Abstract
With their high-energy density, high-power density, long life, and low self-discharge, lithium-ion capacitors are a novel form of electrochemical energy storage devices which are extensively utilized in electric vehicles, energy storage systems, and portable electronic gadgets. Li-ion capacitor aging mechanisms and life prediction techniques, however, continue to be active research areas. This paper examines the aging process for Li-ion batteries, covering the alterations in cell composition, the effect of the electrode charge state, temperature effects, and electrolyte deterioration. Additionally, this research offers approaches for predicting the lifespan of lithium-ion batteries, including those based on physical models, machine learning, and artificial intelligence. In this work, cycle life testing techniques are also discussed, including accelerated aging experiments for lithium-ion capacitors. The paper concludes by discussing future directions for the creation of aging mechanisms and lithium-ion capacitor life prediction techniques.
1. Introduction
As global climate change intensifies and renewable energy sources develop, the lack of environmentally friendly and efficient new energy storage technologies has become a major issue. As conventional fuel vehicles are replaced with electric vehicles and new energy sources such as wind and solar energy are being used in large numbers on land, a suitable energy currency must be sought [1,2,3]. Traditional lithium-ion batteries (LIBs) have a high-energy density and a long life in this industry, but they are slow to charge and discharge and pose safety risks for rapid charging and discharging. While electric double-layer capacitors (EDLCs) have the benefits of high-power density, a long cycle life, and quick charging and discharging capacities, their energy density is low. Therefore, to address the respective limitations of conventional LIBs and EDLCs in terms of energy storage, the development of a hybrid energy storage device that eliminates the disadvantages while maintaining the advantages of both becomes the goal of research. The lithium-ion capacitor (LIC) was then born [4,5,6]. Lithium-ion capacitors consist of three parts: the diaphragm, the negative electrode, and the positive electrode. The positive electrode is made of lithium-ion embedded material, the negative electrode is made of activated carbon material, and the diaphragm is made of polypropylene film. Inside the cell, lithium ions migrate along the diaphragm through the electrolyte, charging and discharging between the positive and negative electrodes, and providing or consuming electrical energy through the circuit [7], as shown in Figure 1. With the benefits of high-energy density, high-power density, a long cycle life, and fast charging and discharging capacities, it combines the benefits of LIBs and EDLCs and finds a balance between the two. It also has a wide variety of application prospects [8,9].
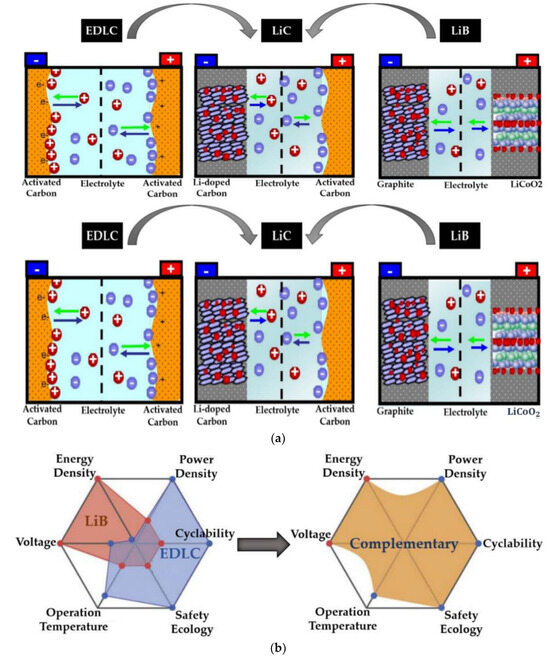
Figure 1.
(a) Storage mechanisms and chemical structures of LIBs, LICs, and EDLCs (b) Comparison of the properties of LIBs, EDLCs, and LICs.
As the LIC undergoes a variety of aging mechanisms and complex life degradation phenomena during use, such as decreasing capacity, increasing internal resistance, and deteriorating polarization characteristics, these problems seriously affect the performance and life of the LIC [10]. Therefore, in order to improve their service life and guarantee their reliability, it becomes crucial to study the aging principle of lithium-ion capacitors and establish an effective life predictive model, so that corresponding measures can be taken to repair or replace LICs in a timely manner. In addition, by studying the aging mechanism and life failure prediction method of LICs, it is also possible to understand the influence of its usage conditions on its life failure, and at the same time provide a reference for the design and optimization of LICs to facilitate their development and application [11]. Therefore, it is of great theoretical and practical significance to study the aging mechanism and life failure prediction method of lithium-ion capacitors.
2. Description of Aging Principle of Li-Ion Capacitors
An in-depth investigation of the aging mechanism of Li-ion capacitors can pinpoint the crucial elements and mechanisms at play, laying the groundwork for the creation of design and production approaches that will increase the functionality and longevity of Li-ion capacitors. It also provides a reliable research direction to accurately predict the remaining life of Li-ion capacitors by uncovering the aging characteristics during the declining life of Li-ion capacitors. However, as the LIC is still considered to be a new technology, its aging mechanism is still under continuous research.
2.1. Changes in the Structure of Battery Materials
2.1.1. Positive Pole
Similar to bilayer capacitors, since the positive electrode in the LIC is formed of activated carbon, the aging process of the SC’s positive electrode serves as a valuable benchmark for the LIC. Indeed, with chemically and thermally treated carbon electrodes, some functional groups may reside on their surface [12]. The electrodes are subjected to these processes in order to pore them out and increase their capacitance. Redox reactions take place between these functional groups and the electrolyte constituents at high temperatures and pressures. As a result, solid products can be deposited on the activated carbon’s surface, resulting in pore blockage [13]. The reduction in porosity leads to a reduction in the total capacitance of the capacitor. In addition, due to the increase in pressure, gases may be generated which swell the pack. Ionic conductivity may be decreased as a result of these products adhering to the activated carbon surface or even to the separator. As a result, the LIC’s overall resistance may be considerably higher. The capacitive package’s high voltage could cause electrode cracking or collector deterioration. The SC’s series resistance is likewise significantly impacted by this. Using field effect scanning electron microscopy, it has been shown that during SC aging, a considerable change occurs in the surface morphology of the positive electrode [14], as depicted in Figure 2. The removed graphite-based electrodes from the 2.2 V aging battery have a surface that is very comparable to that of the new electrodes, as can be shown. On the surface, there are a few stray filaments visible. The particles in the image corresponding to the 3.0 V old cell seem to be protected by a surface layer. Fine filamentous white deposits can be seen in some places. On the surface of the negative electrode for the cell that has been aged at 3.8 V, filamentary deposits are also visible. On a macroscopic level, they are not visible and do not blanket the surface. There are other spots where the particles appear to be iridescent. The table in Table 1 displays the specific observations.
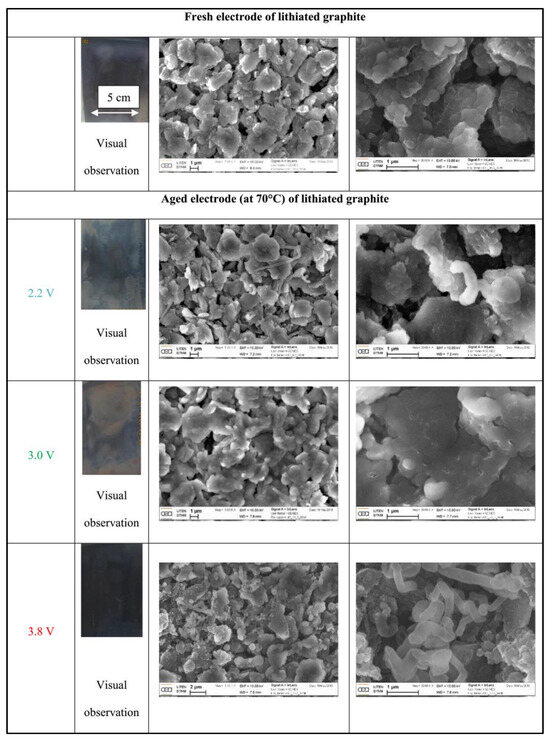
Figure 2.
Comparison of SEM images of the negative electrodes extracted from the brand-new cell and the aged cells.

Table 1.
Visual inspections of the negative electrodes of LICs after calendar aging tests.
2.1.2. Negative Pole
The formation of the negative passivation layer during lithiation, also known as the solid electrolyte interface (SEI), is the primary method by which the LIB ages [15,16,17,18]. Its aging can be a major issue because the LIC has a similar negative electrode. The electrolyte decomposes on the negatively charged graphite surface because it lacks thermodynamic stability. A passivation layer is created on the electrode surface by the organic or inorganic byproducts of the electrolyte breakdown. The SEI becomes unstable at high temperatures and some parasitic reactions may take place, producing solid products. The SEI may develop throughout the life of the cell and even encroach into the pores of the electrode or separator, as shown in Figure 3. As a result, the ions can no longer reach as much surface area. During the aging process, the formation of the SEI also causes an increase in impedance [19]. Additionally, the processes that help the layer form could result in the production of gases that harm the electrode. The negative electrode may also plate with lithium, especially if its potential is relatively low. Thus, the drop in capacitance during cycling results in the rise of its internal resistance as well as the depletion of recyclable lithium ions.
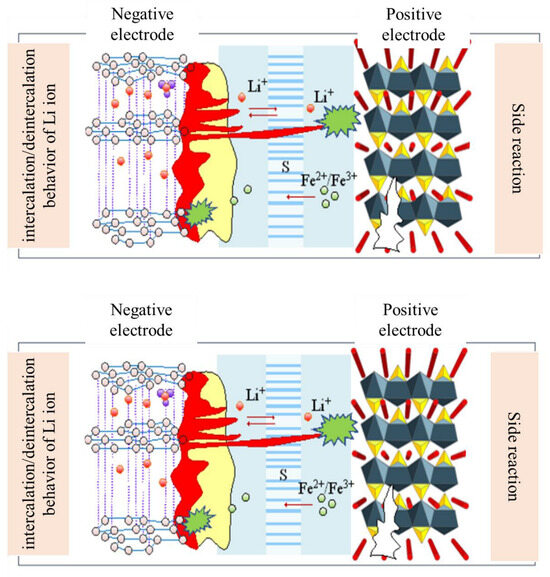
Figure 3.
The aging mechanism of LIB and growth process of SEI.
Other aging processes, such as graphite shedding from solventized lithium ion embedding, may potentially have an impact on the cathode of lithium-ion batteries. Additionally, especially at low temperatures, metallic lithium could be deposited on the graphite electrode at lower Li/Li+ potentials. An increased risk of thermal runaway and internal short circuits in the battery can result from persistent dendritic lithium deposition [20].
These pathways for electrode aging may also exist in the LIC. The capacity of the negative electrode is much greater as compared to the positive electrode; therefore, the influence of SEI growth on negative electrode aging is anticipated to be diminished. In [21], multiple size methodologies were evaluated to determine the ideal positive to negative electrode mass ratio. The findings demonstrate that the higher the state of charge during storage, without surpassing a level that causes severe capacitance degradation, the larger the negative electrode.
2.2. Effect of Electrode Charge State
The LIC operates according to a particular theory. A varied chemical makeup can be seen in each of the cell’s potentials. One can see that an anode of the galvanized carbon is almost neutral at 3 V, which is the open-circuit voltage used to build commercial LICs. A few of the lithium ions that have been pre-embedded in the cathode during the discharge from 3 V to 2.2 V are released from the carbon layer and go to an anode, where they accumulate. The anions from the electrolyte salt, however, build on the surface of the activated carbon when the cell is charged 3–3.8 V, and the salt’s cations are introduced into the negative electrode. Consequently, the double layer is formed at 2.2 V and 3.8 V. The aging mechanism of LIC is shown in Figure 4.
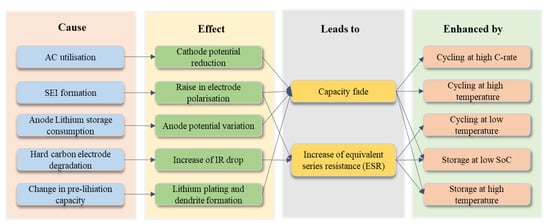
Figure 4.
Overview of LIC aging mechanisms.
It is obvious that a 2.2 V cathode is less lithiated than a 3.8 V cathode, and a comparison of the composition of the cells at 2.2 V and 3.8 V is depicted in Figure 5. At 2.2 V, the positive electrode surface begins to accumulate Li+ cations, while at 3.8 V, it begins to accumulate PF-6 anions. It was shown in [22] that the rate of the capacitance loss increased with the decreasing negative electrode voltage during calendar aging. Whereas lithiation reduces the potential of the electrode, it can be deduced that the LIC cathode at 2.2 V has a shorter aging time than the cathode at 3.8 V because it is less lithiated. In the literature, it has been experimentally shown that a battery aged at 2.2 V has a greater drop in capacitance than a battery aged at 3.8 V. Therefore, it has been determined that the presence of Li+ cations on the cathode surface accounts for the majority of the decrease in battery capacitance at 2.2 V with age.
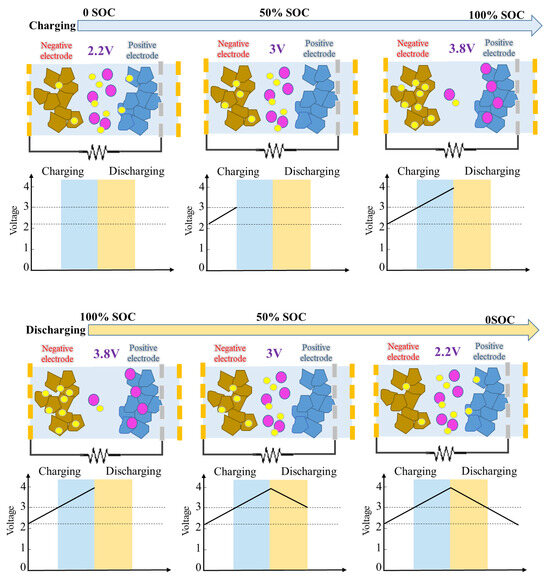
Figure 5.
LIC’s chemical make-up at 2.2, 3, and 3.8 V.
The collection of Li+ cations on the activated carbon is a process unique to LICs; limited studies have been conducted on the implications of this process. An SC comprises a couple of activated carbon electrodes that have an electrolyte employed in LICs; this has been explored in [23]. After numerous cycles, it was discovered that the activated carbon electrodes may have drifted. One of the major reasons there is a decline in performance is because there is an irreversible adsorption of the Li+ cations by parasitic clusters on the surface of the activated carbon. The products of these reactions diminish the active surface and clog the pores on the positive side of the activated carbon. These kinds of conditions must be avoided, especially for the storage system, as the LICs would not be discharged at the lowest voltage. It is recommended that keep the voltage level at 3 V.
The mechanism by which battery performance decreases when aged at 3.8 V is different. Fully lithiated negative electrodes in LICs aged at 3.8 V will be affected by the SEI [24]. As mentioned previously, the thickening of the SEI will reduce the active surface available for the ions and reduce the rise of the overall cell’s impedance. Additionally, the loss of lithium ions will raise the potential of the aging negative electrode. In order to maintain a total voltage of 3.8 V, the potential of the anode will thereafter drift to a higher positive value. This results in the breakage of electrolyte molecules at the cell’s positive electrode. They might then react with the parasitic groups on the activated carbon cathode, resulting in the production of additional gas and the expansion of the LIC.
The degradation of the aged cells is minimal at 3 V. An activated carbon anode is in a neutral condition at this voltage level, as depicted in Figure 2. Ultimately, the unfavorable mechanism brought on by the ion buildup on the electrode’s surface can be suppressed. The findings indicate that these cells’ decline in capacitance and rise in resistance are substantially less pronounced than those of the LICs aged at 2.2 V and 3.8 V. Overall, the cathode of an electrode is responsible for the cell’s deterioration at 3 V. Similarly, although with less impact, by aging the electrode at 3.8 V, the major result would be the formation of the SEI layer. In actuality, the negative electrode’s lithiation level is lower at 3 V than it is at 3.8 V. It is obvious that a negative electrode that is aged at 3.8 V causes extra damage compared to a negative electrode that is aged at 3 V.
2.3. Influence of Other Factors
The electrolyte is another important component in lithium-ion capacitors. Throughout the charge and discharge cycles, the electrolyte’s solvents and salts undergo a series of electrochemical processes that cause the electrolyte to degrade. The degradation of the electrolyte causes the chemical reactions within the capacitor to speed up, resulting in a drop in capacity and an increase in internal resistance. Temperature also plays a vital role in the chemical stability and electrochemical performance of capacitors [1]. It also accelerates aging tests in the literature [25]; using commercial LICs from different manufacturers, aging tests at 60 C and 3.8 V caused a 10% capacity drop within 5000 h, while aging tests at 0 C and 3.8 V resulted in less than a 2% capacity drop. As a result, it was discovered that the higher the temperature, the LIC’s degradation becomes more severe. Furthermore, cyclic aging studies have revealed that the depth of discharge has no effect on LIC capacity degradation.
3. Accelerated Aging Test for Lithium-Ion Capacitors
Accelerated aging tests are critical in the study of lithium-ion capacitor life prediction. On the one hand, accelerated aging tests can shorten the aging time of capacitors under different environmental conditions, thus improving the efficiency of the study. On the other hand, accelerated aging tests can collect a large amount of data on the gradual aging, decline, and failure of capacitors. The data can be utilized to create a capacitor life model and provide life forecasts. Simultaneously, the experimental findings may be compared and analyzed with the capacitor failure data from the actual application process, confirming the accuracy of the developed life model and improving the model’s predictive capability. Sun et al. conducted constant current charge/discharge testing and performed an analysis of the electrochemical impedance. This study investigated the effect of temperature on the electrochemical performance of LICs. A comparison of the performance of LIC cells with varying degrees of anode pre-lithiation and cathode compositions was performed. The results of accelerated aging experiments of 12 LIC samples have been detailed in the literature [26]. Aging tests were performed at temperatures of 60 °C and 70 °C for the voltage values up to 20 months. This setup was designed to explore the impacts of the maximum (3.8 V) and minimum voltages (2.2 V) on capacitive decay. Furthermore, it was experimentally proven that spectroscopy is one of the best and precise measurement tools for revealing the actual level of degradation within the LIC battery and serves as a significant inspiration for modeling the lifetime of lithium-ion capacitors.
Calendar aging tests, cycle aging tests, or both are frequently used in battery aging test procedures. A strategy for distinguishing between calendar and cyclic aging is suggested by Redondo et al. in their report on the results of accelerated aging testing. The interaction between calendar and cycle aging during battery cycling was considered using this analytical approach, and cycle tests made up of gentle, brief charge/discharge cycles coupled with relatively long rest periods were considered to demonstrate the non-linearity of calendar and cycle aging superimposed in electric vehicle applications. Furthermore, in order to analyze and predict the mechanisms affecting the electrode and internal cell composition under different conditions, El Ghossein et al. [25] looked into how floating aging affected LIC performance. Over the course of a year, 18 samples were evaluated at three distinct voltage levels and two different temperatures. Impedance measurements were used to track the performance of the test cells throughout the aging process.
Floating aging experiments involve placing lithium-ion capacitors under a constant voltage and constant charging to simulate the operating conditions in which lithium-ion capacitors are placed during actual use or long-term storage. Through floating charging over a long period of time, changes in parameters such as the leakage current, capacitance loss, and internal resistance of lithium-ion capacitors can be observed, and their performance and lifetime performance can be assessed accordingly. The research in the literature [14] examines the application of several accelerated aging tests to track the progression of aging and to identify and contrast the underlying aging mechanisms through a postmortem examination. The experiments that were executed used floating aging. At either high or low terminal voltages, Song et al.’s [27] floating aging tests on commercial LICs were sped up by high temperatures. In a high greenhouse, separate aging conditions of 4 V and 2.6 V were set to speed up aging.
4. Lifetime Models and Prediction Methods
4.1. Lifetime Model
Aging assessment, also known as State of Health (SoH) monitoring, is a type of condition evaluation that is required to guarantee the secure and long-lasting operation of LIC systems [28]. The two primary signs of LIC aging are an increase in Equivalent Series Resistance (ESR) and a decrease in capacitance. These are frequently used to denote the conclusion of the life cycle of an LIC.
A 20 percent drop in capacity or a 100 percent rise in ESR are typical conditions. Modeling the life cycle of LICs is crucial for predicting the life of the capacitors. The life prediction of LICs is the process of estimating and predicting their useful life.
4.1.1. Calendar Aging Analysis
The term “calendar life” of a lithium-ion battery refers to the number of years that pass from the date of production to the end of the battery’s life, taking into account storage, aging, high and low temperatures, cycling, simulating operating conditions, and other factors. Lithium-ion battery aging is a very complex process, and in order to anticipate a battery’s life with more accuracy, one needs to build models based on the battery’s aging mechanism in addition to empirical battery values and the accumulation of data. The lithium-ion battery calendar life projection has a significant impact on how battery maintenance is guided, how long the batteries last, the reliability and stability of the performance of the capacitor, and how risky it is to use the batteries.
El Ghossein et al. [14] performed an accelerated aging investigation on LICs over a 20-month period at elevated temperatures (60 °C and 70 °C) and varying voltage levels (2.2, 3, and 3.8 V, corresponding to 0, 50, and 100% SOC, respectively). They did not, however, offer any model that could foretell the end of the life of the batteries at these temperatures. Soltani et al. [10] studied a thorough life model for LIC 2300F batteries that could forecast the end of life (EOL) for calendar life tests at various temperatures. In this model, the lifetime was examined in relation to several stress factors, such as temperature, storage time, and the SOC. The capacity and internal resistance progression of the LIC utilized for the calendar life test are depicted in Figure 6.
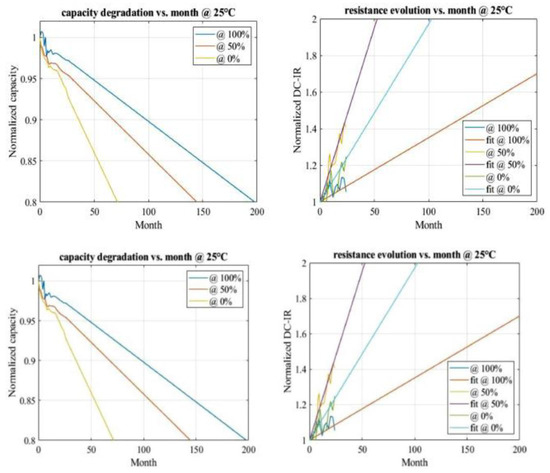
Figure 6.
Linear extrapolation for capacity and resistance for different SOCs at 25 °C.
4.1.2. Cyclic Aging Analysis
The number of charging and discharging cycles a battery can endure before its capacity degrades to a specific value under a specific charging and discharging regime is known as the standard cycle life of a battery. The capacitor life and cycle stability can be assessed by modelling performance changes under various cycle durations, offering a crucial benchmark for predicting the lifespan of capacitors.
Using an accelerated aging technique, Uno et al. [29] studied the aging behavior of LICs. They offered a life cycle model with the capacity to estimate its end of life. Accelerated aging tests were used to deal with slow life tests and to provide predictive models for energy storage system designers since it is impractical to conduct life tests under real-time time spans and operating conditions, and since the optimal design of the system is not possible without an accurate life model. To test if LICs could take the role of LIBs, the authors cycled three different LICs from different manufacturers at varying depths of discharge (DoD) (0% (floating), 40%, and 80%) as well as three distinct temperatures (cold, medium, and hot), but at extremely low current rates (1 C). A satellite energy storage system uses the chosen cycle characteristics. They also took into account how two alternative beginning states of charge (SOC) might affect the cyclic aging behavior for the 40% DoD. The authors’ lifespan model demonstrates that cycling has a minimal impact on the deterioration of two of the SOCs but a large impact on the degradation of the third SOC. The model can be expanded to arbitrary temperatures using activation energy values and aging acceleration factors. High current rates have an impact on LICs, which are mostly utilized for high-power applications, and the model cannot be regarded as comprehensive if the impact of the current rate on the lifetime model is not taken into account. The evolution of the internal resistance, the impact of the cell’s surface, and the influence of the temperature on the lifespan of the LICs were not assessed by the authors, who also failed to tie the electrical and thermal behavior to the lifetime model. Because LICs and LIBs share some attributes, the current rate appears to have an effect on longevity. In the case of LIBs, Omar et al. [30] have shown that the cycle profile affects the increase in ESR more so than the capacitance loss. In order to forecast the EOL, Soltani et al. [10] looked into the aging behavior of cycling applications at various temperatures. This model looked into how various stress parameters, including temperature, current flow, and the number of EQ cycles, affected the cycling lifetime. The DoD has been demonstrated to have no impact on the degradation of the LICs that were chosen. Using dynamic loading profiles at various temperatures, the model has been verified. With a relative error of less than 5%, the simulated findings and the experimental data accord well.
4.2. Prediction Methods
LICs are a new type of asymmetric electrochemical supercapacitor combining EDLCs and LIBs, and monitoring and assessing the LIC’s actual performance is crucial. A crucial component of prognosis and health management, remaining useful life (RUL) is the number of cycles left before hitting the EOL (End of Life) criterion (usually a 20% reduction in capacity from the rated value) [31]. However, as the aging mechanisms of Li-ion capacitors are still under continuous research, most of the methods proposed for their life decline prediction are inspired by the life prediction methods of Li-ion batteries and supercapacitors, to verify their applicability to Li-ion capacitors or to develop new Li-ion capacitor life models based on them.
4.2.1. Lifetime Prediction for Lithium-Ion Batteries
In this section we will explore the model-based and data-driven RUL prediction approaches for Li-ion batteries. We can categorize these into two groups by creating mathematical models to depict the relationship between the battery features and influences. These models also focus on analyses of the process of predicting Li-ion battery life decline. For the purpose of predicting the RUL of batteries, Wang and Zhang et al. [32,33] created the particle filter (PF) approach. The PF approach and the Dempster–Shafer theory were utilized by Lucu et al. [34] to calculate the battery capacity decline. For the purpose of predicting the RUL of batteries, Xu et al. [35] combined logistic regression and Gaussian process regression models. The authors of [36] used odorless Kalman filters for the prediction of the RUL of batteries. The application of various filtering methods for predicting the RUL of lithium-ion batteries can reduce the influence of noise on the prediction results and obtain a reliable prediction accuracy. In contrast, electrochemical models offer higher accuracy and reliability than methods based on statistical analysis and allow for a detailed study of several aspects of the physical properties inside the battery. A particle filtering (PF) based structure has been demonstrated by Liu et al. [37] for predicting the RUL of LIBs and a particular electrochemical model has also been demonstrated. A specially created current excitation can be utilized to identify the parameters of the simplified electrochemical model (SEM), which are employed as state variables in the PF algorithm. As shown in Figure 7, the observational equation in the PF algorithmic framework is based on the SEM-based capability simulation procedure. When predicting RUL, this method fully considers the battery’s mechanism.
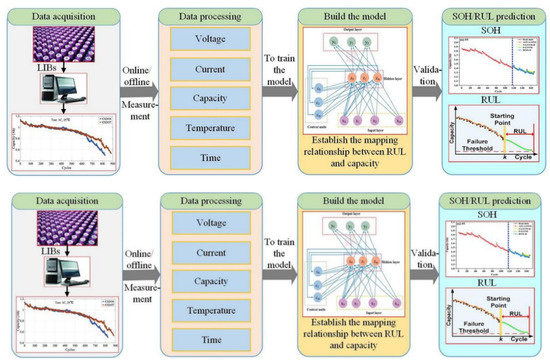
Figure 7.
Flowchart of the data-driven method for RUL prediction.
The data-driven strategy is distinguished by avoiding assumptions about the mechanism of battery life decrease and instead analyzing a significant amount of experimental data to forecast the battery life decline. It can analyze previous lithium-ion battery operating data and uncover patterns and trends in the depletion of various batteries under the same load using techniques like data mining and machine learning. The establishment of a prediction model is based on these trends and patterns. It is capable of producing precise forecasting results for the present battery rule. Improved neural network algorithms based on deep learning have gradually become a research hotspot for prediction problems in recent years, and Liu et al. [38] proposed an RUL prediction method for lithium-ion batteries based on a long short-term memory (LSTM) network optimized by the Improved Sparrow Search Algorithm (ISSA) in the field of RUL prediction. The suggested RUL prediction approach was also compared to the support vector regression (SVR), convolutional neural network (CNN), recurrent neural network (RNN), and linear support vector machine (LSTM). The experimental findings demonstrate how much more accurate and reliable the proposed RUL prediction approach is. However, relying too much on data-driven models prevents us from fully utilizing the aging characteristics. Taking into account the capacity regeneration phenomenon in Li-ion battery RUL prediction can help the model perform better at making predictions. The separation of global deterioration and local regeneration aspects of battery capacity sequences using the multi-scale wavelet decomposition technique (WDT) was proposed by Pang et al. [39] as a new method for predicting the RUL of Li-ion batteries. The suggested approach not only captures the capacity renewal phenomenon well, but also has good forecast accuracy and is less affected by various prediction starting points. Furthermore, to fully exploit the time-series properties of the lithium-ion battery data, Gao et al. [40] combined a convolutional neural network (CNN) and a bidirectional long and short-term memory (BiLSTM) neural network to extract deep features of battery capacity data, and they chose the memory function of the BiLSTM neural network to retain key information in the data and predict the trend of variations in the RUL of batteries. It is crucial to remember that the aging battery data drastically restricts the precision and stability of the data-driven algorithm. For the mitigation of the aging effects an adequate precaution on the data-driven method must be implemented while forming battery RUL prediction algorithms. For instance, regular battery maintenance and replacement can be implemented to ensure the data’s quality and freshness. Alternately, more sophisticated and advanced algorithms may be taken into consideration to lessen the influence of data elements.
4.2.2. Lifetime Prediction of Supercapacitors
The secret to an ultracapacitor’s dependable use in electric cars is accurate service life prediction. The RUL prediction method is summarized in Figure 8. Xu et al. created a classical Arrhenius model for predicting the capacitive decay; this model includes variables such as temperature, current strength, and number of cycles [41]. This is because the aging of supercapacitors is primarily related to factors like operating temperature, current strength, operating voltage, and number of cycles. A variety of supercapacitors of various design configurations have been used; however, the model has been limited to the temperature range of 25 °C to 55 °C, because temperature differences have an impact on the supercapacitor’s initial aging mechanism. Liu et al. proposed a technique to forecast the lifespan of supercapacitors since the degradation trend was reliable with the power function [42]. The outcomes demonstrate that when the extrapolation approach is applied to predict the life of supercapacitors, the accuracy of the prediction is positively associated with the number of cycles. As a result, the choice of the correct number of cycles may increase the accuracy of the prediction. When developing the ECM or selection function model, the model-based approach must completely consider the supercapacitor’s actual working conditions. It should also be updated online to increase the model’s adaptability [43,44]. The selection or creation of suitable intelligence algorithms is crucial to completing the prediction, and an accurate identification of model parameters is the key to increasing the forecast accuracy.
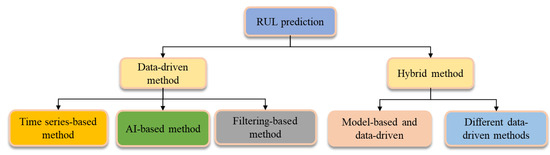
Figure 8.
Classification of RUL prediction methods.
The data-based method does not require a complex mathematical model to mimic the internal aging mechanisms of the supercapacitor, unlike the model-based method, which must advance its operating principle and internal structure. The relevant neural network-based model only requires the input of a significant amount of SC aging parameters connected to its age trend data or the attenuation of data from ultracapacitors for strength and threshold modification. Future scenarios can be evaluated using a network of practices that has been in place for a while. Using fully connected neural networks, Weigert et al. [45] estimated the life cycle of a supercapacitor which had been designed for HEVs and investigated the primary causes of aging. To forecast the RUL of a supercapacitor that uses short charging and discharging curves, a new recursive neural network (SIM RNN) has been developed. The drawback of this SIM RNN algorithm is the long-term dependency of the iteration process. It can be simplified since, if data has been retained for a long period, the gradient becomes diminished and the network is unable to learn new information. A hybrid evolutionary algorithm that mixes long- and short-term memory neural networks was proposed by Zhou et al. The LSTM structure, as depicted in Figure 9, has a memory unit that enables it to retain the crucial states of earlier phases. The LSTM also incorporates an oblivion gate that clears out unnecessary information and resets the memory unit. The long-term dependence and correlations between successive samples of sequential data can be learned by the LSTM. A novel t-time RNN approach that follows time values and adds to the input sequence of a conventional network is discussed in Liu et al.’s study [46], which employed a stacked bidirectional LSTM-RNN model. Researchers have offered a novel deep learning-based technique. This technique, named the deep belief network (DBN), which is a hybrid of Bayesian optimization and Hyperband (BOHB) approaches, has been proposed by Haris et al. When supercapacitor degradation occurs in its early stages, this technique is utilized to forecast the RUL. The RUL prediction model’s development took 54% less time than in earlier experiments. The technique is able to rapidly shorten the input cycle and still needs a lot of information to extract. As a result of the artificial features’ inescapable loss of aging information, the accuracy of the predictive findings is constrained. Additionally, manual feature extraction is laborious because it needs domain-specific expertise and takes a long time to process. A CNN-based end-to-end regularization prediction method is suggested in the literature [47]. This approach uses far less input data and is more accurate. The requirement for data is effectively reduced by this method, and the prediction accuracy is increased, both of which are beneficial for supercapacitor diagnosis and prediction. Predicting energy storage life and discharge actions via a data-driven tactic does not entail physical component modeling. The main goal is to find out the relationship of measured data and the respective element degradation. This method does not require a thorough examination of the internal chemicals or side reaction interference.
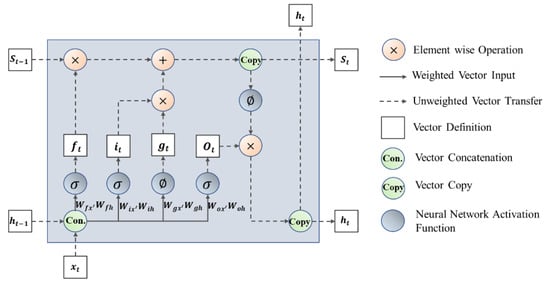
Figure 9.
The structure of AST-LSTM NN.
4.2.3. Life Prediction of Li-Ion Capacitors
The prediction of LIHC lifetime decline is a complex task, and many factors need to be considered. Various methods based on physical models and data-driven methodologies, similar to Li-ion battery and supercapacitor lifetime prediction methods, have been developed throughout the years. However, due to the lack of a deep understanding of the aging principle of Li-ion capacitors and insufficient experimental data for data training, these two relatively mature prediction methods are not easy to achieve in the field of life prediction for lithium-ion capacitors. Based on this, Li et al. [48] suggested a multi-state joint estimation approach. The effect of temperature on important LIHC parameters was investigated. The dynamic characteristics of LIHCs were described using the first-order resistance-capacitance equivalent circuit model. The polymorphism and LIHC parameter coupling relationship was deduced. To update model parameters adaptively, the recursive least squares algorithm with a variable forgetting factor (VFF-RLS) was utilized. Then, an artificial neural network model with long- and short-term memory (LSTM) and an ampere-hour integration approach were utilized to correct the open circuit voltage (OCV) of the online recognition. The estimation of the state of charge (SOC), remaining useable energy (RUE), and energy state (SOE) under dynamic operating conditions was obtained throughout a large temperature range, allowing for the development of a physical model for Li-ion capacitor lifetime prediction. The data used in this approach were based on FUDS and DST tests performed at 10 °C, 25 °C, and 50 °C. In addition, the cycle life test in the literature [49] was conducted on commercial off-the-shelf (COTS) LIC batteries sourced from three different manufacturers and applied to the LIC life cycle prediction model developed for EDLC in previous work. The activation energy of the deterioration rate was estimated using the Arrhenius equation based on the capacitance retention trend, and the aging acceleration factor was determined. The calculated acceleration factor varies from manufacturer to manufacturer, suggesting that the appropriate burn-in acceleration factor for each manufacturing unit should be determined based on cycle life tests, rather than simply applying the accepted rules of thumb for LIBs and EDLCs. Soltani et al. [10] proposed a lifetime model, which can predict the EOL of batteries stored or applied in cycles at different temperatures. This model can depict the increase in resistance caused by aging, which is extremely valuable in the design of thermal management systems. Cycle and calendar aging effects are separated in this model by employing two independent capacity degradation lookup tables and two independent internal resistance growth lookup tables. Yang et al. suggested an RUL prediction approach for LICs based on the data-driven method. The framework is shown in Figure 10. In contrast to earlier techniques, the proposed network will be trained using the de-noised data, which will enable the network to learn information from the time series.
Figure 10.
Flow diagram of the RUL prediction algorithm.
5. Conclusions and Suggestions for Future Research
Lithium-ion capacitors are high-performance energy storage technological devices that are widely utilized in contemporary electronic products. These capacitors have good qualities like a high-energy density, a long lifespan, and a low self-discharge rate. However, the life of Li-ion capacitors might degrade with prolonged use. Therefore, it is critical to research the lifetime decline predictions for Li-ion capacitors. The lifetime of Li-ion capacitors can be successfully extended and their performance can be optimized with accurate lifetime prediction. This study provided a comprehensive summary of Li-ion capacitor aging principles and an analysis of a number of aging-related variables. The life model of Li-ion capacitors was the focus of this paper, which also examined the most recent research findings on the life decline prediction of Li-ion capacitors in recent years. This paper summarized the concepts and techniques offered by the remaining life prediction methods of Li-ion batteries and supercapacitors to build a life prediction model of Li-ion capacitors. As for the data required by the model, various accelerated aging tests of Li-ion capacitors were summarized and compared.
Although many models and methods have reaped certain results in the life decline prediction of Li-ion batteries, with the development of new energy technologies demanding more and more performance from Li-ion capacitors, we still need to explore more stable and reliable Li-ion capacitor life prediction models, which can be considered in the following ways in future research.
Lifetime decline mechanism research: many hypotheses on the aging mechanism of Li-ion capacitors have been proposed, but more experiments and modelling are needed to verify and improve these hypotheses. This includes factors such as lithium-ion concentration fields, diffusion coefficients, electrode surface topography, and chemical reactions near the electrode/electrolyte interface.
Data acquisition and processing techniques: an accurate multi-physics field and charge/discharge process simulations require more advanced and accurate sensors and measurement methods. As more data and information is accumulated, artificial intelligence (AI) algorithms can better analyze this information to identify patterns and regularities that may lead to lithium-ion capacitor failure.
Combining multiple predictive models: predictive methods based on data analyses with those based on physical models can be combined to create multiple predictive models in order to make the predictions more accurate and reliable.
Author Contributions
Writing—original draft, S.C.; methodology, S.C.; investigation, S.R.; writing—review and editing, K.W.; project administration, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Youth Fund of Shandong Province Natural Science Foundation (No. ZR2020QE212), Key Projects of Shandong Province Natural Science Foundation (No. ZR2020KF020), the Guangdong Provincial Key Lab of Green Chemical Product Technology (GC202111), Zhejiang Province Natural Science Foundation (No. LY22E070007), and National Natural Science Foundation of China (No. 52007170).
Data Availability Statement
The data and materials used to support the results of this study cannot be obtained due to privacy reasons. Data are contained within the article.
Conflicts of Interest
Shuhui Cui and Kai Wang. have received research grants from School of Electrical Engineering, Qingdao University, Weihai Innovation Research Institute and Shandong Suoxiang Intelligent Technology Co., Ltd. Saleem Riaz has been involved as a consultant and expert in School of Automation, Northwestern Polytechnical University.
References
- Karimi, D.; Behi, H.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Lithium-Ion Capacitor Technology: Theory, Development, Modeling, Thermal Management Systems, and AppLICations. Molecules 2022, 27, 3119. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shang, Y.; Zheng, L.; Wang, K. Application of nanogenerator in the field of acoustics. ACS Appl. Electron. Mater. 2023, 5, 5240–5248. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, D.; Wang, K. State-of-health estimation of lithium-ion batteries based on electrochemical impedance spectroscopy: A review. Prot. Control. Mod. Power Syst. 2023, 8, 41. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.B.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.P.; Xu, J.; Yang, S. Lithium ion capacitors (LICs): Development of the materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Zhang, Y.; Wang, L.; Wang, K. Summary of Health-State Estimation of Lithium-Ion Batteries Based on Electrochemical Impedance Spectroscopy. Energies 2023, 16, 5682. [Google Scholar] [CrossRef]
- Zuo, W.H.; Li, R.Z.; Zhou, C.; Li, Y.Y.; Xia, J.L.; Liu, J.P. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, Z.; Yin, K.; Wang, L.; Wang, K. Sensing as the key to the safety and sustainability of new energy storage devices. Prot. Control. Mod. Power Syst. 2023, 8, 27. [Google Scholar] [CrossRef]
- Soltani, M.; Ronsmans, J.; Van Mierlo, J. Cycle life and calendar life model for lithium-ion capacitor technology in a wide temperature range. J. Energy Storage 2020, 31, 101659. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, C.; Chen, R.; Jiang, J. Three-Leg Quasi-Z-Source Inverter with Input Ripple Suppression for Renewable Energy Application. Energies 2023, 16, 4393. [Google Scholar] [CrossRef]
- Richner, R.; Muller, S.; Wokaun, A. Grafted and crosslinked carbon black as an electrode material for double layer capacitors. Carbon 2002, 40, 307–314. [Google Scholar] [CrossRef]
- Azais, P.; Duclaux, L.; Florian, P.; Massiot, D.; Lillo-Rodenas, M.A.; Linares-Solano, A.; Peres, J.P.; Jehoulet, C.; Beguin, F. Causes of supercapacitors ageing in organic electrolyte. J. Power Sources 2007, 171, 1046–1053. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P.; Genies, S.; Azais, P. Post-Mortem Analysis of Lithium-Ion Capacitors after Accelerated Aging Testes. J. Energy Storage 2021, 33, 102039. [Google Scholar] [CrossRef]
- Hu, S.D.; Liang, Z.P.; He, X.N. Ultracapacitor-Battery Hybrid Energy Storage System Based on the Asymmetric Bidirectional Z-Source Topology for EV. IEEE Trans. Power Electron. 2016, 31, 7489–7498. [Google Scholar] [CrossRef]
- Barre, A.; Deguilhem, B.; Grolleau, S.; Gerard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive appLICations. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Huang, Y.L.; Zhao, Y.; Gong, Q.M.; Weng, M.Y.; Bai, J.F.; Liu, X.; Jiang, Y.Q.; Wang, J.J.; Wang, D.Z.; Shao, Y.; et al. Experimental and Correlative Analyses of the Ageing Mechanism of Activated Carbon Based Supercapacitor. Electrochim. Acta 2017, 228, 214–225. [Google Scholar] [CrossRef]
- Parvini, Y.; Siegel, J.B.; Stefanopoulou, A.G.; Vahidi, A. Supercapacitor Electrical and Thermal Modeling, Identification, and Validation for a Wide Range of Temperature and Power AppLICations. IEEE Trans. Ind. Electron. 2016, 63, 1574–1585. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P. Effects of the Hybrid Composition of Commercial Lithium-Ion Capacitors on Their Floating Aging. IEEE Trans. Power Electron. 2019, 34, 2292–2299. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Li, L.; Wang, L.; Wang, K. SnO2/TiO2 Nanocomposite Prepared by Pulsed Laser Deposition as Anode Material for Flexible Quasi-solid-state Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 11709–11722. [Google Scholar] [CrossRef]
- Dsoke, S.; Fuchs, B.; Gucciardi, E.; Wohlfahrt-Mehrens, M. The importance of the electrode mass ratio in a Li-ion capacitor based on activated carbon and Li4Ti5O12. J. Power Sources 2015, 282, 385–393. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries I. Impact of the Graphite Anode on Capacity Fade. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Zhang, T.; Fuchs, B.; Secchiaroli, M.; Wohlfahrt-Mehrens, M.; Dsoke, S. Electrochemical behavior and stability of a commercial activated carbon in various organic electrolyte combinations containing Li-salts. Electrochim. Acta 2016, 218, 163–173. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P. Lifetime Prediction of Lithium-Ion Capacitors Based on Accelerated Aging Tests. Batteries 2019, 5, 28. [Google Scholar] [CrossRef]
- Redondo-Iglesias, E.; Venet, P.; Pelissier, S. Calendar and cycling ageing combination of batteries in electric vehicles. Microelectron. Reliab. 2018, 88–90, 1212–1215. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; An, Y.B.; Sun, X.Z.; Ma, Y.W. Experimental Study on Calendar Aging of Commercial Lithium-ion Capacitors. In Proceedings of the 23rd International Conference on Electrical Machines and Systems (ICEMS), Electr Network, Hamamatsu, Japan, 24–27 November 2020; Electr Network, 2020. pp. 1587–1591. [Google Scholar]
- Zhang, M.; Wang, W.; Xia, G.; Wang, L.; Wang, K. Self-Powered Electronic Skin for Remote Human–Machine Synchronization. ACS Appl. Electron. Mater. 2023, 5, 498–508. [Google Scholar] [CrossRef]
- Uno, M.; Kukita, A. Cycle Life Evaluation Based on Accelerated Aging Testing for Lithium-Ion Capacitors as Alternative to Rechargeable Batteries. IEEE Trans. Ind. Electron. 2016, 63, 1607–1617. [Google Scholar] [CrossRef]
- Omar, N.; Gualous, H.; Salminen, J.; Mulder, G.; Samba, A.; Firouz, Y.; Monem, M.A.; Bossche, P.; Van Mierlo, J. Electrical double-layer capacitors: Evaluation of ageing phenomena during cycle life testing. Appl. Electrochem. 2014, 44, 509–522. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.L.; Foley, A.M.; Zulke, A.; Berecibar, M.; Nanini-Maury, E.; Van Mierlo, J.; Hoster, H.E. Data-driven health estimation and lifetime prediction of lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2019, 113, 109254. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.F.; Zhao, Y.; Tsui, K.L. Battery remaining useful life prediction at different discharge rates. Microelectron. Reliab. 2017, 78, 212–219. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xiong, R.; He, H.W.; Pecht, M. Validation and verification of a hybrid method for remaining useful life prediction of lithium-ion batteries. J. Clean. Prod. 2019, 212, 240–249. [Google Scholar] [CrossRef]
- Lucu, M.; Martinez-Laserna, E.; Gandiaga, I.; Camblong, H. A critical review on self-adaptive Li-ion battery ageing models. J. Power Sources 2018, 401, 85–101. [Google Scholar] [CrossRef]
- Xu, D.; Sui, S.B.; Zhang, W.; Xing, M.L.; Chen, Y.; Kang, R. RUL prediction of electronic controller based on multiscale characteristic analysis. Mech. Syst. Signal Process. 2018, 113, 253–270. [Google Scholar] [CrossRef]
- Zheng, X.J.; Fang, H.J. An integrated unscented kalman filter and relevance vector regression approach for lithium-ion battery remaining useful life and short-term capacity prediction. Reliab. Eng. Syst. Saf. 2015, 144, 74–82. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Zhang, J.Y.; Li, K.; Lv, C. The Remaining Useful Life Prediction by Using Electrochemical Model in the Particle Filter Framework for Lithium-Ion Batteries. IEEE Access 2020, 8, 126661–126670. [Google Scholar] [CrossRef]
- Liu, Y.W.; Sun, J.; Shang, Y.L.; Zhang, X.D.; Ren, S.; Wang, D.T. A novel remaining useful life prediction method for lithium-ion battery based on long short-term memory network optimized by improved sparrow search algorithm. J. Energy Storage 2023, 61, 106645. [Google Scholar] [CrossRef]
- Pang, X.Q.; Huang, R.; Wen, J.; Shi, Y.H.; Jia, J.F.; Zeng, J.C. A Lithium-ion Battery RUL Prediction Method Considering the Capacity Regeneration Phenomenon. Energies 2019, 12, 2247. [Google Scholar] [CrossRef]
- Gao, D.; Liu, X.; Yang, Q. Remaining Useful Life Prediction of Lithium-Ion Battery Based on CNN and BiLSTM Fusion. Inf. Control 2022, 51, 318. [Google Scholar]
- El Mejdoubi, A.; Chaoui, H.; Sabor, J.; Gualous, H. Remaining Useful Life Prognosis of Supercapacitors Under Temperature and Voltage Aging Conditions. IEEE Trans. Ind. Electron. 2018, 65, 4357–4367. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.; Lu, X. Prediction of the cycle life of supercapacitor. Battery Bimon. 2018, 48, 159–162. [Google Scholar]
- Naseri, F.; Farjah, E.; Ghanbari, T.; Kazemi, Z.; Schaltz, E.; Schanen, J.L. Online Parameter Estimation for Supercapacitor State-of-Energy and State-of-Health Determination in Vehicular AppLICations. IEEE Trans. Ind. Electron. 2020, 67, 7963–7972. [Google Scholar] [CrossRef]
- Feng, F.; Hu, X.S.; Hu, L.; Hu, F.L.; Li, Y.; Zhang, L. Propagation mechanisms and diagnosis of parameter inconsistency within Li-Ion battery packs. Renew. Sustain. Energy Rev. 2019, 112, 102–113. [Google Scholar] [CrossRef]
- Weigert, T.; Tian, Q.; Lian, K. Cycle Life Prediction of Battery-Supercapacitor Hybrids Using Artificial Neural Networks, Symposium on Batteries for Renewable Energy Storage. In Proceedings of the 217th Meeting of the Electrochemical-Society (ECS), Vancouver, BC, Canada, 25–30 April 2010; pp. 35–42. [Google Scholar]
- Liu, C.L.; Zhang, Y.; Sun, J.R.; Cui, Z.H.; Wang, K. Stacked bidirectional LSTM RNN to evaluate the remaining useful life of supercapacitor. Int. J. Energy Res. 2022, 46, 3034–3043. [Google Scholar] [CrossRef]
- Haris, M.; Hasan, M.N.; Qin, S.Y. Early and robust remaining useful life prediction of supercapacitors using BOHB optimized Deep Belief Network. Appl. Energy 2021, 286, 116541. [Google Scholar] [CrossRef]
- Wang, C.X.; Xiong, R.; Tian, J.P.; Lu, J.H.; Zhang, C.M. Rapid ultracapacitor life prediction with a convolutional neural network. Appl. Energy 2022, 305, 117819. [Google Scholar] [CrossRef]
- Li, X.Y.; Long, T.; Tian, J.D.; Tian, Y. Multi-state joint estimation for a lithium-ion hybrid capacitor over a wide temperature range. J. Power Sources 2020, 479, 228677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).