Promising Bioalcohols for Low-Emission Vehicles
Abstract
:1. Introduction
2. Why Bioalcohols?
2.1. Methanol (CH3OH)
2.2. Ethanol (C2H5OH)
2.3. Butanol (C4H9OH)
2.4. Pentanol (C5H11OH)
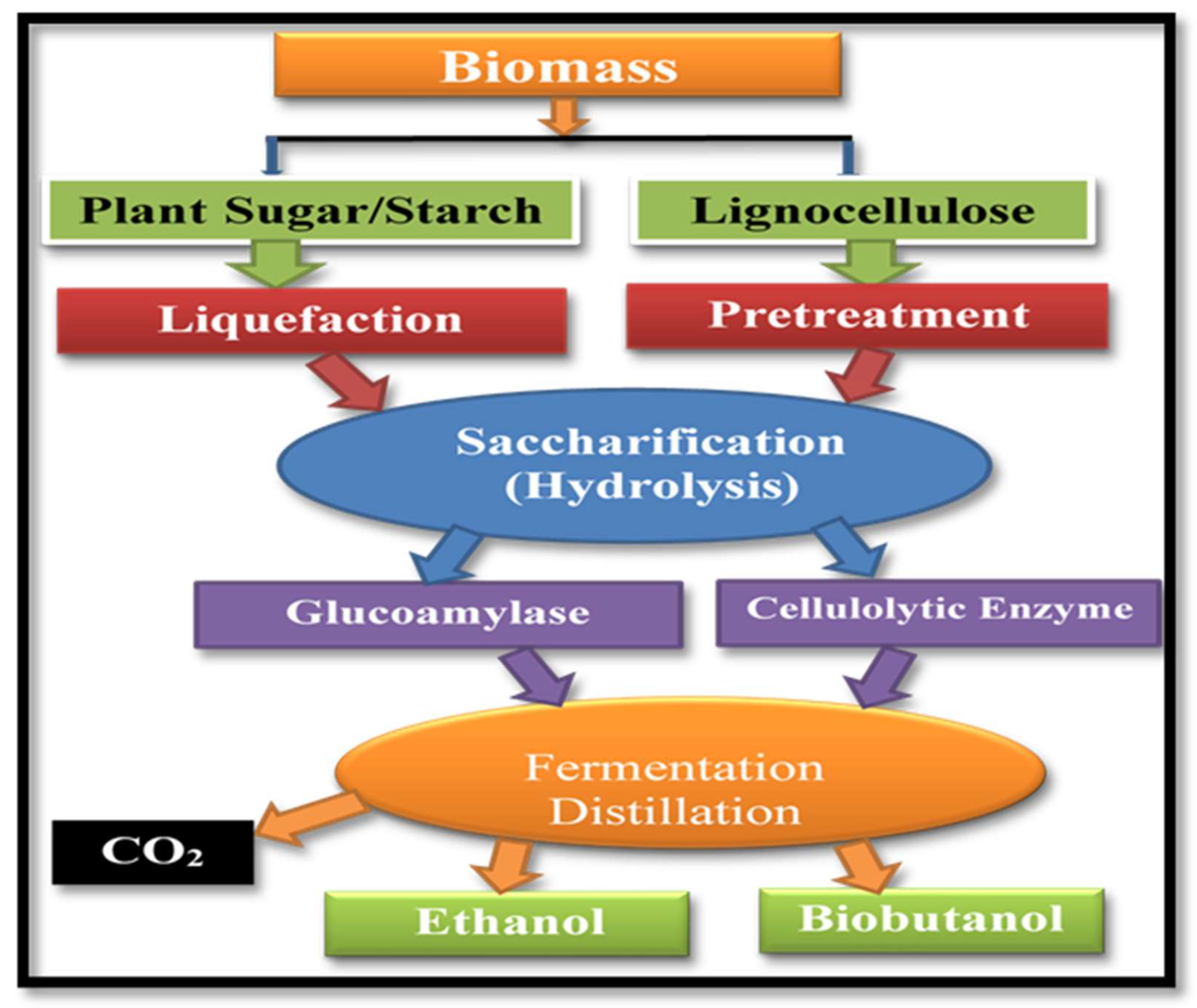
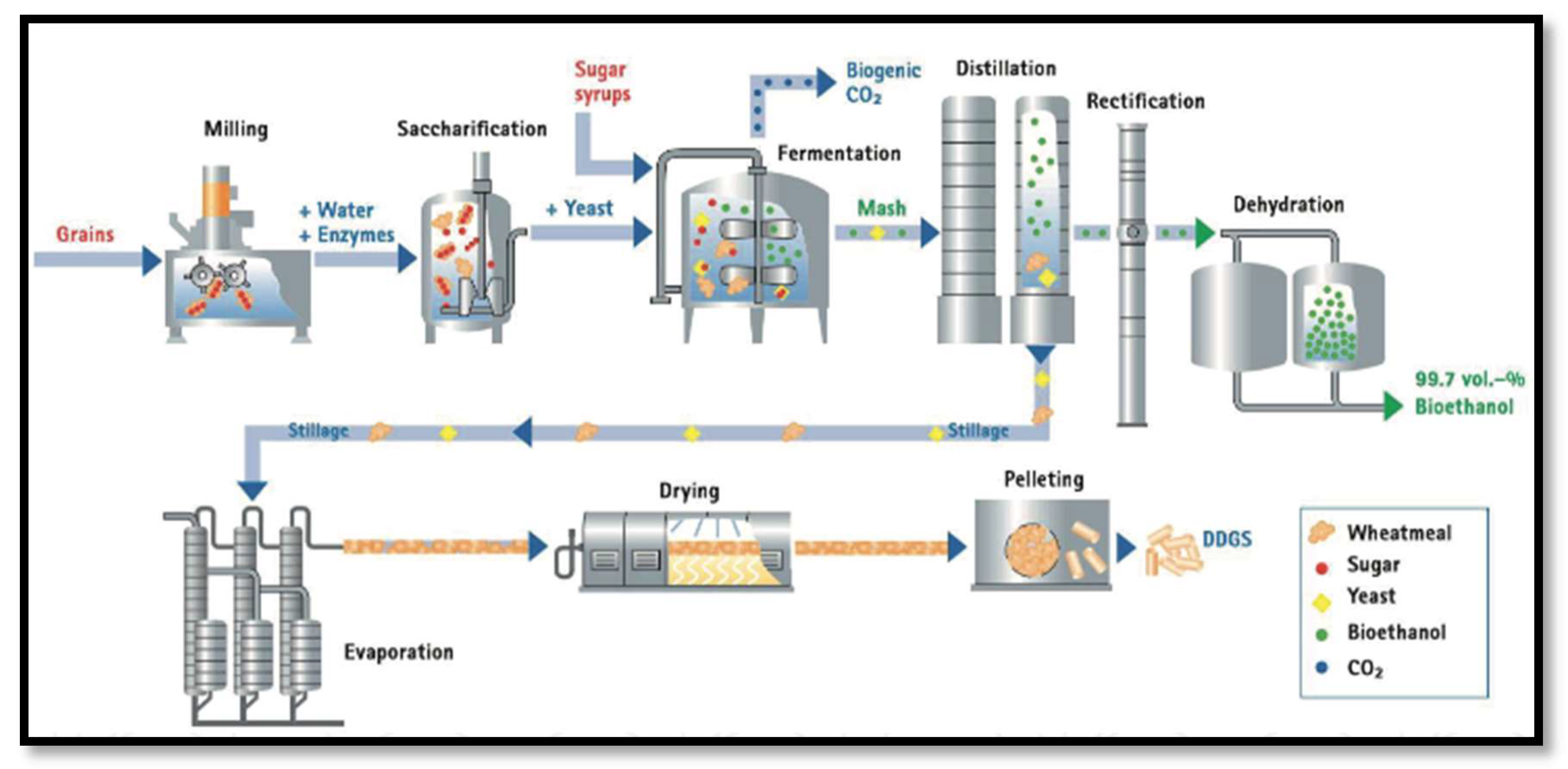
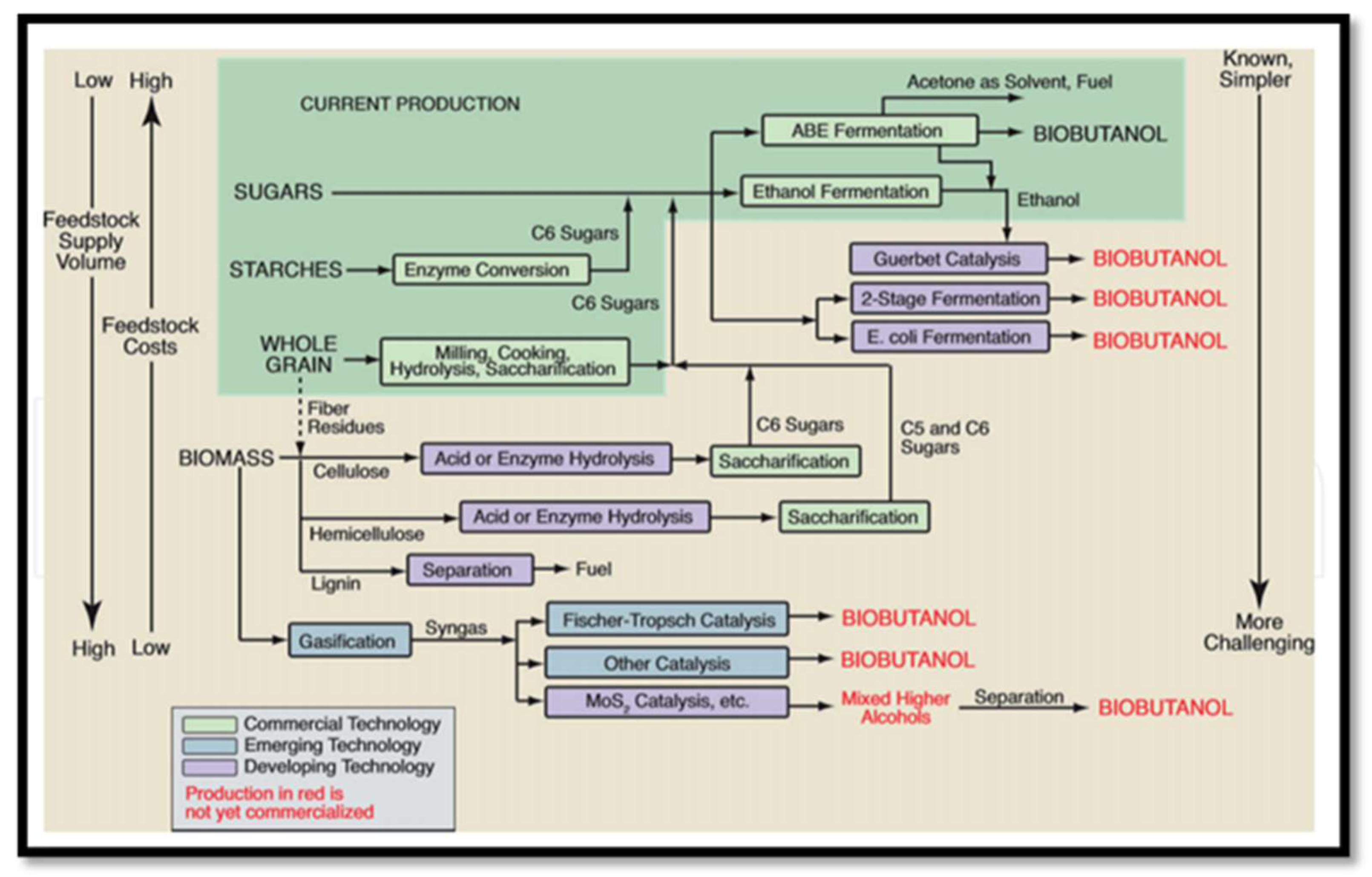
2.5. Production of Bioalcohols
2.6. Properties of Bioalcohols
3. Effects of Bioalcohols on Emissions
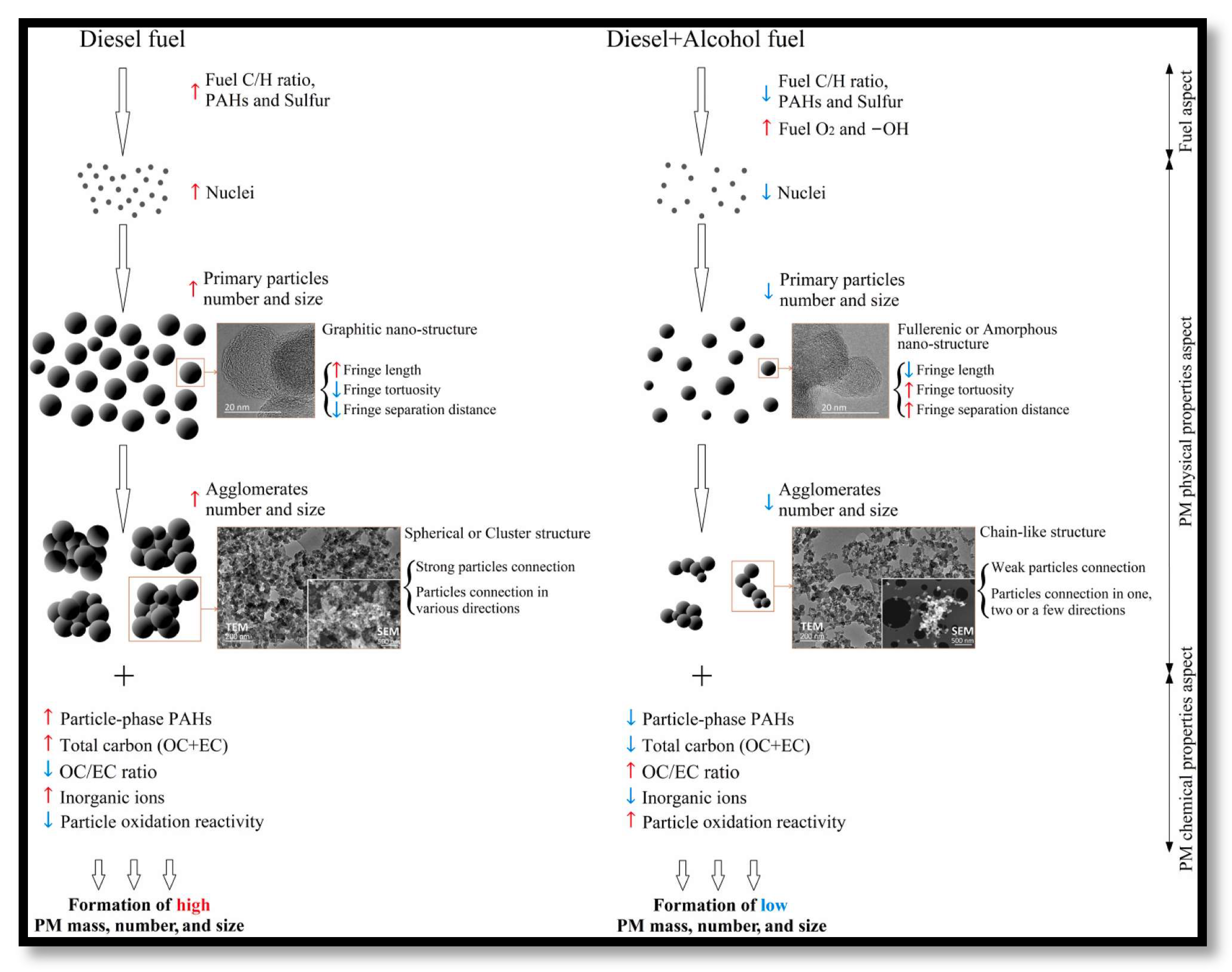
- Both lower alcohols and higher alcohols can significantly change the composition and structure of fine dust, which leads to an average reduction of about 50% (weight) of fine dust, 60% (weight) of fine dust (TNC), and 30% (by weight) under various conditions of fine dust size (GMD). Under the same alcohol consumption (by volume or weight), lower alcohols, especially methanol and ethanol, are more effective than higher alcohols in reducing PM. This is because compared with the characteristics of higher alcohols, the difference in characteristics of lower alcohols significantly affects the formation of PM (especially when the carbon content is low, and the oxygen content is high).
- Mixed alcohol has a greater impact on PM reduction than fumigated alcohol, especially when running at low engine temperature (i.e., low speed or low torque). Evaporation of fumigation alcohol can cause poor combustion. In addition, part of the fumigated alcohol droplets will enter the low-temperature cooling area of the combustion chamber wall/tank, resulting in unburned fuel. In addition, spraying alcohol with a low-pressure fuel injection will produce large drops of alcohol, reducing the chance of complete combustion and further particle formation.
- Because alcohol has a lower weight, quantity, size, elemental carbon (about −60%) and flash point (about −8%) as well as higher fine dust oxidation reaction activity (about increased performance and service life) it can be used in a catalytic converter, especially a DPF.
| Reference Fuel | Alcohol Used | Fuelling Type | Engine Tested | Operating Conditions | Test Results | Ref. |
|---|---|---|---|---|---|---|
| Diesel | Methanol | Fumigation (achieving 10, 20, and 30% of desired engine load by fumigated methanol) | 4-cylinder, NA, diesel engine | Japanese 13-mode test cycle | Decrease in PM mass (up to −21.2%, on average of different conditions for 30% fumigated methanol) with increase in methanol ratio | [117] |
| Diesel | Ethanol | Blend (mixing 5, 10, 15, and 20% of ethanol by volume with diesel) | 6-cylinder, heavy-duty, turbocharged diesel engine | European 13-mode test cycle | Decrease in dry soot but increase in SOF resulting in no obvious effect of ethanol on the PM mass | [118] |
| Diesel | Butanol | Blend (mixing 5, 10, 15, and 20% of butanol by volume with diesel) | Single-cylinder, DI stationary diesel engine | 25, 50, and 75% of maximum engine power at a speed of 3000 rpm | Decrease in PM mass (up to −25%) with increase in butanol ratio | [119] |
| Diesel | n-Pentanol | Blend (mixing 10, 20, and 30% of n-pentanol by volume with diesel) | 4-cylinder, NA, DI diesel engine | Five engine loads (BMEP of 0.84, 2.06, 4.15, 6.20, and 7.10 bar) at a speed of 1800 rpm | Decrease in PM mass (up to about −70%) with increase in pentanol ratio (on average of five loads, −42% reduction for 30% pentanol blend) | [120] |
| Fuel Used | Engine Tested | Parameter Investigated | Ref. |
|---|---|---|---|
| Gasoline; diesel | SI and CI | PM mass; TNC; GMD; nanostructure of particles; OC-EC; ions; metal and elements | [121] |
| Gasoline; diesel | SI and CI | Micro- and nanostructure of particles | [122] |
| Ethanol | SI and CI | PM mass; PAHs | [123] |
| Methanol | SI and CI | PM mass; PAHs | [123] |
| Diesel | CI | PM mass; TNC; GMD; morphology, micro- and nanostructures of particles; OC-EC; metals and elements; ions; oxidation reactivity | [107] |
| Alcohol (methanol, ethanol, butanol, and pentanol) | CI | Soot; PAHs | [124] |
| Reference Fuel | Alcohol Used | Fuelling Type | Engine Tested | Operating Conditions | Test Results | Ref. |
|---|---|---|---|---|---|---|
| Diesel | CH3OH | Blend (mixing 11.5% methanol by volume with 88.5% diesel) | 4-cylinder, turbocharged diesel engine | One engine load (BMEP of 12 bar) at a speed of 1400 rpm | Decrease in the radius of gyration (−25%), fractal dimension (about −10%), number of primary particles (−51%), and primary particle diameter (7.5–42.5 nm for methanol and 9.5–50 nm for diesel) | [86] |
| Diesel | C2H5OH | Blend (mixing 10 and 20% of ethanol with diesel) | 4-cylinder, supercharged diesel engine | One engine load (full load) at full engine speed | Decrease in the agglomerates and primary particles sizes with an increase in ethanol ratio | [114] |
| Diesel | n-Butanol | Blend (mixing 10 and 20% of butanol by volume with diesel) | 4-cylinder, turbocharged diesel engine | One engine load (78.4 Nm) at a speed of 2000 rpm | Decrease in the agglomerates and primary particles sizes | [104] |
| Diesel | n-Pentanol | Blend (mixing 15 and 30% of pentanol by volume with diesel) | 4-cylinder, turbocharged diesel engine | One engine load of 130 Nm at a speed of 2000 rpm | No significant differences in the overall morphology of particles (near-spherical shape) and agglomerates (branched-chain structures) for all the fuels tested; decrease in primary particles size (up to − 8.2%) with an increase in pentanol ratio | [105] |
4. Conclusions
- Compared to methanol, ethanol has a higher energy density, is nontoxic, and is available in large quantities, reducing racers’ vulnerability to price increases and supply disruptions. Methanol can be made from organic materials, such as biomass and domestic waste; at some point, it may even be made from coal. The top five countries in the world’s coal reserves are indeed the US, Russia, Australia, China, and India, and these reserves will be very abundant in the next few years. As compared with gasoline, the molar ratio of alcohol combustion products to reactants is higher, so higher combustion pressure is generated in the combustion chamber of the internal combustion engine, thereby improving energy production and thermal efficiency [125].
- Because alcohol fuel has higher volumetric efficiency, alcohol has better combustion characteristics and performance, making methanol the preferred fuel for racing cars. The acceleration time decreases as the power increases.
- Alcohol has better visibility in the event of a fire. It is used for evacuation, rescue, minor suffocation, cold flames, and low heat, resulting in minor burns, minor smoke damage, and easy removal of residues. Water can be used to extinguish fires, and it is easier to use powder foam.
- Alcohol fuel has lower vapour emissions. When alcohol fuel is used, less harmful byproducts are released into the atmosphere.
- Because the carbon content in alcohol fuel is very low, the combustion of internal combustion engines will produce a small amount of soot, which is discharged into the atmosphere.
- Alcohol fuel is liquid, so it only needs to be modified slightly to use the same transportation and infrastructure management as traditional fuels.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (IEA) World Energy Balances: Overview. Available online: https://www.iea.org/reports/world-energy-balances-overview/world (accessed on 21 February 2022).
- Sadeghinezhad, E.; Kazi, S.N.; Badarudin, A.; Oon, C.S.; Zubir, M.N.M.; Mehrali, M. A Comprehensive Review of Bio-Diesel as Alternative Fuel for Compression Ignition Engines. Renew. Sustain. Energy Rev. 2013, 28, 410–424. [Google Scholar] [CrossRef]
- International Energy Agency (IEA) Global Energy Review 2021: Oil. Available online: https://www.iea.org/reports/global-energy-review-2021/oil (accessed on 14 March 2022).
- Arbab, M.I.; Masjuki, H.H.; Varman, M.; Kalam, M.A.; Imtenan, S.; Sajjad, H. Fuel Properties, Engine Performance and Emission Characteristic of Common Biodiesels as a Renewable and Sustainable Source of Fuel. Renew. Sustain. Energy Rev. 2013, 22, 133–147. [Google Scholar] [CrossRef]
- Doustdar, O.; Wyszynski, M.L.; Mahmoudi, H.; Tsolakis, A. Enhancing the Properties of Fischer-Tropsch Fuel Produced from Syngas over Co/SiO2 Catalyst: Lubricity and Calorific Value. IOP Conf. Ser. Mater. Sci. Eng. 2016, 148, 012092. [Google Scholar] [CrossRef]
- Prado, C.M.R.; Antoniosi Filho, N.R. Production and Characterization of the Biofuels Obtained by Thermal Cracking and Thermal Catalytic Cracking of Vegetable Oils. J. Anal. Appl. Pyrolysis 2009, 86, 338–347. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Kazi, S.N.; Sadeghinejad, F.; Badarudin, A.; Mehrali, M.; Sadri, R.; Reza Safaei, M. A Comprehensive Literature Review of Bio-Fuel Performance in Internal Combustion Engine and Relevant Costs Involvement. Renew. Sustain. Energy Rev. 2014, 30, 29–44. [Google Scholar] [CrossRef]
- Duarte Souza Alvarenga Santos, N.; Rückert Roso, V.; Teixeira Malaquias, A.C.; Coelho Baêta, J.G. Internal Combustion Engines and Biofuels: Examining Why This Robust Combination Should Not Be Ignored for Future Sustainable Transportation. Renew. Sustain. Energy Rev. 2021, 148, 111292. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Analysis of Blended Fuel Properties and Engine Performance with Palm Biodiesel–Diesel Blended Fuel. Renew. Energy 2016, 86, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and Comparison of Fuel Properties, Engine Performance, and Emission Characteristics of Biodiesel from Various Non-Edible Vegetable Oils: A Review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.H.; Ishak, W.F.W.; Shaiful, A.I.M. Alcohol Based Automotive Fuels from First Four Alcohol Family in Compression and Spark Ignition Engine: A Review on Engine Performance and Exhaust Emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Sidik, N.A.C.; Yusaf, T.; Kadirgama, K.; Kettner, M. Alcohol and Ether as Alternative Fuels in Spark Ignition Engine: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2586–2605. [Google Scholar] [CrossRef]
- No, S.-Y. Application of Liquid Biofuels to Internal Combustion Engines; Zentrum für Sonnenenergie-und Wasserstoff-Forschung Baden-Württemberg (ZSW), Ed.; Green Energy and Technology; Springer: Singapore, 2019; ISBN 978-981-13-6736-6. [Google Scholar]
- Sridhar, R.; Jeevahan, J.; Chandrasekaran, M. Effect of the Addition of 1-Pentanol on Engine Performance and Emission Characteristics of Diesel and Biodiesel Fuelled Single Cylinder Diesel Engine. Int. J. Ambient Energy 2020, 41, 58–63. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and Emission Assessment of Diesel–Biodiesel–Ethanol/Bioethanol Blend as a Fuel in Diesel Engines: A Review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Solubility of Things Alcohol Solubility-Methanol, Ethanol, Propanol Etc. in Solubility OF Things. Available online: https://www.solubilityofthings.com/water/alcohols (accessed on 2 February 2022).
- Cheung, C.S.; Zhu, L.; Huang, Z. Regulated and Unregulated Emissions from a Diesel Engine Fueled with Biodiesel and Biodiesel Blended with Methanol. Atmos. Environ. 2009, 43, 4865–4872. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; Soave, G.; Gamba, S.; Langè, S. Economic Analysis of a Combined Energy–Methanol Production Plant. Appl. Energy 2011, 88, 4891–4897. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Fidalgo, B.; Arenillas, A.; Menéndez, J.A. CO2 Reforming of Coke Oven Gas over a Ni/ΓAl2O3 Catalyst to Produce Syngas for Methanol Synthesis. Fuel 2012, 94, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Specht, M.; Bandi, A. “The Methanol Cycle”—Sustainable Supply of Liquid Fuels; Zentrum für Sonnenenergie-und Wasserstoff-Forschung Baden-Württemberg (ZSW): Stuttgart, Germany, 1999. [Google Scholar]
- Wang, C.; Chen, W.; Wang, W.; Wu, Y.; Chi, R.; Tang, Z. Experimental Study on Methanol Recovery through Flashing Vaporation in Continuous Production of Biodiesel via Supercritical Methanol. Energy Convers. Manag. 2011, 52, 1454–1458. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An Overview on the Production of Bio-Methanol as Potential Renewable Energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Leduc, S.; Lundgren, J.; Franklin, O.; Dotzauer, E. Location of a Biomass Based Methanol Production Plant: A Dynamic Problem in Northern Sweden. Appl. Energy 2010, 87, 68–75. [Google Scholar] [CrossRef]
- Renó, M.L.G.; Lora, E.E.S.; Palacio, J.C.E.; Venturini, O.J.; Buchgeister, J.; Almazan, O. A LCA (Life Cycle Assessment) of the Methanol Production from Sugarcane Bagasse. Energy 2011, 36, 3716–3726. [Google Scholar] [CrossRef]
- Holmgren, K.M.; Berntsson, T.; Andersson, E.; Rydberg, T. System Aspects of Biomass Gasification with Methanol Synthesis–Process Concepts and Energy Analysis. Energy 2012, 45, 817–828. [Google Scholar] [CrossRef]
- Clausen, L.R.; Houbak, N.; Elmegaard, B. Technoeconomic Analysis of a Methanol Plant Based on Gasification of Biomass and Electrolysis of Water. Energy 2010, 35, 2338–2347. [Google Scholar] [CrossRef] [Green Version]
- Hall, J. Potential Air Quality Benefits of Methanol as a Vehicle Fuel. Energy 1985, 10, 733–736. [Google Scholar] [CrossRef]
- Dürre, P. Biobutanol: An Attractive Biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Ethanol Fuel Basics. Available online: https://afdc.energy.gov/fuels/ethanol_fuel_basics.html (accessed on 5 February 2022).
- Renewable Fuels Association (RFA) The Impact of Accidental Ethanol Releases on the Environment. Available online: https://ethanolrfa.org/ethanol-101/environment (accessed on 15 March 2022).
- Liquid Transportation Fuels from Coal and Biomass; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13712-6.
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R.L. Challenges in Biobutanol Production: How to Improve the Efficiency? Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative Butanol Production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef]
- Grana, R.; Frassoldati, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Cattolica, R.; Seshadri, K. An Experimental and Kinetic Modeling Study of Combustion of Isomers of Butanol. Combust. Flame 2010, 157, 2137–2154. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in Gasoline: Environmental Impacts and Sustainability Review Article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Singh, G.N.; Bharj, R.S. Study of Physical-Chemical Properties for 2nd Generation Ethanol-Blended Diesel Fuel in India. Sustain. Chem. Pharm. 2019, 12, 100130. [Google Scholar] [CrossRef]
- Babu, V.; Murthy, M.; Rao, A.P. Butanol and Pentanol: The Promising Biofuels for CI Engines—A Review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar] [CrossRef]
- Wu, H.; Nithyanandan, K.; Zhou, N.; Lee, T.H.; Lee, C.F.; Zhang, C. Impacts of Acetone on the Spray Combustion of Acetone–Butanol–Ethanol (ABE)-Diesel Blends under Low Ambient Temperature. Fuel 2015, 142, 109–116. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Z.; Liu, J.; Lee, C. Combustion and Emissions Characteristics of High N-Butanol/Diesel Ratio Blend in a Heavy-Duty Diesel Engine and EGR Impact. Energy Convers. Manag. 2014, 78, 787–795. [Google Scholar] [CrossRef]
- Tian, Z.; Zhen, X.; Wang, Y.; Liu, D.; Li, X. Combustion and Emission Characteristics of N-Butanol-Gasoline Blends in SI Direct Injection Gasoline Engine. Renew. Energy 2020, 146, 267–279. [Google Scholar] [CrossRef]
- Gao, K.; Boiano, S.; Marzocchella, A.; Rehmann, L. Cellulosic Butanol Production from Alkali-Pretreated Switchgrass (Panicum Virgatum) and Phragmites (Phragmites Australis). Bioresour. Technol. 2014, 174, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Procentese, A.; Raganati, F.; Olivieri, G.; Elena Russo, M.; Marzocchella, A. Pre-Treatment and Enzymatic Hydrolysis of Lettuce Residues as Feedstock for Bio-Butanol Production. Biomass Bioenergy 2017, 96, 172–179. [Google Scholar] [CrossRef]
- Killol, A.; Reddy, N.; Paruvada, S.; Murugan, S. Experimental Studies of a Diesel Engine Run on Biodiesel N-Butanol Blends. Renew. Energy 2019, 135, 687–700. [Google Scholar] [CrossRef]
- Wei, H.; Feng, D.; Pan, M.; Pan, J.; Rao, X.; Gao, D. Experimental Investigation on the Knocking Combustion Characteristics of N-Butanol Gasoline Blends in a DISI Engine. Appl. Energy 2016, 175, 346–355. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Saravanan, S. Effects of Iso-Butanol/Diesel and n-Pentanol/Diesel Blends on Performance and Emissions of a DI Diesel Engine under Premixed LTC (Low Temperature Combustion) Mode. Fuel 2016, 170, 49–59. [Google Scholar] [CrossRef]
- Zheng, J.; Tashiro, Y.; Wang, Q.; Sonomoto, K. Recent Advances to Improve Fermentative Butanol Production: Genetic Engineering and Fermentation Technology. J. Biosci. Bioeng. 2015, 119, 1–9. [Google Scholar] [CrossRef]
- No, S.-Y. Utilization of Pentanol as Biofuels in Compression Ignition Engines. Front. Mech. Eng. 2020, 6, 15. [Google Scholar] [CrossRef]
- Lee, Y.J. Utilisation of Bio-Ethanol as Automobile Fuels and Technology Development. Available online: https://www.konetic.or.kr/main/REPORT/REPORT_VIEW.asp?PARENT_NUM=1055&MENU1=4024 (accessed on 12 May 2022).
- Yun, Y. Alcohol Fuels: Current Status and Future Direction. In Alcohol Fuels-Current Technologies and Future Prospect; Yun, Y., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-043-7. [Google Scholar]
- Renewable Fuels Association (RFA) Annual Ethanol Production: U.S. and World Ethanol Production. Available online: https://ethanolrfa.org/markets-and-statistics/annual-ethanol-production (accessed on 12 May 2022).
- Cascone, R. Biobutanol: A Replacement for Bioethanol? Chem. Eng. Prog. 2008, 104, 4. [Google Scholar]
- CropEnergies Bioethanol Production Processes. Available online: https://www.cropenergies.com/en/products/ethanol (accessed on 17 March 2022).
- Kandasamy, M.; Hamawand, I.; Bowtell, L.; Seneweera, S.; Chakrabarty, S.; Yusaf, T.; Shakoor, Z.; Algayyim, S.; Eberhard, F. Investigation of Ethanol Production Potential from Lignocellulosic Material without Enzymatic Hydrolysis Using the Ultrasound Technique. Energies 2017, 10, 62. [Google Scholar] [CrossRef]
- Gregg, J.; Bolwig, S.; Hansen, T.; Solér, O.; Ben Amer-Allam, S.; Pladevall Viladecans, J.; Klitkou, A.; Fevolden, A. Value Chain Structures That Define European Cellulosic Ethanol Production. Sustainability 2017, 9, 118. [Google Scholar] [CrossRef] [Green Version]
- Cotana, F.; Cavalaglio, G.; Pisello, A.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable Ethanol Production from Common Reed (Phragmites Australis) through Simultaneuos Saccharification and Fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef] [Green Version]
- Eggert, H.; Greaker, M. Promoting Second Generation Biofuels: Does the First Generation Pave the Road? Energies 2014, 7, 4430–4445. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, J.; Lin, G.; Jiang, D.; Yan, X. Switchgrass-Based Bioethanol Productivity and Potential Environmental Impact from Marginal Lands in China. Energies 2017, 10, 260. [Google Scholar] [CrossRef] [Green Version]
- Hansdah, D.; Murugan, S.; Das, L.M. Experimental Studies on a DI Diesel Engine Fueled with Bioethanol-Diesel Emulsions. Alex. Eng. J. 2013, 52, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Jin, Y.; Zhang, G.; Fang, Y.; Xiao, Y.; Zhao, H. Improving Production of Bioethanol from Duckweed (Landoltia Punctata) by Pectinase Pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Rajoli, S.; Taherzadeh, M. Techno-Economic Analysis of Integrating First and Second-Generation Ethanol Production Using Filamentous Fungi: An Industrial Case Study. Energies 2016, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Connolly, D.; Mathiesen, B.V.; Ridjan, I. A Comparison between Renewable Transport Fuels That Can Supplement or Replace Biofuels in a 100% Renewable Energy System. Energy 2014, 73, 110–125. [Google Scholar] [CrossRef]
- Tsolakis, A.; Bogarra, M.; Herreros, J. Environmental Impacts of Road Vehicles. In Environmental Impacts of Road Vehicles: Past, Present and Future; Harrison, R.M., Hester, R.E., Eds.; Issues in Environmental Science and Technology; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–24. ISBN 978-1-78262-892-7. [Google Scholar]
- Lapuerta, M.; García-Contreras, R.; Agudelo, J.R. Lubricity of Ethanol-Biodiesel-Diesel Fuel Blends. Energy Fuels 2010, 24, 1374–1379. [Google Scholar] [CrossRef]
- Blumberg, P.N.; Bromberg, L.; Kang, H.; Tai, C. Simulation of High Efficiency Heavy Duty SI Engines Using Direct Injection of Alcohol for Knock Avoidance. SAE Int. J. Engines 2008, 1, 1186–1195. [Google Scholar] [CrossRef]
- Gong, C.; Deng, B.; Wang, S.; Su, Y.; Gao, Q.; Liu, X. Combustion of a Spark-Ignition Methanol Engine during Cold Start under Cycle-by-Cycle Control. Energy Fuels 2008, 22, 2981–2985. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.-M.; Su, Y.; Dou, H.-L.; Liu, X.-J. Effect of Injection and Ignition Timings on Performance and Emissions from a Spark-Ignition Engine Fueled with Methanol. Fuel 2010, 89, 3919–3925. [Google Scholar] [CrossRef]
- Gong, C.-M.; Huang, K.; Jia, J.-L.; Su, Y.; Gao, Q.; Liu, X.-J. Regulated Emissions from a Direct-Injection Spark-Ignition Methanol Engine. Energy 2011, 36, 3379–3387. [Google Scholar] [CrossRef]
- Brusstar, M.J.; Gray, C.L. High Efficiency with Future Alcohol Fuels in a Stoichiometric Medium Duty Spark Ignition Engine. In Proceedings of the Powertrain & Fluid Systems Conference and Exhibition, San Diego, CA, USA, 29 October 2007. [Google Scholar]
- Rajesh Kumar, B.; Saravanan, S. Use of Higher Alcohol Biofuels in Diesel Engines: A Review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Muthukkumar, T.; Krishnamoorthy, V.; Saravanan, S. A Comparative Evaluation and Optimization of Performance and Emission Characteristics of a DI Diesel Engine Fueled with N-Propanol/Diesel, n-Butanol/Diesel and n-Pentanol/Diesel Blends Using Response Surface Methodology. RSC Adv. 2016, 6, 61869–61890. [Google Scholar] [CrossRef]
- Herreros, J.M.; Schroer, K.; Sukjit, E.; Tsolakis, A. Extending the Environmental Benefits of Ethanol–Diesel Blends through DGE Incorporation. Appl. Energy 2015, 146, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Hergueta, C.; Tsolakis, A.; Herreros, J.M.; Bogarra, M.; Price, E.; Simmance, K.; York, A.P.E.; Thompsett, D. Impact of Bio-Alcohol Fuels Combustion on Particulate Matter Morphology from Efficient Gasoline Direct Injection Engines. Appl. Energy 2018, 230, 794–802. [Google Scholar] [CrossRef]
- Hellier, P.; Purton, S.; Ladommatos, N. Molecular Structure of Photosynthetic Microbial Biofuels for Improved Engine Combustion and Emissions Characteristics. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Cho, J.H.; Park, J.; Moon, I. Advances in Diesel–Alcohol Blends and Their Effects on the Performance and Emissions of Diesel Engines. Renew. Sustain. Energy Rev. 2013, 22, 46–72. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.D.; García-Contreras, R.; Tsolakis, A. The Effect of the Addition of Individual Methyl Esters on the Combustion and Emissions of Ethanol and Butanol -Diesel Blends. Energy 2012, 42, 364–374. [Google Scholar] [CrossRef]
- Sukjit, E. Synergistic Effects of Alcohol Based Renewable Fuels: Fuel Properties and Emissions. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2013. [Google Scholar]
- Hellier, P.; Ladommatos, N.; Yusaf, T. The Influence of Straight Vegetable Oil Fatty Acid Composition on Compression Ignition Combustion and Emissions. Fuel 2015, 143, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Cann, A.F.; Liao, J.C. Pentanol Isomer Synthesis in Engineered Microorganisms. Appl. Microbiol. Biotechnol. 2010, 85, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Fernández, J.; Arnal, J.M.; Gómez, J.; Dorado, M.P. A Comparison of Performance of Higher Alcohols/Diesel Fuel Blends in a Diesel Engine. Appl. Energy 2012, 95, 267–275. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Teoh, Y.H.; How, H.G. Higher Alcohol–Biodiesel–Diesel Blends: An Approach for Improving the Performance, Emission, and Combustion of a Light-Duty Diesel Engine. Energy Convers. Manag. 2016, 111, 174–185. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Xiao, J. Combustion and Emission Characteristics of Diesel Engine Fueled with Diesel/Biodiesel/Pentanol Fuel Blends. Fuel 2015, 156, 211–218. [Google Scholar] [CrossRef]
- Rajesh kumar, B.; Saravanan, S. Effect of Exhaust Gas Recirculation (EGR) on Performance and Emissions of a Constant Speed DI Diesel Engine Fueled with Pentanol/Diesel Blends. Fuel 2015, 160, 217–226. [Google Scholar] [CrossRef]
- Fayad, M.A.; Tsolakis, A.; Fernández-Rodríguez, D.; Herreros, J.M.; Martos, F.J.; Lapuerta, M. Manipulating Modern Diesel Engine Particulate Emission Characteristics through Butanol Fuel Blending and Fuel Injection Strategies for Efficient Diesel Oxidation Catalysts. Appl. Energy 2017, 190, 490–500. [Google Scholar] [CrossRef]
- Fayad, M.A.; Herreros, J.M.; Martos, F.J.; Tsolakis, A. Role of Alternative Fuels on Particulate Matter (PM) Characteristics and Influence of the Diesel Oxidation Catalyst. Environ. Sci. Technol. 2015, 49, 11967–11973. [Google Scholar] [CrossRef] [Green Version]
- Sukjit, E.; Herreros, J.M.; Piaszyk, J.; Dearn, K.D.; Tsolakis, A. Finding Synergies in Fuels Properties for the Design of Renewable Fuels–Hydroxylated Biodiesel Effects on Butanol-Diesel Blends. Environ. Sci. Technol. 2013, 47, 3535–3542. [Google Scholar] [CrossRef]
- Choi, B.; Jiang, X. Individual Hydrocarbons and Particulate Matter Emission from a Turbocharged CRDI Diesel Engine Fueled with n -Butanol/Diesel Blends. Fuel 2015, 154, 188–195. [Google Scholar] [CrossRef]
- Fayad, M.A.; Fernández-Rodríguez, D.; Herreros, J.M.; Lapuerta, M.; Tsolakis, A. Interactions between Aftertreatment Systems Architecture and Combustion of Oxygenated Fuels for Improved Low Temperature Catalysts Activity. Fuel 2018, 229, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Campos-Fernandez, J.; Arnal, J.M.; Gomez, J.; Lacalle, N.; Dorado, M.P. Performance Tests of a Diesel Engine Fueled with Pentanol/Diesel Fuel Blends. Fuel 2013, 107, 866–872. [Google Scholar] [CrossRef]
- Xing-cai, L.; Jian-guang, Y.; Wu-gao, Z.; Zhen, H. Effect of Cetane Number Improver on Heat Release Rate and Emissions of High Speed Diesel Engine Fueled with Ethanol–Diesel Blend Fuel. Fuel 2004, 83, 2013–2020. [Google Scholar] [CrossRef]
- Lapuerta, M.; García-Contreras, R.; Campos-Fernández, J.; Dorado, M.P. Stability, Lubricity, Viscosity, and Cold-Flow Properties of Alcohol−Diesel Blends. Energy Fuels 2010, 24, 4497–4502. [Google Scholar] [CrossRef]
- Pinzi, S.; Redel-Macías, M.D.; Carmona-Cabello, M.; Cubero, A.; Herreros, J.M.; Dorado, M.P. Influence of 1-Butanol and 1-Pentanol Addition to Diesel Fuel on Exhaust and Noise Emissions under Stationary and Transient Conditions. Fuel 2021, 301, 121046. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Fernández-Rodríguez, D.; Cova-Bonillo, A. Autoignition of Blends of N-Butanol and Ethanol with Diesel or Biodiesel Fuels in a Constant-Volume Combustion Chamber. Energy 2017, 118, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol Combustion Chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- No, S.-Y. Application of Biobutanol in Advanced CI Engines—A Review. Fuel 2016, 183, 641–658. [Google Scholar] [CrossRef]
- Lapuerta, M.; Sánchez-Valdepeñas, J.; Sukjit, E. Effect of Ambient Humidity and Hygroscopy on the Lubricity of Diesel Fuels. Wear 2014, 309, 200–207. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Fernández-Rodríguez, D.; Patiño-Camino, R. Cold Flow and Filterability Properties of N-Butanol and Ethanol Blends with Diesel and Biodiesel Fuels. Fuel 2018, 224, 552–559. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, D.; Lapuerta, M.; German, L. Progress in the Use of Biobutanol Blends in Diesel Engines. Energies 2021, 14, 3215. [Google Scholar] [CrossRef]
- Wichmann, H.-E. Diesel Exhaust Particles. Inhal. Toxicol. 2007, 19, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, J.; Chow, J.C.; Watson, J.G.; An, Z.; Jin, Z.; Fung, K.; Liu, S. Evaluation of the Thermal/Optical Reflectance Method for Discrimination between Char- and Soot-EC. Chemosphere 2007, 69, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Li, S.; Roskilly, A.P.; Yu, H.; Li, H. Experimental Investigation on the Performance and Emissions of a Diesel Engine Fuelled with Ethanol–Diesel Blends. Appl. Therm. Eng. 2009, 29, 2484–2490. [Google Scholar] [CrossRef]
- Abou-Rachid, H.; Marrouni, K.E.; Kaliaguine, S. DFT Studies of the Hydrogen Abstraction from Primary Alcohols by O2 in Relation with Cetane Number Data. J. Mol. Struct. THEOCHEM 2003, 631, 241–250. [Google Scholar] [CrossRef]
- Al-Hasan, M.I.; Al-Momany, M. The effect of iso-butanol-diesel blends on engine performance. Transport 2008, 23, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Myung, C.L.; Park, S. Review on Characterization of Nano-Particle Emissions and PM Morphology from Internal Combustion Engines: Part 2. Int. J. Automot. Technol. 2014, 15, 219–227. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, H.; Jiang, D.; Zeng, K.; Liu, B.; Zhang, J.; Wang, X. Combustion Behaviors of a Compression-Ignition Engine Fuelled with Diesel/Methanol Blends under Various Fuel Delivery Advance Angles. Bioresour. Technol. 2004, 95, 331–341. [Google Scholar] [CrossRef]
- Sayin, C.; Ilhan, M.; Canakci, M.; Gumus, M. Effect of Injection Timing on the Exhaust Emissions of a Diesel Engine Using Diesel–Methanol Blends. Renew. Energy 2009, 34, 1261–1269. [Google Scholar] [CrossRef]
- Chao, H.R.; Lin, T.C.; Chao, M.R.; Chang, F.H.; Huang, C.I.; Chen, C.B. Effect of Methanol-Containing Additive on the Emission of Carbonyl Compounds from a Heavy-Duty Diesel Engine. J. Hazard. Mater. 2000, 73, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Cheung, C.S.; Chan, T.L.; Yao, C.D. Experimental Investigation on Regulated and Unregulated Emissions of a Diesel/Methanol Compound Combustion Engine with and without Diesel Oxidation Catalyst. Sci. Total Environ. 2010, 408, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, M.; Liu, Y.; Xie, M. Numerical Study on the Combustion and Emission Characteristics of a Methanol/Diesel Reactivity Controlled Compression Ignition (RCCI) Engine. Appl. Energy 2013, 106, 184–197. [Google Scholar] [CrossRef]
- Cheng, C.H.; Cheung, C.S.; Chan, T.L.; Lee, S.C.; Yao, C.D. Experimental Investigation on the Performance, Gaseous and Particulate Emissions of a Methanol Fumigated Diesel Engine. Sci. Total Environ. 2008, 389, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, L.; Pan, W.; Yao, C. Investigation of Operating Range in a Methanol Fumigated Diesel Engine. Fuel 2015, 140, 164–170. [Google Scholar] [CrossRef]
- Sayin, C.; Ozsezen, A.N.; Canakci, M. The Influence of Operating Parameters on the Performance and Emissions of a DI Diesel Engine Using Methanol-Blended-Diesel Fuel. Fuel 2010, 89, 1407–1414. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tsang, K.S.; Cheung, C.S.; Chan, T.L.; Yao, C.D. Effect of Fumigation Methanol and Ethanol on the Gaseous and Particulate Emissions of a Direct-Injection Diesel Engine. Atmos. Environ. 2011, 45, 2001–2008. [Google Scholar] [CrossRef]
- Geng, P.; Yao, C.; Wei, L.; Liu, J.; Wang, Q.; Pan, W.; Wang, J. Reduction of PM Emissions from a Heavy-Duty Diesel Engine with Diesel/Methanol Dual Fuel. Fuel 2014, 123, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Bai, Y. Oxidation Behaviors and Nanostructure of Particulate Matter Produced from a Diesel Engine Fueled with N-Pentanol and 2-Ethylhexyl Nitrate Additives. Fuel 2021, 288, 119844. [Google Scholar] [CrossRef]
- Najafi, G.; Yusaf, T.F. Experimental Investigation of Using Methanol–Diesel Blended Fuels in Diesel Engine. In Proceedings of the Proceedings of the Fourth International Conference on Thermal Engineering: Theory and Applications, Abu Dhabi, United Arab Emirates, 12–14 January 2009; pp. 1–5. [Google Scholar]
- Sayin, C. Engine Performance and Exhaust Gas Emissions of Methanol and Ethanol–Diesel Blends. Fuel 2010, 89, 3410–3415. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Balasubramanian, R. Influence of Butanol–Diesel Blends on Particulate Emissions of a Non-Road Diesel Engine. Fuel 2014, 118, 130–136. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.S.; Huang, Z. Effect of N-Pentanol Addition on the Combustion, Performance and Emission Characteristics of a Direct-Injection Diesel Engine. Energy 2014, 70, 172–180. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, Y.; Pan, M.; Zhuang, Y.; Qiu, L.; Zhou, T.; Liu, Y. Morphology Analysis of Soot Particles from a Modern Diesel Engine Fueled with Different Types of Oxygenated Fuels. Fuel 2020, 267, 117248. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Li, L.; Chen, L.; Li, R. Particulate Status of Diesel Engine Fueled with Ethanol/Diesel Blends. Nongye Jixie Xuebao/Transactions Chinese Soc. Agric. Mach. 2013, 44, 28–32. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A. Effect of Alcohol Blend and Fumigation on Regulated and Unregulated Emissions of IC Engines—A Review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Bai, Y.; Wang, P.; Zhao, Y. An Overview of Physical and Chemical Features of Diesel Exhaust Particles. J. Energy Inst. 2019, 92, 1864–1888. [Google Scholar] [CrossRef]
- Hua, Y.; Liu, F.; Wu, H.; Lee, C.F.; Li, Y. Effects of Alcohol Addition to Traditional Fuels on Soot Formation: A Review. Int. J. Engine Res. 2020, 22, 1395–1420. [Google Scholar] [CrossRef]
- Song, C.L.; Zhou, Y.C.; Huang, R.J.; Wang, Y.Q.; Huang, Q.F.; Lü, G.; Liu, K.M. Influence of Ethanol–Diesel Blended Fuels on Diesel Exhaust Emissions and Mutagenic and Genotoxic Activities of Particulate Extracts. J. Hazard. Mater. 2007, 149, 355–363. [Google Scholar] [CrossRef] [PubMed]
- BP Statistical Review of World Energy 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 5 July 2022).
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; Deangelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Balasubramanian, R. Effects of Oxygenated Fuel Blends on Carbonaceous Particulate Composition and Particle Size Distributions from a Stationary Diesel Engine. Fuel 2015, 141, 1–8. [Google Scholar] [CrossRef]
- Winberry W., T.; Jungclaus, G. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-13A Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]


| Property | Diesel | Methanol | Ethanol | Butanol | Pentanol |
|---|---|---|---|---|---|
| Molecular formula | C12H22 | CH3OH | C2H5OH | C4H9OH | C5H11OH |
| Molecular weight (kg·kmol−1) | 166.3 | 32.04 | 46.7 | 74.112 | 88.15 |
| Cetane number | 52 | 5 | 8 | 17 | 18.2 |
| Density at 15 °C (kg·m−3) | 834.8 | 791.3 | 789.4 | 811.5 | 814.8 |
| Kinematic viscosity at 40 °C (cSt) | 2.627 | 0.58 | 1.13 | 2.17 | 2.74 |
| Lower heating value (MJ·kg−1) | 45.97 | 19.58 | 26.83 | 33.81 | 34.65 |
| Self-ignition temperature (°C) | 254–300 | 463 | 420 | 345 | 300 |
| Vapour pressure (mmHg) | 0.4 | 127 | 55 | 7 | 6 |
| Latent heat of evaporation (kJ/kg) | 270–375 | 1162.64 | 918.42 | 581.4 | 656 |
| Carbon (wt%) | 86.44 | 37.48 | 52.14 | 64.86 | 68.13 |
| Hydrogen (wt%) | 13.56 | 12.48 | 13.02 | 13.51 | 13.72 |
| Oxygen (wt%) | 0 | 49.93 | 34.73 | 21.62 | 18.15 |
| Water content (mg·kg−1) | 41.7 | 11.46 | 29.7 | ||
| Boiling point (°C) | 180–360 | 64.7 | 78.3 | 117.4 | 137.9 |
| Property | Methods | Ethanol (C2H5OH) | Butanol (C4H9OH) |
|---|---|---|---|
| Purity (% v/v) | 99.7 | 99 | |
| Density at 15 °C (Kg/m3) | EN ISO 3675 | 789.4 | 809.7 |
| Kinematic viscosity at 40 °C (cSt) | EN ISO 3104 | 1.13 | 2.22 |
| Lower heating value (MJ/kg) | UNE 51123 | 26.83 | 33.09 |
| C (wt%) | 52.14 | 64.82 | |
| H (wt%) | 13.13 | 13.60 | |
| O (wt%) | 34.73 | 21.59 | |
| Water content (ppm wt) | EN ISO 12937 | 2024 | 1146 |
| Molecular weight (kg/kmol) | 46.07 | 74.12 | |
| Boiling point (°C) | 78.3 | 117.5 | |
| Stoichiometric fuel/air ratio | 1/9.01 | 1/11.19 | |
| Cold filter plugging point (°C) | EN 116 | <−51 | <−51 |
| Lubricity (WSD) (µm) | EN ISO 12156-1 | 1057 | 591 |
| Cetane number | 8 [100,101] | 17 [94,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanwar, M.D.; Torres, F.A.; Alqahtani, A.M.; Tanwar, P.K.; Bhand, Y.; Doustdar, O. Promising Bioalcohols for Low-Emission Vehicles. Energies 2023, 16, 597. https://doi.org/10.3390/en16020597
Tanwar MD, Torres FA, Alqahtani AM, Tanwar PK, Bhand Y, Doustdar O. Promising Bioalcohols for Low-Emission Vehicles. Energies. 2023; 16(2):597. https://doi.org/10.3390/en16020597
Chicago/Turabian StyleTanwar, Manju Dhakad, Felipe Andrade Torres, Ali Mubarak Alqahtani, Pankaj Kumar Tanwar, Yashas Bhand, and Omid Doustdar. 2023. "Promising Bioalcohols for Low-Emission Vehicles" Energies 16, no. 2: 597. https://doi.org/10.3390/en16020597







