Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review
Abstract
:1. Introduction
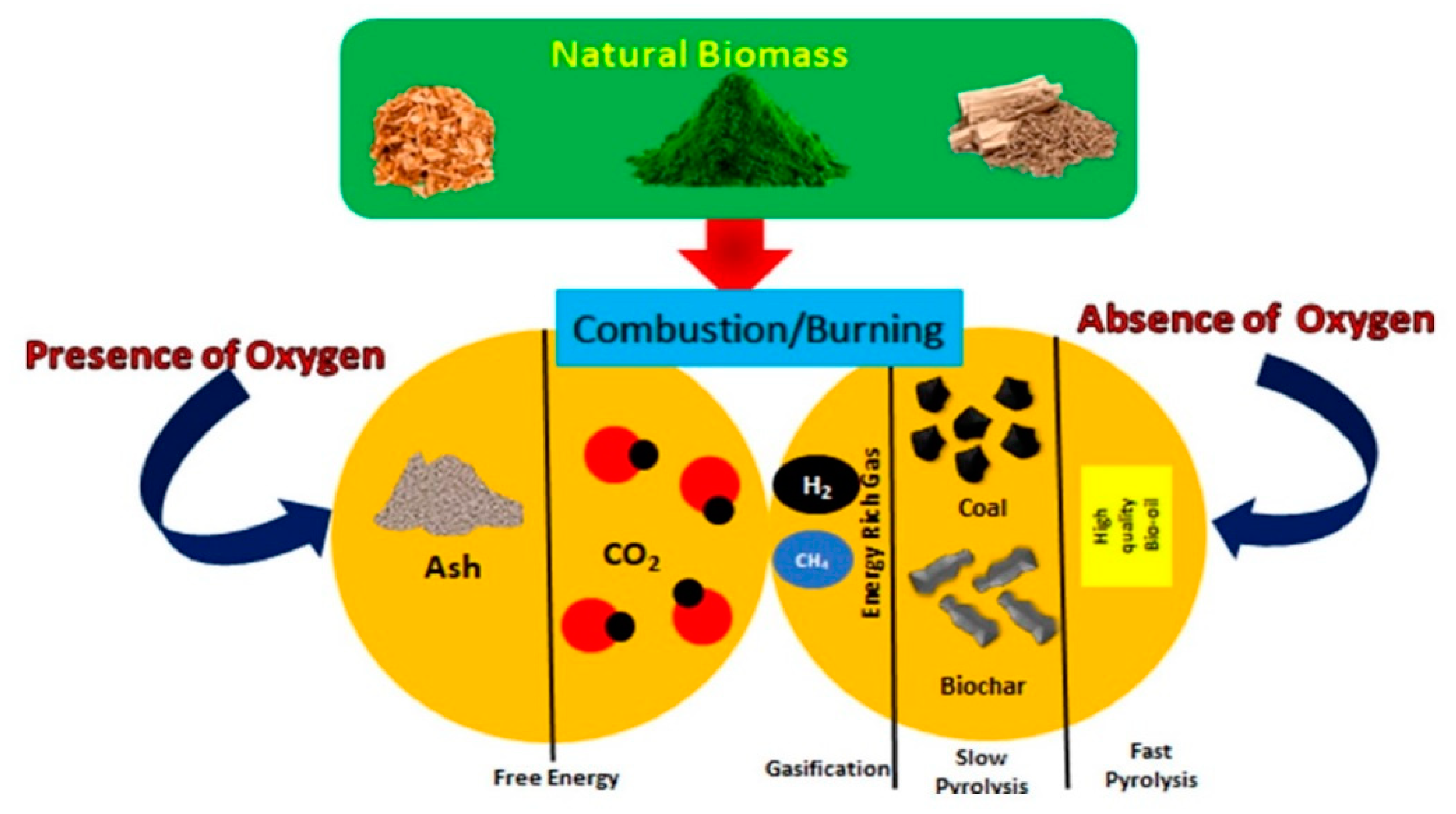
2. Methods of Biochar Production
2.1. Pyrolysis
- Low resistance to heat and corrosion as well as a loss of solids in the oil;
- Char catalysis causes a gradual increase in viscosity;
- The oil absorbs the char’s dissolved alkali and generates pyrolytic water.
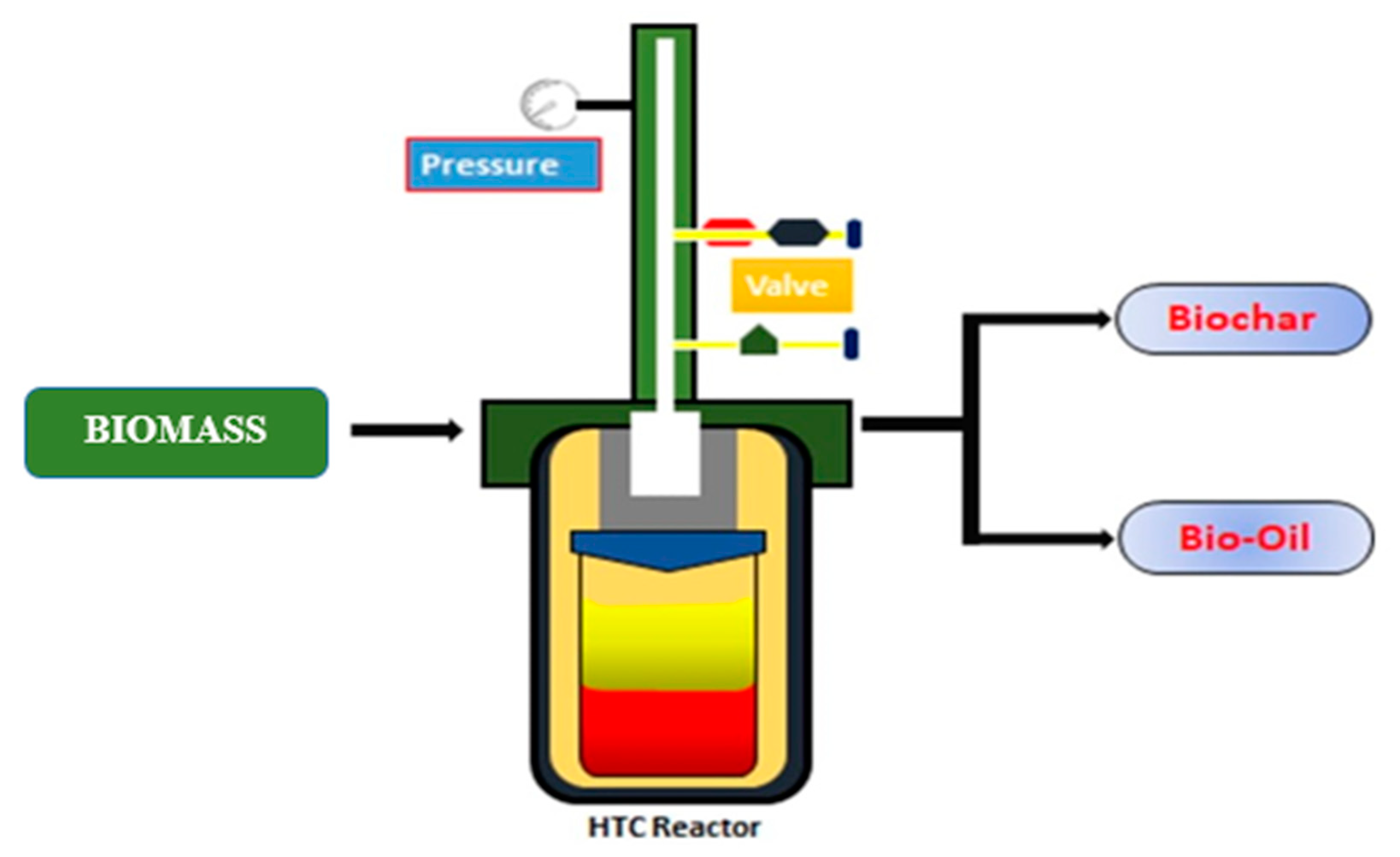
2.2. Hydrothermal Carbonization
2.3. Torrefaction
2.4. Hydrothermal Liquefaction
3. Biochar Composition
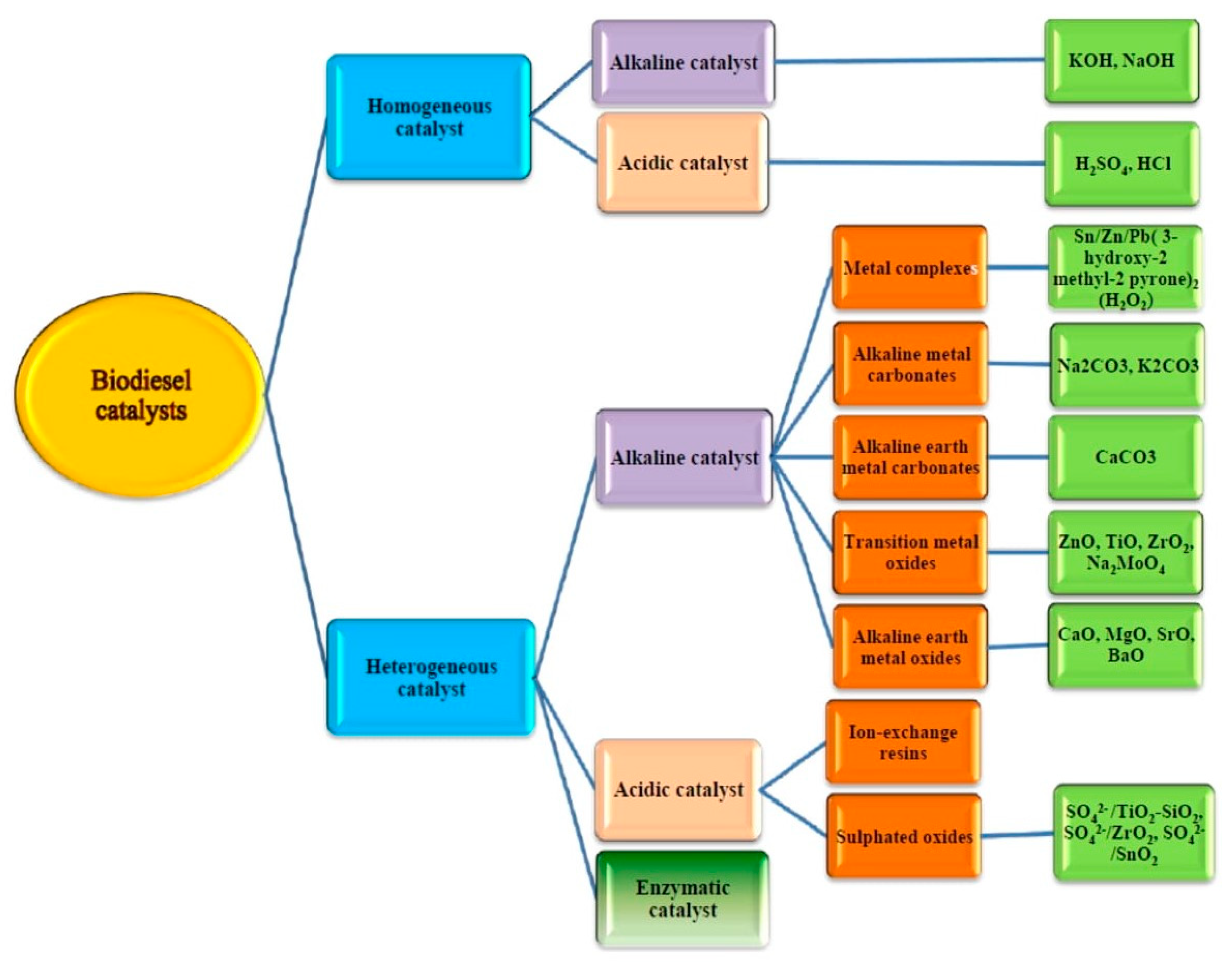
4. Biochar-Based Catalysts
5. Biochar as a Catalyst for Fuel Production
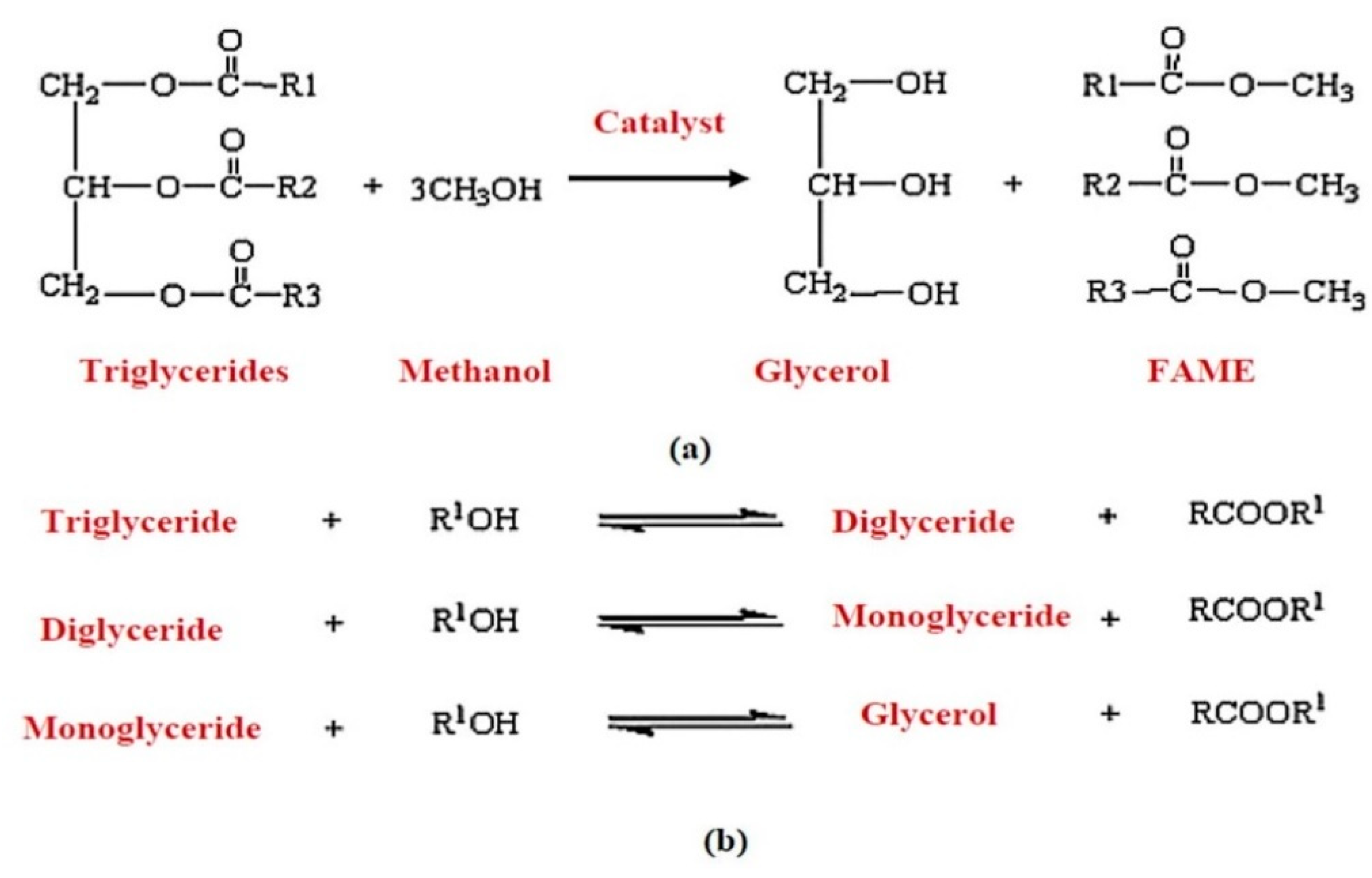
5.1. Transesterification and Esterification
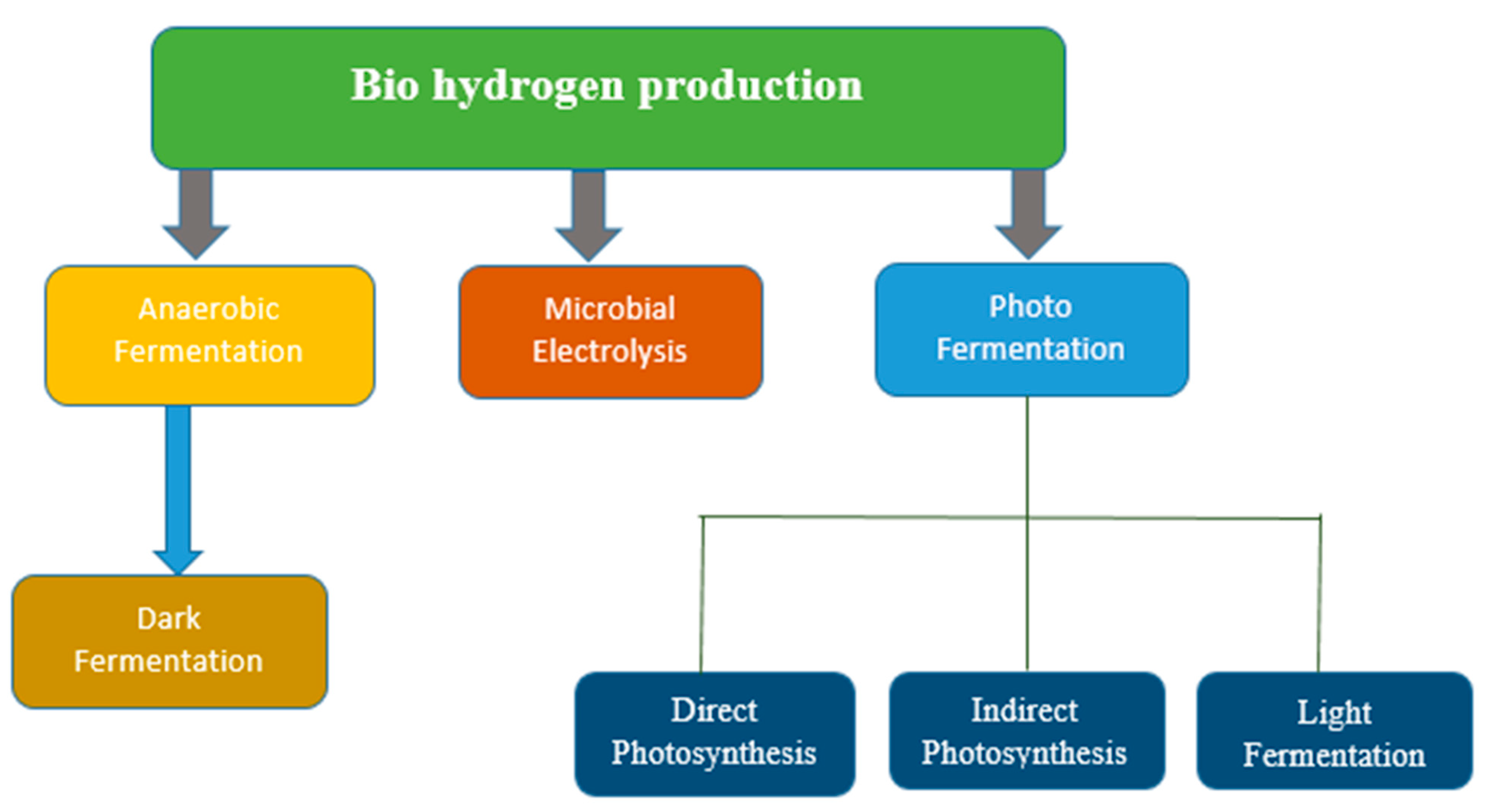
5.2. Biohydrogen Production
5.3. Biomass Hydrolysis
6. Conclusions, Future Prospects, and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IEA | International Energy Agency |
| HTC | Hydrothermal carbonization |
| Syngas | Synthesis gas |
| FFA | Free fatty acid |
| FAME | Fatty acid methyl ester |
| CCUS | Carbon capture, utilization, and storage |
| CAC | Conventional acid catalyst |
| FAEE | Fatty acid ethyl ester |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray powder diffraction |
| MBC | Magnetic biochar catalyst |
References
- Afolabi, O.O.; Sohail, M.; Cheng, Y.-L. Optimisation and characterisation of hydrochar production from spent coffee grounds by hydrothermal carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Akinfalabi, S.I.; Rashid, U.; Yunus, R.; Taufiq-Yap, Y.H. Synthesis of biodiesel from palm fatty acid distillate using sulfonated palm seed cake catalyst. Renew. Energy 2017, 111, 611–619. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Abro, R.; Harijan, K.; Zhao, Z.; Bazmi, A.A.; Abbas, T.; Yu, G. Progress in the production of biomass-to-liquid biofuels to decarbonize the transport sector—Prospects and challenges. RSC Adv. 2016, 6, 32140–32170. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Anto, S.; Karpagam, R.; Renukadevi, P.; Jawaharraj, K.; Varalakshmi, P. Biomass enhancement and bioconversion of brown marine microalgal lipid using heterogeneous catalysts mediated transesterification from biowaste derived biochar and bionanoparticle. Fuel 2019, 255, 115789. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Helmis, M.; Mumme, J.; Diakit’e, M.; Nehls, I. Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour. Technol. 2013, 130, 621–628. [Google Scholar] [CrossRef]
- Behera, S.; Singh, R.; Arora, R.; Sharma, N.K.; Shukla, M.; Kumar, S. Scope of algae as third generation biofuels. Front. Bioeng. Biotechnol. 2015, 2, 90. [Google Scholar] [CrossRef]
- Bohlouli, A.; Mahdavian, L. Catalysts used in biodiesel production: A review. Biofuels 2019, 12, 885–898. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and energy efficient hydrogen production via glycerol reforming techniques: A review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.-V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Currents challenges, innovative insights, and future perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [Green Version]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chin. J. Chem. Eng. 2018, 26, 2654–2663. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Chen, X.; Lin, Q.; He, R.; Zhao, X.; Li, G. Hydrochar production from watermelon peel by hydrothermal carbonization. Bioresour. Technol. 2017, 241, 236–243. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Chin, L.; Abdullah, A.; Hameed, B. Sugar cane bagasse as solid catalyst for synthesis of methyl esters from palm fatty acid distillate. Chem. Eng. J. 2012, 183, 104–107. [Google Scholar] [CrossRef]
- Choudhary, T.K.; Khan, K.S.; Hussain, Q.; Ahmad, M.; Ashfaq, M. Feedstock-induced changes in composition and stability of biochar derived from different agricultural wastes. Arab. J. Geosci. 2019, 12, 617. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrol. 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Central composite design approach towards optimization of flamboyant pods derived steam activated carbon for its use as heterogeneous catalyst in transesterification of Hevea brasiliensis oil. Energy Convers. Manag. 2015, 100, 277–287. [Google Scholar] [CrossRef]
- Dong, T.; Gao, D.; Miao, C.; Yu, X.; Degan, C.; Garcia-Pérez, M.; Rasco, B.; Sablani, S.S.; Chen, S. Two-step microalgal biodiesel production using acidic catalyst generated from pyrolysis-derived biochar. Energy Convers. Manag. 2015, 105, 1389–1396. [Google Scholar] [CrossRef]
- Dora, S.; Bhaskar, T.; Singh, R.; Naik, D.V.; Adhikari, D. Effective catalytic conversion of cellulose into high yields of methyl glucosides over sulfonated carbon based catalyst. Bioresour. Technol. 2012, 120, 318–321. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Li, Q.; Zhang, Y.; Zhou, H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass. Mater. Today Sustain. 2022, 19, 100209. [Google Scholar] [CrossRef]
- Jewiarz, M.; Wróbel, M.; Mudryk, K.; Szufa, S. Impact of the Drying Temperature and Grinding Technique on Biomass Grindability. Energies 2020, 13, 3392. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Pussayanavin, T.; Polprasert, C. Hydrochar production by hydrothermal carbonization of faecal sludge. J. Water Sanit. Hyg. Dev. 2015, 5, 439–447. [Google Scholar] [CrossRef]
- Fu, X.; Li, D.; Chen, J.; Zhang, Y.; Huang, W.; Zhu, Y.; Yang, J.; Zhang, C. A microalgae residue based carbon solid acid catalyst for biodiesel production. Bioresour. Technol. 2013, 146, 767–770. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- González, M.; Cea, M.; Reyes, D.; Romero-Hermoso, L.; Hidalgo, P.; Meier, S.; Benito, N.; Navia, R. Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production. Energy Convers. Manag. 2017, 137, 165–173. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Xu, C.C.; Smith, R.L. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci. 2012, 38, 672–690. [Google Scholar] [CrossRef]
- Hamad, M.A.; Radwan, A.M.; Heggo, D.A.; Moustafa, T. Hydrogen rich gas production from catalytic gasification of biomass. Renew. Energy 2016, 85, 1290–1300. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Morgan, T.; Wilson, M.; Crocker, M.; Zhang, J.; Liu, K.; Stork, J.; Debolt, S. Microalgae as a renewable fuel source: Fast pyrolysis of Scenedesmus sp. Renew. Energy 2013, 60, 625–632. [Google Scholar] [CrossRef]
- Hernandez-Mena, L.; Pecora, A.; Beraldo, A. Slow pyrolysis of bamboo biomass: Analysis of biochar properties. Chem. Eng. Transact. 2014, 37, 115–120. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I.; Saidur, R. Latest development in microalgae-biofuel production with nano-additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Italiano, C.; Vita, A.; Fabiano, C.; Laganà, M.; Pino, L. Bio-hydrogen production by oxidative steam reforming of biogas over nanocrystalline Ni/CeO2 catalysts. Int. J. Hydrogen Energy 2015, 40, 11823–11830. [Google Scholar] [CrossRef]
- Jien, S.-H. Physical Characteristics of Biochars and Their Effects on Soil Physical Properties. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–35. [Google Scholar] [CrossRef]
- Yu, S.; Xie, M.; Li, Q.; Zhang, Y.; Zhou, H. Evolution of kraft lignin during hydrothermal treatment under different reaction conditions. J. Energy Inst. 2022, 103, 147–153. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, P.; Yang, X.; Li, Q.; Zhang, Y.; Zhou, H. Formation and evolution of pectin-derived hydrothermal carbon from pectin. Fuel 2022, 326, 124997. [Google Scholar] [CrossRef]
- Jung, J.-M.; Oh, J.-I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Kabadayi Catalkopru, A.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef]
- Limousy, L.; Jeguirim, M.; Labaki, M. Energy applications of coffee processing by-products. In Handbook of Coffee Processing By-Products; Academic Press: Cambridge, MA, USA, 2017; pp. 323–367. [Google Scholar] [CrossRef]
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Fundamentals of Gasification and Pyrolysis. In Gasification of Waste Materials Technologies for Generating Energy, Gas, and Chemicals from Municipal Solid Waste, Biomass, Nonrecycled Plastics, Sludges, and Wet Solid Wastes; Academic Press: Cambridge, MA, USA, 2018; pp. 13–36. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Zhang, L.; Jiang, S.P.; Yang, S.; Wang, S.; Sun, H.; Johannessen, B.; Liu, S. Cobalt Single Atoms Embedded in Nitrogen-Doped Graphene for Selective Oxidation of Benzyl Alcohol by Activated Peroxymonosulfate. Small 2021, 17, e2004579. [Google Scholar] [CrossRef] [PubMed]
- Kavindi, G.A.G.; Lei, Z. Development of activated hydrochar from paddy straw for nutrient adsorption and crop water management. Water Resour. Manag. 2019, 229, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Konwar, L.J.; Chutia, S.; Boro, J.; Kataki, R.; Deka, D. Biochar supported Cao as heterogeneous catalyst for biodiesel production. Int. J. Innov. Res. Dev. 2012, 1, 186–195. [Google Scholar]
- Krylova, A.Y.; Zaitchenko, V.M. Hydrothermal Carbonization of Biomass: A Review. Solid Fuel Chem. 2018, 52, 91–103. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Ok, Y.S.; Kwon, E.E. Sustainable approach to biodiesel synthesis via thermally induced transesterification using biochar as surrogate porous media. Energy Convers. Manag. 2017, 151, 601–606. [Google Scholar] [CrossRef]
- Li, S.; Gu, Z.; Bjornson, B.E.; Muthukumarappan, A. Biochar based solid acid catalyst hydrolyze biomass. J. Environ. Chem. Eng. 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Yu, R.; Ji, X.; Guo, M. Hydrothermal carbonization of holocellulose into hydrochar: Structural, chemical characteristics, and combustion behavior. Bioresour. Technol. 2018, 263, 508–516. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Wu, G.; Huang, Y.; Zheng, Z.; Garces, H.F.; Yan, K. Fabrication of ultrathin lily-like NiCo2O4 nanosheets via mooring NiCo bimetallic oxide on waste biomass-derived carbon for highly efficient removal of phenolic pollutants. Chem. Eng. J. 2022, 441, 136066. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H.; Yu, H.-Q. Harvest of Cu NP anchored magnetic carbon materials from Fe/Cu preloaded biomass: Their pyrolysis, characterization, and catalytic activity on aqueous reduction of 4-nitrophenol. Green Chem. 2014, 16, 4198–4205. [Google Scholar] [CrossRef]
- Lu, J.-H.; Chen, C.; Huang, C.; Leu, S.-Y.; Lee, D.-J. Glucose fermentation with biochar amended consortium: Sequential fermentations. Bioresour. Technol. 2020, 303, 122933. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Mardhiah, H.H.; Ong, M.H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Mathimani, T.; Uma, L.; Prabaharan, D. Homogeneous acid catalysed transesterification of marine microalga Chlorella sp. BDUG 91771 lipid—An efficient biodiesel yield and its characterization. Renew. Energy 2015, 81, 523–533. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bachmann, R.; Loh, S.; Manroshan, S.; Ong, S. Effect of Pyrolysis Temperature and Time on Properties of Palm Kernel Shell-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2019, 548, 12020. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.; Six, J.; Parikh, S.J. Use of Chemical and Physical Characteristics to Investigate Trends in Biochar Feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Muradov, N.; Fidalgo, B.; Gujar, A.C.; Garceau, N.; T-Raissi, A. Production and characterization of Lemna minor bio-char and its catalytic application for biogas reforming. Biomass Bioenergy 2012, 42, 123–131. [Google Scholar] [CrossRef]
- Nakason, K.; Panyapinyopol, B.; Kanokkantapong, V.; Viriya-Empikul, N.; Kraithong, W.; Pavasant, P. Hydrothermal carbonization of unwanted biomass materials: Effect of process temperature and retention time on hydrochar and liquid fraction. J. Energy Inst. 2018, 91, 786–796. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F. Catalytic Effect of Functional and Fe Composite Biochars on Biofuel and Biochemical Derived from the Pyrolysis of Green Marine Biomass. Fermentation 2018, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Ormsby, R.; Kastner, J.R.; Miller, J. Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal. Today 2012, 190, 89–97. [Google Scholar] [CrossRef]
- Park, K.Y.; Lee, K.; Kim, D. Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour. Technol. 2018, 258, 119–124. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Castro AM de Coelho, M.A.Z.; Freire, D.M.G. Production and use of lipases in bioenergy: A review from the feedstocks to biodiesel production. Enzym. Res. 2011, 2011, 615803. [Google Scholar] [CrossRef] [Green Version]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the Art of Catalysts for Biodiesel Production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Ryu, Y.-J.; Kim, Z.-H.; Lee, S.G.; Yang, J.-H.; Shin, H.-Y.; Lee, C.-G. Development of Carbon-Based Solid Acid Catalysts Using a Lipid-Extracted Alga, Dunaliella tertiolecta, for Esterification. J. Microbiol. Biotechnol. 2018, 28, 732–738. [Google Scholar] [CrossRef]
- Saravanan, A.P.; Pugazhendhi, A.; Mathimani, T. A comprehensive assessment of biofuel policies in the BRICS nations: Implementation, blending target and gaps. Fuel 2020, 272, 117635. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Abdollahifar, M. Hydrogen production via reforming of biogas over nanostructured Ni/Y catalyst: Effect of ultrasound irradiation and Ni-content on catalyst properties and performance. Mater. Res. Bull. 2014, 60, 328–340. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent progress in synthesis and surface functionalization of mesoporous acidic heterogeneous catalysts for esterification of free fatty acid feedstocks: A review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; Di Maria, F.; Signoretto, M.; Menegazzo, F.; Di Michele, A. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Van Boi, L.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Titiladunayo, I.F.; McDonald, A.G.; Fapetu, O.P. Effect of Temperature on Biochar Product Yield from Selected Lignocellulosic Biomass in a Pyrolysis Process. Waste Biomass Valorization 2012, 3, 311–318. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Tag, A.T.; Duman, G.; Yanik, J. Influences of feedstock type and process variables on hydrochar properties. Bioresour. Technol. 2018, 250, 337–344. [Google Scholar] [CrossRef]
- Vieira, F.R.; Luna, C.M.R.; Arce, G.L.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass-Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Gan, X.; Zheng, L.; Peng, C.; Wang, B.; Li, C.; Zeng, G. Evaluation of the clean characteristics and combustion behavior of hydrochar derived from food waste towards solid biofuel production. Bioresour. Technol. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, C.; Xie, J.; Bu, Q. Study on reaction mechanism of superior bamboo biochar catalyst production by molten alkali carbonates pyrolysis and its application for cellulose hydrolysis. Sci. Total. Environ. 2020, 712, 136435. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Yu, S.; Hao, N.; Wells, T.; Meng, X.; Li, M.; Pu, Y.; Liu, S.; Ragauskas, A.J. Characterization of products from hydrothermal carbonization of pine. Bioresour. Technol. 2017, 244, 78–83. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Dehkhoda, A.M.; Ellis, N. Development of Biochar-based Catalyst for Transesterification of Canola Oil. Energy Fuels 2011, 25, 337–344. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Zhao, D.; Huang, H.; Noriyuki, K.; Chen, Y. Influence of temperature on product distribution and biochar properties by municipal sludge pyrolysis. J. Mater. Cycles Waste Manag. 2013, 15, 357–361. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef]
- Zama, E.F.; Zhu, Y.-G.; Reid, B.J.; Sun, G.-X. The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J. Clean. Prod. 2017, 148, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, C.; Zang, L. Improvement of hydrogen production from glucose by ferrous iron and biochar. Bioresour. Technol. 2017, 245, 98–105. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Karmakar, B.; Ghosh, S.; Halder, G. Parametric optimisation of biodiesel synthesis from waste cooking oil via Taguchi approach. J. Environ. Chem. Eng. 2018, 6, 3971–3980. [Google Scholar] [CrossRef]
- Nata, I.F.; Putra, M.D.; Irawan, C.; Lee, C.-K. Catalytic performance of sulfonated carbon-based solid acid catalyst on esterification of waste cooking oil for biodiesel production. J. Environ. Chem. Eng. 2017, 5, 2171–2175. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Taher, H.; Al Dhaheri, S.; Wajeeh, S.; Nour, M.; El-Najjar, E. Biodiesel Production from Oils Extracted from Date Pits. Green Sustain. Chem. 2017, 07, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Angkawijaya, A.E.; Irawaty, W.; Ju, Y.-H.; Tran-Nguyen, P.L.; Hartono, S.B. Utilization of waste capiz shell—Based catalyst for the conversion of leather tanning waste into biodiesel. J. Environ. Chem. Eng. 2020, 8, 104012. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A Critical Review of the Production and Advanced Utilization of Biochar via Selective Pyrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jung, J.; Liu, M.; Li, X.; Goel, A.; Chen, J.; Song, S.; Anderson, C.; Chen, D.; Leong, K.; et al. Gasification Biochar from HorticulturalWaste: An Exemplar of the Circular Economy in Singapore. Sci. Total Environ. 2021, 781, 146573. [Google Scholar] [CrossRef]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of Torrefaction on Biomass Properties Depending on Temperature and Operation Time. Sci. Total Environ. 2020, 740, 140086. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Bevan, E.; Fu, J.; Zheng, Y. Challenges and Opportunities of Hydrothermal Carbonisation in the UK.; Case Study in Chirnside. RSC Adv. 2020, 10, 31586–31610. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Sebestyén, Z.; Blazsó, M.; Berényi, B.; Kumar, J.; Krishna, B.B.; Bhaskar, T.; Czégény, Z. Comparison of Hydrothermal Carbonization and Torrefaction of Azolla Biomass: Analysis of the Solid Products. J. Anal. Appl. Pyrolysis 2020, 149, 104844. [Google Scholar] [CrossRef]


| Feedstock | Biochar Production Method | Catalyst Production Method | Type of Catalyst | References |
|---|---|---|---|---|
| Peanut hulls, glucose, oat hulls, coconut husks, | Carbonization | Sulfonation | Acidic | [23] |
| Wood saw dust | Slow pyrolysis | Sulfonation | Acidic | [24] |
| Pamela fruit skin, palm nut shells | Carbonization | Calcination Wet impregnation | Alkaline | [25] |
| Husk of rice | Carbonization | ChemicalActivation | Acidic | [26] |
| Peat | Carbonization | Wet impregnation | Alkaline | [27] |
| Banana | Carbonization | Wet impregnation | Alkaline | [28] |
| Type of Biomass Process Parameters Time Yield of Biochar (%) | References |
|---|---|
| Pyrolysis | |
| Safflower seeds T = 400 °C 34.2 | [51] |
| Waste water sludge T = 300 °C 90.2 | [52] |
| Olive husk T = 177 °C 44.6 | [53] |
| Poultry waste T = 300 °C 79 | [54] |
| Rice husk T = 300 °C 37.8 | [55] |
| Peanut kernel shells T = 350 °C 45.8 | [56] |
| Palm shell T = 400 °C 43.3 | [57] |
| Bamboo T = 300 °C 81 | [58] |
| Bio-sludge from pulp mill T = 200 °C | [59] |
| Time = 120 min | |
| Paddy straw T = 100 °C | [60] |
| Time = 60 min 82 | |
| Watermelon peel T = 260 °C | |
| Time = 60 min 55 | [61] |
| Coconut husk T = 140 °C | |
| Time = 240 mins >76 | [62] |
| Municipal solid waste T = 120 °C | |
| Time = 180 min 89 | [63] |
| Catalyst Used | TiO2 and Cu | CH3-O-CO-R3 | Acid Biochar and Murumuru Kernel Shell | Flamboyant Pods | KOH/Al2O3 | Al2O3 Supported Coconut Chaff | Na2CO3 and Ca(NO3)2 |
|---|---|---|---|---|---|---|---|
| Oil used | Palm oil | Sunflower and soybean oil | Jupati oil | Hevea-brasiliensis oil | Waste cooking oil | Waste frying oil | Soybean oil |
| Biodiesel yield (%) | 90.9 | 93.4 | 91.8 | 89.8 | 98.2 | 91.06 | 99 |
| Time (minutes) | 45 | 52 | 45 | 60 | 60 | 150 | 240 |
| Temperature of reaction (°C) | 45 | 50 | 135 | 55 | 70 | 65 | 60 |
| References | [70] | [71] | [72] | [73] | [74] | [75] | [76] |
| Biochar Types | Activation Conditions | Elemental Composition (wt.%) | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Applications | References |
|---|---|---|---|---|---|---|---|
| Woody biomass | Sulfonation, activation temperature 150 °C | C = 75.03 H = 0.82 N ≤ 0.3 O = 15.16 S = 0.36 | 839 | 3.48 | 0.86 | Esterification and transesterification | |
| Peanut hull | Sulfonation, activation temperature 100 °C | C = 63.5 N = 1.5 S = 0.14 | 243 | 1.05 | 0.12 | Esterification | |
| Woody biomass | Sulfonation, activation temperature 875 °C | C = 81.39 H ≤ 0.3 N = 1.01 O = 6.64 S = 1.21 | 1412 | 2.19 | 0.75 | Transesterification | [94] |
| Sugarcane bagasse | Sulfonation, activation temperature 150 °C | C = 74.18 O = 21.61 S = 4.21 | 55.02 | 2.8 | -- | Esterification | |
| Dried leaves mixture | Charring temperature—470 °C | C = 78.26 O = 13.50 | 19.23 | 2.84 | 0.02 | Transesterification | [95] |
| Lemna minor (duckweed) | Sulfonation | C = 40.11 H = 6.13 O = 36.74 N = 5.52 S = 0.67 | 11.9 | ---- | 0.015 | Biogas reforming | |
| Rice husk | KOH activation, activation temperature—650 °C | -- | 1059 | 2.4 | 0.61 | Syngas methanation | [96] |
| Pomelo peel | KOH activation | C = 76.1 O = 15 | 277.8 | --- | 0.156 | Transesterification | |
| Corn-bran residue | Sulfonation | C = 74.36 H = 2.78 O = 18.39 N = 4.47 | 59.34 | ---- | --- | [97] | |
| Irul wood biomass | Sulfonation | C = 30.98 H = 2.71 N = 0.22 S = 6.62 O = 60.47 | 3.4 | 100.89 | 0.006 | Esterification and Transesterification | [98] |
| Municipal wood waste | Sulfonation | – | 184 | --- | 0.0072 | MFC (oxygen reduction) | |
| Sewage sludge | Sulfonation | C = 34.96 O = 35.50 | 43.9 | 8.7 | --- | MFC (oxygen reduction) | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Soomro, S.A.; Harijan, K.; Uqaili, M.A.; Kumar, L. Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review. Energies 2023, 16, 644. https://doi.org/10.3390/en16020644
Kumar S, Soomro SA, Harijan K, Uqaili MA, Kumar L. Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review. Energies. 2023; 16(2):644. https://doi.org/10.3390/en16020644
Chicago/Turabian StyleKumar, Sooraj, Suhail Ahmed Soomro, Khanji Harijan, Mohammad Aslam Uqaili, and Laveet Kumar. 2023. "Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review" Energies 16, no. 2: 644. https://doi.org/10.3390/en16020644
APA StyleKumar, S., Soomro, S. A., Harijan, K., Uqaili, M. A., & Kumar, L. (2023). Advancements of Biochar-Based Catalyst for Improved Production of Biodiesel: A Comprehensive Review. Energies, 16(2), 644. https://doi.org/10.3390/en16020644







