Influence of RDF Composition on Mercury Release during Thermal Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Samples
2.2. Determination of the Morphological Composition of the RDF Samples
2.3. Sample Preparation
2.4. Characterization of the RDF Samples
2.5. Procedure for Thermal Pretreatment of RDF Samples
- ΔHg is the mercury release index (%);
- Δm is the mass loss of the sample (%);
- Hg0 is the mercury content in the raw sample (µg/kg);
- Hgt is the mercury content in the sample after thermal treatment (µg/kg).
2.6. Procedure for Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Mercury Content in the RDF Samples
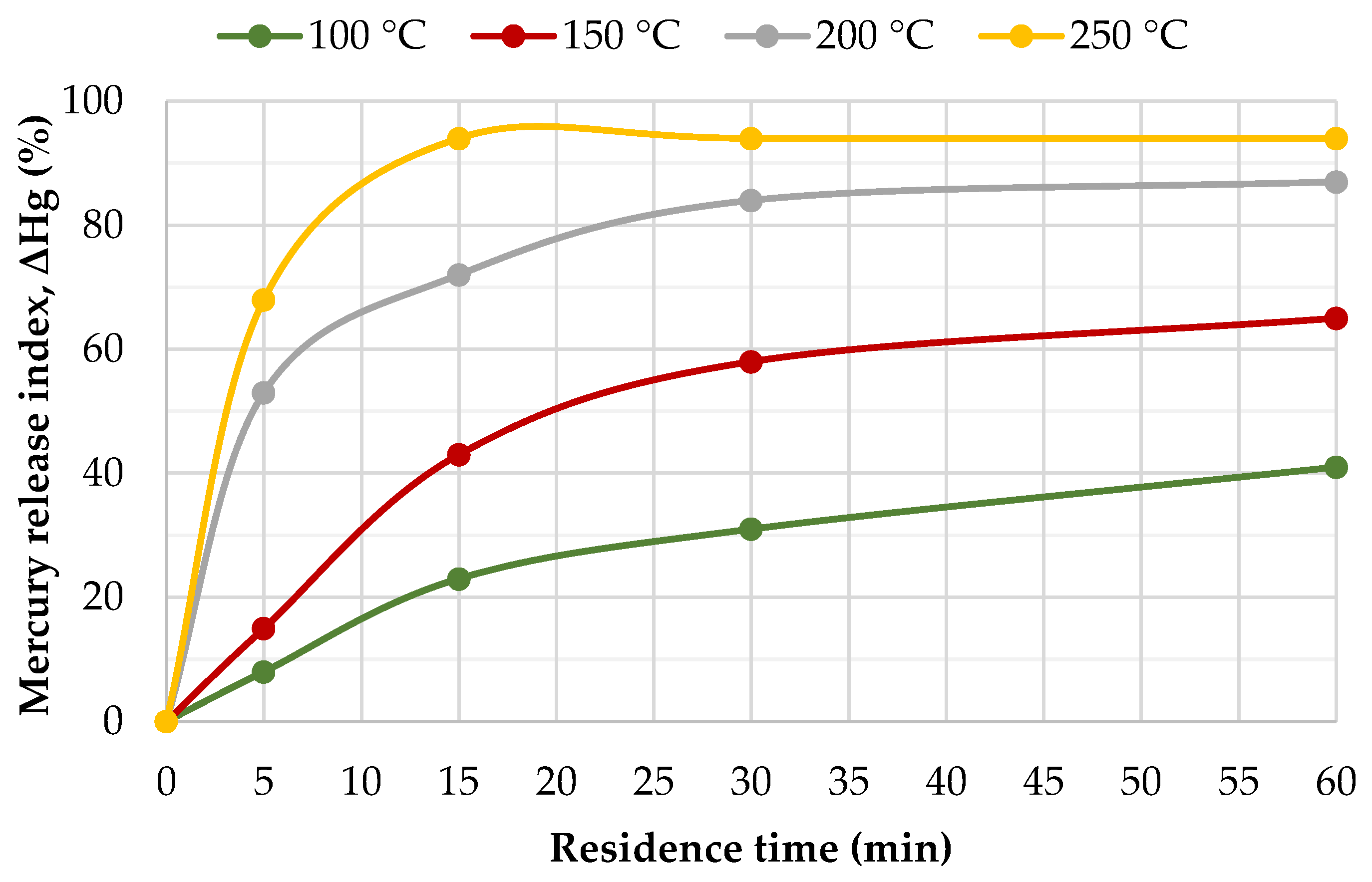
3.2. Effect of Temperature on the Release of Mercury from the RDF Samples
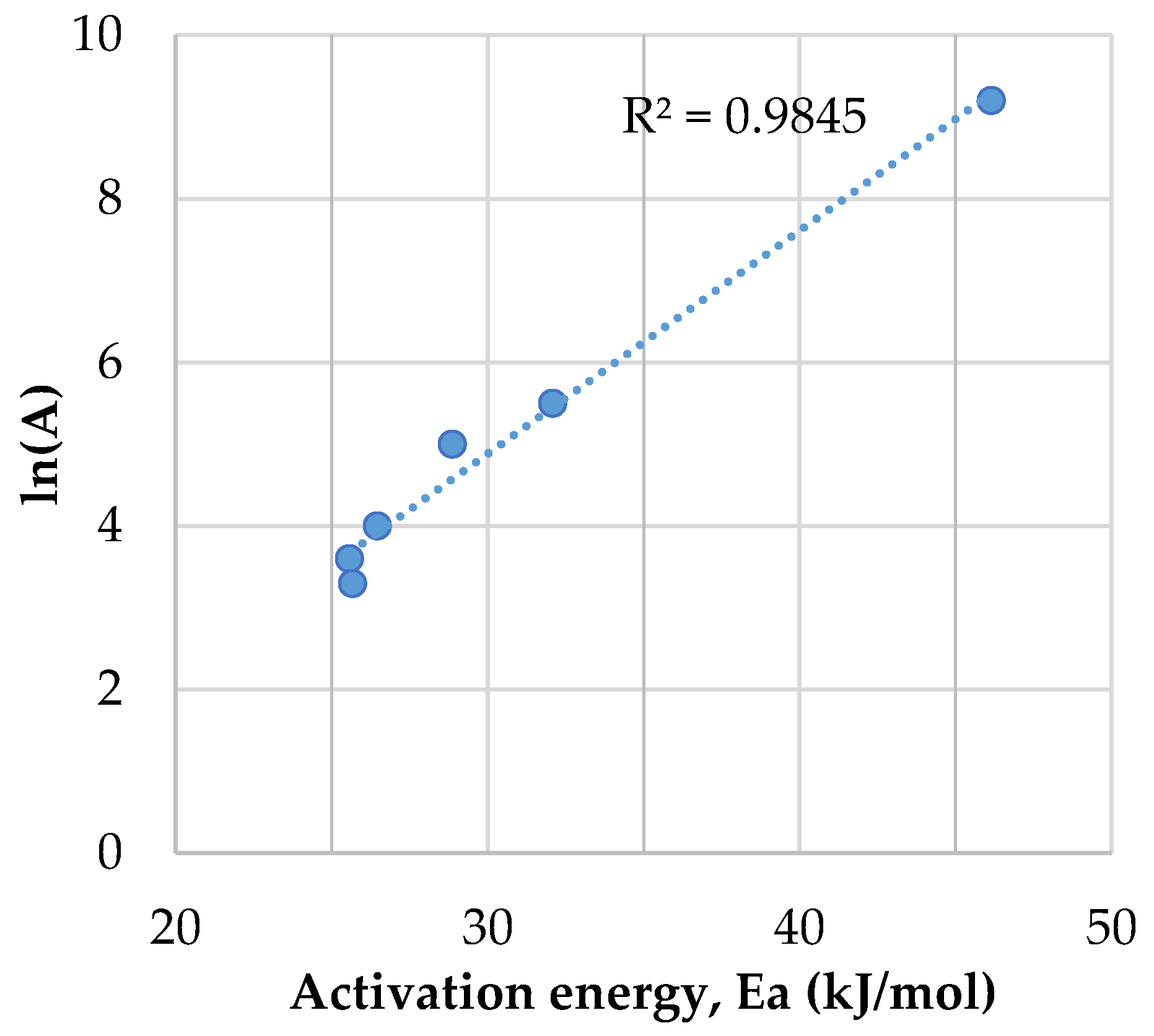
3.3. Determination of the Activation Energy for the Release of Mercury from the RDF Samples
- ΔHg is the mercury release after time t (-);
- ΔHgmax is the maximum mercury release (-);
- t is the reaction time (min);
- k is the mercury release coefficient (min−1).
- Ea is the activation energy (kJ/mol);
- A is the pre-exponential factor (min−1);
- R is the universal gas constant (kJ/(mol·K));
- T is the temperature (K).
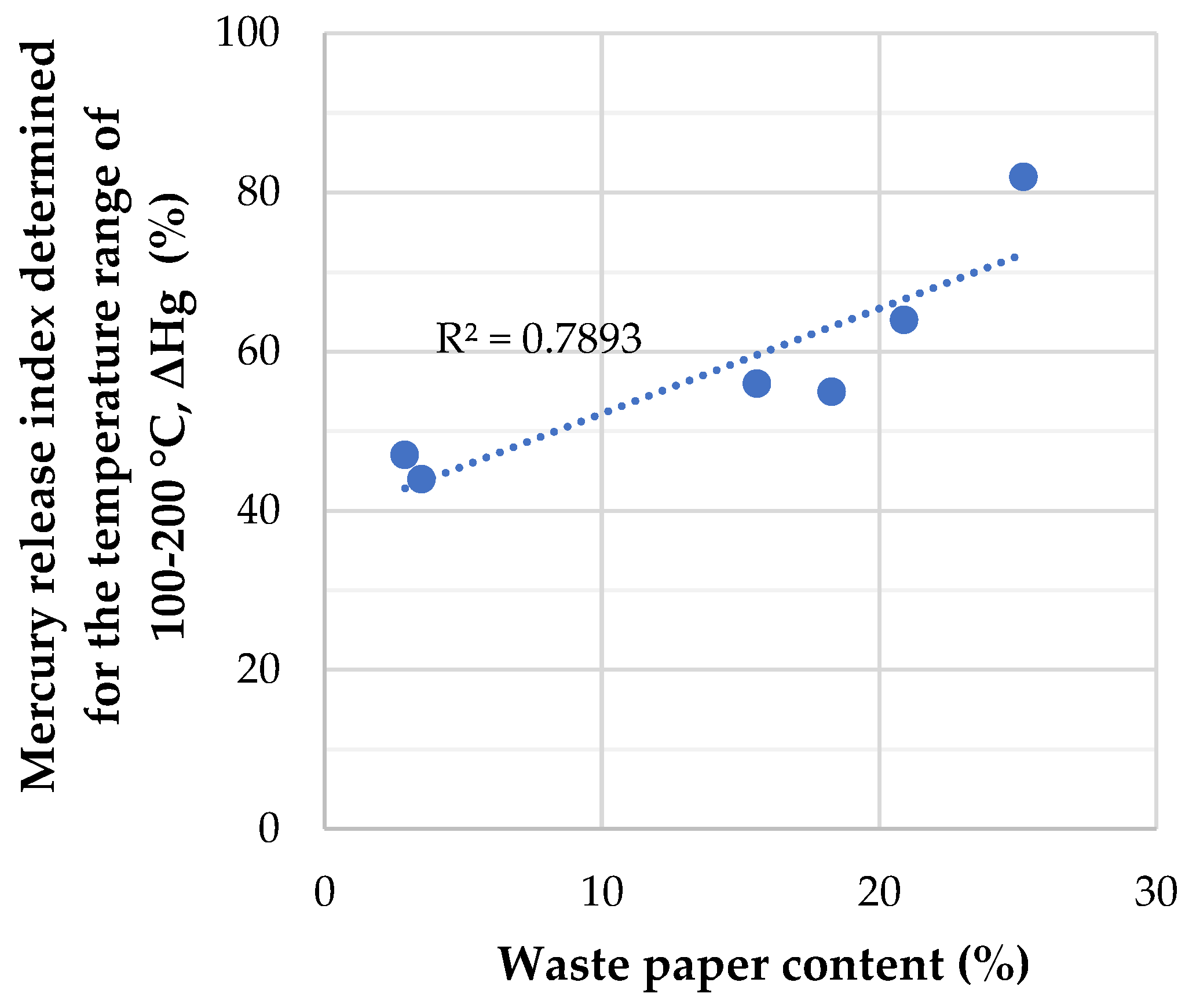
3.4. Statistical Analysis of the Influence of the RDF Samples’ Composition on the Amount of Mercury Released
3.5. Limitations for Mercury Removal from RDF in Thermal Pretreatment Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; van Woerden, F. What a Waste 2.0. In What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- World Energy Outlook 2021—Analysis—IEA. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 14 December 2022).
- Psomopoulos, C.S.; Kiskira, K.; Kalkanis, K.; Leligou, H.C.; Themelis, N.J. The Role of Energy Recovery from Wastes in the Decarbonization Efforts of the EU Power Sector. IET Renew. Power Gener. 2022, 16, 48–64. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.B.; Tarelho, L.A.C.; Cardoso, J.S.; Eusébio, D. Snapshot Review of Refuse-Derived Fuels. Util. Policy 2022, 74, 101316. [Google Scholar] [CrossRef]
- Jewiarz, M.; Mudryk, K.; Wróbel, M.; Fraczek, J.; Dziedzic, K. Parameters Affecting RDF-Based Pellet Quality. Energies 2020, 13, 910. [Google Scholar] [CrossRef] [Green Version]
- Gerassimidou, S.; Velis, C.A.; Williams, P.T.; Komilis, D. Characterisation and Composition Identification of Waste-Derived Fuels Obtained from Municipal Solid Waste Using Thermogravimetry: A Review. Waste Manag. Res. 2020, 38, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Dziok, T.; Bury, M.; Bytnar, K.; Burmistrz, P. Possibility of Using Alternative Fuels in Polish Power Plants in the Context of Mercury Emissions. Waste Manag. 2021, 126, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Yazdanpanah, F.; Lim, C.J.; Sokhansanj, S. Pelletization Properties of Refuse-Derived Fuel—Effects of Particle Size and Moisture Content. Fuel Process. Technol. 2020, 205, 106437. [Google Scholar] [CrossRef]
- Pawłowski, P.; Bałazińska, M.; Ignasiak, K.; Robak, J. Preparation of the Selected Groups of Waste Their Energy Use—Fuel from Waste Type SRF—Piece Przemysłowe & Kotły—Tom Nr 4 (2016)—BazTech—Yadda. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-e47428b1-ef35-4fce-81b4-5d4f7cce3866 (accessed on 14 December 2022).
- Chatziaras, N.; Psomopoulos, C.S.; Themelis, N.J. Use of Waste Derived Fuels in Cement Industry: A Review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193. [Google Scholar] [CrossRef]
- Kogut, K.; Górecki, J.; Burmistrz, P. Opportunities for Reducing Mercury Emissions in the Cement Industry. J. Clean. Prod. 2021, 293, 126053. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Dziok, T.; Magdziarz, A.; Nowak, W. Composition and Properties of Fly Ash Collected from a Multifuel Fluidized Bed Boiler Co-Firing Refuse Derived Fuel (RDF) and Hard Coal. Energy 2021, 234, 121229. [Google Scholar] [CrossRef]
- Chaliki, P.; Psomopoulos, C.S.; Themelis, N.J. WTE Plants Installed in European Cities: A Review of Success Stories. Manag. Environ. Qual. 2015, 27, 606–620. [Google Scholar] [CrossRef]
- Psomopoulos, C. Residue Derived Fuels as an Alternative Fuel for the Hellenic Power Generation Sector and Their Potential for Emissions Reduction. AIMS Energy 2014, 2, 321–341. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy Recovery from Municipal Solid Waste Using Pyrolysis Technology: A Review on Current Status and Developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Bijak, K. The Effect of Temperature-Pressure Conditions on the RDF Gasification in the Atmosphere of Steam and Carbon Dioxide. Energies 2021, 14, 7502. [Google Scholar] [CrossRef]
- Hilber, T.; Maier, J.; Scheffknecht, G.; Agraniotis, M.; Grammelis, P.; Kakaras, E.; Glorius, T.; Becker, U.; Derichs, W.; Schiffer, H.P.; et al. Advantages and Possibilities of Solid Recovered Fuel Cocombustion in the European Energy Sector. J. Air Waste Manag. Assoc. 2012, 57, 1178–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisler, R. Mercury Hazards to Living Organisms. In Mercury Hazards to Living Organisms; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–312. [Google Scholar] [CrossRef]
- BAT-LCP, Commission Implementing Decision (EU) 2017/1442 of 31 July 2017 Establishing Best Available Techniques (BAT) Conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for Large Combustion Plants. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017D1442 (accessed on 14 December 2022).
- Ummatin, K.K.; Arifianti, Q.A.M.O.; Hani, A.; Annissa, Y. Quality Analysis of Refused-Derived Fuel as Alternative Fuels in the Cement Industry and Its Evaluation on Production. In Proceedings of the 2019 International Conference on Engineering, Science, and Industrial Applications, ICESI 2019, Tokyo, Japan, 22–24 August 2019. [Google Scholar] [CrossRef]
- Hryb, W.; Matyasik, P. Mercury Content in Refuse-Derived Fuels. Arch. Environ. Prot. 2018, 44, 65–72. [Google Scholar] [CrossRef]
- Bhatt, M.; Chakinala, A.G.; Joshi, J.B.; Sharma, A.; Pant, K.K.; Shah, K.; Sharma, A. Valorization of Solid Waste Using Advanced Thermo-Chemical Process: A Review. J. Envrion. Chem. Eng. 2021, 9, 105434. [Google Scholar] [CrossRef]
- Zhang, Z.; Ju, R.; Zhou, H.; Chen, H. Migration Characteristics of Heavy Metals during Sludge Pyrolysis. Waste Manag. 2021, 120, 25–32. [Google Scholar] [CrossRef]
- Gao, N.; Wang, F.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire Pyrolysis Char: Processes, Properties, Upgrading and Applications. Prog. Energy Combust. Sci. 2022, 93, 101022. [Google Scholar] [CrossRef]
- Stępień, P.; Banik, C.; Koziel, J.A.; Białowiec, A. Emission of Volatile Organic Compounds from a Carbonized Refuse-Derived Fuel. Przemysł Chem. 2019, 98, 103–105. [Google Scholar] [CrossRef]
- Edo, M.; Skoglund, N.; Gao, Q.; Persson, P.E.; Jansson, S. Fate of Metals and Emissions of Organic Pollutants from Torrefaction of Waste Wood, MSW, and RDF. Waste Manag. 2017, 68, 646–652. [Google Scholar] [CrossRef]
- PN-ENISO 21640:2021; Solid Recovered Fuels—Specifications and Classes. ISO: Geneva, Switzerland, 2021.
- Dziok, T.; Kołodziejska, E.K.; Kołodziejska, E.L. Mercury Content in Woody Biomass and Its Removal in the Torrefaction Process. Biomass Bioenergy 2020, 143, 105832. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, Y.; Wang, L. An Improved Method for Assessing Environmental Impacts Caused by Chemical Pollutants: A Case Study in Textiles Production. Toxicol. Ind. Health 2020, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Xue, M.G.; Wang, S.F.; Huang, C.X. Determination of Heavy Metals(Pb, Cd, Cr and Hg) in Printed Paper as Food Packaging Materials and Analysis of Their Sources. CIESC J. 2010, 12, 32. [Google Scholar]
- Turner, A.; Filella, M. Hazardous Metal Additives in Plastics and Their Environmental Impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef]
- Dziok, T.; Bury, M.; Burmistrz, P. Mercury Release from Municipal Solid Waste in the Thermal Treatment Process. Fuel 2022, 329, 125528. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Geng, Y.; Wang, N.; Mao, Y.; Cai, Y. Occurrence, Speciation and Fate of Mercury in the Sewage Sludge of China. Ecotoxicol. Environ. Saf. 2019, 186, 109787. [Google Scholar] [CrossRef]
- Iwashita, A.; Tanamachi, S.; Nakajima, T.; Takanashi, H.; Ohki, A. Removal of Mercury from Coal by Mild Pyrolysis and Leaching Behavior of Mercury. Fuel 2004, 83, 631–638. [Google Scholar] [CrossRef]
- Bury, M.; Dziok, T. Mercury Content in Paper Waste and Possibility of Its Reduction. Przemysł Chem. 2021, 100, 77–79. [Google Scholar] [CrossRef]
- Pilar, L.; Borovec, K.; Szeliga, Z.; Górecki, J. Mercury Emission from Three Lignite-Fired Power Plants in the Czech Republic. Fuel Process. Technol. 2021, 212, 106628. [Google Scholar] [CrossRef]
- Rujido-Santos, I.; Herbello-Hermelo, P.; Barciela-Alonso, M.C.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Metal Content in Textile and (Nano)Textile Products. Int. J. Environ. Res. Public Health 2022, 19, 944. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Rivera-Hernández, J.R.; Rodrigues, J.P.; Moltó, V. Interaction of Mercury with Beached Plastics with Special Attention to Zonation, Degradation Status and Polymer Type. Mar. Chem. 2020, 222, 103788. [Google Scholar] [CrossRef]
- Xin, F.; Xiao, R.; Zhao, Y.; Zhang, J. Surface Sulfidation Modification of Magnetospheres from Fly Ash for Elemental Mercury Removal from Coal Combustion Flue Gas. Chem. Eng. J. 2022, 436, 135212. [Google Scholar] [CrossRef]
- Kawamoto, K. Adsorption Characteristics of the Carbonaceous Adsorbents for Organic Compounds in a Model Exhaust Gas from Thermal Treatment Processing. J. Air Waste Manag. Assoc. 2022, 72, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, J.; Chen, G.; Yan, B.; Cheng, Z. Distribution of Hg during Sewage Sludge and Municipal Solid Waste Co-Pyrolysis: Influence of Multiple Factors. Waste Manag. 2020, 107, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Eom, Y.; Lee, T.G. Mercury Recovery from Mercury-Containing Wastes Using a Vacuum Thermal Desorption System. Waste Manag. 2017, 60, 546–551. [Google Scholar] [CrossRef]
- Rezić, I. Historical Textiles and Their Characterization; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2022. [Google Scholar]
- Stępień, P.; Pulka, J.; Serowik, M.; Białowiec, A. Thermogravimetric and Calorimetric Characteristics of Alternative Fuel in Terms of Its Use in Low-Temperature Pyrolysis. Waste Biomass Valorization 2019, 10, 1669–1677. [Google Scholar] [CrossRef]
- Merdes, A.C.; Keener, T.C.; Khang, S.J.; Jenkins, R.G. Investigation into the Fate of Mercury in Bituminous Coal during Mild Pyrolysis. Fuel 1998, 77, 1783–1792. [Google Scholar] [CrossRef]
- Koukouch, A.; Idlimam, A.; Asbik, M.; Sarh, B.; Izrar, B.; Bostyn, S.; Bah, A.; Ansari, O.; Zegaoui, O.; Amine, A. Experimental Determination of the Effective Moisture Diffusivity and Activation Energy during Convective Solar Drying of Olive Pomace Waste. Renew. Energy 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Sotiropoulou, R.E.P.; Serafidou, M.; Skodras, G. Thermal Mercury Removal from Coals: Effect of Pyrolysis Conditions and Kinetic Analysis. Fuel 2019, 238, 44–50. [Google Scholar] [CrossRef]
- Czop, M.; Błaszczyk, E. Determination of the Fuel Properties of Selected Packaging Waste from the Municipal Sector. Arch. Gospod. Odpad. Ochr. Środowiska 2015, 17, 131–138. [Google Scholar]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V.A. An Overview on Biocatalysts Immobilization on Textiles: Preparation, Progress and Application in Wastewater Treatment. Chemosphere 2021, 279, 130481. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, G.; Zhang, Q.; Cui, W.; Li, Z.; Zhang, S. Potential Hazards of Novel Waste-Derived Sorbents for Efficient Removal of Mercury from Coal Combustion Flue Gas. J. Hazard. Mater. 2021, 412, 125226. [Google Scholar] [CrossRef] [PubMed]
- Misztal, E.; Chmielniak, T.; Mazur, I.; Sajdak, M. The Release and Reduction of Mercury from Solid Fuels through Thermal Treatment Prior to Combustion. Energies 2022, 15, 7987. [Google Scholar] [CrossRef]
- Shen, A.; Wang, Y.; Wang, R.; Duan, Y.; Tao, J.; Gu, X.; Wang, P.; Xu, Z. Numerical Simulation of Sorbent Injection into Flue Gas for Mercury Removal in Coal-Fired Power Plant. Part 2. Operational Parameters and Optimization. Fuel 2022, 326, 124990. [Google Scholar] [CrossRef]
- Chmielniak, T. Reduction of Mercury Emissions to the Atmosphere from Coal Combustion Processes of Using Low-Temperature Pyrolysis—A Concept of Process Implementation on a Commercial Scale. Rynek Energii 2011, 93, 176–181. [Google Scholar]
- Jastrząb, K.; Mazurek, I. The Study of Thermal Desorption of Mercury Compounds from Spent Active Cokes Used for Exhaust Gas Treatment in Waste Incineration Plants. Inżynieria Ochr. Sr. 2013, 16, 273–285. [Google Scholar]
- Antuña-Nieto, C.; Rodríguez, E.; Lopez-Anton, M.A.; García, R.; Martínez-Tarazona, M.R. Noble Metal-Based Sorbents: A Way to Avoid New Waste after Mercury Removal. J. Hazard. Mater. 2020, 400, 123168. [Google Scholar] [CrossRef]
- Monni, S. From Landfilling to Waste Incineration: Implications on GHG Emissions of Different Actors. Int. J. Greenh. Gas Control 2012, 8, 82–89. [Google Scholar] [CrossRef]
- Wasielewski, R.; Bałazińska, M. Energy Recovery from Waste in the Aspect of qualifications of Electricity and Heat as Coming from Renewable Energy and to Participate in the Emissions Trading System. Polityka Energetyczna—Energy Policy J. 2018, 21, 129–142. [Google Scholar]
- Batool, I.; Munir, S.; Khalid, A.; Tahir, A.; Munir, S. Effectiveness of Different Binders on RDF Potential from Agro Waste. Tech. J. UET Taxila 2021, 26, 38–44. [Google Scholar]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Properties of Densified-Refuse Derived Fuel Using Glycerin as a Binder. Procedia Eng. 2015, 100, 505–510. [Google Scholar] [CrossRef]


| Sample Number | Share of Fractions Separated (%) | |||||
|---|---|---|---|---|---|---|
| Plastic Foil | Plastics | Waste Paper | Textiles | Aluminum | Other | |
| RDF-1 | 50.0 | 22.8 | 2.9 | 2.0 | 1.0 | 21.3 |
| RDF-2 | 21.5 | 30.2 | 15.6 | 29.0 | 2.7 | 1.0 |
| RDF-3 | 25.6 | 23.9 | 25.2 | 22.7 | 0.0 | 2.6 |
| RDF-4 | 35.2 | 16.8 | 20.9 | 18.4 | 4.0 | 4.7 |
| RDF-5 | 16.4 | 40.1 | 18.3 | 21.4 | 3.7 | 0.1 |
| RDF-6 | 18.2 | 38.8 | 3.5 | 1.5 | 1.8 | 36.3 |
| Average | 27.7 | 28.8 | 14.4 | 15.8 | 2.2 | 11.1 |
| Parameter | Method of Measurement | Principle behind the Method | Procedure/ Standard |
|---|---|---|---|
| Moisture (Mad) | Moisture balance MA 110.R by Radwag | Gravimetric method | Standard PN-ISO 589:2006 |
| Ash (Aad) | Muffle furnace: 550 °C | Gravimetric method | PN-EN 15403:2011 |
| Volatile matter (Vad) | Muffle furnace: 900 °C | Gravimetric method | PN-EN 15402:2011 |
| Gross calorific value (qV.gr.ad) | IKA C 6000 calorimeter by ELTRA | Combustion in a calorimetric bomb in oxygen at a pressure of 3 MPa | ISO 1928 |
| Carbon (Cad) | CHS-580 analyzer by ELTRA | High-temperature combustion method | PKN-ISO/TS 12902:2007 |
| Hydrogen (Had) | CHS-580 analyzer by ELTRA | High-temperature combustion method | PKN-ISO/TS 12902:2007 |
| Sulfur (St ad) | CHS-580 analyzer by ELTRA | High-temperature combustion method | Standard ASTM D-4239 |
| Mercury (Hgad) | DMA-80 mercury analyzer by Milestone | Atomic absorption spectrometry | Milestone procedure |
| Sample Number | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad (%) | Aad (%) | Vad (%) | qp.net.ad (MJ/kg) | Cad (%) | Had (%) | St ad (%) | Hg ad (µg/kg) | Hgad/ qp,net,ad (µg/MJ) | |
| RDF-1 | 1.1 | 11.0 | 87.66 | 33.538 | 71.4 | 13.40 | 0.07 | 56 | 1.7 |
| RDF-2 | 2.9 | 17.2 | 70.46 | 23.713 | 56.0 | 8.44 | 0.64 | 764 | 32.2 |
| RDF-3 | 2.5 | 12.0 | 78.41 | 24.077 | 52.8 | 7.19 | 0.16 | 849 | 35.3 |
| RDF-4 | 3.1 | 19.6 | 75.14 | 18.022 | 47.6 | 6.22 | 0.32 | 89 | 4.9 |
| RDF-5 | 1.9 | 11.7 | 78.50 | 26.916 | 59.2 | 8.41 | 0.11 | 686 | 25.5 |
| RDF-6 | 3.3 | 21.8 | 62.84 | 16.623 | 40.5 | 6.39 | 0.25 | 45 | 2.7 |
| Waste Type | Mercury Release (%) | Temperature of Thermal Pretreatment (°C) | Ref. |
|---|---|---|---|
| RDF | 96 | 250 | [33] |
| RDF + demolition and construction wood | 82 | 220 | [26] |
| Sewage sludge + MSW | 80 | 300 | [42] |
| Sewage sludge | 91 | 250 | [33] |
| Paper | 63–93 | 300 | [33,36] |
| Cardboards | 93 | 300 | [33] |
| Plastics | 48 | 350 | [33] |
| Plastic foils | 81 | 300 | [33] |
| Textiles | 94 | 250 | [33] |
| Car tires | 85 | 300 | [33] |
| Mercury-containing waste from various industrial facilities | 97 | 400 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bury, M.; Dziok, T.; Borovec, K.; Burmistrz, P. Influence of RDF Composition on Mercury Release during Thermal Pretreatment. Energies 2023, 16, 772. https://doi.org/10.3390/en16020772
Bury M, Dziok T, Borovec K, Burmistrz P. Influence of RDF Composition on Mercury Release during Thermal Pretreatment. Energies. 2023; 16(2):772. https://doi.org/10.3390/en16020772
Chicago/Turabian StyleBury, Marcelina, Tadeusz Dziok, Karel Borovec, and Piotr Burmistrz. 2023. "Influence of RDF Composition on Mercury Release during Thermal Pretreatment" Energies 16, no. 2: 772. https://doi.org/10.3390/en16020772






