Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions
Abstract
:1. Introduction
2. Methods
2.1. Electricity Grid Mix and Energy
2.2. Life Cycle Analysis of Steel Production Processes
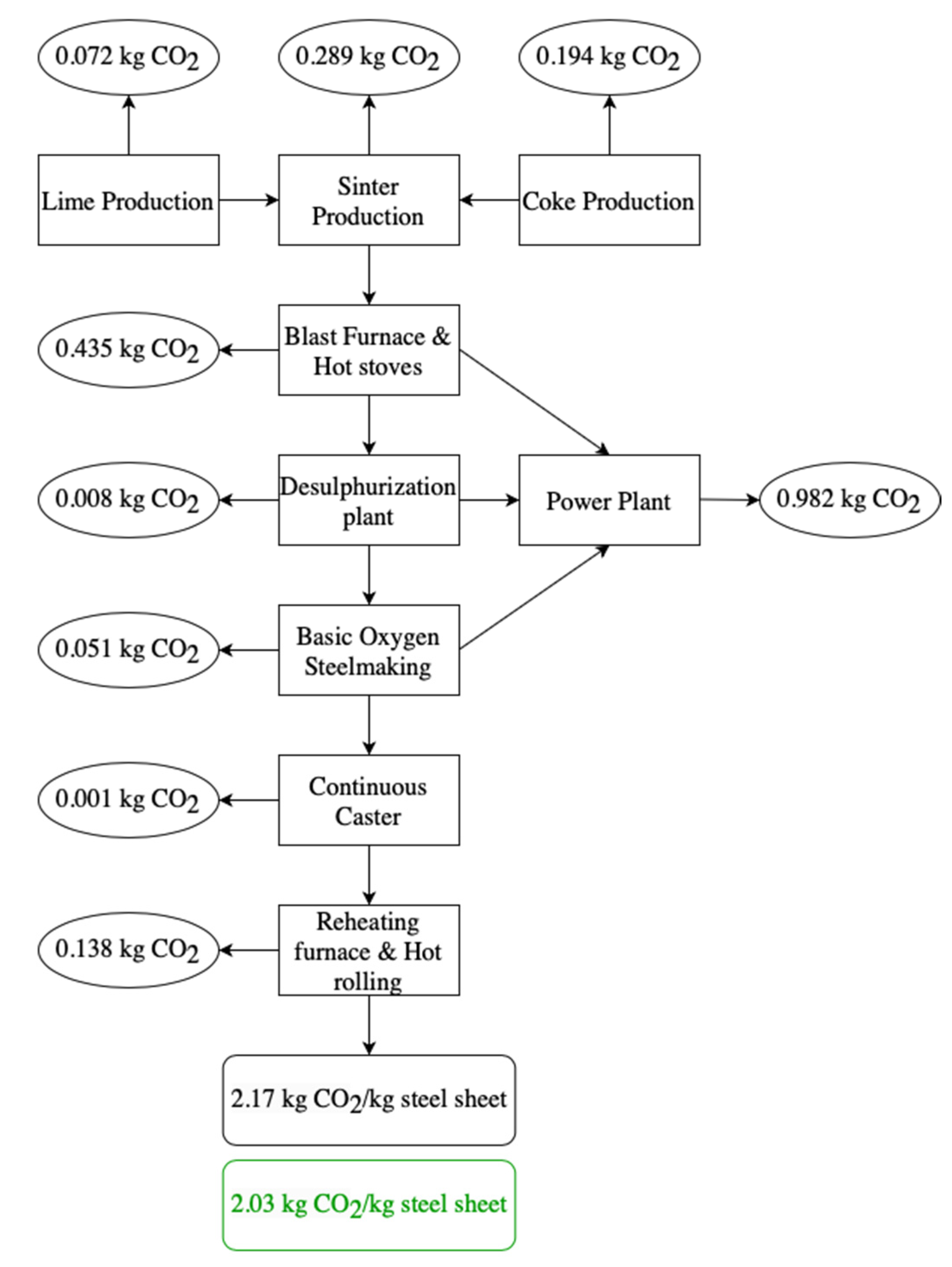
2.2.1. Blast Furnace and Basic Oxygen Furnace
2.2.2. Electric Arc Furnace
2.2.3. Direct Reduction of Iron
2.3. Decarbonization Methods for Steel
2.3.1. BF-BOF with Carbon Capture
2.3.2. EAF Route
2.3.3. H2-DRI (Using Hydrogen Generated from Renewable Electricity Sources)
2.4. Life Cycle Analysis of Aluminum Production
2.4.1. Primary Aluminum Production
2.4.2. Secondary Aluminum Production
2.5. Decarbonization Methods for Aluminum
2.5.1. Primary Aluminum Production and Carbon Capture
2.5.2. Decarbonized Aluminum
2.6. LCA for Glass Fiber Composites
2.7. LCA for CFRC
2.8. Manufacturing Cost Analysis
Carbon Capture
2.9. Normalization Using Material Properties
3. Results
3.1. Comparison of Materials’ Global Warming Potentials, Raw and Normalized
3.2. Comparison of Materials’ Manufacturing Costs, and Potential Assessed Carbon Tariffs
4. Discussion
- –
- The lowest nGWPs belong to secondary aluminum production and the 100% scrap-based EAF route. However, there are two important aspects that make 100% scrap-based EAF scenario only a virtual option for automotive application. First, availability of scrap varies greatly between different geographical locations; the price of high quality (low impurity) scrap also varies. Second, the quality of the steel is tremendously dependent on the scrap elements content. The main problem is caused by the presence of copper in scrap, which is almost impossible to remove. The level of impurities present in the final steel product can vary depending on the steel grade being produced, allowing for copper impurities in the range of 0.04–0.10 wt.% for steel sheet, while for rebar Cu content can be as high as 0.8 wt.% [99]. Current scrap quality achievable almost inevitably requires a high portion of pig iron or DRI for the dilution to obtain automotive sheet steel grade. To achieve the desired quality of the scrap, more efficient sorting is required, which would most likely add cost. Furthermore, the scrap supply is unlikely to meet the worldwide demand for steel to make a significant contribution for worldwide decarbonization.
- –
- In the case of natural gas DRI-EAF with CCS, most of the CO2 emissions from NG can be eliminated by CCS, while the following EAF emissions will be mainly a function of the electricity grid mix. Under the assumption of low-CO2 electricity generation and electrical heating in upstream mining and downstream hot rolling mill, the obtained steel can be produced almost CO2-free. Surprisingly, the H2-DRI-EAF production route has substantially higher GHG emissions compared to NG-DRI when using the current US grid mix. Complete decarbonization of the electricity production could improve the balance further. It should be mentioned that wide adoption of DRI technologies is not backed with the prospective sufficient supply of necessary DRI-grade iron ore pellets [100].
- –
- BF-BOF with CCS in the present study has only slightly higher emissions than NG-DRI-EAF with the CCS. Such an outcome is obtained due to the assumption that CCS has 90% efficiency, and it is applied to the sintering, blast furnace, and power plant exhaust gases balance of CO2. Indeed, it is technically possible to capture emissions from BF and BOF. However, some parts of the CO2 will be hard to capture (e.g., iron ore mining, ore transportation, hot rolling of the metal sheet).
- –
- Traditional BF-BOF steelmaking routes show relatively high GHG emissions—about 2 t CO2 eq/t steel. However, steel remains competitive compared with alternative materials, such as primary aluminum as well as glass and carbon fibers. Even CCS for primary aluminum will not outcompete traditional steelmaking. Only complete decarbonization of Al production helps it to compete with the current state of steelmaking. However, decarbonization of Al production does not achieve the GHG potential of any decarbonized version of steelmaking. The electrolysis process, also known as the Hall–Héroult process, is the most carbon-intensive during Al production. The process requires a significant amount of electricity, and the electricity used is often generated from fossil fuels, which leads to the emission of carbon dioxide. Recycling of Al can compete with the best steelmaking options in terms of nGWP. When compared to producing aluminum from raw materials, recycling aluminum saves at least 90% of the energy needed for its production. The aluminum recycling rate in the United States is 49.8%, while in Europe it is 76.3% [101]. Increasing recycling incentives could help increase reuse of aluminum, reduce the demand for virgin aluminum production, and therefore, decrease CO2 emissions. However, the same issue arises as for steel recycling, with the dilution requirements with virgin metal for achieving necessary quality.
- –
- CO2 capture and subsequent geological storage is expected to be limited to specific regions with appropriate geological structures. Furthermore, transport of captured CO2 requires significant investment in pipeline and booster pump infrastructure. This transport and sequestration infrastructure currently exists in Texas, USA, so it is insightful to compare hypothetical scenarios involving CCUS there. For illustration’s sake, assume one tonne of steel is produced via the hydrogen direct reduction process close to the customer (no transportation GWP or costs). Then consider one tonne of steel produced via natural gas direct reduction, EAF, and CCS in Texas. The latter steel is assumed to be transported by rail freight approximately 2500 km—the average distance from Austin, TX to each of the capitals of the 48 contiguous states. Assume rail transport GWP is 0.015 kg CO2 eq/t per km (primarily diesel fuel combustion) [0.048 lbs CO2 eq/ton-mile, and its cost is USD 0.05/ton-mile [76,102]. The rail transport of the NG-CCS steel from Texas to an “average customer” would add 37 kg CO2 eq/t steel and USD 82/t steel. Neither of these items is significant enough to change our current rank order results showing that NG-DRI-EAF with CCS has lower manufacturing cost and lower total cost (when including an USD 80/t carbon tax) versus hydrogen DRI-EAF per tonne of steel produced. The GWP for NG-DRI-EAF with CCS and rail transport is lower than local H2-DRI-EAF when assuming a traditional electricity grid and 5% higher when assuming the renewables-based grid. This indicates that a regional/national CCS hub with available natural gas could support a steel manufacturing “cluster” which outperforms environmentally and economically against geographically distributed hydrogen DRI siting.
- –
- In the case of glass fiber reinforced composite production, the most carbon-intensive step is resin production. This process involves heating the raw materials followed by melting and refining, requiring a significant amount of energy and resulting in the release of CO2 through the decomposition of the batch materials.
- –
- Due to high baking temperatures (above 1000 °C) and use of natural gas as an energy source, resin production during CFRC manufacturing is considered the most carbon-intensive. About 10.96 kg is emitted to produce 1 kg of carbon-fiber-reinforced composite. If carbon capture technologies would be applied to the resin production, the balance of CO2 might reduce. However, CFRC remains not competitive with steel neither in terms of nGWP nor in terms of normalized cost.
- –
- The solution of the short-term strategy to decarbonize steelmaking processes is highly depended on the local conditions, such as energy grid mix, existing metallurgical infrastructure, potential for CCS, and scrap availability. Government policies and regulations (e.g., carbon pricing, taxes or subsidies for clean energy, and emissions standards) can encourage or mandate the use of low-carbon steelmaking processes. Based on LCA, to radically reduce CO2 emissions from steel it is highly recommended to apply carbon capture to BF-BOF process or use the natural gas DRI-EAF route with carbon capture.
- –
- The best two options, from GHG emission point of view, are steel from recycling (EAF) and recycled aluminum, with an nGWP of a little less than 0.2 kg/t steel equivalent. However, both these options are not the most cost-effective and remain hypothetical for automotive sheet application due to the quality limitations discussed previously and limitations in supply to meet the growing demand.
- –
- NG-DRI-EAF with CCS appears to be the most optimal solution, with 0.33 kg CO2 eq/kg steel and lowest overall cost. In this scenario, the high efficiency of the CCS is removing the lions’ share of the CO2 from the DRI production, while decarbonized electricity generation removes the environmental burden of electric arc furnace melting of the DRI and production of steel. The remaining CO2 emissions are the efficiency limit of CCS (0.07 kg CO2 eq/kg steel), CO2 embedded in carbon capture unit (0.04 kg CO2 eq/kg steel), the CO2 of renewable grid mix used for MIDREX shaft reactor operations (0.04 kg CO2 eq/kg steel), EAF (0.06 kg CO2 eq/kg steel) and the upstream emissions (mining and transportation), and those of the hot rolling mill with gas-powered reheating, i.e., 0.07 kg CO2 eq/kg steel.
- –
- The second best cost-performing GWP scenario is H2-DRI-EAF with 0.31 kg CO2 eq/kg steel. Decarbonization of electricity generation contributes substantially to both iron ore reduction by eliminating majority of carbon footprint of electrolysis and to EAF processing of DRI. The remaining balance of CO2 emissions is attributed to electrolysis (0.08 kg CO2 eq/kg steel), EAF (0.06 kg CO2 eq/kg steel), reheating furnace for hot rolling and pelletizing (both 0.06 kg CO2/kg steel), and shaft reactor operations, mining, and transportation (0.01 kg CO2 eq/kg steel for each). Other steelmaking options are at least twice as carbon-intensive as NG-DRI-EAF with CCUS and H2-DRI-EAF routes. The assumed cost of hydrogen is one of the most important cost drivers in the hydrogen DRI scenario. As discussed previously, there is great uncertainty around the cost of scaling up the necessary electrical generation and electrolyzer capacity. We have selected a middle value of USD 4.5/kg for total landed cost of hydrogen (Figure 12). This is far above the United States Department of Energy “Hydrogen Shot” target of USD 1/kg [103]. Achieving that target would dramatically reduce the cost of the H2-DRI-EAF process and would make this production route by far the most optimal. Aside from cost reduction potential, production of hydrogen is more flexible towards geographic location compared to CCUS projects needed for NG-DRI. These two aspects imply that H2-DRI-EAF has a better long-term potential over NG-DRI-EAF with CCUS and should be prioritized as an investment strategy.
- –
- Recycled aluminum showed emissions of 0.19 kg CO2 eq/kg steel eq. Most of the remaining carbon footprint is coming from the scrap collection and transportation, and from the smelting of the scrap and rolling the Al sheet. Maximum decarbonized primary aluminum production is still lagging behind the recycling route with 0.58 kg of CO2 eq/kg steel eq. Even primary aluminum production of Al sheet with CCS, due to the renewable grid, substantially improves its carbon footprint from 3.42 kg to 0.83 kg of CO2 eq/kg steel eq. However, it remains among the heaviest from environmental footprint.
- –
- Both glass fiber and carbon fiber composite materials production require implementation of carbon capture solutions along the production chain to be competitive in terms of nGWP potential with the decarbonized steelmaking and with decarbonized aluminum. Even without CCUS implementation, these materials are not cost-competitive with steel and aluminum.
5. Conclusions
- –
- When comparing various steel decarbonization methods, in order of highest to lowest impact on GWP reduction, 100% scrap-based production through EAF resulted in an 81.5% reduction, followed by natural gas DRI-EAF with carbon capture with a 68.8% reduction, and BF-BOF with carbon capture (CCS) with 68%. Hydrogen-based DRI with EAF offers only a 24.6% reduction owing to the required electric power derived from current grids. Among them, natural gas DRI-EAF (USD 533/t) and natural gas DRI-EAF with CCS (USD 546/t) as well as 100% scrap-based EAF (USD 564.5/t) are the cheapest.
- –
- Using 100% recycling in production of Al is the most environmentally friendly option for sheet decarbonization with the potential to cut baseline GWP by 90% by achieving nGWP of 0.22 CO2 eq/kg hot-rolled sheet. Secondary Al (USD 668.25/t) is the cheapest, while primary Al with CCS (USD 1082.55/t) and decarbonized primary Al (USD 1257.15) are too expensive to compare to steel equivalent.
- –
- Glass-fiber-reinforced composite with a nGWP of 1.39 CO2 eq/kg hot-rolled sheet instead of conventional steel has a potential to reduce GWP by 36%. At the same time, GFRC and CFRC are not cost-competitive with their production costs of USD 2633.88/t and USD 5677/t, respectively.
- –
- Neither primary aluminum nor carbon-fiber-reinforced composites offer decarbonization potential compared to the current baseline of BF-BOF without carbon capture.
- –
- When comparing various steel decarbonization methods, in order of highest to lowest impact on GWP reduction, 100% scrap-based EAF steel remained the highest with a 91% reduction, followed by hydrogen-based DRI-EAF (84.7% reduction), and natural gas DRI-EAF with CCUS (83.7% reduction) and BF-BOF with CCUS (79% reduction). Natural gas DRI-EAF (USD 506.8/t) and natural gas DRI-EAF with CCS (USD 517.5/t) as well as 100% scrap-based EAF (USD 546.7/t) remain the cheapest;
- –
- Using 100% recycling of Al is on par with 100% steel recycling, resulting in a 91% nGWP reduction;
- –
- Primary aluminum becomes more favorable when a renewable grid is used. Without any decarbonization technologies, it has an nGWP of 1.64 CO2 eq/kg hot-rolled sheet eq, which is a 19% decrease from the baseline BF-BOF route. Application of CCS lowers CO2 emissions to 0.83 CO2 eq/kg hot-rolled sheet eq (59% decrease). Complete decarbonization of Al with 0.58 CO2 eq/kg hot-rolled sheet eq brings the emissions down 71.4%. Although secondary Al (USD 665.1/t) is still the cheapest Al production route, primary Al with CCS (USD 875.1/t) and decarbonized primary Al (USD 945.4) will also significantly drop in price with the renewable grid mix.
- –
- Carbon-fiber-reinforced composite (CFRC) offers about a 24% decrease (1.55 CO2 eq/kg hot-rolled sheet eq);
- –
- Glass-fiber-reinforced composite (GFRC) offers about a 43% reduction of nGWP with 1.15 CO2 eq/kg hot-rolled sheet eq;
- –
- Costs for future decarbonized production pathways of GFRC and CCRC are USD 2614.04/t and USD 5369.35/t, respectively, which are prohibitive for mass production.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEL | Alkaline Electrolysis |
| BF | Blast Furnace |
| BMI | Bismaleimide |
| BOF | Basic Oxygen Furnace |
| CCS | Carbon Capture and Storage |
| CCUS | Carbon Capture, Usage, and Storage |
| CFRC | Carbon-Fiber-Reinforced Composite |
| CO2 | Carbon Dioxide |
| CPI-U | Consumer Price Index for all Urban Consumers |
| DRI | Direct Reduced Iron |
| E | Elastic modulus (GPa) |
| EAF | Electric Arc Furnace |
| E-glass | Electrical Glass |
| eq/t | Equivalent/tonne |
| (g) | The substance is in a gaseous state |
| GFRC | Glass-Fiber-Reinforced Composite |
| GWP | Global Warming Potential |
| H2 | Hydrogen gas |
| IEA | International Energy Agency |
| K | Stiffness (N/m) |
| kWh/t | Kilowatt-hours/tonne |
| LCA | Life Cycle Analysis |
| MEA | Mono-Ethanolamine |
| Mfg. Cost | Manufacturing Cost |
| Mtpa | Million tonnes per annum |
| NG | Natural Gas |
| nGWP | normalized Global Warming Potential |
| O&G | Oil and Gas |
| PAN | Polyacrylonitrile |
| PEMEL | Polymer Electrolyte Membrane Electrolysis |
| (s) | The substance is in a solid state |
| SMC | Sheet Molding Compound |
| SOEL | Solid Oxide Electrolysis |
| t | Sheet thickness (cm) |
| t [unit] | Metric ton; tonne |
| TEA | Techno-Economic Analysis |
References
- The United States Department of State. The United States Executive Office of the President The Long-Term Strategy of the United States, Pathways to Net-Zero Greenhouse Gas Emissions by 2050. 2021. Available online: https://www.whitehouse.gov/wp-content/uploads/2021/10/US-Long-Term-Strategy.pdf (accessed on 21 April 2023).
- European Commission. Regulation of the European Parliament and of the Council; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative Emerging Ironmaking Technologies for Energy-Efficiency and Carbon Dioxide Emissions Reduction: A Technical Review. Renew. Sustain. Energy Rev. 2014, 33, 645–658. [Google Scholar] [CrossRef]
- Bataille, C. Low and Zero Emissions in the Steel and Cement Industries. In OECD Green Growth Papers; OECD Publishing: Paris, France, 2020; Volume 2. [Google Scholar] [CrossRef]
- Neugebauer, S.; Finkbeiner, M.; Volkhausen, W.; Mecke, S.; Endemann, G.; Aldenhoff, T. Environmental evaluation of steel—Advanced life cycle assessment considers multiple recycling of steel. Stahl Und Eisen 2013, 133, 49–55. [Google Scholar]
- Burchart-Korol, D. Life Cycle Assessment of Steel Production in Poland: A Case Study. J. Clean. Prod. 2013, 54, 235–243. [Google Scholar] [CrossRef]
- Backes, J.G.; Suer, J.; Pauliks, N.; Neugebauer, S.; Traverso, M. Life Cycle Assessment of an Integrated Steel Mill Using Primary Manufacturing Data: Actual Environmental Profile. Sustainability 2021, 13, 3443. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Iron and Steel Technology Roadmap Towards More Sustainable Steelmaking; Part of the Energy Technology Perspectives Series; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Kirschen, M.; Badr, K.; Pfeifer, H. Influence of Direct Reduced Iron on the Energy Balance of the Electric Arc Furnace in Steel Industry. Energy 2011, 36, 6146–6155. [Google Scholar] [CrossRef]
- Birat, J.P.; Hanrot, F.; Danloy, G. CO2 mitigation technologies in the steel industry: A benchmarking study based on process calculations. Stahl Und Eisen 2003, 123, 69–72. [Google Scholar]
- Hornby, S. Future Green Steelmaking Technologies. In Proceedings of the 60th Electric Furnace Conference, San Antonio, TX, USA, 10–13 November 2002; pp. 175–194. [Google Scholar]
- Kopfle, J.; Metius, G. Environmental Benefits of Natural Gas Direct Reduction; American Iron and Steel Society: Pittsburgh, PA, USA, 2010. [Google Scholar]
- Nduagu, E.I.; Yadav, D.; Bhardwaj, N.; Elango, S.; Biswas, T.; Banerjee, R.; Rajagopalan, S. Comparative Life Cycle Assessment of Natural Gas and Coal-Based Directly Reduced Iron (DRI) Production: A Case Study for India. J. Clean. Prod. 2022, 347, 131196. [Google Scholar] [CrossRef]
- Barati, M. Application of Slag Engineering Fundamentals to Continuous Steelmaking. In Treatise on Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 305–357. ISBN 978-0-08-096984-8. [Google Scholar]
- Ameling, D.; Endemann, G.; Igelbüscher, A. Carbon Dioxide: Curse or Future? Steel Institute VDEh: Düsseldorf, Germany, 2011. [Google Scholar]
- SSAB. HYBRIT: Milestone Reached—Pilot Facility for Hydrogen Storage up and Running. Available online: https://www.ssab.com/en-us/news/2022/09/hybrit-milestone-reached--pilot-facility-for-hydrogen-storage-up-and-running (accessed on 10 April 2023).
- HYBRIT. A Fossil-Free Future. Available online: https://www.hybritdevelopment.se/en/a-fossil-free-future/ (accessed on 10 April 2023).
- U.S. Department of Energy. Office of Energy Efficiency and Renewable Energy NEPA Determination. Grid-Interactive Steelmaking with Hydrogen (GISH). 2021. Available online: https://www.energy.gov/sites/prod/files/2021/01/f82/101996.pdf (accessed on 12 April 2023).
- Nuber, D.; Eichberger, H.; Rollinger, B. Circored Fine Ore Direct Reduction. Stahl Und Eisen 2006, 126, 47–51. [Google Scholar]
- Rechberger, K.; Spanlang, A.; Sasiain Conde, A.; Wolfmeir, H.; Harris, C. Green Hydrogen-Based Direct Reduction for Low-Carbon Steelmaking. Steel Res. Int. 2020, 91, 2000110. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-Carbon Production of Iron and Steel: Technology Options, Economic Assessment, and Policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Jinlong Wang The Environmental Footprint of Semi-Fabricated Aluminum Products in North America; Aluminum Association: Arlington, VA, USA, 2022; p. 158.
- Stolz, P.; Frischknecht, R. Life Cycle Inventories of Alumini-Um and Aluminium Profiles; Schweizerische Zentrale Fenster Und Fassaden (SZFF): Uster, Switzerland, 2016; Available online: http://www.dflca.ch/inventories/Hintergrund/Stolz_Frischknecht_2016-Oekobilanz-Aluminium-Bauprodukte_v1.0-Web.pdf (accessed on 20 April 2023).
- Georgitzikis, K.; Mancini, L.; d’Elia, E.; Vidal-Legaz, B. Sustainability Aspects of Bauxite and Aluminium Climate Change, Environmental, Socio-Economic and Circular Economy Considerations; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Hodgson, D.; Vass, T. Aluminium; IEA: Paris, France, 2022. [Google Scholar]
- Hydro Carbon Footprint of Recycled Aluminium. Available online: https://www.climateaction.org/news/carbon-footprint-of-recycled-aluminium (accessed on 15 April 2023).
- Hasanbeigi, A.; Springer, C.; Shi, D. Aluminum Climate Impact. An International Benchmarking of Energy and CO2 Intensities; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Kawajiri, K.; Sakamoto, K. Environmental Impact of Carbon Fibers Fabricated by an Innovative Manufacturing Process on Life Cycle Greenhouse Gas Emissions. Sustain. Mater. Technol. 2022, 31, e00365. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R.; Gutowski, T.G. Life Cycle Energy Analysis of Fiber-Reinforced Composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1257–1265. [Google Scholar] [CrossRef]
- Tchana Toffe, G.; Oluwarotimi Ismail, S.; Montalvão, D.; Knight, J.; Ren, G. A Scale-up of Energy-Cycle Analysis on Processing Non-Woven Flax/PLA Tape and Triaxial Glass Fibre Fabric for Composites. J. Manuf. Mater. Process. 2019, 3, 92. [Google Scholar] [CrossRef]
- Roussanaly, S.; Berghout, N.; Fout, T.; Garcia, M.; Gardarsdottir, S.; Nazir, S.M.; Ramirez, A.; Rubin, E.S. Towards Improved Cost Evaluation of Carbon Capture and Storage from Industry. Int. J. Greenh. Gas Control 2021, 106, 103263. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. How Much Carbon Dioxide Is Produced per Kilowatthour of U.S. Electricity Generation? Available online: https://www.eia.gov/tools/faqs/faq.php?id=74&t=11 (accessed on 20 April 2023).
- National Energy Technology Laboratory. Carbon Dioxide Utilization. In NETL CO2U LCA GUIDANCE TOOLKIT 2022; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2022. Available online: https://www.netl.doe.gov/LCA/CO2U (accessed on 23 April 2023).
- U.S. Department of Energy. Office of Energy Efficiency and Renewable Energy NEPA Determination What Is U.S. Electricity Generation by Energy Source? Available online: https://www.eia.gov/tools/faqs/faq.php?id=427&t=3 (accessed on 23 April 2023).
- Worldsteel Association. World Steel in Figures 2022. Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/#crude-steel-production-by-process-2021 (accessed on 23 April 2023).
- Santos, S. Iron and Steel CCS Study (Techno-Economics Integrated Steel Mill); IEA Greenhouse Gas R&D Programme (IEAGHG): Cheltenham, UK, 2013. [Google Scholar]
- Atsushi, M.; Uemura, H.; Sakaguchi, T. The Midrex Process. Kobelco Technol. Rev. 2010, 29, 50–57. [Google Scholar]
- Energiron. Technology General Overview. 2012. Available online: https://www.energiron.com/wp-content/uploads/2019/05/ENERGIRON-DR-Technology-Overview.pdf (accessed on 10 April 2023).
- Gazzani, M.; Romano, M.C.; Manzolini, G. CO2 Capture in Integrated Steelworks by Commercial-Ready Technologies and SEWGS Process. Int. J. Greenh. Gas Control 2015, 41, 249–267. [Google Scholar] [CrossRef]
- Steel Manufacturers Association (SMA). Emissions Analysis Executive Summary; CRU International Ltd.: Washington, DC, USA, 2022; Available online: https://steelnet.org/steelmaking-emissions-report-2022/ (accessed on 24 April 2023).
- Hornby, S. Hydrogen-Based DRI EAF Steelmaking—Fact or Fiction? In Proceedings of the AISTech 2021-Proceedings of the Iron & Steel Technology Conference, Nashville, TN, USA, 29 June–1 July 2021; Association for Iron and Steel Technology: Nashville, TN, USA, 2021; pp. 249–261. [Google Scholar]
- Languna, J.C.; Dureinck, J.; Meinke-Hubeny, F.; Meinke-Hubeny, F. Carbon-Free Steel Production: Cost Reduction Options and Usage of Existing Gas Infrastructure; Scientific Foresight Unit (STOA), European Parliamentary Research Service (EPRS), European Union: Brussels, Belgium, 2021. [Google Scholar] [CrossRef]
- Rajat, G.; Shirish, S.; Naveen, U.; Divy, M. Decarbonising India: Charting a Pathway for Sustainable Growth; McKinsey & Company: Mumbai, India, 2022. [Google Scholar]
- Kim, J.; Lee, H.; Lee, B.; Kim, J.; Oh, H.; Lee, I.B.; Yoon, Y.S.; Lim, H. An Integrative Process of Blast Furnace and SOEC for Hydrogen Utilization: Techno-Economic and Environmental Impact Assessment. Energy Convers. Manag. 2021, 250, 114922. [Google Scholar] [CrossRef]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of Hydrogen Direct Reduction for Fossil-Free Steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Jacobasch, E.; Herz, G.; Rix, C.; Müller, N.; Reichelt, E.; Jahn, M.; Michaelis, A. Economic Evaluation of Low-Carbon Steelmaking via Coupling of Electrolysis and Direct Reduction. J. Clean. Prod. 2021, 328, 129502. [Google Scholar] [CrossRef]
- Nunez, P.; Jones, S. Cradle to Gate: Life Cycle Impact of Primary Aluminium Production. Int. J. Life Cycle Assess. 2016, 21, 1594–1604. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Galitsky, C. World Best Practice Energy Intensity Values for Selected Industrial Sectors; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2008.
- Dai, Q.; Kelly, J.C.; Burnham, A.; Elgowainy, A. Updated Life Cycle Analysis of Aluminum Production and Semi Fabrication for GREET Model; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2015. [CrossRef]
- Jilvero, H.; Mathisen, A.; Eldrup, N.H.; Normann, F.; Johnsson, F.; Müller, G.I.; Melaaen, M.C. Techno-Economic Analysis of Carbon Capture at an Aluminum Production Plant—Comparison of Post-Combustion Capture Using MEA and Ammonia. Energy Procedia 2014, 63, 6590–6601. [Google Scholar] [CrossRef]
- Solheim, A. Inert Anodes—The Blind Alley to Environmental Friendliness? In Light Metals 2018; Springer International Publishing: New York, NY, USA, 2018; pp. 1253–1260. [Google Scholar] [CrossRef]
- Xianxi, W. Inert Anodes for Aluminum Electrolysis, 1st ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-28912-6. [Google Scholar]
- Dai, Q.; Kelly, J.; Sullivan, J.; Elgowainy, A. Life-Cycle Analysis Update of Glass and Glass Fiber for the GREETTM Model. Environ. Sci. 2015. Available online: https://www.semanticscholar.org/paper/Life-Cycle-Analysis-Update-of-Glass-and-Glass-Fiber-Dai-Kelly/763916041bb2e72f736a58e549fcba2f7f692242 (accessed on 24 April 2023).
- Hill, C.; Jch, A.N. LCA Database of Environmental Impacts to Inform Material Selection Process; DACOMAT: Paris, France, 2018. [Google Scholar]
- U.S. Department of Energy. Bandwidth Study on Energy Use and Potential Energy Saving Opportunities in U.S. Carbon Fiber Reinforced Polymer Manufacturing. 2017. Available online: https://www.energy.gov/sites/default/files/2019/05/f62/GFRP_bandwidth_study_2017.pdf (accessed on 30 April 2023).
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Fuchs, E.R.H.; Field, F.R.; Roth, R.; Kirchain, R.E. Strategic Materials Selection in the Automobile Body: Economic Opportunities for Polymer Composite Design. Compos. Sci. Technol. 2008, 68, 1989–2002. [Google Scholar] [CrossRef]
- Steelonthenet.com. BOF Steelmaking Cost Model year 2022. Basic Oxygen Furnace Route Steelmaking Costs 2022. Available online: https://www.steelonthenet.com/cost-bof.html (accessed on 13 March 2023).
- S&P Global. Latest Oil, Energy & Metals News, Market Data and Analysis. Available online: https://www.spglobal.com/commodityinsights/en (accessed on 13 March 2023).
- U.S. Energy Information Administration. Texas Natural Gas Industrial Price (Dollars per Thousand Cubic Feet). Available online: https://www.eia.gov/dnav/ng/hist/n3035tx3m.htm (accessed on 13 March 2023).
- Hooey, L.; Tobiesen, A.; Johns, J.; Santos, S. Techno-Economic Study of an Integrated Steelworks Equipped with Oxygen Blast Furnace and CO2 Capture. Energy Procedia 2013, 37, 7139–7151. [Google Scholar] [CrossRef]
- Schuff Steel. The Steel Supply Chain: FAQs; Schuff Steel: Portland, OR, USA, 2021. [Google Scholar]
- Obaidat, M.; Al-Ghandoor, A.; Phelan, P.; Villalobos, R.; Alkhalidi, A. Energy and Exergy Analyses of Different Aluminum Reduction Technologies. Sustainability 2018, 10, 1216. [Google Scholar] [CrossRef]
- Alumina Limited. Alumina Limited 2019 Annual Report; 2020. Available online: https://www.aluminalimited.com/wp-content/uploads/ayudacms/f/0/ALU8972_Annual_Report_FA2_ASX.pdf (accessed on 13 March 2023).
- 2023 Gravel Prices|Crushed Stone Cost (Per Ton, Yard & Load). Available online: https://homeguide.com/costs/gravel-prices (accessed on 13 March 2023).
- Caustic Soda Prices: Pricing, Chart, Latest Price, Market Analysis. Available online: https://www.procurementresource.com/resource-center/caustic-soda-price-trends (accessed on 21 November 2022).
- Inert Anode Technology for Aluminium Smelters | Climate Technology Centre & Network | Tue, 11/08/2016. Available online: https://www.ctc-n.org/technologies/inert-anode-technology-aluminium-smelters (accessed on 13 March 2023).
- Blomberg, J. Essays on the Economics of the Aluminium Industry. Ph.D. Thesis, Luleå University of Technology, Department of Business Administration and Social Sciences, Luleå, Sweden, June 2007. Available online: https://www.diva-portal.org/smash/get/diva2:989892/FULLTEXT01.pdf (accessed on 13 March 2023).
- Kawatra, S.K.; Young, C. SME Mineral Processing & Extractive Metallurgy Handbook; Society for Mining, Metallurgy & Exploration, Inc.: Englewood, CO, USA, 2019; ISBN 978-0-87335-385-4. [Google Scholar]
- Zare, R. Life Cycle Assessment of Secondary Extruded Aluminum Production Process in Industrial City of Arak. Appl. Ecol. Environ. Res. 2016, 14, 125–135. [Google Scholar] [CrossRef]
- American Recycling. Available online: http://americanrecycling.info/home/Pricing (accessed on 1 December 2022).
- Garside, M. Iron Oxide Price in the United States from 2013 to 2022. Available online: https://www.statista.com/statistics/881746/average-us-iron-oxide-price/ (accessed on 13 March 2023).
- Shah, D.U.; Schubel, P.J.; Clifford, M.J. Can Flax Replace E-Glass in Structural Composites? A Small Wind Turbine Blade Case Study. Compos. Part B Eng. 2013, 52, 172–181. [Google Scholar] [CrossRef]
- Das, S.; Warren, J.; West, D.; Laboratory, O.R.N. Global Carbon Fiber Composites Supply Chain Competitiveness Analysis. Technical Report; 2016. Available online: https://www.nrel.gov/docs/fy16osti/66071.pdf (accessed on 13 March 2023).
- Laska, A. Freight Rail’s Role in a Net-Zero Economy – Third Way. 7 June 2021. Available online: https://www.thirdway.org/memo/freight-rails-role-in-a-net-zero-economy (accessed on 13 March 2023).
- Association of American Railroads. Average U.S. Freight Rail Rates. Available online: https://www.aar.org/data/average-u-s-freight-rail-rates-since-deregulation/ (accessed on 13 March 2023).
- Google Maps. Available online: https://www.google.com/maps/@45.7252364,-122.6195851,14z (accessed on 13 March 2023).
- Damodaran, A. Cost of Equity and Capital (US). Available online: https://pages.stern.nyu.edu/~adamodar/New_Home_Page/datafile/wacc.html (accessed on 13 March 2023).
- Secard, R. Nucor to Build New $2.7 Billion Mill in West Virginia. Available online: https://www.industryweek.com/leadership/growth-strategies/article/21214007/nucor-to-build-new-27-billion-steel-mill-in-west-virginia (accessed on 13 March 2023).
- CLIFFS. Producing High Quality HBI in Toledo. Available online: https://www.clevelandcliffs.com/sustainability/environment/producing-high-quality-hbi-in-toledo (accessed on 13 March 2023).
- MIDREX Plants. Available online: https://www.midrex.com/about-midrex/midrex-plants-map/ (accessed on 13 March 2023).
- England, R. Cleveland-Cliffs Reports Lower Carbon Intensity for 2021. 6 June 2022. Available online: https://www.fastmarkets.com/insights/cleveland-cliffs-reports-lower-carbon-intensity-for-2021 (accessed on 13 March 2023).
- DataWeb. Available online: https://dataweb.usitc.gov/ (accessed on 14 March 2023).
- U.S. Bureau of Labor Statistics. CPI Inflation Calculator. Available online: https://www.bls.gov/data/inflation_calculator.htm (accessed on 1 April 2023).
- Kusic, S. Industry Statistics. Iron Steel Technol. 2023, 20, 16–17. [Google Scholar]
- European Commission. Taxation and Customs Union Carbon Border Adjustment Mechanism. Available online: https://taxation-customs.ec.europa.eu/green-taxation-0/carbon-border-adjustment-mechanism_en (accessed on 30 April 2023).
- Tsupari, E.; Kärki, J.; Arasto, A.; Pisilä, E. Post-Combustion Capture of CO2 at an Integrated Steel Mill—Part II: Economic Feasibility. Int. J. Greenh. Gas Control 2013, 16, 278–286. [Google Scholar] [CrossRef]
- Arasto, A.; Tsupari, E.; Kärki, J.; Pisilä, E.; Sorsamäki, L. Post-Combustion Capture of CO2 at an Integrated Steel Mill—Part I: Technical Concept Analysis. Int. J. Greenh. Gas Control 2013, 16, 271–277. [Google Scholar] [CrossRef]
- Arasto, A.; Tsupari, E.; Kärki, J.; Sihvonen, M.; Lilja, J. Costs and Potential of Carbon Capture and Storage at an Integrated Steel Mill. Energy Procedia 2013, 37, 7117–7124. [Google Scholar] [CrossRef]
- Wiley, D.E.; Ho, M.T.; Bustamante, A. Assessment of Opportunities for CO2 Capture at Iron and Steel Mills: An Australian Perspective; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 2654–2661. [Google Scholar]
- Gaffney Cline National Petroleum Council (NPC). Capture Facility Reference Costs. Available online: https://www.gaffneycline.com/calculator/npc-scenarios-table (accessed on 12 February 2023).
- Grant, T.; Morgan, D.; Poe, A.; Valenstein, J.; Lawrence, R.; Simpson, J. Which Reservoir for Low Cost Capture, Transportation, and Storage? Energy Procedia 2014, 63, 2663–2682. [Google Scholar] [CrossRef]
- Grant, T.; Shih, C.Y. Cost Analysis of Carbon Capture, Transport, and Storage; National Energy Technology Laboratory (NETL): Pittsburg, PA, USA, 2018.
- Kawachi, T.; Kimoto, N.; Tsunemi, Y. Stiffness Increase and Weight Reduction Based on Stiffness Evaluation Techniques. Nippon Steel Tech. Rep. 2019, 122, 93–98. Available online: https://www.nipponsteel.com/en/tech/report/pdf/122-15.pdf (accessed on 4 May 2023).
- Tata Steel Team. Weight Reduction by Use of Aluminium in Automobile Manufacturing; Zoom Meeting; Tata Steel Team: Kharagpur, India, 2022; p. 15. [Google Scholar]
- AZO. Materials E-Glass/Epoxy Resin, Borosilicate Glass Reinforced Epoxy Composites—Properties—Supplier Data by Goodfellow. Available online: https://www.azom.com/article.aspx?ArticleID=2453 (accessed on 4 May 2023).
- MatWeb Database Overview of Materials for Epoxy/Carbon Fiber Composite. Available online: https://www.matweb.com/search/datasheet_print.aspx?matguid=39e40851fc164b6c9bda29d798bf3726 (accessed on 4 May 2023).
- Toktarova, A.; Karlsson, I.; Rootzén, J.; Göransson, L.; Odenberger, M.; Johnsson, F. Pathways for Low-Carbon Transition of the Steel Industry—A Swedish Case Study. Energies 2020, 13, 3840. [Google Scholar] [CrossRef]
- Jones, J. Raw Material and Technology Consideration for Low-C EAF Steelmaking. AIST 2023, 1, 50–64. [Google Scholar]
- Doyle, A.; Voet, T. The DRI Dilemma: Could Raw Material Shortages Hinder the Steel Industry’s Green Transition? Available online: https://www.mckinsey.com/industries/metals-and-mining/our-insights/the-dri-dilemma-could-raw-material-shortages-hinder-the-steel-industrys-green-transition (accessed on 4 May 2023).
- Reinsch, W.A.; Benson, E. Decarbonizing Aluminum Rolling Out a More Sustainable Sector; Center for Strategic & International Studies (CSIS). 2022. Available online: https://www.csis.org/analysis/decarbonizing-aluminum-rolling-out-more-sustainable-sector (accessed on 20 April 2023).
- Kaack, L.H.; Vaishnav, P.; Morgan, M.G.; Azevedo, I.L.; Rai, S. Decarbonizing Intraregional Freight Systems with a Focus on Modal Shift. Environ. Res. Lett. 2018, 13, 083001. [Google Scholar] [CrossRef]
- Office of Energy Efficiency & Renewable Energy. Hydrogen Shot. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-shot (accessed on 4 May 2023).











| Processes | Traditional Electricity (kg CO2 eq/kg Steel Sheet) | Renewable Electricity (kg CO2 eq/kg Steel Sheet) |
|---|---|---|
| Scrap processing | 0.04 [40] | 0.04 |
| Transportation | 0.02 [40] | 0.02 |
| Direct emissions | 0.04 [8] | 0.04 |
| Emissions from electricity (indirect emissions) | 0.16 [41] | 0.01 |
| Reheating furnace | 0.06 [36] | 0.06 |
| Sheet rolling | 0.08 [40] | 0.01 |
| Total | 0.40 | 0.18 |
| Steel Production Scenario | Published or Forecast Year | Iron Ore Cost Assumption (USD/t) | Electricity Cost (USD/kg) | Green H2 Cost (USD/kg) | Estimated Steel Mfg. Cost (USD/t) | References |
|---|---|---|---|---|---|---|
| Crude Steel (H2-DRI) | 2022 | 110 | N/A | 4.3 | 660 | [43] |
| Crude Steel (H2-DRI) | 2030 | 110 | N/A | 1.8 | 550 | [43] |
| Crude Steel (H2-DRI) | 2040 | 110 | N/A | 1.3 | 520 | [43] |
| Steel Hot Metal (H2 Injection BF-BOF) | 2021 | N/A | 0.045 | 5.57 | + 153 vs. BF-BOF | [22] |
| Steel Hot Metal (100% H2-DRI) | 2021 | N/A | 0.045 | 5.57 | + 465 vs. NG-DRI | [21] |
| Steel Hot Metal (H2 Injection BF-BOF) | 2021 | N/A | 0.062 | 8.88 | 360 | [44] |
| Steel Hot Metal (H2 Injection BF-BOF) (Low) | 2050 | N/A | 0.025 | 4.04 | 410 | [44] |
| Steel Hot Metal (H2 Injection BF-BOF) (Mid) | 2050 | N/A | 0.025 | 2.13 | 360 | [44] |
| Steel Hot Metal (H2 Injection BF-BOF) (High) | 2050 | N/A | 0.025 | 1.45 | 340 | [44] |
| Liquid Steel (100% H2-DRI) (Low) a | 2018 | 116 | 0.024 | 1.7 | 420 | [45] |
| Liquid Steel (100% H2-DRI) (Mid) a | 2018 | 116 | 0.072 | 3.8 | 580 | [45] |
| Liquid Steel (100% H2-DRI) (High) a | 2018 | 116 | 0.120 | 6.0 | 742 | [45] |
| Crude Steel (H2-DRI) b | 2020 | 174 | 0.070 | 4.8 | 790 | [42] |
| Crude Steel (H2-DRI) b | 2030 | 174 | 0.079 | 3.7 | 799 | [42] |
| Crude Steel (H2-DRI) b | 2050 | 174 | 0.055 | 1.3 | 678 | [42] |
| Crude Steel (SOEL) c | 2021 | 98 | 0.089 | N/A | 1056 | [46] |
| Crude Steel (PEMEL) c | 2021 | 98 | 0.089 | N/A | 954 | [46] |
| Crude Steel (AEL) c | 2021 | 98 | 0.089 | N/A | 829 | [46] |
| Crude Steel (SOEL) c | 2050 | 98 | 0.063 | N/A | 628 | [46] |
| Crude Steel (PEMEL) c | 2050 | 98 | 0.063 | N/A | 615 | [46] |
| Crude Steel (AEL) c | 2050 | 98 | 0.063 | N/A | 632 | [46] |
| Processes | Energy Input (kWh/kg Al Sheet) | CO2 from Process Heat (kg CO2 eq/kg Al) | CO2 from Electricity (kg CO2 eq/kg Al) | Direct Emissions (kg CO2 eq/kg Al) | Total (kg CO2 eq/kg Al) |
|---|---|---|---|---|---|
| Bauxite mining | 0.32 [47] | 0.10 | 0.10 | ||
| Bayer’s process | 5.56 [48] | 1.04 | 0.15 | 1.19 | |
| Anode production | 0.34 [48] | 0.06 | 0.02 | 0.08 | |
| Electrolysis | 13 [48] | 5.01 | 1.53 | 6.54 | |
| Hot rolling | 1.04 [49] | 0.11 | 0.20 | 0.31 | |
| Total | 20.27 | 1.21 | 5.38 | 1.63 | 8.22 |
| Processes | Conventional Aluminum (kg CO2 eq/kg Al Sheet) | Decarbonized Aluminum (kg CO2 eq/kg Al Sheet) | Technologies |
|---|---|---|---|
| Bauxite mining | 0.10 | 0.10 | No Change |
| Bayer process | 1.19 | 0.18 | Thermal Energy from Renewable Electricity |
| Anode production | 0.08 | 0.25 | Inert Anodes |
| Electrolysis | 6.54 | 0.51 | Inert Anodes |
| Hot rolling | 0.31 | 0.13 | Renewable electricity |
| Total | 8.22 | 1.16 |
| Process | Energy (kWh/ kg GFRC) | CO2 from Process Heat (kg CO2 eq/ kg GFRC) | CO2 from Electricity (kg CO2 eq/ kg GFRC) | Direct Emissions (kg CO2 eq/ kg GFRC)) | Total (kg CO2 eq/ kg GFRC) |
|---|---|---|---|---|---|
| Batch preparation | 0.36 [53] | 0.14 | 0.14 | ||
| Melting and refining | 2.60 [53] | 0.53 | 0.17 | 0.70 | |
| Forming | 1.21 [53] | 0.47 | 0.47 | ||
| Post-forming | 1.07 [53] | 0.22 | 0.22 | ||
| Total | 5.23 | 1.52 | |||
| Epoxy resin production | 21.11 [54] | 4.7 | 4.70 | ||
| Resin: reinforcement = 50:50 | |||||
| Fabrication using sheet molding compound (SMC) | 0.97 [55] | 0.37 | 0.37 | ||
| Materials used | 13.17 | 3.11 | |||
| Total | 14.15 | 3.48 | |||
| Process | Energy (kWh/kg) | Natural Gas (%) | Electric (%) |
|---|---|---|---|
| Polymerization | 14.00 | 84.70 | 15.30 |
| Spinning | 19.02 | 95.70 | 4.30 |
| Oxidation/Carbonization * | 38.31 | 43.40 | 56.60 |
| Finishing | 6.73 | 0.00 | 100 |
| Resin production | 21.11 | 91.90 | 8.10 |
| Fabrication by SMC | 0.97 | 0.00 | 100 |
| Processes | Emissions from Process Heat (kg CO2 eq/kg CFRC) | Emissions from Electricity (kg CO2 eq/kg CFRC) | Total Emissions (kg CO2 eq/kg CFRC) |
|---|---|---|---|
| Polymerization | 2.39 | 0.82 | 3.22 |
| Spinning | 3.68 | 0.31 | 3.99 |
| Oxidation/Carbonization | 3.36 | 8.35 | 11.71 |
| Finishing | 0.00 | 2.59 | 2.59 |
| Total | 9.43 | 12.08 | 21.51 |
| Epoxy resin production | 4.70 | 4.70 | |
| Resin: reinforcement = 65:35 | |||
| Fabrication by SMC | 0.37 | 0.37 | |
| Materials used | 10.58 | ||
| Total | 10.96 | ||
| Scenario | Cost (USD/t CO2 | Published Year/Forecast Year | Assumptions | References |
|---|---|---|---|---|
| Carbon capture for steel | 68.7 | 2013 | Post-combustion capture | [89,90] |
| Carbon capture for steel | 65.1–119.2 | 2013 | Post-combustion capture | [91] |
| Carbon capture for steel | 78.5 | 2011 | Post-combustion capture with MEA of blast furnace flue gas | [92] |
| Carbon capture for steel | 104.21 | 2020 | N/A | [93] |
| Carbon capture for aluminum (4%) | 123.51 | 2013 | MEA-based carbon capture, concentration of CO2 in flue gas is 4% | [50] |
| Carbon capture for aluminum (7%) | 115.84 | 2013 | MEA-based carbon capture, concentration of CO2 in flue gas is 7% | [50] |
| Carbon capture for aluminum (10%) | 110.52 | 2013 | MEA-based carbon capture, concentration of CO2 in flue gas is 10% | [50] |
| Transport | 3.1 | 2014 | 3.2 Mtpa CO2 over 100 miles, onshore | [94] |
| Transport | 4.9 | 2018 | 3 Mtpa CO2 over 155 miles, onshore pipeline | [95] |
| Storage | 4.32 | 2015 | Depleted O&G Field—reusing wells onshore | [31] |
| Storage | 5.76 | 2015 | Depleted O&G Field—no reusing wells onshore | [31] |
| Storage | 7.2 | 2015 | Saline formations onshore | [31] |
| Storage | 8.64 | 2015 | Depleted O&G Field—reusing wells offshore | [31] |
| Storage | 14.4 | 2015 | Depleted O&G Field—no reusing wells offshore | [31] |
| Storage | 20.16 | 2015 | Saline formations offshore | [31] |
| Material | E: Elastic Modulus (GPa) | ρ: Density (g/cc) | Equivalent Factor (Material/kg Steel) | Weight Savings(%) |
|---|---|---|---|---|
| Steel | 207 [97] | 7.8 [97] | 1 | NA |
| Aluminum | 71 [97] | 2.8 [97] | 0.5 | 50 |
| Glass-Fiber-Reinforced Composite | 41 [98] | 1.9 [98] | 0.4 | 60 |
| Carbon-Fiber-Reinforced Composite | 95.5 [99] | 1.4 [99] | 0.23 | 77 |
| Traditional Electricity Grid | ||||
|---|---|---|---|---|
| Process/Material | CCS (t CO2 Captured) | Global Warming Potential (GWP) (t CO2 eq/t Material) | Stiffness Normalization Factor (t Material/t Steel) | Normalized GWP (nGWP) (t CO2 eq/t Steel Sheet eq) |
| BF-BOF Steel | 0 | 2.17 | 1.00 | 2.17 |
| Electric Arc Furnace Steel (100% scrap) | 0 | 0.40 | 1.00 | 0.40 |
| Natural Gas DRI-EAF Steel | 0 | 1.25 | 1.00 | 1.25 |
| Hydrogen DRI-EAF Steel | 0 | 1.64 | 1.00 | 1.64 |
| BF-BOF Steel with CCS | 1.42 | 0.70 | 1.00 | 0.70 |
| NG-DRI-EAF Steel with CCS | 0.64 | 0.68 | 1.00 | 0.68 |
| Primary Aluminum | 0 | 8.22 | 0.50 | 4.11 |
| Secondary Aluminum (100% scrap) | 0 | 0.45 | 0.50 | 0.22 |
| Primary Aluminum with CCS | 2.32 | 6.84 | 0.50 | 3.42 |
| Decarbonized Aluminum | 0 | 8.96 | 0.50 | 4.48 |
| Glass Fiber Composites (Epoxy) | 0 | 3.48 | 0.40 | 1.39 |
| Carbon Fiber Composites (Epoxy) | 0 | 10.96 | 0.23 | 2.52 |
| Renewables-Based Electricity Grid | ||||
|---|---|---|---|---|
| Process/Material | CCS (t CO2 Captured) | Global Warming Potential (GWP) (t CO2 eq/t Material) | Stiffness Normalization Factor (t Material/t Steel) | Normalized GWP (nGWP) (t CO2 eq/t Steel Sheet eq) |
| BF-BOF Steel | 0 | 2.03 | 1.00 | 2.03 |
| Electric Arc Furnace Steel (from 100% scrap) | 0 | 0.18 | 1.00 | 0.18 |
| Natural Gas DRI-EAF Steel | 0 | 0.92 | 1.00 | 0.92 |
| Hydrogen DRI-EAF Steel | 0 | 0.31 | 1.00 | 0.31 |
| BF-BOF Steel with CCS | 1.42 | 0.43 | 1.00 | 0.43 |
| NG-DRI-EAF Steel with CCS | 0.64 | 0.33 | 1.00 | 0.33 |
| Primary Aluminum | 0 | 3.28 | 0.50 | 1.64 |
| Secondary Aluminum (from 100% scrap) | 0 | 0.37 | 0.50 | 0.18 |
| Primary Aluminum with CCS | 2.32 | 1.65 | 0.50 | 0.83 |
| Decarbonized Aluminum | 0 | 1.16 | 0.50 | 0.58 |
| Glass Fiber Composites (Epoxy) | 0 | 2.86 | 0.40 | 1.15 |
| Carbon Fiber Composites (Epoxy) | 0 | 6.74 | 0.23 | 1.55 |
| Process/Material | GWP of Current Studies (t CO2 eq/t Finished Sheet) | GWP from Literature (t CO2 eq/t Material) | References | |
|---|---|---|---|---|
| Traditional Grid | Renewable Grid | |||
| BF- BOF Steel | 2.17 | 2.03 | 1.7–2.5 | [5,6,7] |
| Electric Arc Furnace Steel (100% scrap) | 0.40 | 0.18 | 0.3–0.47 | [8,9,10,11,12] |
| Natural Gas DRI-EAF Steel | 1.25 | 0.92 | 0.82–1.16 | [13,14,15] |
| Hydrogen DRI-EAF Steel | 1.64 | 0.31 | 1.1 | [20,21] |
| BF-BOF Steel with CCS | 0.70 | 0.43 | ||
| NG-DRI-EAF Steel with CCS | 0.68 | 0.33 | ||
| Primary Aluminum | 8.22 | 3.28 | 8.2–11.2 | [22,23,24] |
| Secondary Aluminum (100% scrap) | 0.45 | 0.37 | 0.2–0.85 | [22,23,24] |
| Primary Aluminum with CCS | 6.84 | 1.65 | ||
| Decarbonized Aluminum | 8.96 | 1.16 | ||
| Glass-Fiber-Reinforced Composite | 1.52 | 0.96 | 1.8–4.6 | [30] |
| Carbon-Fiber-Reinforced Composite | 21.51 | 10.43 | 28.83–42.32 | [28] |
| Resin | 4.7 | 4.7 | 4.9 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajulwar, V.V.; Shyrokykh, T.; Stirling, R.; Jarnerud, T.; Korobeinikov, Y.; Bose, S.; Bhattacharya, B.; Bhattacharjee, D.; Sridhar, S. Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions. Energies 2023, 16, 6904. https://doi.org/10.3390/en16196904
Rajulwar VV, Shyrokykh T, Stirling R, Jarnerud T, Korobeinikov Y, Bose S, Bhattacharya B, Bhattacharjee D, Sridhar S. Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions. Energies. 2023; 16(19):6904. https://doi.org/10.3390/en16196904
Chicago/Turabian StyleRajulwar, Vaishnavi Vijay, Tetiana Shyrokykh, Robert Stirling, Tova Jarnerud, Yuri Korobeinikov, Sudip Bose, Basudev Bhattacharya, Debashish Bhattacharjee, and Seetharaman Sridhar. 2023. "Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions" Energies 16, no. 19: 6904. https://doi.org/10.3390/en16196904
APA StyleRajulwar, V. V., Shyrokykh, T., Stirling, R., Jarnerud, T., Korobeinikov, Y., Bose, S., Bhattacharya, B., Bhattacharjee, D., & Sridhar, S. (2023). Steel, Aluminum, and FRP-Composites: The Race to Zero Carbon Emissions. Energies, 16(19), 6904. https://doi.org/10.3390/en16196904







