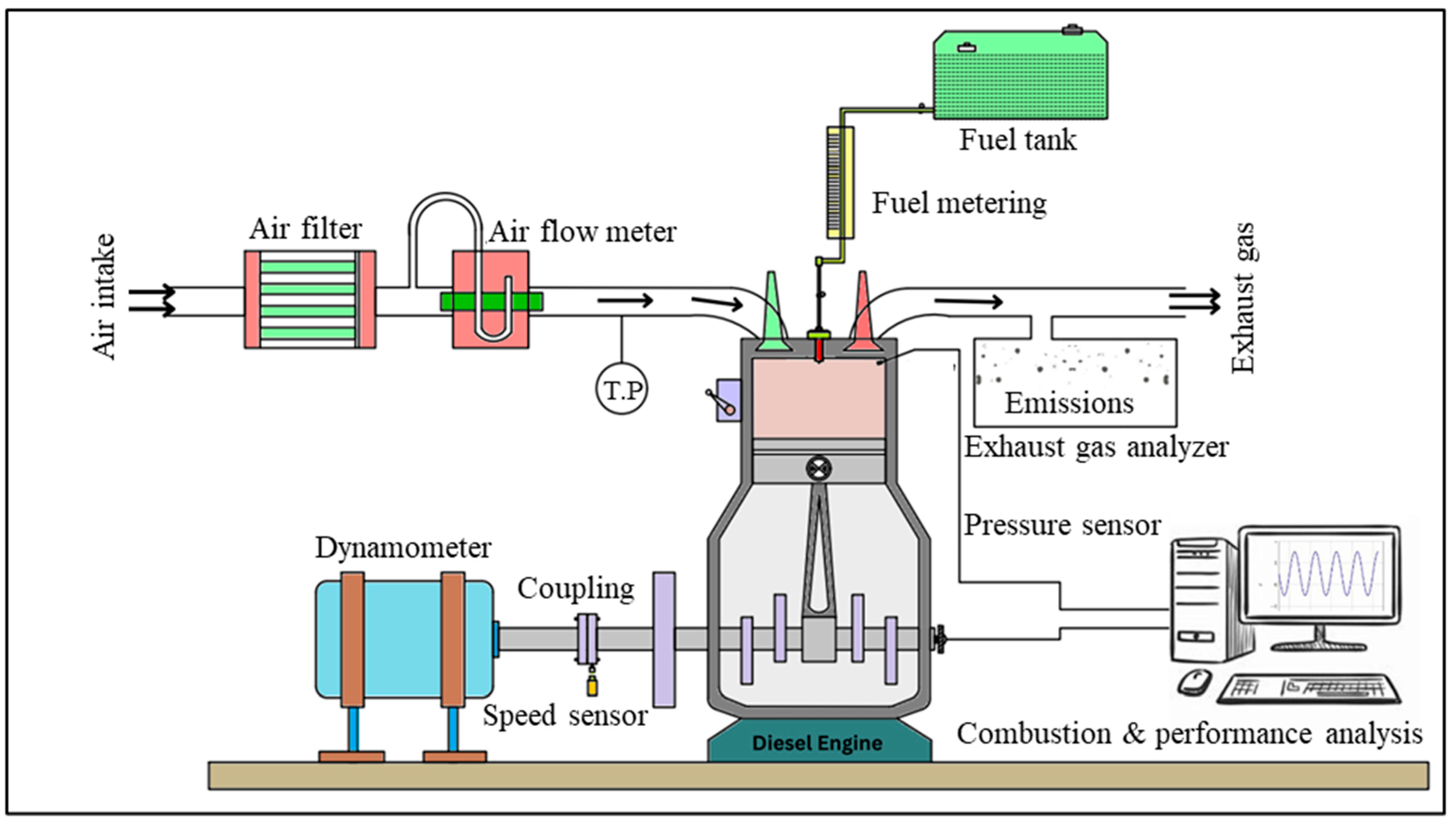
2.3. Test Engine and Facilities
2.4. Experimental Procedures
2.5. Error and Uncertainty Analysis
3. Results and Discussion
3.1. Combustion Analysis
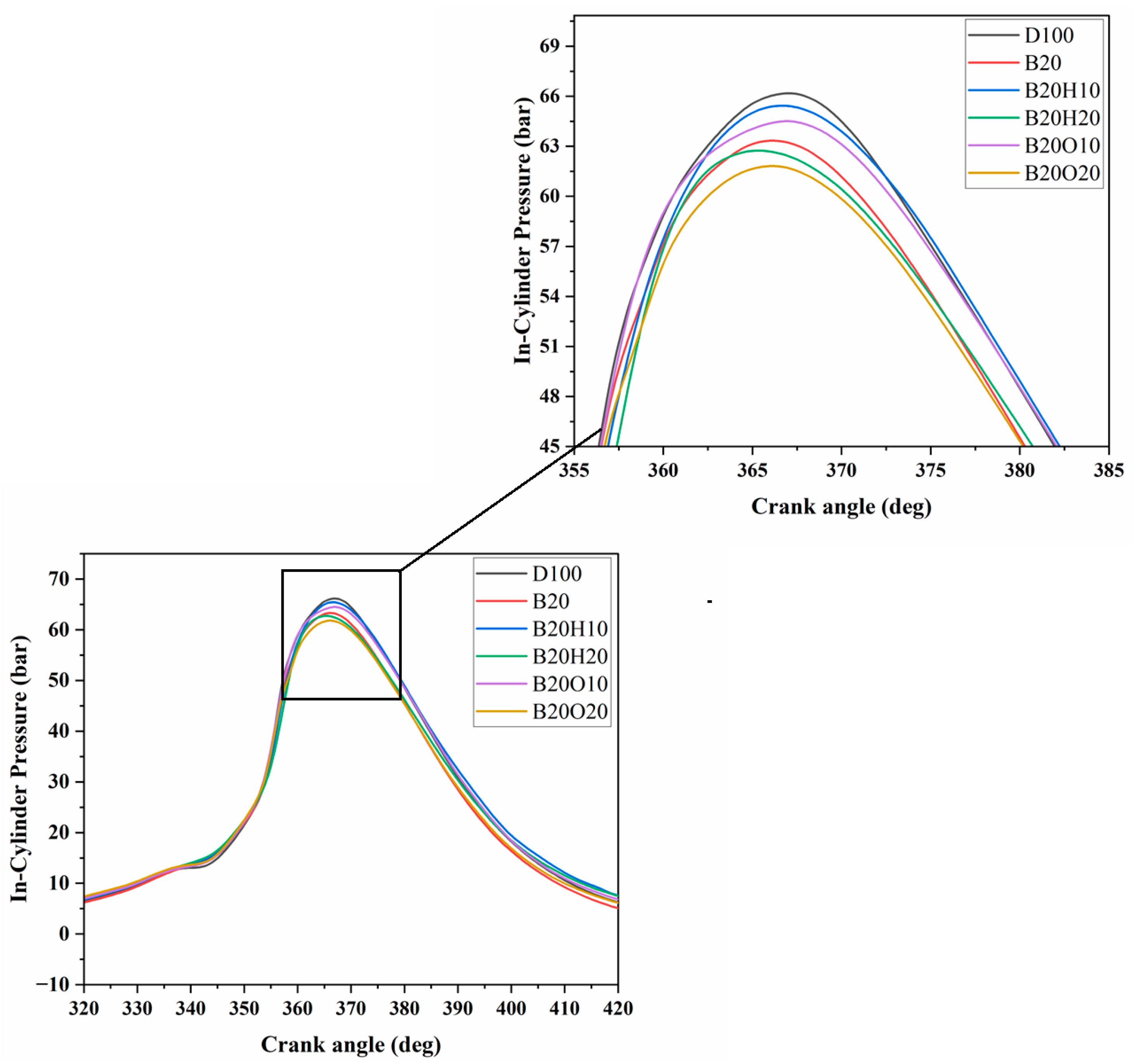
3.1.1. In-Cylinder Pressure
3.1.2. Net Heat Release Rate
3.2. Engine Performance
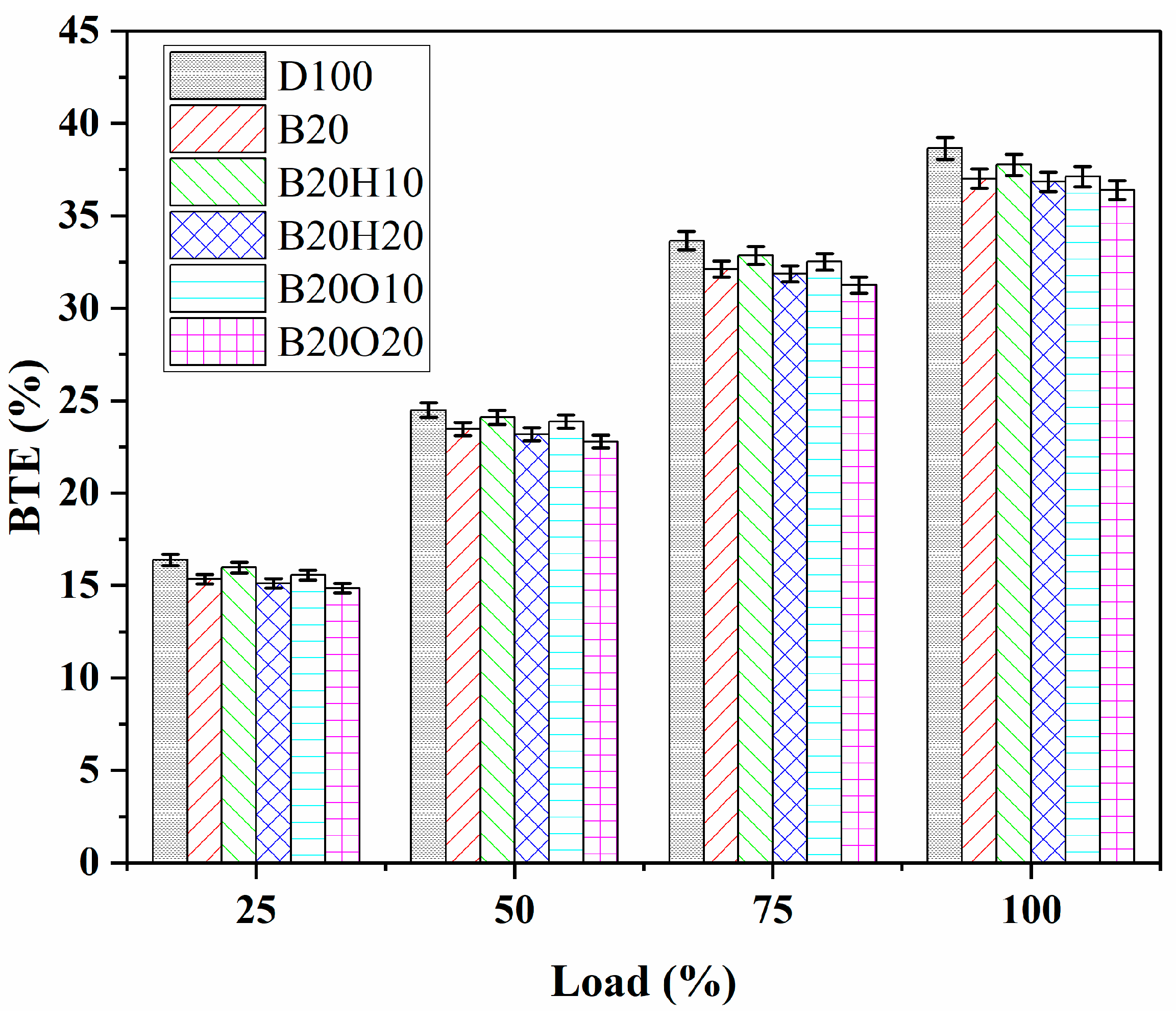
3.2.1. Brake Thermal Efficiency (BTE)
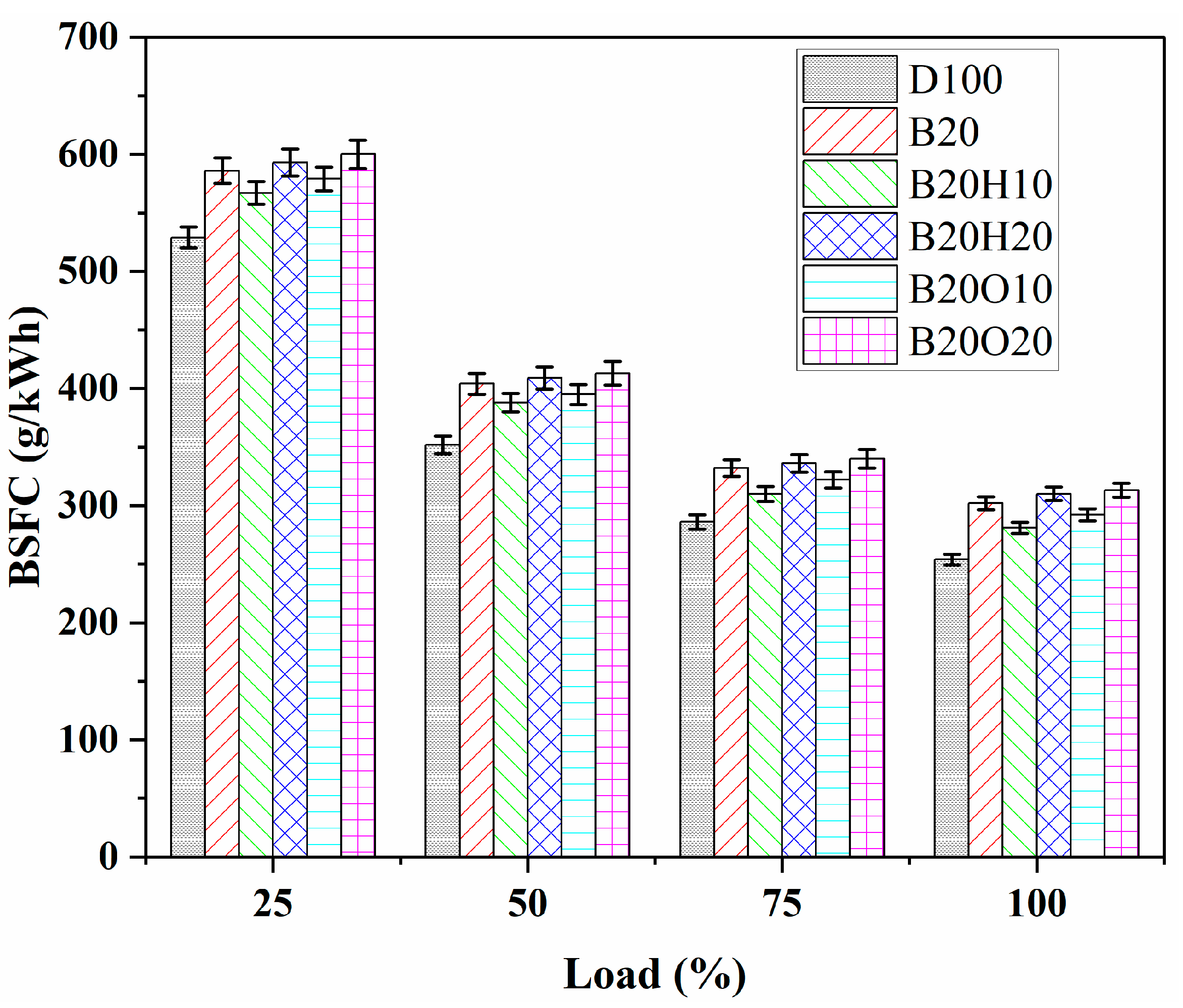
3.2.2. Break-Specific Fuel Consumption (BSFC)
3.3. Exhaust Emissions
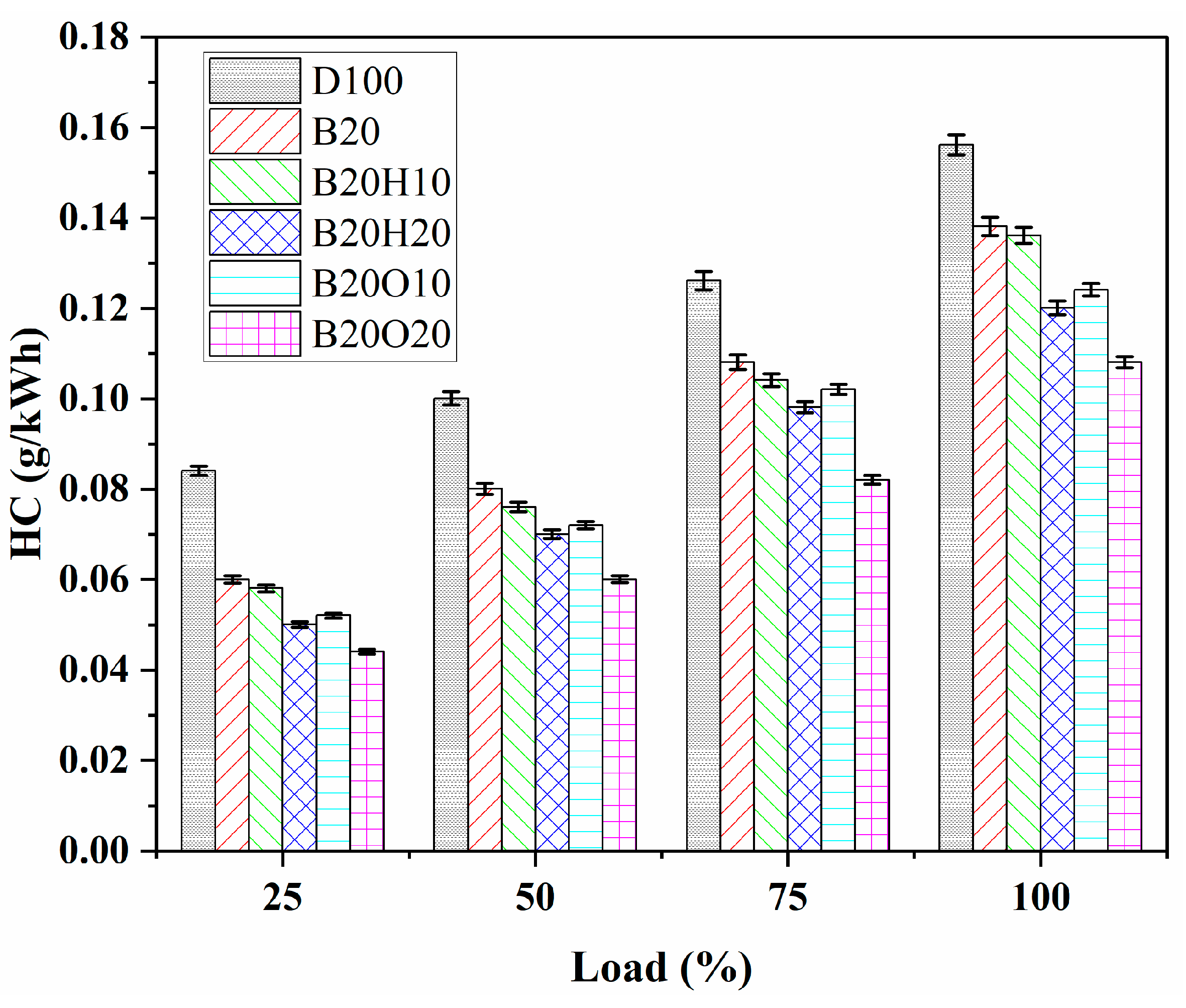
3.3.1. HC Emission
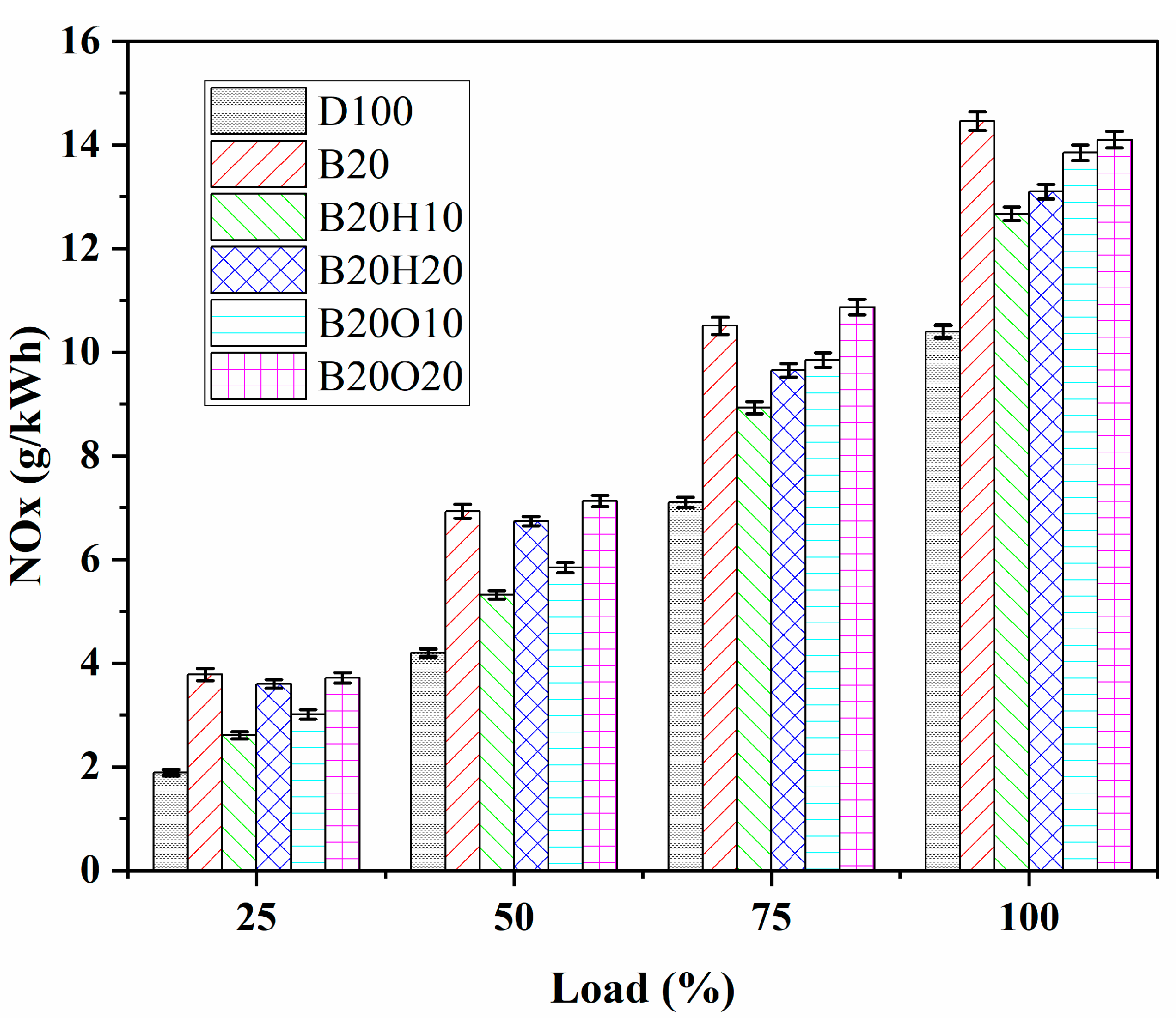
3.3.2. NOx Emission
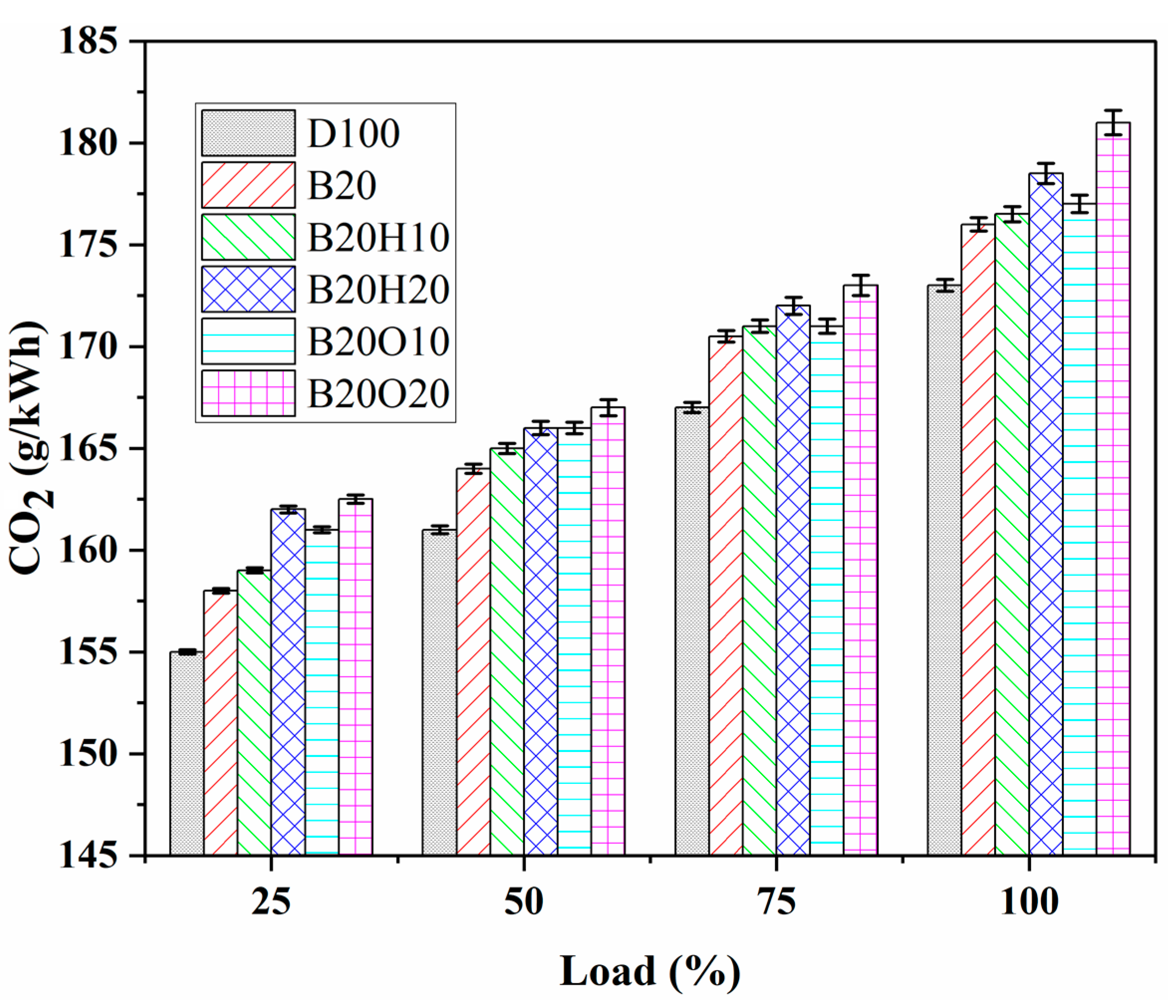
3.3.3. CO2 Emission
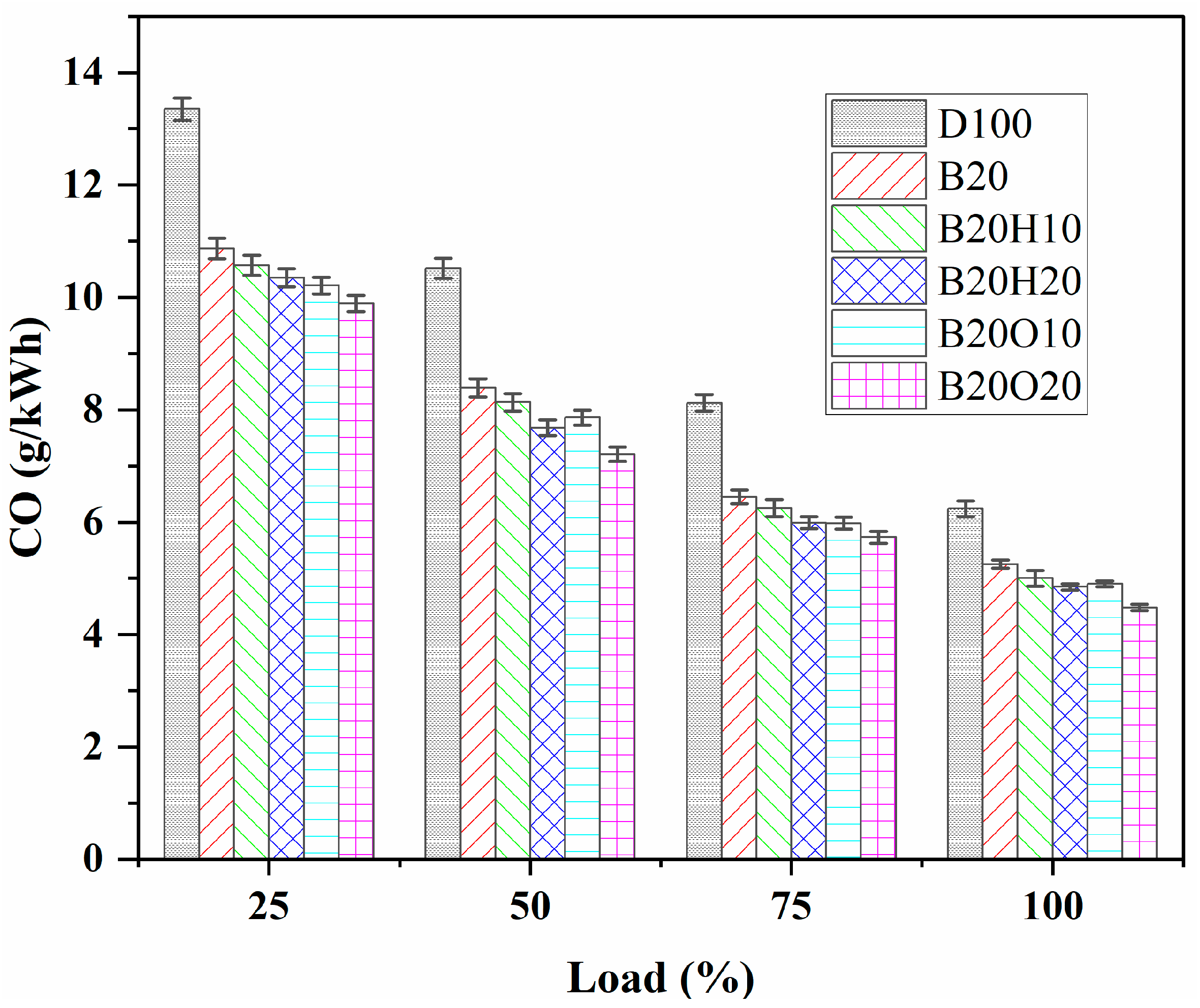
3.3.4. CO Emission
4. Conclusions
- The introduction of lower percentages of 1-heptanol and n-octanol in the diesel/biodiesel fuel blend resulted in higher peak in-cylinder gas pressure compared to the binary blend. However, increasing the percentage to 20% in the diesel/biodiesel fuel blend led to a reduction in CP.
- The low percentage of heptanol in the diesel/biodiesel provided the highest heat released per cycle at all tested conditions compared to that of the low percentage of octanol fuel blends and B20. Additionally, Heptanol/diesel blends showed the highest combustion efficiency among the tested fuels.
- Diesel fuel demonstrated higher BTE owing to its superior heating value. The introduction of lower percentages of 1-heptanol and n-octanol in the blend resulted in higher BTE compared to binary blends but decreased with higher proportions of these alcohols. The BTE decrease followed the sequence B20O20 < B20H20 < B20 < B20O10 < B20H10. B20O20 experienced the smallest BTE decrease compared to diesel.
- Diesel fuel exhibited a lower BSFC compared to binary blends and ternary blends of 1-heptanol and n-octanol due to its higher energy content and better thermal efficiency. Fuels with higher proportions of 1-heptanol and n-octanol (B20H20 and B20O20) displayed higher BSFC values compared to B20H10 and B20O10. The lower BSFC may be attributed to the higher enthalpy of evaporation in heptanol (574 kJ/kg) compared to octanol (315 kJ/kg). Various factors, including the latent heat of evaporation, calorific value, viscosity, and density, influenced engine performance and combustion characteristics.
- Ternary blends with n-octanol showed lower HC emissions than diesel fuel at all loads. However, the amount of n-octanol in the blend affected HC levels, with higher percentages leading to overlean mixtures and increased oxygen content. Adding 1-heptanol to biodiesel/diesel blends reduced HC emissions compared to pure diesel but remained higher than n-octanol-blended fuel. The maximum HC emissions were in the order of D100 > B20 > B20H10 > B20O10 > B20H20 > B20O20.
- Diesel fuel resulted in the lowest NOx emissions at all loads. The addition of n-octanol to biodiesel/diesel fuel (B20O20) increased NOx emissions due to higher oxygen content, resulting in weaker combustion conditions. The ternary blend B20H10 showed the lowest NOx emissions. However, it still had higher NOx emissions than diesel fuel, increasing by 21.8% at full load. The maximum NOx emissions are in the order of B20 > B20O20 > B20H20 > B20O10 > B20H10 > D100.
- The B20O20 fuel blend had the highest CO2 emissions at 181.11 g/kWh due to its native oxygen atoms readily reacting with CO to form CO2. CO2 emissions for other blends ranged from 173.23 g/kWh to 181.11 g/kWh. Blends with higher alcohols showed similar CO2 emissions, with a difference of around 7 to 8%. The addition of alcohol reduced CO2 emissions compared to the B20 blend, with 1-heptanol playing a significant role in CO2 formation. The maximum CO2 emissions were in the order of B20O20 > B20H20 > B20O10 > B20H10 > B20 > D100.
- CO emissions were reduced significantly more with the addition of alcohol than diesel and B20 blends. Ternary blends of 1-heptanol (B20H10 and B20H20) exhibited 19.71% and 22.27% lower CO emissions than pure diesel oil at full load. Similarly, ternary blends of n-octanol (B20O10 and B20O20) showed 21.31% and 28.2% lower CO emissions than pure diesel oil at full load. The higher oxygen content of n-octanol contributed to this effect, and alcohols’ fewer carbon atoms in the chemical bond resulted in less CO formation. The least CO emissions were in the order of B20O20 < B20O10 < B20H20 < B20H10 < B20 < D100.
- The obtained results with ternary blends ensure an alternate promising next-generation ecofriendly fuel to run in diesel engines without significant modifications. The detailed insights could help to prepare ternary blends at an industrial scale. This research work can be extended in the following directions: 1. Higher alcohols, such as hexanol and decanal, can be investigated as fuel additives for diesel engines. 2. Engine parameters such as injection timing, injection pressure, compression ratio, and engine speed can be altered and analysed for better performance, combustion, and emission characteristics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ASTM | American Society for Testing and Materials |
| B100 | 100% Biodiesel fuel |
| D100/DF | Reference diesel fuel |
| B20 | Diesel (80%) + Biodiesel (20%) by vol. |
| B20H10 | Diesel (70%) + Biodiesel (20%) + 1-heptanol (10%) by vol. |
| B20H20 | Diesel (60%) + Biodiesel (20%) + 1-heptanol (20%) by vol. |
| B20O10 | Diesel (70%) + Biodiesel (20%) + n-octanol (10%) by vol. |
| B20O20 | Diesel (60%) + Biodiesel (20%) + n-octanol (20%) by vol. |
| CN | Cetane number |
| NaOH | Sodium hydroxide |
| HHV | Higher heating value |
| VCR | Variable compression ratio |
| CA, θ | Crank angle |
| NOx | Nitrogen oxides |
| HC | Unburnt hydrocarbons |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| PM | Particulate matter |
| BSFC | Brake-specific fuel consumption |
| BTE | Brake thermal efficiency |
| C4–C8 | Carbon chain length |
| CI | Compression-ignition |
| CRDI | Common rail direct injection |
| VCR | Variable compression ratio |
| HBO | Hybrid biodiesel oil |
| CHO | Crude hybrid oil |
| CP | Cylinder Pressure |
| DF | Diesel fuel |
| ICEs | Internal combustion engines |
| TDC | Top dead centre |
| NHRR | Net heat release rate |
| CR | Compression ratio |
| NO | Nitrogen monoxide |
| NO2 | Nitrogen dioxide |
| N2O2 | Dinitrogen dioxide |
| N2O3 | Dinitrogen trioxide |
| N2O4 | Dinitrogen tetroxide |
| N2O5 | Dinitrogen pentoxide |
| OS | Oxidation stability |
References
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Beretta, G.P. World energy consumption and resources: An outlook for the rest of the century. Int. J. Environ. Technol. Manag. 2007, 7, 99–112. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- De Almeida D’Agosto, M. Energy sources for transportation. In Transportation, Energy Use and Environmental Impacts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–225. [Google Scholar] [CrossRef]
- Kumar Kadian, A.; Khan, M.; Sharma, R.P.; Mozammil hasnain, S.M. Performance enhancement and emissions mitigation of DI-CI engine fuelled with ternary blends of jatropha biodiesel-diesel-heptanol. Mater. Sci. Energy Technol. 2022, 5, 145–154. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; Kosaka, H.; Hassan, H.; Sato, S. Combustion and emission characteristics of a common rail diesel engine and RCEM fueled by n-heptanol-diesel blends and carbon nanomaterial additives. Energy Convers. Manag. 2019, 196, 370–394. [Google Scholar] [CrossRef]
- Sarma, C.J.; Sharma, P.; Bora, B.J.; Bora, D.K.; Senthilkumar, N.; Balakrishnan, D.; Ayesh, A.I. Improving the combustion and emission performance of a diesel engine powered with mahua biodiesel and TiO2 nanoparticles additive. Alex. Eng. J. 2023, 72, 387–398. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. Intensified transesterification of mixture of edible and nonedible oils in reverse flow helical coil reactor for biodiesel production. Renew. Energy 2019, 134, 509–525. [Google Scholar] [CrossRef]
- Da Silva Guabiroba, R.C.; da Silva, R.M.; da Silva César, A.; da Silva, M.A.V. Value chain analysis of waste cooking oil for biodiesel production: Study case of one oil collection company in Rio de Janeiro—Brazil. J. Clean. Prod. 2017, 142, 3928–3937. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.J.; Ngadi, M. Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew. Sustain. Energy Rev. 2015, 45, 574–588. [Google Scholar] [CrossRef]
- Ayoob, A.K.; Fadhil, A.B. Valorization of waste tires in the synthesis of an effective carbon based catalyst for biodiesel production from a mixture of non-edible oils. Fuel 2020, 264, 116754. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Aljaafari, A.; Fattah, I.M.R.; Jahirul, M.I.; Gu, Y.; Mahlia, T.M.I.; Islam, M.A.; Islam, M.S. Biodiesel emissions: A state-of-the-art review on health and environmental impacts. Energies 2022, 15, 6854. [Google Scholar] [CrossRef]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M. Performance and emission characteristics of diesel engine fueled with rice bran biodiesel and n-butanol. Energy Rep. 2019, 5, 78–83. [Google Scholar] [CrossRef]
- Samuel, O.D.; Gulum, M. Mechanical and corrosion properties of brass exposed to waste sunflower oil biodiesel-diesel fuel blends. Chem. Eng. Commun. 2019, 206, 682–694. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Hernández, J.J.; Calle-Asensio, A.; Ramos, Á.; Barba, J. Selection of blends of diesel fuel and advanced biofuels based on their physical and thermochemical properties. Energies 2019, 12, 2034. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Parthasarathy, M.; Lalvani, J.S.C.I.J.; Mehboob, H.; Samuel, O.D.; Enweremadu, C.C.; Saleel, C.A.; Afzal, A. Effect of injection timing in reducing the harmful pollutants emitted from CI engine using N-butanol antioxidant blended eco-friendly Mahua biodiesel. Energy Rep. 2021, 7, 6205–6221. [Google Scholar] [CrossRef]
- Emiroğlu, A.O.; Keskin, A.; Şen, M. Experimental investigation of the effects of turkey rendering fat biodiesel on combustion, performance and exhaust emissions of a diesel engine. Fuel 2017, 216, 266–273. [Google Scholar] [CrossRef]
- Nour, M.; Attia, A.M.A.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Karthickeyan, V. Effect of cetane enhancer on Moringa oleifera biodiesel in a thermal coated direct injection diesel engine. Fuel 2018, 235, 538–550. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. A detailed investigation on the performance, combustion, and exhaust emission characteristics of a diesel engine running on the blend of diesel fuel, biodiesel and 1-heptanol (C7 alcohol) as a next-generation higher alcohol. Fuel 2020, 275, 117893. [Google Scholar] [CrossRef]
- Tripathi, S.; Subramanian, K.A. Experimental investigation of utilization of Soya soap stock based acid oil biodiesel in an automotive compression ignition engine. Appl. Energy 2017, 198, 332–346. [Google Scholar] [CrossRef]
- Shelke, P.S.; Sakhare, N.M.; Lahane, S. Investigation of Combustion Characteristics of a Cottonseed Biodiesel Fuelled Diesel Engine. Procedia Technol. 2016, 25, 1049–1055. [Google Scholar] [CrossRef]
- Gürü, M.; Artukoǧlu, B.D.; Keskin, A.; Koca, A. Biodiesel production from waste animal fat and improvement of its characteristics by synthesized nickel and magnesium additive. Energy Convers. Manag. 2009, 50, 498–502. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, H.; Yoon, S.; Choi, N. Reducing volatile organic compound emissions from diesel engines using canola oil biodiesel fuel and blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. The effects of the fuel injection pressure on the performance and emission characteristics of a diesel engine fuelled with waste cooking oil biodiesel-diesel blends. Renew. Energy 2019, 132, 649–666. [Google Scholar] [CrossRef]
- Santhoshkumar, A.; Thangarasu, V.; Anand, R. Performance, combustion, and emission characteristics of DI diesel engine using mahua biodiesel. In Advanced Biofuels Applications, Technologies and Environmental Sustainability; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2019; pp. 291–327. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M.; Lim, H.C. A study on the performance and emission of a diesel engine fueled with Karanja biodiesel and its blends. Energy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Altaie, M.A.H.; Janius, R.B.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R.; Zakaria, R.; Adam, N.M. Performance and exhaust emission characteristics of direct-injection diesel engine fueled with enriched biodiesel. Energy Convers. Manag. 2015, 106, 365–372. [Google Scholar] [CrossRef]
- Bueno, A.V.; Pereira, M.P.B.; de Oliveira Pontes, J.V.; de Luna, F.M.T.; Cavalcante, C.L. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Nabi, M.N.; Zare, A.; Hossain, F.M.; Ristovski, Z.D.; Brown, R.J. Reductions in diesel emissions including PM and PN emissions with diesel-biodiesel blends. J. Clean. Prod. 2017, 166, 860–868. [Google Scholar] [CrossRef]
- Cheikh, K.; Sary, A.; Khaled, L.; Abdelkrim, L.; Mohand, T. Experimental assessment of performance and emissions maps for biodiesel fueled compression ignition engine. Appl. Energy 2016, 161, 320–329. [Google Scholar] [CrossRef]
- Bari, S.; Saad, I. Optimization of vane numbers through simulation and experiment, and investigation of the effect on the performance and emissions of a CI (compression ignition) engine run with biodiesel. Energy 2015, 79, 248–263. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. A comprehensive study on measurement and prediction of viscosity of biodiesel-diesel-alcohol ternary blends. Energy 2018, 148, 341–361. [Google Scholar] [CrossRef]
- Zaharin, M.S.M.; Abdullah, N.R.; Najafi, G.; Sharudin, H.; Yusaf, T. Effects of physicochemical properties of biodiesel fuel blends with alcohol on diesel engine performance and exhaust emissions: A review. Renew. Sustain. Energy Rev. 2017, 79, 475–493. [Google Scholar] [CrossRef]
- Ibrahim, A. Performance and combustion characteristics of a diesel engine fuelled by butanol–biodiesel–diesel blends. Appl. Therm. Eng. 2016, 103, 651–659. [Google Scholar] [CrossRef]
- Pereira, L.G.; Dias, M.O.S.; Mariano, A.P.; Maciel Filho, R.; Bonomi, A. Economic and environmental assessment of n-butanol production in an integrated first and second generation sugarcane biorefinery: Fermentative versus catalytic routes. Appl. Energy 2015, 160, 120–131. [Google Scholar] [CrossRef]
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel production in micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.W.; Cheung, C.S. Investigation on the combustion characteristics and particulate emissions from a diesel engine fueled with diesel-biodiesel-ethanol blends. Energy 2015, 83, 343–350. [Google Scholar] [CrossRef]
- Khoobbakht, G.; Najafi, G.; Karimi, M.; Akram, A. Optimization of operating factors and blended levels of diesel, biodiesel and ethanol fuels to minimize exhaust emissions of diesel engine using response surface methodology. Appl. Therm. Eng. 2016, 99, 1006–1017. [Google Scholar] [CrossRef]
- Jamrozik, A. The effect of the alcohol content in the fuel mixture on the performance and emissions of a direct injection diesel engine fueled with diesel-methanol and diesel-ethanol blends. Energy Convers. Manag. 2017, 148, 461–476. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. A comparative analysis of n-butanol/diesel and 1-pentanol/diesel blends in a compression ignition engine. Fuel 2018, 234, 161–169. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Aydin, M. Experimental investigation on the performance, combustion and exhaust emission characteristics of a compression-ignition engine fueled with cottonseed oil biodiesel/diethyl ether/diesel fuel blends. Energy Convers. Manag. 2019, 205, 112355. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Saravanan, S.; Rana, D.; Anish, V.; Nagendran, A. Effect of a sustainable biofuel—N-octanol—on the combustion, performance and emissions of a di diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Energy Convers. Manag. 2016, 118, 275–286. [Google Scholar] [CrossRef]
- Doǧan, O. The influence of n-butanol/diesel fuel blends utilization on a small diesel engine performance and emissions. Fuel 2011, 90, 2467–2472. [Google Scholar] [CrossRef]
- Campos-Fernández, J.; Arnal, J.M.; Gómez, J.; Dorado, M.P. A comparison of performance of higher alcohols/diesel fuel blends in a diesel engine. Appl. Energy 2012, 95, 267–275. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; Kayatas, Z.; Hawi, M.; Kosaka, H.; He, Z. Combustion and emission characteristics of a rapid compression-expansion machine operated with N-heptanol-methyl oleate biodiesel blends. Renew. Energy 2020, 147, 2064–2076. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Wang, X. Densities and viscosities for binary mixtures of dimethyl carbonate with 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol. J. Chem. Thermodyn. 2021, 157, 106404. [Google Scholar] [CrossRef]
- Nour, M.; Nada, S.; Li, X. Experimental study on the combustion performance of a stationary CIDI engine fueled with 1-heptanol-diesel mixtures. Fuel 2021, 312, 122902. [Google Scholar] [CrossRef]
- Li, J.; Deng, X.; Zhao, W.; Wang, D. A comparative study for the n-butanol/n-octanol and n-butanol/ di-n-butylether fueled dual-fuel engines with different injection timings. Fuel 2023, 338, 127339. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Saravanan, B.; Somasundaram, P.; Jegadheesan, C.; Chaturvedi, B.; Sharma, S.; Patni, G. A novel study on the effect lemon peel oil as a fuel in CRDI engine at various injection strategies. Energy Convers. Manag. 2018, 172, 517–528. [Google Scholar] [CrossRef]
- Karthickeyan, V.; Ashok, B.; Nanthagopal, K.; Thiyagarajan, S.; Geo, V.E. Investigation of novel Pistacia khinjuk biodiesel in DI diesel engine with post combustion capture system. Appl. Therm. Eng. 2019, 159, 113969. [Google Scholar] [CrossRef]
- Bharti, A.; Banerjee, T. Reactive force field simulation studies on the combustion behavior of n-octanol. Fuel Process. Technol. 2016, 152, 132–139. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Akasyah, M.K.; Jamlos, M.F.; Yusop, A.F. Evaluation of engine combustion and exhaust emissions characteristics using diesel/butanol blended fuel. Appl. Therm. Eng. 2019, 156, 209–219. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.; Nagappan, B.; Choubey, G. Study on the effect on combining long-chain additive with neat bio-diesel fueled engine to examine its ignition characteristics. Fuel 2020, 279, 118400. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Eryilmaz, T.; Arslan, M. A comparative analysis of the engine performance, exhaust emissions and combustion behaviors of a compression ignition engine fuelled with biodiesel/diesel/1-butanol (C4 alcohol) and biodiesel/diesel/n-pentanol (C5 alcohol) fuel blends. Energy 2018, 165, 1332–1351. [Google Scholar] [CrossRef]
- Vigneshwar, V.; Krishnan, S.Y.; Kishna, R.S.; Srinath, R.; Ashok, B.; Nanthagopal, K. Comprehensive review of Calophyllum inophyllum as a feasible alternate energy for CI engine applications. Renew. Sustain. Energy Rev. 2019, 115, 109397. [Google Scholar] [CrossRef]
- Khan, M.M.; Kumar Kadian, A.; Sharma, R.P. Investigation of high fuel injection pressure variation on compression ignition engines powered by jatropha oil methyl ester-heptanol-diesel blends. Alex. Eng. J. 2023, 65, 675–688. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. The examination of a compression-ignition engine powered by peanut oil biodiesel and diesel fuel in terms of energetic and exergetic performance parameters. Fuel 2020, 278, 118319. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; He, Z.; Kosaka, H. Combustion and emission characteristics of a common rail diesel engine run with n-heptanol-methyl oleate mixtures. Energy 2021, 214, 118972. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Cakmak, A. An extensive investigation of utilization of a C8 type long-chain alcohol as a sustainable next-generation biofuel and diesel fuel blends in a CI engine—The effects of alcohol infusion ratio on the performance, exhaust emissions, and combustion characteristics. Fuel 2021, 305, 121453. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Xie, G.; Li, J.; Xu, W.; Jiang, F.; Huang, Y.; Tan, D. Investigation on the combustion and emission characteristics of diesel engine fueled with diesel/methanol/n-butanol blends. Fuel 2022, 314, 123088. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Lv, J.; Wang, S.; Zhong, Y.; Dong, R.; Gao, S.; Cao, C.; Tan, D. Investigation on combustion, performance and emission characteristics of a diesel engine fueled with diesel/alcohol/n-butanol blended fuels. Fuel 2022, 320, 123975. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Dong, R.; Zou, Z.; Gao, S.; Tan, D. Performance, combustion and emission characteristics investigations on a diesel engine fueled with diesel/ethanol/n-butanol blends. Energy 2022, 249, 123733. [Google Scholar] [CrossRef]
- Ramesh, A.; Ashok, B.; Nanthagopal, K.; Pathy, M.R.; Tambare, A.; Mali, P.; Phuke, P.; Patil, S.; Subbarao, R. Influence of hexanol as additive with Calophyllum Inophyllum biodiesel for CI engine applications. Fuel 2019, 249, 472–485. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumar, M. Experimental investigations of oxidation stability of biodiesel produced from Prunus armeniaca oil (apricot oil) and effect of various antioxidants on stability, engine performance and emissions. Fuel 2018, 216, 861–869. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Ashok, B.; Saravanan, B.; Pathy, M.R.; Sahil, G.; Ramesh, A.; Nabi, M.N.; Rasul, M.G. Study on decanol and Calophyllum Inophyllum biodiesel as ternary blends in CI engine. Fuel 2019, 239, 862–873. [Google Scholar] [CrossRef]
- Emiroğlu, A.O.; Şen, M. Combustion, performance and emission characteristics of various alcohol blends in a single cylinder diesel engine. Fuel 2018, 212, 34–40. [Google Scholar] [CrossRef]
- Awad, O.I.; Ali, O.M.; Mamat, R.; Abdullah, A.A.; Najafi, G.; Kamarulzaman, M.K.; Yusri, I.M.; Noor, M.M. Using fusel oil as a blend in gasoline to improve SI engine efficiencies: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 69, 1232–1242. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z.H.; Ren, X.C.H. Performance and combustion characteristics of biodiesel–diesel–methanol blend fuelled engine. Appl. Energy 2010, 87, 1679–1686. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sarıdemir, S.; Karagöz, M. Experimental investigation of fusel oil (isoamyl alcohol) and diesel blends in a CI engine. Fuel 2020, 267, 117042. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, H.; Jiang, D.; Zeng, K.; Liu, B.; Zhang, J.; Wang, X. Combustion behaviors of a compression-ignition engine fuelled with diesel/methanol blends under various fuel delivery advance angles. Bioresour. Technol. 2004, 95, 331–341. [Google Scholar] [CrossRef]
- Tutak, W.; Lukács, K.; Szwaja, S.; Bereczky, Á. Alcohol–diesel fuel combustion in the compression ignition engine. Fuel 2015, 154, 196–206. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Candan, F.; Ciniviz, M.; Ors, I. Effect of cetane improver addition into diesel fuel: Methanol mixtures on performance and emissions at different injection pressures. Therm. Sci. 2017, 21, 555–566. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.S.; Huang, Z. Effect of n-pentanol addition on the combustion, performance and emission characteristics of a direct-injection diesel engine. Energy 2014, 70, 172–180. [Google Scholar] [CrossRef]
- Fu, J.; Shu, J.; Zhou, F.; Liu, J.; Xu, Z.; Zeng, D. Experimental investigation on the effects of compression ratio on in-cylinder combustion process and performance improvement of liquefied methane engine. Appl. Therm. Eng. 2017, 113, 1208–1218. [Google Scholar] [CrossRef]
- Imran, A.; Varman, M.; Masjuki, H.H.; Kalam, M.A. Review on alcohol fumigation on diesel engine: A viable alternative dual fuel technology for satisfactory engine performance and reduction of environment concerning emission. Renew. Sustain. Energy Rev. 2013, 26, 739–751. [Google Scholar] [CrossRef]
- Atmanlı, A.; İleri, E.; Yüksel, B. Effects of higher ratios of n-butanol addition to diesel–vegetable oil blends on performance and exhaust emissions of a diesel engine. J. Energy Inst. 2015, 88, 209–220. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yuksel, B.; Yilmaz, N. Extensive analyses of diesel–vegetable oil–n-butanol ternary blends in a diesel engine. Appl. Energy 2015, 145, 155–162. [Google Scholar] [CrossRef]
- Rahimi, H.; Ghobadian, B.; Yusaf, T.; Najafi, G.; Khatamifar, M. Diesterol: An environment-friendly IC engine fuel. Renew. Energy 2009, 34, 335–342. [Google Scholar] [CrossRef]
- Yilmaz, N. Comparative analysis of biodiesel–ethanol–diesel and biodiesel–methanol–diesel blends in a diesel engine. Energy 2012, 40, 210–213. [Google Scholar] [CrossRef]
- Sayin, C.; Canakci, M. Effects of injection timing on the engine performance and exhaust emissions of a dual-fuel diesel engine. Energy Convers. Manag. 2009, 50, 203–213. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Anand, V.; Aravind, K.M.; Jeevanantham, A.K.; Balusamy, S. Effects of n-octanol as a fuel blend with biodiesel on diesel engine characteristics. Fuel 2019, 235, 363–373. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, N.; Karnwal, A.; Gupta, D.; Vibhanshu, V.; Sharma, A.; Patel, J.S. Assessment of the Performance and Emission Characteristics of 1-Octanol/Diesel Fuel Blends in a Water Cooled Compression Ignition Engine; SAE Technical Paper 2014-01-2830; SAE International: Warrendale, PA, USA, 2014. [Google Scholar] [CrossRef]
- Mahalingam, Y.; Munuswamy, A.; Devarajan, D.B. Emission and performance analysis on the effect of exhaust gas recirculation in alcohol-biodiesel aspirated research diesel engine. Environ. Sci. Pollut. Res. 2018, 25, 12641–12647. [Google Scholar] [CrossRef]
- Babu, D.; Anand, R. Effect of biodiesel-diesel-n-pentanol and biodiesel-diesel-n-hexanol blends on diesel engine emission and combustion characteristics. Energy 2017, 133, 761–776. [Google Scholar] [CrossRef]
- Phoon, L.Y.; Mustaffa, A.A.; Hashim, H.; Mat, R.; Manan, Z.A.; Yunus, N.A. Performance and emission characteristics of green diesel blends containing diethyl-succinate and 1-octanol. J. Clean. Prod. 2017, 161, 1192–1202. [Google Scholar] [CrossRef]
- Mohammadi, M.; Neshat, E. Accurate prediction of NOx emissions from diesel engines considering in-cylinder ion current. Environ. Pollut. 2020, 266, 115347. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, T.; Nalini, R. Experimental investigation on performance, combustion and emission characteristics of four stroke diesel engine using diesel blended with alcohol as fuel. Energy 2014, 78, 356–363. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Ashok, B.; Saravanan, B.; Patel, D.; Sudarshan, B.; Aaditya Ramasamy, R. An assessment on the effects of 1-pentanol and 1-butanol as additives with Calophyllum Inophyllum biodiesel. Energy Convers. Manag. 2018, 158, 70–80. [Google Scholar] [CrossRef]
- Soloiu, V.; Moncada, J.D.; Gaubert, R.; Muiños, M.; Harp, S.; Ilie, M.; Zdanowicz, A.; Molina, G. LTC (low-temperature combustion) analysis of PCCI (premixed charge compression ignition) with n-butanol and cotton seed biodiesel versus combustion and emissions characteristics of their binary mixtures. Renew. Energy 2018, 123, 323–333. [Google Scholar] [CrossRef]
- Akar, M.A. Performance and emission characteristics of compression ignition engine operating with false flax biodiesel and butanol blends. Adv. Mech. Eng. 2016, 8, 1687814016632677. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Xiao, J. Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel 2015, 156, 211–218. [Google Scholar] [CrossRef]
- Randazzo, M.L.; Sodré, J.R. Exhaust emissions from a diesel powered vehicle fuelled by soybean biodiesel blends (B3–B20) with ethanol as an additive (B20E2–B20E5). Fuel 2011, 90, 98–103. [Google Scholar] [CrossRef]




| Properties | Units | D100 a | HBO | 1-Heptanol b | n-Octanol c |
|---|---|---|---|---|---|
| Density | kg/m3 | 828 | 880.4 | 822 | 827 |
| Viscosity at 40 °C | mm2/s | 2.578 | 4.33 | 5.75 | 7.59 |
| Heating Value | MJ/kg | 42.54 | 38.91 | 39.92 | 38.53 |
| Cetane Number | 40–55 | 53 | 23 | 39 | |
| Flash Point | °C | 65–70 | 159 | 70 | 81 |
| Cloud Point | °C | −11.9 | −8 | −5 to −10 | - |
| Pour Point | °C | −15.8 | −15 | - | - |
| Molecular formula | C14–C25 | -- | C7H15OH | C8H17OH | |
| Molecular weight | wt. | 198.4 | -- | 116.19 | 130.21 |
| Carbon | wt.% | 87.05 | 77.2 | 72.16 | 73.72 |
| Hydrogen | wt.% | 12.95 | 11.4 | 13.71 | 13.82 |
| Oxygen content | wt.% | 6.72 | 11.3 | 14.13 | 15.7 |
| Boiling point | °C | 180–370 | -- | 175 | 195 |
| Latent heating at 25 °C | kJ/kg | 270 | -- | 574 | 315 |
| Autoignition temperature | °C | 210–260 | 275 | 253 | 270 |
| Description | Specification |
|---|---|
| Product | Code 244, CRDI-VCR research engine test setup |
| Type | Kirloskar make, engine one-cylinder, 4-stroke |
| Cooling Type | Water-cooled |
| Power | 3.5 kilo Watt |
| Varying CR | 12:1 to 18:1 |
| Common Rail | With pressure regulation and pressure sensor |
| Dynamometer | Eddy current with loading |
| ECU | Nira i7r with programmable and solenoid injector driver |
| Fuel Injector | Solenoid-driven |
| Airbox | With an orifice meter (MS-fabricated) and manometer |
| Fuel Tank | With fuel metering pipe of glass (15-L capacity) |
| Calorimeter | Pipe-in-pipe |
| Data Acquisition Software | Engine soft. performance and analysis software |
| Rotameter | Cooling (40–400) Iph, calorimeter (25–250) lph, Eureka make |
| Propeller Shaft | Universal joints, Hindustan Hardy make |
| Sensor/Transmitter | Measurement Range | Make | Relative Uncertainty |
|---|---|---|---|
| Piezo pressure sensor | 0 to 350 bar | PCB USA make | ±1% FS |
| Crank angle sensor | Resolution 1 Deg, speed 5500 RPM with TDC pulse | Kubler Germany make | ±0.06 |
| Temperature sensor | RTD Type and Type K thermocouple | Radix make | ±1 °C |
| Load sensor | 0–50 kg | VPG Sensotronics make | ±0.85 |
| Temperature transmitter | Range 0–100 °C, Output 4–20 mA | Abustek USA make | ±1 °C |
| Fuel flow transmitter | range 0–500 mm WC | Yokogawa Japan make | |
| Air flow transmitter | Pressure transmitter, range (-) 250 mm WC | Wika Germany make | ±1% FS |
| Overall dimensions | W 2000 × D 2500 × H 1500 | Kirloskar | - |
| Particulars | Measurement | Resolution |
|---|---|---|
| CO | 0–15% Vol | 0.001% Vol |
| HC | 0–20,000 ppm Vol | 1 ppm (0–2000 ppm) |
| CO2 | 0–20% Vol | 0.1% Vol |
| O2 | 0–26% Vol | 0.01% Vol |
| NO | 0–6000 ppm | 1 ppm Vol |
| Engine Speed | 400–6000 rpm | 1 rpm |
| Oil Temperature | 0 °C–125 °C | 1 °C |
| Lambda | 0–9.999 | 0.001 |
| Description | Details | |
| Display | LCD display | |
| Interface | USB | |
| Operating Voltage | 100–300 V AC, 50–60 Hz | |
| Power Consumption | Max. 10 W | |
| Dimensions (w × h × l). | 270 × 85 × 320 mm | |
| Weight | 4.5 kg | |
| Parameter | Measurement Range | Accuracy | Uncertainty (%) |
|---|---|---|---|
| Engine speed | 400–6000 rpm | ±1 rpm | ±0.06 |
| Brake power | 0–3.5 kW | ±0.3 kW | ±0.8355 |
| Engine load | 0–12 kg | ±0.1 kg | ±0.85 |
| Mass of the fuel | - | - | ±1.553 |
| temperature | 0 °C to 125 °C | ±1 °C | ±1 |
| Time | - | ||
| BSFC | - | ±5 g/kWh | 1.7641 |
| Burette system | 0–100 cc | ±0.1 cc | ±0.1 |
| Brake thermal efficiency | - | ±0.5% | ±1.76 |
| CO | 0–15% vol | ±0.001% | ±1.026 |
| HC | 0–20,000 ppm | ±1 ppm | ±1.478 |
| CO2 | 0–20% vol | ±0.1% | ±0.907 |
| NOx | 0–6000 ppm | ±1 ppm | ±0.837 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thippeshnaik, G.; Prakash, S.B.; Suresh, A.B.; Chandrashekarappa, M.P.G.; Samuel, O.D.; Der, O.; Ercetin, A. Experimental Investigation of Compression Ignition Engine Combustion, Performance, and Emission Characteristics of Ternary Blends with Higher Alcohols (1-Heptanol and n-Octanol). Energies 2023, 16, 6582. https://doi.org/10.3390/en16186582
Thippeshnaik G, Prakash SB, Suresh AB, Chandrashekarappa MPG, Samuel OD, Der O, Ercetin A. Experimental Investigation of Compression Ignition Engine Combustion, Performance, and Emission Characteristics of Ternary Blends with Higher Alcohols (1-Heptanol and n-Octanol). Energies. 2023; 16(18):6582. https://doi.org/10.3390/en16186582
Chicago/Turabian StyleThippeshnaik, Ganesha, Sajjal Basanna Prakash, Ajith Bintravalli Suresh, Manjunath Patel Gowdru Chandrashekarappa, Olusegun David Samuel, Oguzhan Der, and Ali Ercetin. 2023. "Experimental Investigation of Compression Ignition Engine Combustion, Performance, and Emission Characteristics of Ternary Blends with Higher Alcohols (1-Heptanol and n-Octanol)" Energies 16, no. 18: 6582. https://doi.org/10.3390/en16186582
APA StyleThippeshnaik, G., Prakash, S. B., Suresh, A. B., Chandrashekarappa, M. P. G., Samuel, O. D., Der, O., & Ercetin, A. (2023). Experimental Investigation of Compression Ignition Engine Combustion, Performance, and Emission Characteristics of Ternary Blends with Higher Alcohols (1-Heptanol and n-Octanol). Energies, 16(18), 6582. https://doi.org/10.3390/en16186582










