The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study
Abstract
:1. Introduction
2. Models and Methods
2.1. Calculation Models
2.2. Calculation Methods
3. Results and Discussion
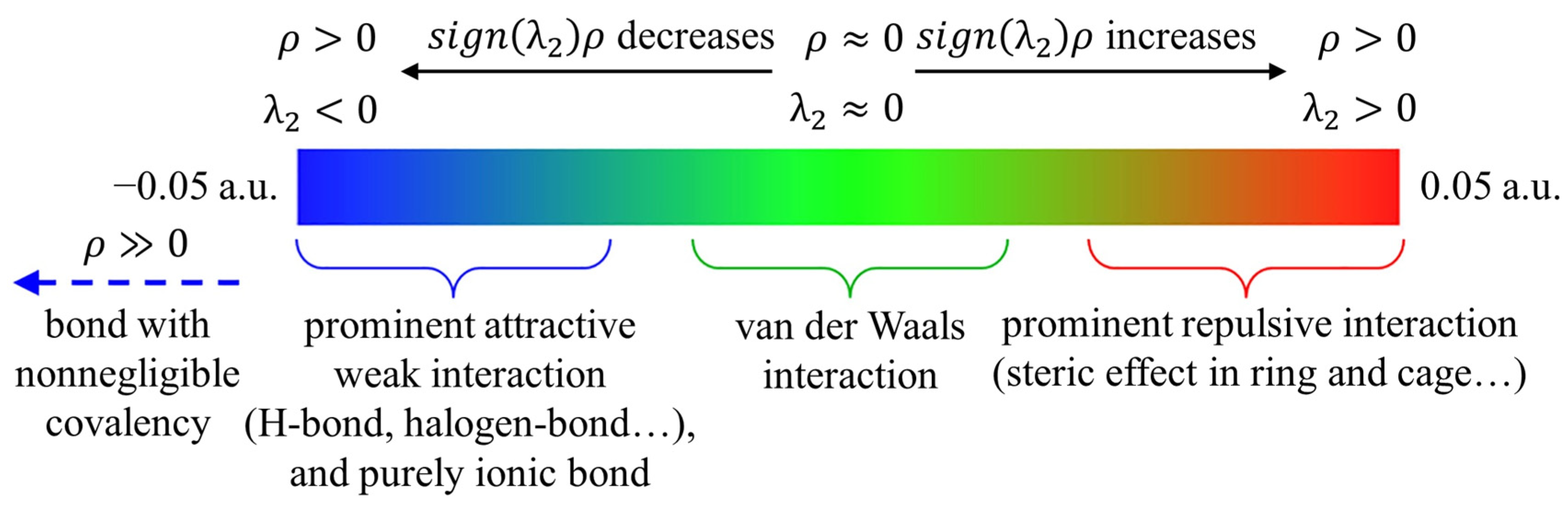
3.1. ESP Analysis
3.2. Adsorption Simulation of C2H6
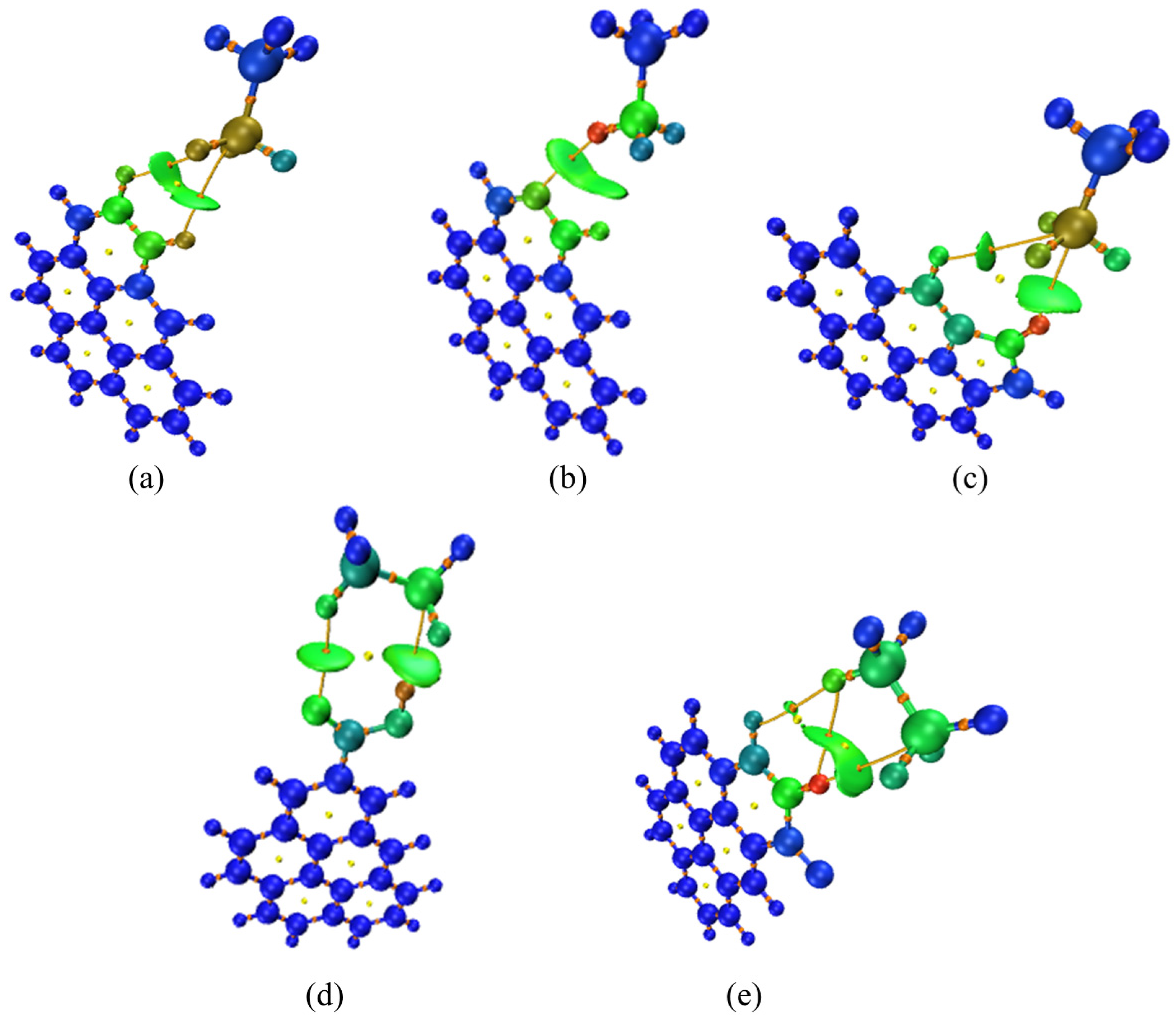
3.3. Co-Adsorption Simulation of C2H6 and SO2
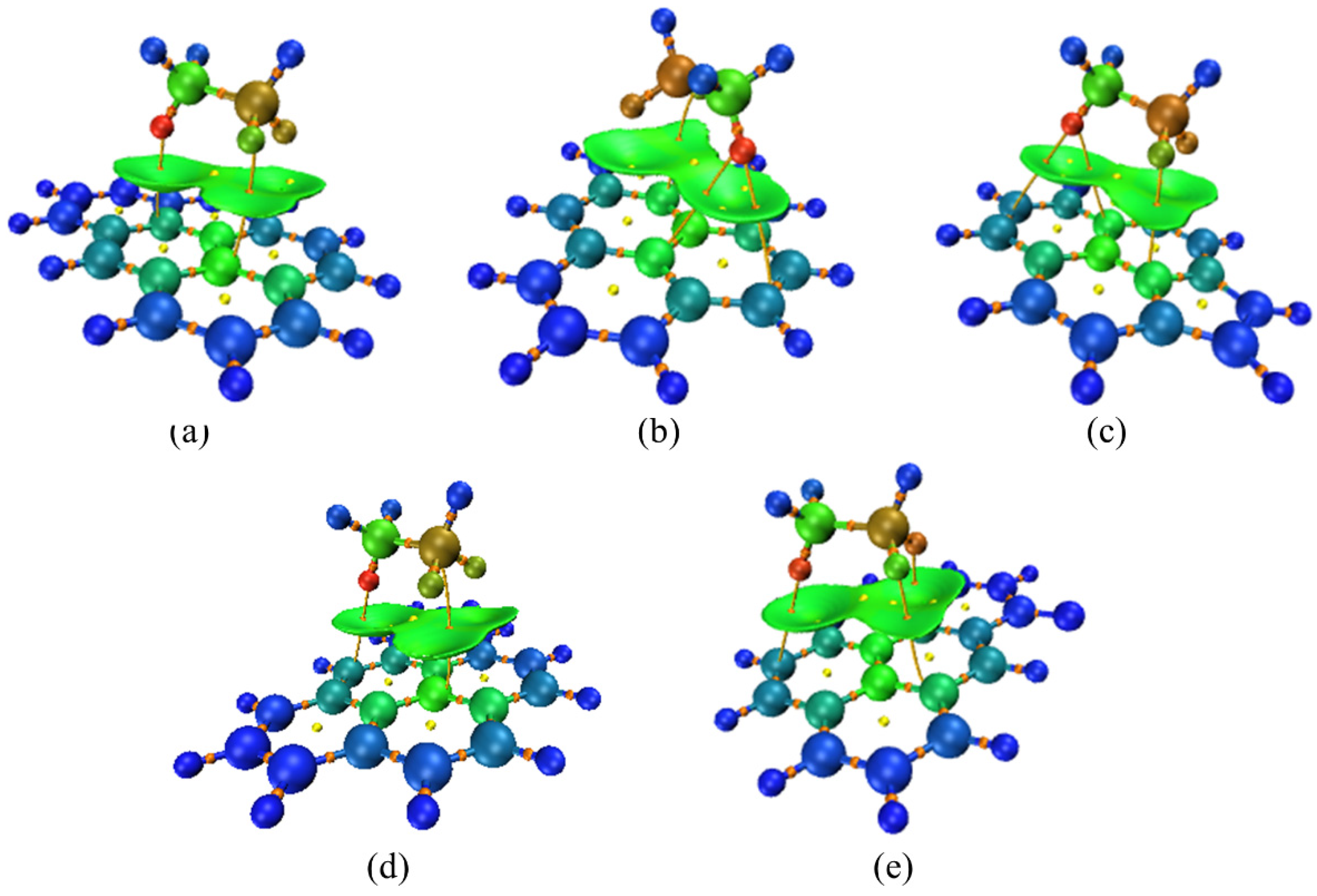
3.4. Co-Adsorption Simulation of C2H6 and NO
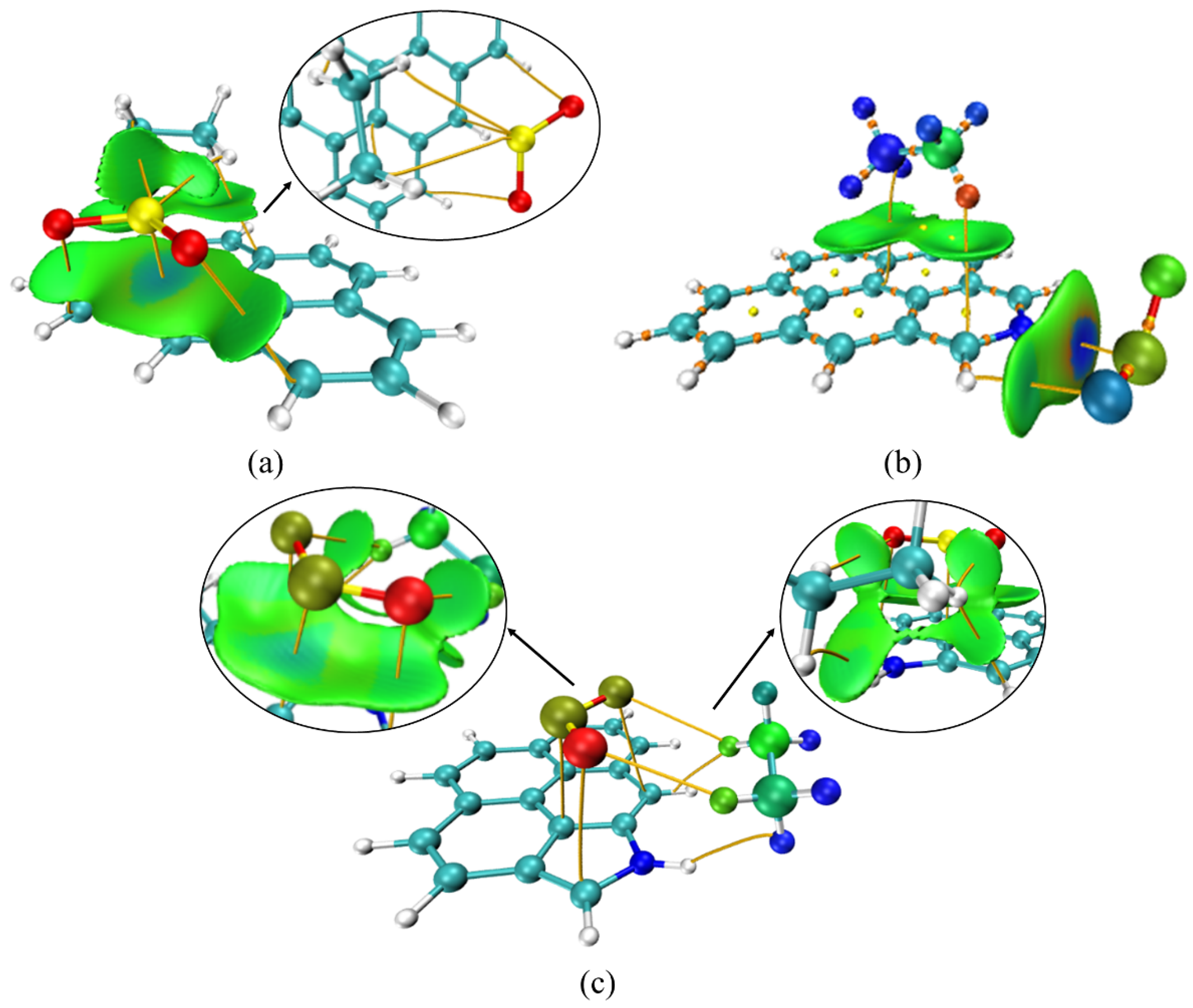
3.5. Co-Adsorption Simulation of C2H6, SO2 and NO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Acevedo, S.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of CO2 on activated carbons prepared by chemical activation with cupric nitrate. ACS Omega 2020, 5, 10423–10432. [Google Scholar] [CrossRef]
- Tzompa-Sosa, Z.A.; Mahieu, E.; Franco, B.; Keller, C.A.; Turner, A.J.; Helmig, D.; Fried, A.; Richter, D.; Weibring, P.; Walega, J.; et al. Revisiting global fossil fuel and biofuel emissions of ethane. J. Geophys. Res. Atmos. 2017, 122, 2493–2512. [Google Scholar] [CrossRef]
- Meng, X.; Sun, R.; Zhou, W.; Liu, X.; Yan, Y.; Ren, X. Effects of corn ratio with pine on biomass co-combustion characteristics in a fixed bed. Appl. Therm. Eng. 2018, 142, 30–42. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, A.; Xing, Y.; Gao, Y.; He, F. From concept to action: A review of research on co-benefts and co-control of grenhouse gases and local air pollutants reductions. Clim. Chang. Res. 2021, 17, 255–267. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Room temperature adsorptive removal of thiophene over zinc oxide-based adsorbents. J. Mater. Eng. Perform. 2018, 27, 2661–2667. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process. Saf. Environ. Prot. 2023, 172, 395–407. [Google Scholar] [CrossRef]
- Almahri, A.; Abou-Melha, K.S.; Katouah, H.A.; Al-Bonayan, A.M.; Saad, F.A.; El-Desouky, M.G.; El-Bindary, A.A. Adsorption and removal of the harmful pesticide 2,4-dichlorophenylacetic acid from an aqueous environment via coffee waste biochar: Synthesis, characterization, adsorption study and optimization via Box–Behnken design. J. Mol. Struct. 2023, 1293, 136238. [Google Scholar] [CrossRef]
- AAl-Wasidi, A.S.; AlZahrani, I.I.; Thawibaraka, H.I.; Naglah, A.M.; El-Desouky, M.G.; El-Bindary, M.A. Adsorption studies of carbon dioxide and anionic dye on green adsorbent. J. Mol. Struct. 2022, 1250, 131736. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Liu, X.; Gao, J.; Qie, Z.; Zhao, G. The effect of nitrogen-containing functional groups on SO2 adsorption on carbon surface: Enhanced physical adsorption interactions. Surf. Sci. 2018, 677, 78–82. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Hao, M.; Zhang, Y.; Ren, X.; Cao, F.; Zhang, L.; Sun, Q.; Wennersten, R. Effect of functional groups on VOCs adsorption by activated carbon: DFT study. Surf. Sci. 2023, 736, 122352. [Google Scholar] [CrossRef]
- Pi, X.; Sun, F.; Qu, Z.; Gao, J.; Wang, A.; Zhao, G.; Liu, H. Producing elemental sulfur from SO2 by calcium loaded activated coke: Enhanced activity and selectivity. Chem. Eng. J. 2020, 401, 126022. [Google Scholar] [CrossRef]
- Singh, S.B.; Haskin, N.; Dastgheib, S.A. Coal-based graphene oxide-like materials: A comprehensive review. Carbon 2023, 215, 118447. [Google Scholar] [CrossRef]
- Alomair, N.A.; Al-Aqeel, N.S.; Alabbad, S.S.; Kochkar, H.; Berhault, G.; Younas, M.; Jomni, F.; Hamdi, R.; Ercan, I. The role of the ferroelectric polarization in the enhancement of the photocatalytic response of copper-doped graphene oxide–TiO2 nanotubes through the addition of strontium. ACS Omega 2023, 8, 8303–8319. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z. DFT study of NO adsorption on pristine graphene. RSC Adv. 2017, 7, 13082–13091. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, W.; Liu, J.; Guo, J.; Yin, H.; Ning, P. Adsorption and oxidation of SO2 by graphene oxides: A van der Waals density functional theory study. Appl. Surf. Sci. 2015, 324, 61–67. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, M.; Wu, H.; Lv, X.; Zhou, Z. DFT study of the effect of Ca on NO heterogeneous reduction by char. Fuel 2020, 265, 116995. [Google Scholar] [CrossRef]
- Pi, X.; Sun, F.; Gao, J.; Qu, Z.; Wang, A.; Qie, Z.; Wang, L.; Liu, H. A new insight into the SO2 adsorption behavior of oxidized carbon materials using model adsorbents and DFT calculations. Phys. Chem. Chem. Phys. 2019, 21, 9181–9188. [Google Scholar] [CrossRef]
- Rubeš, M.; Kysilka, J.; Nachtigall, P.; Bludský, O. DFT/CC investigation of physical adsorption on a graphite (0001) surface. Phys. Chem. Chem. Phys. 2010, 12, 6438–6444. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zhang, M.; Ma, C. Effect of N-doping on NO2 adsorption and reduction over activated carbon: An experimental and computational study. Fuel 2019, 258, 116109. [Google Scholar] [CrossRef]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y.A. Levendis. Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Process. Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Liu, X.; Sun, F.; Qu, Z.; Gao, J.; Wu, S. The effect of functional groups on the SO2 adsorption on carbon surface I: A new insight into noncovalent interaction between SO2 molecule and acidic oxygen-containing groups. Appl. Surf. Sci. 2016, 369, 552–557. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on basis sets beautiful: Seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Multiwfn_3.8_dev.pdf. Beijing Kein Research Center for Natural Sciences. Available online: http://sobereva.com/multiwfn/ (accessed on 10 October 2023).




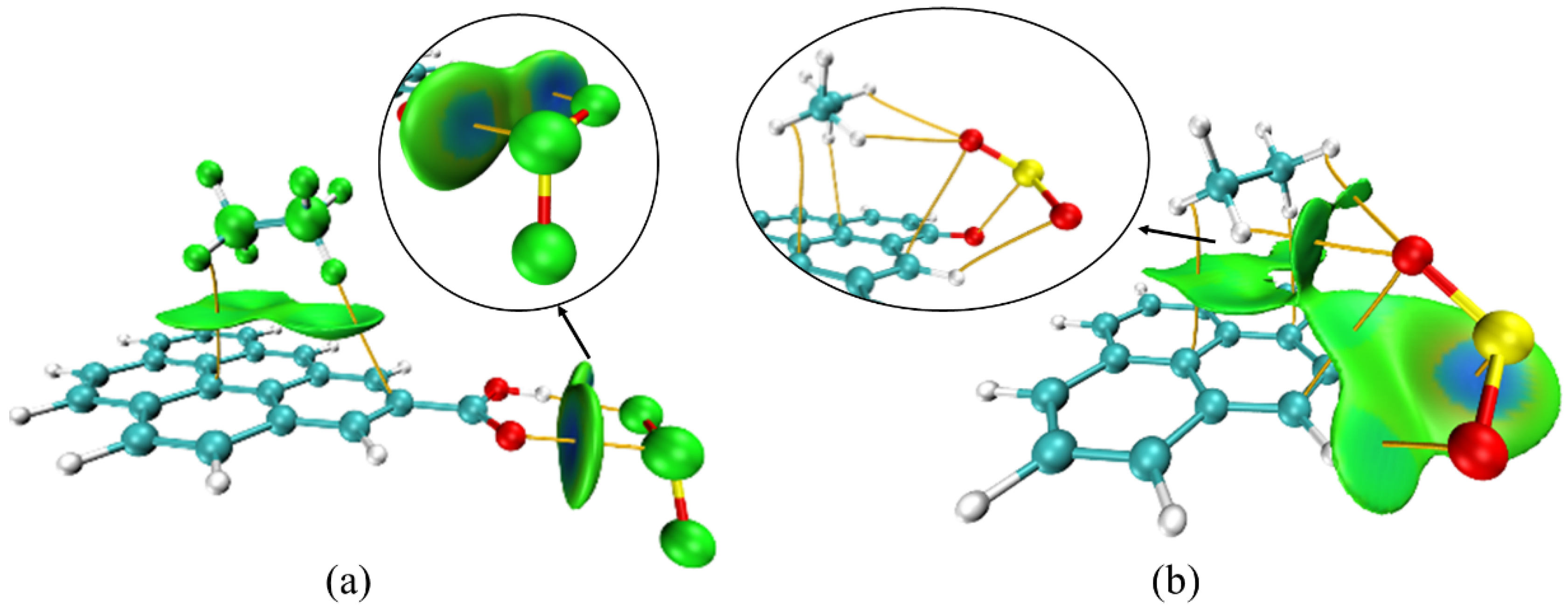
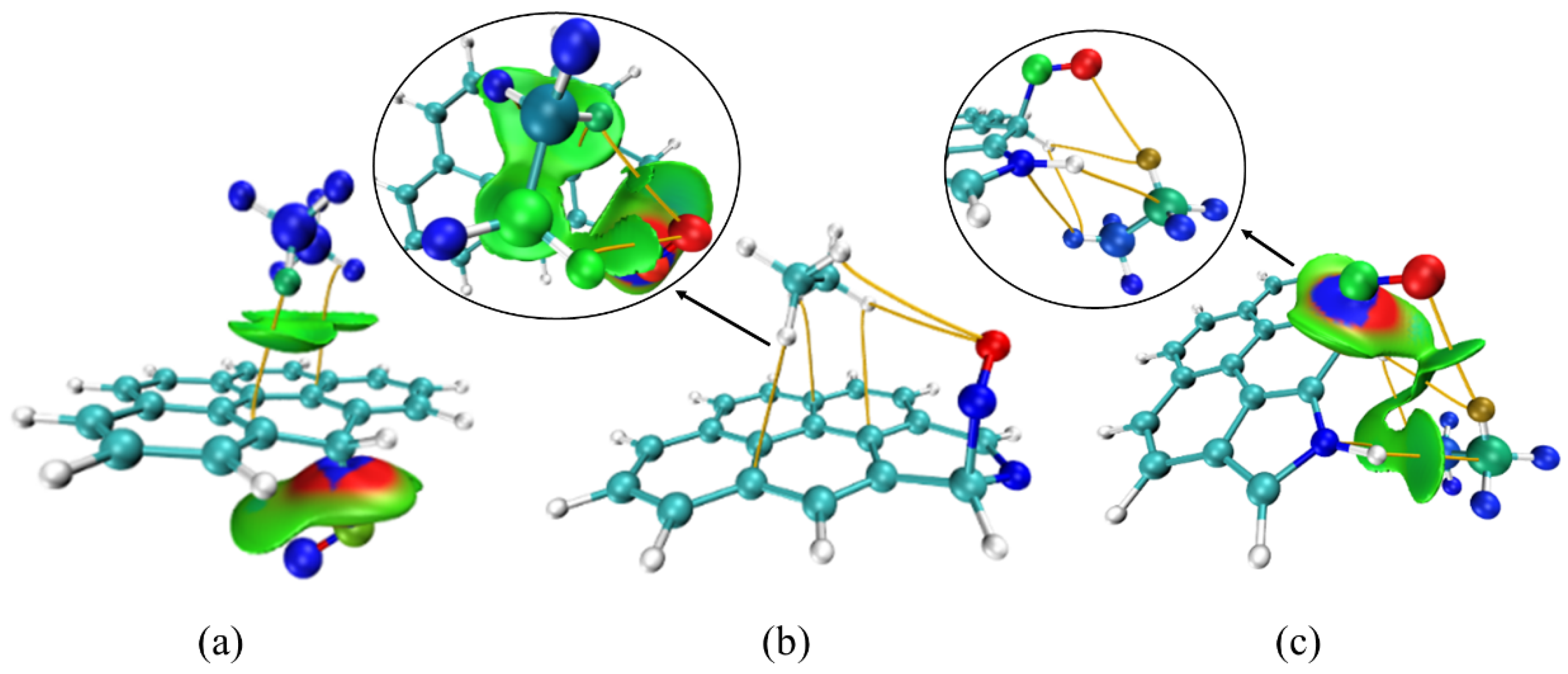



| Activated Carbon Model | Adsorbate | Calculation Method | Adsorption Energy (kJ/mol) |
|---|---|---|---|
| Activated carbon model without group | SO2 | BLYP/def2-SVP | −13.4 [9] |
| SO2 | Opt:M06-2X/6-31G(d, p) Sp: M06-2X/6-311+G G(d, p) | −5.7 [18] | |
| C2H6 | MP2/A-VTZ | −24.44 [19] | |
| C2H6 | DFT/CC | −17.49 [19] | |
| NO | B3LYP/6-311G** | −4.4 [20] | |
| Activated carbon model with pyridine group | SO2 | BLYP/def2-SVP | −52.9 [9] |
| NO | B3LYP/6-311G** | −12.3 [20] | |
| SO2/H2O/O2 | Opt: B3LYP/6–311G**+D3(BJ) Sp: M062X/jun-cc-pVTZ+D3 | −85.0/79.8 [21] | |
| Activated carbon model with pyrrole group | SO2 | BLYP/def2-SVP | −33.3 [9] |
| NO | B3LYP/6-311G** | −7.3 [20] | |
| SO2/H2O/O2 | Opt: B3LYP/6–311G**+D3(BJ) Sp: M062X/jun-cc-pVTZ+D3 | −68.7/43.1 [21] | |
| Activated carbon model with carboxyl group | SO2 | Opt: BLYP/def2-SVP Sp: B3LYP/def2-QZVP | −7.4 [22] |
| SO2 | Opt:M06-2X/6-31G(d, p) Sp: M06-2X/6-311+G G(d, p) | −34.95 [18] |
| Edge | Plane | |
|---|---|---|
| OG | 19.85 | 34.44 |
| PD | 20.34 | 34.41 |
| PR | 23.14 | 35.04 |
| CBX | 24.39 | 34.51 |
| CBN | 19.58 | 33.70 |
| C2H6/SO2 | C2H6/NO | C2H6/SO2/NO | |
|---|---|---|---|
| AC | 62.41 | 79.72 | 74.26 |
| PD | 75.94 | 57.97 | 86.19 |
| PR | 59.40 | 59.09 | 60.06 |
| CBX | 71.07 | 99.24 | 88.86 |
| CBN | 73.13 | 47.16 | 75.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, S.; Xu, S.; Ren, X.; Zhang, Y.; Cao, F.; Sun, Q.; Wennersten, R.; Yang, L. The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study. Energies 2023, 16, 7537. https://doi.org/10.3390/en16227537
Zhang L, Zhang S, Xu S, Ren X, Zhang Y, Cao F, Sun Q, Wennersten R, Yang L. The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study. Energies. 2023; 16(22):7537. https://doi.org/10.3390/en16227537
Chicago/Turabian StyleZhang, Lei, Shuhui Zhang, Shaofeng Xu, Xiaohan Ren, Yan Zhang, Fan Cao, Qie Sun, Ronald Wennersten, and Li Yang. 2023. "The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study" Energies 16, no. 22: 7537. https://doi.org/10.3390/en16227537
APA StyleZhang, L., Zhang, S., Xu, S., Ren, X., Zhang, Y., Cao, F., Sun, Q., Wennersten, R., & Yang, L. (2023). The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study. Energies, 16(22), 7537. https://doi.org/10.3390/en16227537








