Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future
Abstract
:1. Introduction
2. Hydrogen Production from Bio-Alcohols: Focus on Bioethanol
2.1. Reforming
| Technology | Temperature | Pressure | Feed Ratio | Ref. |
|---|---|---|---|---|
| Steam Reforming | 450–650 °C | 2–25 bar | Water/Ethanol = 3–6 | [8,23] |
| Partial Oxidation | 200–400 °C | 1–10 bar | Air/Ethanol = 4 | [42,45] |
| Autothermal Reforming | 600–900 °C | 1–10 bar | Ethanol/Air/Water = 1/0.3–0.5/2–3 | [42,46] |
2.2. Photocatalysis
2.3. Electrolysis
3. Hydrogen Storage
3.1. Traditional and Innovative Methods for Hydrogen Storage
3.2. Liquid Organic Hydrogen Carriers (LOHC): Formic Acid and Formate
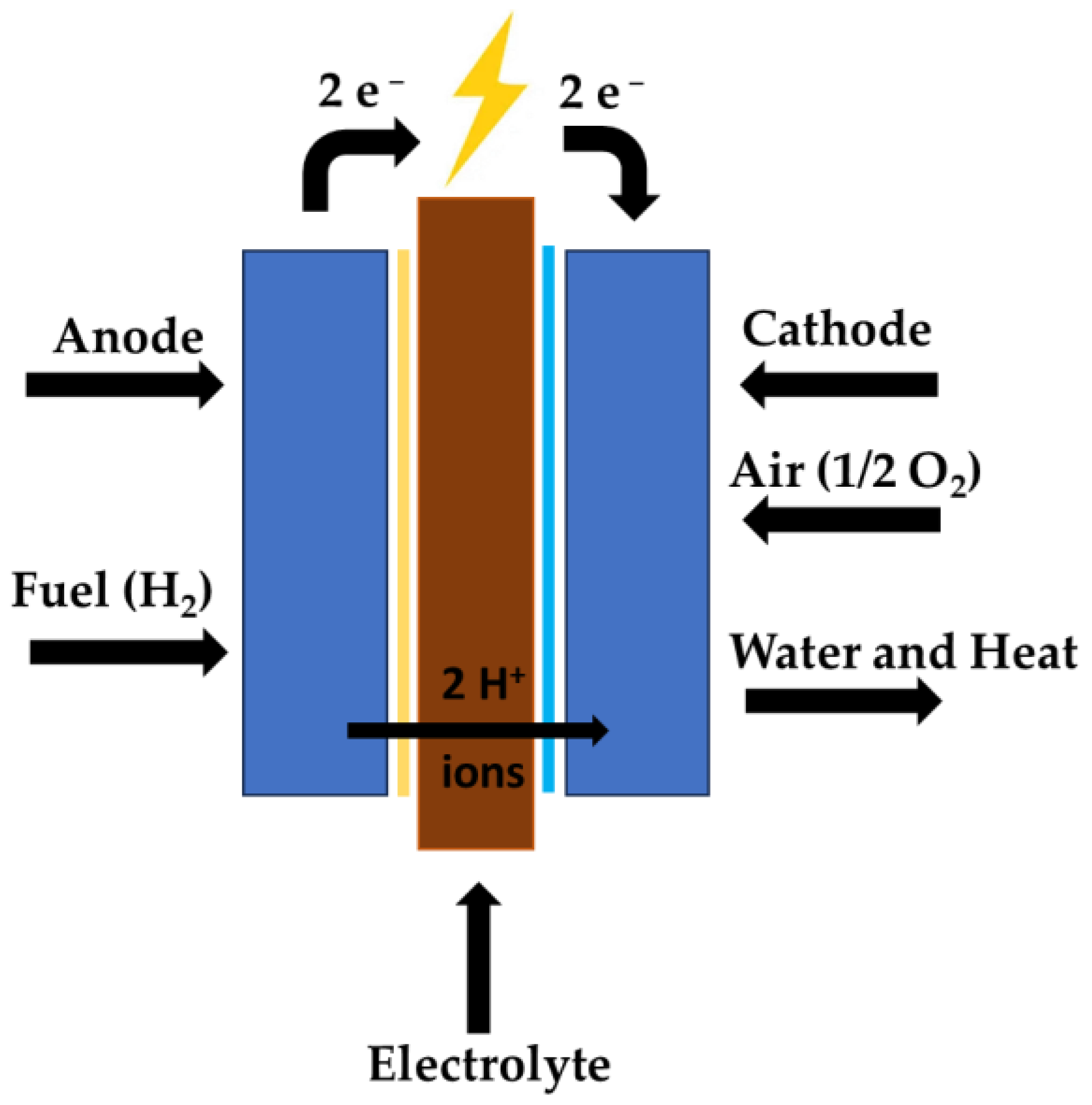
4. H2 in Fuel Cells
4.1. Comparison with Other Energy Conversion Systems Based on H2
4.1.1. Internal Combustion Engines (ICEs) and Gas Turbines against Hydrogen Fuel Cells (FCs)
4.1.2. Hydrogen Fuel Cells vs. Batteries
4.1.3. Solar and Wind Energy against Hydrogen Fuel Cells
4.2. Main Advantages and Disadvantages Related to FCs
- I.
- High efficiency. Since FCs directly convert chemical energy into electrical energy, they can reach higher efficiencies than energy conversion systems based on conventional combustion, up to 60%.
- II.
- Low emissions. FCs produce fewer emissions compared to traditional fossil fuel-based energy systems, making them a more environmentally friendly option. Indeed, hydrogen fuel cells (HFCs) emit only water vapor and heat as by-products; accordingly, they stand as a clean energy source. Furthermore, unwanted byproducts such as NOx and SOx or particulate emissions, are theoretically eliminated.
- III.
- Quiet operation: FCs operate quietly compared to traditional fossil fuel-based energy systems. This makes them an attractive option for applications where noise pollution is a concern, such as residential areas.
- IV.
- Modular design: FC systems can be designed and scaled to meet a variety of energy demands. This allows for the customization of fuel cell systems to meet the specific energy needs of different applications.
- V.
- High costs: FC systems can be costly to develop and deploy, limiting their widespread use. The cost of fuel cell systems is caused by several factors, including material costs, manufacturing methods, and a lack of economies of scale.
- VI.
- Limited durability: Due to their limited durability, fuel cells may require frequent maintenance and replacement, thus increasing their lifecycle cost. This is due to their sensitivity to temperature, humidity, and pollutants, all of which can have an impact on their function over time.
- VII.
- Hydrogen storage and distribution safety issues: Hydrogen storage and distribution is a critical obstacle for the development of fuel cell technology. Hydrogen is a highly combustible gas that must be handled and stored with caution.
- VIII.
- Inadequate infrastructure: The absence of infrastructure to support fuel cell technology, such as hydrogen refueling stations, as well as the high costs associated with their setup and maintenance, hinder widespread implementation.
4.3. Challenges (Pt Replacing, Design Parameters, and Scalability)
4.4. Biowaste as the Most Sustainable Choice for Carbon Electrocatalyst Production
- I.
- Reduce the dependence on platinum and other precious metals;
- II.
- Improve the performance and durability of the electrodes by providing a larger surface area, good electrical conductivity, and high resistance to corrosion;
- III.
- Enhance the catalytic activity and selectivity by introducing heteroatoms (such as nitrogen, sulphur, or phosphorus) or functional groups into the carbon structure;
- IV.
- Lower the cost and environmental impact of fuel cell production by recycling and valorizing waste materials.
- I.
- Low purity and uniformity compared to synthetic materials;
- II.
- Require additional treatments to remove impurities or contaminants that may affect the catalytic performance or poison the catalysts;
- III.
- Limited availability or variability depending on the source and season.
| Biowaste | Active Phase | Application | Performance | Ref |
|---|---|---|---|---|
| Peanut shell | Nitrogen-doped porous carbon | High-performance supercapacitators | -Specific capacitance: 310.59 F/g at 0.5 A/g (three-electrode system) -Specific capacitance: 300.6 F/g at 0.5 A/g (two-electrode system) -Specific capacitance: 246.4 F/g at 10 A/g (two-electrode system) -Capacitance retention: 81.98% from 0.5 to 10 A/g (two-electrode system) -Energy density: 40.92 Wh/kg at 990 W/kg (two-electrode system) -Cycle stability: 90.14% capacitance retention after 5000 cycles (two-electrode system) | [166] |
| Collagen | Nitrogen-rich carbon nano-onion architectures | Metal-free oxygen reduction reaction (ORR) catalyst | -Onset potential: ~0.32 V vs. Ag/AgCl in O2; -Saturated KOH solution; -4-electron transfer pathway for ORR; -Electron transfer number (n): ~4.1; -Comparable performance to the Pt/C catalyst; -Immunity to methanol crossover poisoning; -Excellent operation stability; -Durability and stability confirmed; | [167] |
| Pomelo peel | Nitrogen-doped porous carbon | ORR applied to fuel cells and metal-air batteries | Half-wave potential (0.86 V vs. RHE) and the kinetic current (10.40 mA/cm2), which are much higher than those obtained from the Pt/C (0.83 V, 5.22 mA/cm2, respectively) | [168] |
| Euonymus japonicus leaves | Nitrogen self-doped porous carbon nanosheets (NPCNS) | ORR and OER Electrocatalytic applications such as fuel cells or metal-air batteries | -ORR activity: onset potential of NPCNS-900 is 0.98 V vs. RHE (more positive than commercial Pt/C catalyst, which is 0.95 V); -OER performance: overpotential of NPCNS-900 is only 1.57 V vs. RHE (current density of 5 mA/cm2, lower than commercial Pt/C, >1.7 V); -Enhanced methanol tolerance compared to commercial Pt/C for ORR | [169] |
| Tea residue | N and F co-doped porous carbon materials | ORR | -Heteroatom (N and F) doping facilitates charge redistribution and electron transfer in ORR; -Higher limited current density and electron transfer number (about 3.8) compared to Pt/C | [170] |
| Poultry bio-waste | Activated carbon electrocatalyst (different chemical activators (KOH, H3PO4, ZnCl2) combined with heat treatment) | ORR | Onset potential changing from −0.02 to −0.20 V; ORR current density (JORR) at 0.4 V (mA cm−2) changing from 0.3 to 1.7 | [171] |
| Biogas | Solid Oxide fuel cell (SOFC) | Electricity generation | SOFC performance improvement with increasing reforming temperature; Ability to achieve 100% renewable operation in a hybrid power plant | [172] |
| Egg | Heteroatom-doped mesoporous carbon | Oxygen reduction in microbial fuel cells | ORR potential: +0.10 V, onset potential: +0.257 V (vs. Ag/AgCl); Electron transfer number: 3.84–3.92 (indicating a four-electron pathway); Maximum power density: 737.1 mW m−2 (comparable to MFC-Pt/C at 704 mW m−2) | [173] |
| Cattle bones | Nitrogen and phosphorus co-doped hierarchically porous carbon (N,P-HPC) | ORR | ORR onset potential: 0.924 V (comparable to commercial Pt/C) ORR half-wave potential: 0.853 V (12 mV higher than that of Pt/C) ORR kinetic current density: 38.2 mA cm−2 at 0.8 V (1.9 times that of Pt/C); Superior electrochemical stability and methanol tolerance compared to Pt/C. | [174] |
| Tea residue | Nitrogen and fluorine co-doped carbon (T-NFC) | ORR | ORR-limited current density is higher than commercial Pt/C; ORR electron transfer number of about 3.8 (indicating an efficient ORR mechanism); Enhanced methanol tolerance in alkaline medium compared to Pt/C; Catalyst with high catalytic activity, excellent stability, and high selectivity | [170] |
| Soybean straw | Honeycomb-like Fe-N co-doped porous carbon (Fe-N-PC) | Fuel cells | ORR performance alkaline: Onset potential: 0.989 V; Half-wave potential: 0.854 V; (Comparable to commercial Pt/C catalyst) ORR performance is acidic: Onset potential: 0.886 V; Half-wave potential: 0.754 V; (Superior to many other Fe and N-doped electrocatalysts) | [175] |
| Golden Shower Pods biomass (GSP) | N-doped porous carbon (N-PC) | Oxygen Reduction Reaction (ORR); Oxygen Evolution Reaction (OER); Hydrogen Evolution Reaction (HER) | ORR: four-electron pathway (average n = 3.6), Tafel slope of 86 mV dec−1, half-wave potential of 0.76 V; OER: better overpotential values (314 mV at 10 mA cm−2), Tafel slope of 132 mV dec−1; HER: better overpotential values (179 mV at 10 mA cm−2), Tafel slope of 98 mV dec−1 | [176] |
| Shrimp-shell | N-doped porous carbon (NPC-800); N-doped carbon nanodots (N-CNs); N-CNs@SiO2 composite subjected to thermal evaporation | ORR | ORR: onset potential of 0.06 V, Half-wave potential of 0.21 V, limiting current density of 5.3 mA/cm2 (at 0.4 V vs. Ag/AgCl) Comparable to commercial Pt/C catalyst: onset potential of 0.03 V; half-wave potential of 0.17 V; limiting current density of 5.5 mA/cm2 (at 0.4 V); Superior durability and high methanol tolerance in alkaline media, better than commercial Pt/C catalyst | [177] |
| Seaweed | Fe2N/C ORR catalyst | ORR in alkaline fuel cells | Outperforms Pt in ORR activity, stability, and methanol tolerance in alkaline media. Onset potential of 0.82 V vs. RHE in 1 M HClO4 solution is comparable to Pt/C (0.91 V vs. RHE). Superior electrical conductivity. | [178] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine Emissions with Air Pollutants and Greenhouse Gases and Their Control Technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report. of the Intergovernmental Panel on Climate Change; Arias, P., Bustamante, M., Elgizouli, I., Flato, G., Howden, M., Méndez-Vallejo, C., Pereira, J.J., Pichs-Madruga, R., Rose, S.K., Saheb, Y., et al., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; D’Ambrosio, D.; Spencer, T. Net Zero by 2050—A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 18 November 2023).
- Schmidt, J.; Gruber, K.; Klingler, M.; Klöckl, C.; Ramirez Camargo, L.; Regner, P.; Turkovska, O.; Wehrle, S.; Wetterlund, E. A New Perspective on Global Renewable Energy Systems: Why Trade in Energy Carriers Matters. Energy Environ. Sci. 2019, 12, 2022–2029. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Barua, T.; Das, B.K. A Comprehensive Review on Techno-Environmental Analysis of State-of-the-Art Production and Storage of Hydrogen Energy: Challenges and Way Forward. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 5905–5937. [Google Scholar] [CrossRef]
- Eljack, F.; Kazi, M.K. Prospects and Challenges of Green Hydrogen Economy via Multi-Sector Global Symbiosis in Qatar. Front. Sustain. 2020, 1, 612762. [Google Scholar] [CrossRef]
- Sarah Safety Risk Analysis Hydrogen. Available online: https://www.dicmapi.unina.it/research/gruppi-di-ricerca/sarah-safety-risk-analysis-hydrogen/ (accessed on 14 November 2023).
- Xiang, H.; Xin, R.; Prasongthum, N.; Natewong, P.; Sooknoi, T.; Wang, J.; Reubroycharoen, P.; Fan, X. Catalytic Conversion of Bioethanol to Value-Added Chemicals and Fuels: A Review. Resour. Chem. Mater. 2022, 1, 47–68. [Google Scholar] [CrossRef]
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Ubando, A.T.; Bandala, A.A.; Concepcion, R.S.; Alipio, M.; Chen, W.H.; Show, P.L.; Chang, J.S. Design of Biorefineries towards Carbon Neutrality: A Critical Review. Bioresour. Technol. 2023, 369, 128256. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the Production of Bioethanol: A Review of Sustainable Methods, Technologies, and Bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Bušić, A.; Mardetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- IRENA. Available online: https://www.irena.org/Energy-Transition/Technology/Transportation-costs/Bioethanol (accessed on 15 November 2023).
- Suer, J.; Traverso, M.; Jäger, N. Carbon Footprint Assessment of Hydrogen and Steel. Energies 2022, 15, 9468. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts—A Review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Kappis, K.; Papavasiliou, J.; Avgouropoulos, G. Methanol Reforming Processes for Fuel Cell Applications. Energies 2021, 14, 8442. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent Progress in Ethanol Steam Reforming Using Non-Noble Transition Metal Catalysts: A Review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A Review on Ethanol Steam Reforming for Hydrogen Production over Ni/Al2O3 and Ni/CeO2 Based Catalyst Powders. Int. J. Hydrogen Energy 2022, 47, 8177–8213. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam Reforming of Ethanol for Hydrogen Production: Influence of Catalyst Composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and Process Conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. A Review of Steam Reforming of Glycerol. Chem. Pap. 2019, 73, 2619–2635. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Azizan, M.T.; Aqsha, A.; Ameen, M.; Syuhada, A.; Klaus, H.; Abidin, S.Z.; Sher, F. Catalytic Reforming of Oxygenated Hydrocarbons for the Hydrogen Production: An Outlook. Biomass Convers. Biorefinery 2020, 13, 8441–8464. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Salgansky, E.A.; Arutyunov, V.S.; Sedov, I.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916. [Google Scholar] [CrossRef]
- Balopi, B.; Moyo, M.; Gorimbo, J. Autothermal Reforming of Bio-Ethanol: A Short Review of Strategies Used to Synthesize Coke-Resistant Nickel-Based Catalysts. Catal. Lett. 2022, 152, 3004–3016. [Google Scholar] [CrossRef]
- Baruah, R.; Dixit, M.; Basarkar, P.; Parikh, D.; Bhargav, A. Advances in Ethanol Autothermal Reforming. Renew. Sustain. Energy Rev. 2015, 51, 1345–1353. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.Q. Thermodynamic Analysis of Steam Reforming of Ethanol for Hydrogen Generation. Int. J. Energy Res. 2008, 32, 1432–1443. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction Pathway for Ethanol Steam Reforming on a Ni/SiO2 Catalyst Including Coke Formation. Int. J. Hydrogen Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam Reforming of Ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Appels, L.; Zhang, H.; Sweygers, N.; Baeyens, J.; Dewil, R. Steam Reforming of Ethanol by Non-Noble Metal Catalysts. Renew. Sustain. Energy Rev. 2023, 175, 113184. [Google Scholar] [CrossRef]
- Grzybek, G.; Greluk, M.; Tarach, K.; Pyra, K.; Słowik, G.; Rotko, M.; Góra-Marek, K. Bioethanol Steam Reforming over Cobalt-Containing USY and ZSM-5 Commercial Zeolite Catalysts. Front. Mater. 2020, 7, 597528. [Google Scholar] [CrossRef]
- Sohn, H.; Ozkan, U.S. Cobalt-Based Catalysts for Ethanol Steam Reforming: An Overview. Energy Fuels 2016, 30, 5309–5322. [Google Scholar] [CrossRef]
- Erdohelyi, A.; Raskó, J.; Kecskés, T.; Tóth, M.; Dömök, M.; Baán, K. Hydrogen Formation in Ethanol Reforming on Supported Noble Metal Catalysts. Catal. Today 2006, 116, 367–376. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 Production for MC Fuel Cell by Steam Reforming of Ethanol over MgO Supported Pd, Rh, Ni and Co Catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Dalena, F.; Giglio, E.; Marino, A.; Aloise, A.; Giorgianni, G.; Migliori, M.; Giordano, G. Steam Reforming of Bioethanol Using Metallic Catalysts on Zeolitic Supports: An Overview. Catalysts 2022, 12, 617. [Google Scholar] [CrossRef]
- Montero, C.; Remiro, A.; Valle, B.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Origin and Nature of Coke in Ethanol Steam Reforming and Its Role in Deactivation of Ni/La2O3-αAl2O3 Catalyst. Ind. Eng. Chem. Res. 2019, 58, 14736–14751. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A Review on Reforming Bio-Ethanol for Hydrogen Production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Song, H.; Zhang, L.; Watson, R.B.; Braden, D.; Ozkan, U.S. Investigation of Bio-Ethanol Steam Reforming over Cobalt-Based Catalysts. Catal. Today 2007, 129, 346–354. [Google Scholar] [CrossRef]
- Contreras, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 Production Using the Ethanol Steam Reforming (a Review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Salge, J.R.; Deluga, G.A.; Schmidt, L.D. Catalytic Partial Oxidation of Ethanol over Noble Metal Catalysts. J. Catal. 2005, 235, 69–78. [Google Scholar] [CrossRef]
- Vita, A.; Pino, L.; Italiano, C.; Palella, A. Steam Reforming, Partial Oxidation, and Autothermal Reforming of Ethanol for Hydrogen Production in Conventional Reactors. In Ethanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–191. ISBN 9780128114582. [Google Scholar]
- Youn, M.H.; Seo, J.G.; Lee, H.; Bang, Y.; Chung, J.S.; Song, I.K. Hydrogen Production by Auto-Thermal Reforming of Ethanol over Nickel Catalysts Supported on Metal Oxides: Effect of Support Acidity. Appl. Catal. B 2010, 98, 57–64. [Google Scholar] [CrossRef]
- Gutierrez, A.; Karinen, R.; Airaksinen, S.; Kaila, R.; Krause, A.O.I. Autothermal Reforming of Ethanol on Noble Metal Catalysts. Int. J. Hydrogen Energy 2011, 36, 8967–8977. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.B.; Dong, C. Di A Critical and Systematic Review of Sustainable Hydrogen Production from Ethanol/Bioethanol: Steam Reforming, Partial Oxidation, and Autothermal Reforming. Fuel 2023, 333, 126526. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Ubando, A.T.; Park, Y.K.; Ashokkumar, V.; Chang, J.S. Design of Experiment for Hydrogen Production from Ethanol Reforming: A State-of-the-Art Review. Fuel 2023, 342, 127871. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A Review of Hydrogen Production Processes by Photocatalytic Water Splitting—From Atomistic Catalysis Design to Optimal Reactor Engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Bowker, M. Sustainable Hydrogen Production by the Application of Ambient Temperature Photocatalysis. Green. Chem. 2011, 13, 2235–2246. [Google Scholar] [CrossRef]
- Escobedo, S.; de Lasa, H. Synthesis and Performance of Photocatalysts for Photocatalytic Hydrogen Production: Future Perspectives. Catalysts 2021, 11, 1505. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current Developments and Future Trends in Photocatalytic Glycerol Valorization: Photocatalyst Development. Ind. Eng. Chem. Res. 2020, 59, 22330–22352. [Google Scholar] [CrossRef]
- Rueda-Navarro, C.M.; Ferrer, B.; Baldoví, H.G.; Navalón, S. Photocatalytic Hydrogen Production from Glycerol Aqueous Solutions as Sustainable Feedstocks Using Zr-Based UiO-66 Materials under Simulated Sunlight Irradiation. Nanomaterials 2022, 12, 3808. [Google Scholar] [CrossRef]
- Augustin, A.; Chuaicham, C.; Shanmugam, M.; Vellaichamy, B.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Recent Development of Organic-Inorganic Hybrid Photocatalysts for Biomass Conversion into Hydrogen Production. Nanoscale Adv. 2022, 4, 2561–2582. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Yoshida, H. Heterogeneous Photocatalytic Hydrogen Production from Water and Biomass Derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic Degradation of Organic Pollutants with Simultaneous Production of Hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Babin, A.; Feilizadeh, M.; Nayernia, Z.; Mahinpey, N.; Iliuta, M.C. Photocatalytic Conversion of Alcohols to Hydrogen and Carbon-Containing Products: A Cleaner Alcohol Valorization Approach. J. Clean. Prod. 2021, 318, 128546. [Google Scholar] [CrossRef]
- Sola, A.C.; Homs, N.; Ramírez de la Piscina, P. Photocatalytic H2 Production from Ethanol (Aq) Solutions: The Effect of Intermediate Products. Int. J. Hydrogen Energy 2016, 41, 19629–19636. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Gómez-Cerezo, M.N.; Kubacka, A.; Fernández-García, M. Boosting Pt/TiO2 Hydrogen Photoproduction through Zr Doping of the Anatase Structure: A Spectroscopic and Mechanistic Study. Chem. Eng. J. 2020, 398, 125665. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Chang, C.H.; Idriss, H. Photo-Catalytic Production of Hydrogen Form Ethanol over M/TiO2 Catalysts (M = Pd, Pt or Rh). Appl. Catal. B 2006, 67, 217–222. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photocatalytic Hydrogen Production under Near-UV Using Pd-Doped Mesoporous TiO2 and Ethanol as Organic Scavenger. Catalysts 2019, 9, 33. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Sreńscek-Nazzal, J.; Llorca, J. Photocatalytic Hydrogen Production from Alcohol Aqueous Solutions over TiO2-Activated Carbon Composites Decorated with Au and Pt. J. Photochem. Photobiol. A Chem. 2022, 425, 113726. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A Critical Review of Metal-Doped TiO2 and Its Structure–Physical Properties–Photocatalytic Activity Relationship in Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx-Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Mahinpey, N.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Kinetic Study of the Effects of PH on the Photocatalytic Hydrogen Production from Alcohols. Int. J. Hydrogen Energy 2019, 44, 32030–32041. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S. Electrochemical Conversion of Alcohols for Hydrogen Production: A Short Overview. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 388–400. [Google Scholar] [CrossRef]
- Lamy, C.; Jaubert, T.; Baranton, S.; Coutanceau, C. Clean Hydrogen Generation through the Electrocatalytic Oxidation of Ethanol in a Proton Exchange Membrane Electrolysis Cell (PEMEC): Effect of the Nature and Structure of the Catalytic Anode. J. Power Sources 2014, 245, 927–936. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Sadasivuni, K.K.; Jayakumar, A.; Ponnamma, D.; Tiwary, C.S.; Sasikumar, G. Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies 2020, 13, 5879. [Google Scholar] [CrossRef]
- Caravaca, A.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Molina-Mora, C.; Dorado, F.; Valverde, J.L. Electrochemical Reforming of Ethanol-Water Solutions for Pure H 2 Production in a PEM Electrolysis Cell. Int. J. Hydrogen Energy 2012, 37, 9504–9513. [Google Scholar] [CrossRef]
- Yu, J.; González-Cobos, J.; Dappozze, F.; Grimaldos-Osorio, N.; Vernoux, P.; Caravaca, A.; Guillard, C. First PEM Photoelectrolyser for the Simultaneous Selective Glycerol Valorization into Value-Added Chemicals and Hydrogen Generation. Appl. Catal. B 2023, 327, 122465. [Google Scholar] [CrossRef]
- De Lucas-Consuegra, A.; Calcerrada, A.B.; De La Osa, A.R.; Valverde, J.L. Electrochemical Reforming of Ethylene Glycol. Influence of the Operation Parameters, Simulation and Its Optimization. Fuel Process. Technol. 2014, 127, 13–19. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhao, S.F.; Guo, S.X.; Bond, A.M.; Zhang, J.; Zhu, G.; Hill, C.L.; Geletii, Y.V. Electrooxidation of Ethanol and Methanol Using the Molecular Catalyst [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10−. J. Am. Chem. Soc. 2016, 138, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Asiri, H.A.; Anderson, A.B. Mechanisms for Ethanol Electrooxidation on Pt(111) and Adsorption Bond Strengths Defining an Ideal Catalyst. J. Electrochem. Soc. 2015, 162, F115–F122. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Gonzalez, E.R. Ethanol Electro-Oxidation on Carbon-Supported Pt-Ru, Pt-Rh and Pt-Ru-Rh Nanoparticles. Electrochim. Acta 2008, 53, 2963–2971. [Google Scholar] [CrossRef]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Qu, X.M.; Zhang, J.M.; Zhang, Z.C.; Liao, H.G.; Zhu, F.C.; Dou, S.X.; Jiang, Y.X.; et al. Platinum-Cobalt Bimetallic Nanoparticles with Pt Skin for Electro-Oxidation of Ethanol. ACS Catal. 2017, 7, 892–895. [Google Scholar] [CrossRef]
- Rizo, R.; Sebastián, D.; Lázaro, M.J.; Pastor, E. On the Design of Pt-Sn Efficient Catalyst for Carbon Monoxide and Ethanol Oxidation in Acid and Alkaline Media. Appl. Catal. B 2017, 200, 246–254. [Google Scholar] [CrossRef]
- Silva, J.C.M.; De Souza, R.F.B.; Parreira, L.S.; Neto, E.T.; Calegaro, M.L.; Santos, M.C. Ethanol Oxidation Reactions Using SnO2@Pt/C as an Electrocatalyst. Appl. Catal. B 2010, 99, 265–271. [Google Scholar] [CrossRef]
- Rizo, R.; Bergmann, A.; Timoshenko, J.; Scholten, F.; Rettenmaier, C.; Jeon, H.S.; Chen, Y.T.; Yoon, A.; Bagger, A.; Rossmeisl, J.; et al. Pt-Sn-Co Nanocubes as Highly Active Catalysts for Ethanol Electro-Oxidation. J. Catal. 2021, 393, 247–258. [Google Scholar] [CrossRef]
- Lamy, C.; Rousseau, S.; Belgsir, E.M.; Coutanceau, C.; Léger, J.M. Recent Progress in the Direct Ethanol Fuel Cell: Development of New Platinum-Tin Electrocatalysts. Electrochim. Acta 2004, 49, 3901–3908. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-Based Ternary Catalysts for the Electrooxidation of Ethanol. Particuology 2021, 58, 169–186. [Google Scholar] [CrossRef]
- Kowal, A.; Li, M.; Shao, M.; Sasaki, K.; Vukmirovic, M.B.; Zhang, J.; Marinkovic, N.S.; Liu, P.; Frenkel, A.I.; Adzic, R.R. Ternary Pt/Rh/SnO2 Electrocatalysts for Oxidizing Ethanol to CO2. Nat. Mater. 2009, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, A.; Dorado, F.; Sánchez, P.; de la Osa, A.R. Boosting Hydrogen and Chemicals Production through Ethanol Electro-Reforming on Pt-Transition Metal Anodes. J. Energy Chem. 2022, 70, 394–406. [Google Scholar] [CrossRef]
- Serrano-Jiménez, J.; de la Osa, A.R.; Rodríguez-Gómez, A.; Sánchez, P.; Romero, A.; de Lucas-Consuegra, A. Electro-Reforming of Bioethanol Produced by Sugar Fermentation on a Pt-Ni Anodic Catalyst Supported on Graphene Nanoplatelets. J. Environ. Chem. Eng. 2023, 11, 109703. [Google Scholar] [CrossRef]
- Khamhaeng, P.; Laosiripojana, N.; Assabumrungrat, S.; Kim-Lohsoontorn, P. Techno-Economic Analysis of Hydrogen Production from Dehydrogenation and Steam Reforming of Ethanol for Carbon Dioxide Conversion to Methanol. Int. J. Hydrogen Energy 2021, 46, 30891–30902. [Google Scholar] [CrossRef]
- Khamhaeng, P.; Kim-Lohsoontorn, P. Performance and Cost Analysis of Hydrogen Production from Steam Reforming and Dehydrogenation of Ethanol. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 991. [Google Scholar]
- Tóth, M.; Varga, E.; Oszkó, A.; Baán, K.; Kiss, J.; Erdohelyi, A. Partial Oxidation of Ethanol on Supported Rh Catalysts: Effect of the Oxide Support. J. Mol. Catal. A Chem. 2016, 411, 377–387. [Google Scholar] [CrossRef]
- Chiu, W.-C.; Horng, R.-F.; Chou, H.-M. Hydrogen Production from an Ethanol Reformer with Energy Saving Approaches over Various Catalysts. Int. J. Hydrogen Energy 2013, 38, 2760–2769. [Google Scholar] [CrossRef]
- Hung, C.C.; Chen, S.L.; Liao, Y.K.; Chen, C.H.; Wang, J.H. Oxidative Steam Reforming of Ethanol for Hydrogen Production on M/Al2O3. Int. J. Hydrogen Energy 2012, 37, 4955–4966. [Google Scholar] [CrossRef]
- Silva Júnior, M.E.; Palm, M.O.; Duarte, D.A.; Catapan, R.C. Catalytic Pt/Al2O3 Monolithic Foam for Ethanol Reforming Fabricated by the Competitive Impregnation Method. ACS Omega 2023, 8, 6507–6514. [Google Scholar] [CrossRef]
- Jovic, V.; Al-Azri, Z.H.N.; Chen, W.T.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Photocatalytic H2 Production from Ethanol-Water Mixtures over Pt/TiO2 and Au/TiO2 Photocatalysts: A Comparative Study. Top. Catal. 2013, 56, 1139–1151. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Jovic, V.; Chen, W.-T.; Sun-Waterhouse, D.; Metson, J.B.; Waterhouse, G.I.N. Performance Evaluation of Pd/TiO2 and Pt/TiO2 for Hydrogen Production from ethanol-Water Mixtures. Int. J. Nanotechnol. 2014, 11, 695–703. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Portarapillo, M.; Landi, G.; Luciani, G. Process for Green Hydrogen Production. WO Patent WO2023105545A1, 7 December 2022. [Google Scholar]
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Luciani, G.; Landi, G.; Ruoppolo, G.; Pezzella, A.; Di Benedetto, A. Cyan Hydrogen Process: A New Route for Simultaneous Hydrogen Production and Carbon Valorisation. ACS Omega, 2023; in press. [Google Scholar]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A Comprehensive Review of the Prospects for Future Hydrogen Storage in Materials-Application and Outstanding Issues. Int. J. Energy Res. 2022, 46, 16150–16177. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhao, J.; Li, H.; Yu, B. Research Progress and Development Trends of Key Technologies for Hydrogen Energy Storage and Transportation. Oil Gas Storage Transp. 2023, 42, 856–872. [Google Scholar]
- Durbin, D.J.; Malardier-Jugroot, C. Review of Hydrogen Storage Techniques for on Board Vehicle Applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Zhangi, Y.; Jia, Z.; Yuan, Z.; Yang, T.; Qi, Y.; Zhao, D. Development and Application of Hydrogen Storage. J. Iron Steel Res. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-Scale Storage of Hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Suenobu, T. Hydrogen Storage and Evolution Catalysed by Metal Hydride Complexes. Dalton Trans. 2013, 42, 18–28. [Google Scholar] [CrossRef]
- Jorschick, H.; Vogl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Hydrogenation of Liquid Organic Hydrogen Carrier Systems Using Multicomponent Gas Mixtures. Int. J. Hydrogen Energy 2019, 44, 31172–31182. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment Based on Chemical and Economic Properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Fellay, C.; Dyson, P.J.; Laurenczy, G. A Viable Hydrogen-Storage System Based on Selective Formic Acid Decomposition with a Ruthenium Catalyst. Angew. Chem. Int. Ed. 2008, 47, 3966–3968. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic Acid, a Biomass-Derived Source of Energy and Hydrogen for Biomass Upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Sun, R.; Liao, Y.; Bai, S.T.; Zheng, M.; Zhou, C.; Zhang, T.; Sels, B.F. Heterogeneous Catalysts for CO2hydrogenation to Formic Acid/Formate: From Nanoscale to Single Atom. Energy Environ. Sci. 2021, 14, 1247–1285. [Google Scholar] [CrossRef]
- Calabrese, M.; Russo, D.; di Benedetto, A.; Marotta, R.; Andreozzi, R. Formate/Bicarbonate Interconversion for Safe Hydrogen Storage: A Review. Renew. Sustain. Energy Rev. 2023, 173, 113102. [Google Scholar] [CrossRef]
- Grubel, K.; Jeong, H.; Yoon, C.W.; Autrey, T. Challenges and Opportunities for Using Formate to Store, Transport, and Use Hydrogen. J. Energy Chem. 2020, 41, 216–224. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sasson, Y. Formate-Bicarbonate Cycle as a Vehicle for Hydrogen and Energy Storage. ChemSusChem 2021, 14, 1258–1283. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, F.; Sun, P.; Chen, T. Au-Pd Alloy Catalyst with High Performance for Hydrogen Generation from Formic Acid-Formate Solution at Nearly 0 °C. RSC Adv. 2014, 4, 44500–44503. [Google Scholar] [CrossRef]
- Russo, D.; Calabrese, M.; Marotta, R.; Andreozzi, R.; Di Benedetto, A. Thermodynamics of the Cyclic Formate/Bicarbonate Interconversion for Hydrogen Storage. Int. J. Hydrogen Energy 2022, 47, 31370–31380. [Google Scholar] [CrossRef]
- Hua, T.Q.; Roh, H.S.; Ahluwalia, R.K. Performance Assessment of 700-Bar Compressed Hydrogen Storage for Light Duty Fuel Cell Vehicles. Int. J. Hydrogen Energy 2017, 42, 25121–25129. [Google Scholar] [CrossRef]
- Koh, K.; Jeon, M.; Chevrier, D.M.; Zhang, P.; Yoon, C.W.; Asefa, T. Novel Nanoporous N-Doped Carbon-Supported Ultrasmall Pd Nanoparticles: Efficient Catalysts for Hydrogen Storage and Release. Appl. Catal. B 2017, 203, 820–828. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Lu, M.; Lin, H. Highly Efficient Hydrogen Storage System Based on Ammonium Bicarbonate/Formate Redox Equilibrium over Palladium Nanocatalysts. ChemSusChem 2015, 8, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.Y.; Lin, J.D.; Liu, Y.M.; Du, X.L.; Wang, J.Q.; He, H.Y.; Cao, Y. An Aqueous Rechargeable Formate-Based Hydrogen Battery Driven by Heterogeneous Pd Catalysis. Angew. Chem. Int. Ed. 2014, 53, 13583–13587. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Miao, X.; Zhang, T.; Wang, W.; Wang, J.; Ji, X. Pd Nanoparticles Supported on N- And P-Co-Doped Carbon as Catalysts for Reversible Formate-Based Chemical Hydrogen Storage. ACS Appl. Nano Mater. 2020, 3, 9209–9217. [Google Scholar] [CrossRef]
- Masuda, S.; Shimoji, Y.; Mori, K.; Kuwahara, Y.; Yamashita, H. Interconversion of Formate/Bicarbonate for Hydrogen Storage/Release: Improved Activity Following Sacrificial Surface Modification of a Ag@Pd/TiO2 Catalyst with a TiO XShell. ACS Appl. Energy Mater. 2020, 3, 5819–5829. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental Aspects of Fuel Cells: A Review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. The Potential Role of Hydrogen as a Sustainable Transportation Fuel to Combat Global Warming. Int. J. Hydrogen Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel Cell Application in the Automotive Industry and Future Perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Chandran, P.; Ghosh, A.; Ramaprabhu, S. High-Performance Platinum-Free Oxygen Reduction Reaction and Hydrogen Oxidation Reaction Catalyst in Polymer Electrolyte Membrane Fuel Cell. Sci. Rep. 2018, 8, 3591. [Google Scholar] [CrossRef]
- Hamada, A.T.; Orhan, M.F.; Kannan, A.M. Alkaline Fuel Cells: Status and Prospects. Energy Rep. 2023, 9, 6396–6418. [Google Scholar] [CrossRef]
- Choudhury, S.R. Phosphoric Acid Fuel Cell Technology. In Recent Trends in Fuel Cell Science and Technology; Springer: New York, NY, USA, 2007; pp. 188–216. [Google Scholar]
- Mehmeti, A.; Santoni, F.; Della Pietra, M.; McPhail, S.J. Life Cycle Assessment of Molten Carbonate Fuel Cells: State of the Art and Strategies for the Future. J. Power Sources 2016, 308, 97–108. [Google Scholar] [CrossRef]
- Andersson, M.; Paradis, H.; Yuan, J.; Sundén, B. Review of Catalyst Materials and Catalytic Steam Reforming Reactions in SOFC Anodes. Int. J. Energy Res. 2011, 35, 1340–1350. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Sun, F. Progress in Finite Time Thermodynamic Studies for Internal Combustion Engine Cycles. Entropy 2016, 18, 139. [Google Scholar] [CrossRef]
- Bauen, A.; Hart, D. Assessment of the Environmental Benefits of Transport and Stationary Fuel Cells. J. Power Sources 2000, 86, 482–494. [Google Scholar] [CrossRef]
- Wilberforce, T.; Baroutaji, A.; El Hassan, Z.; Thompson, J.; Soudan, B.; Olabi, A.G. Prospects and Challenges of Concentrated Solar Photovoltaics and Enhanced Geothermal Energy Technologies. Sci. Total Environ. 2019, 659, 851–861. [Google Scholar] [CrossRef]
- Nazir, M.S.; Mahdi, A.J.; Bilal, M.; Sohail, H.M.; Ali, N.; Iqbal, H.M.N. Environmental Impact and Pollution-Related Challenges of Renewable Wind Energy Paradigm—A Review. Sci. Total Environ. 2019, 683, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel Cell Membranes—Pros and Cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current Progress of Pt and Pt-Based Electrocatalysts Used for Fuel Cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; Wiley: New York, NY, USA, 2016; ISBN 9781119113805. [Google Scholar]
- Zhong, G.; Wang, H.; Yu, H.; Peng, F. Nitrogen Doped Carbon Nanotubes with Encapsulated Ferric Carbide as Excellent Electrocatalyst for Oxygen Reduction Reaction in Acid and Alkaline Media. J. Power Sources 2015, 286, 495–503. [Google Scholar] [CrossRef]
- Yan, X.; Gan, L.; Lin, Y.C.; Bai, L.; Wang, T.; Wang, X.; Luo, J.; Zhu, J. Controllable Synthesis and Enhanced Electrocatalysis of Iron-Based Catalysts Derived from Electrospun Nanofibers. Small 2014, 10, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Qian, G.; Song, L.; Chen, D.; Zhou, X.; Duan, X. On the Nature of Pt-Carbon Interactions for Enhanced Hydrogen Generation. J. Catal. 2020, 389, 492–501. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The Rational Design of Biomass-Derived Carbon Materials towards next-Generation Energy Storage: A Review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Mazánek, V.; Luxa, J.; Matějková, S.; Kučera, J.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Ultrapure Graphene Is a Poor Electrocatalyst: Definitive Proof of the Key Role of Metallic Impurities in Graphene-Based Electrocatalysis. ACS Nano 2019, 13, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Jaleh, B.; Nasri, A.; Eslamipanah, M.; Nasrollahzadeh, M.; Advani, J.H.; Fornasiero, P.; Gawande, M.B. Application of Biowaste and Nature-Inspired (Nano)Materials in Fuel Cells. J. Mater. Chem. A Mater. 2023, 11, 93333–99382. [Google Scholar] [CrossRef]
- Kiciński, W.; Dyjak, S. Transition Metal Impurities in Carbon-Based Materials: Pitfalls, Artifacts and Deleterious Effects. Carbon 2020, 168, 748–845. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, A.; Sharma, S.; Bharti; Ghamouss, F.; Singh, P.; Chawla, V.; Kumar, A.; Kumar, Y. Study of Electrochemical Properties of Activated Carbon Electrode Synthesized Using Bio-Waste for Supercapacitor Applications. Biomass Convers. Biorefinery 2022, 13, 14059–14070. [Google Scholar] [CrossRef]
- Pandey, G. Biomass Based Bio-Electro Fuel Cells Based on Carbon Electrodes: An Alternative Source of Renewable Energy. SN Appl. Sci. 2019, 1, 408. [Google Scholar] [CrossRef]
- Surya, K.; Michael, M.S. Novel Interconnected Hierarchical Porous Carbon Electrodes Derived from Bio-Waste of Corn Husk for Supercapacitor Applications. J. Electroanal. Chem. 2020, 878, 114674. [Google Scholar] [CrossRef]
- Shetty, A.; Molahalli, V.; Sharma, A.; Hegde, G. Biomass-Derived Carbon Materials in Heterogeneous Catalysis: A Step towards Sustainable Future. Catalysts 2022, 13, 20. [Google Scholar] [CrossRef]
- Dong, D.; Wu, Y.; Zhang, X.; Yao, J.; Huang, Y.; Li, D.; Li, C.-Z.; Wang, H. Eggshell Membrane-Templated Synthesis of Highly Crystalline Perovskite Ceramics for Solid Oxidefuelcells. J. Mater. Chem. 2011, 21, 1028–1032. [Google Scholar] [CrossRef]
- Dong, D.; Yao, J.; Wu, Y.; Zhang, X.; Xu, G.; Li, C.Z.; Wang, H. A 3D Fibrous Cathode with High Interconnectivity for Solid Oxide Fuel Cells. Electrochem. Commun. 2011, 13, 1038–1041. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Naveenkumar, M. Enhanced Performance Study of Microbial Fuel Cell Using Waste Biomass-Derived Carbon Electrode. Biomass Convers. Biorefinery 2023, 13, 5921–5929. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.; Yu, H.; Peng, F. From Chicken Feather to Nitrogen and Sulfur Co-Doped Large Surface Bio-Carbon Flocs: An Efficient Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2016, 213, 273–282. [Google Scholar] [CrossRef]
- Tyagi, A.; Yadav, A.; Sinha, P.; Singh, S.; Paik, P.; Kar, K.K. Chicken Feather Rachis: An Improvement over Feather Fiber Derived Electrocatalyst for Oxygen Electroreduction. Appl. Surf. Sci. 2019, 495, 143603. [Google Scholar] [CrossRef]
- Dhelipan, M.; Arunchander, A.; Sahu, A.K.; Kalpana, D. Activated Carbon from Orange Peels as Supercapacitor Electrode and Catalyst Support for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. J. Saudi Chem. Soc. 2017, 21, 487–494. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Friedman, A.; Poli, F.; Petri, E.; Honig, H.; Basile, F.; Fasolini, A.; Lorenzi, R.; Berretti, E.; Bellini, M.; et al. Lignin-Derived Bimetallic Platinum Group Metal-Free Oxygen Reduction Reaction Electrocatalysts for Acid and Alkaline Fuel Cells. J. Power Sources 2023, 556, 232416. [Google Scholar] [CrossRef]
- Zhao, K.; Shu, Y.; Li, F.; Peng, G. Bimetallic Catalysts as Electrocatalytic Cathode Materials for the Oxygen Reduction Reaction in Microbial Fuel Cell: A Review. Green Energy Environ. 2023, 8, 1043–1070. [Google Scholar] [CrossRef]
- Akula, S.; Sahu, A.K. Heteroatoms Co-Doping (N, F) to the Porous Carbon Derived from Spent Coffee Grounds as an Effective Catalyst for Oxygen Reduction Reaction in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2019, 166, F93. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Gu, Y.; Cen, Z.; Wei, L.; Zhou, Y.; Zhang, C.; Yang, C. Trash to Treasure: A Novel Chemical Route to Synthesis of NiO/C for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 16144–16153. [Google Scholar] [CrossRef]
- Karaman, C. Orange Peel Derived-Nitrogen and Sulfur Co-Doped Carbon Dots: A Nano-Booster for Enhancing ORR Electrocatalytic Performance of 3D Graphene Networks. Electroanalysis 2021, 33, 1356–1369. [Google Scholar] [CrossRef]
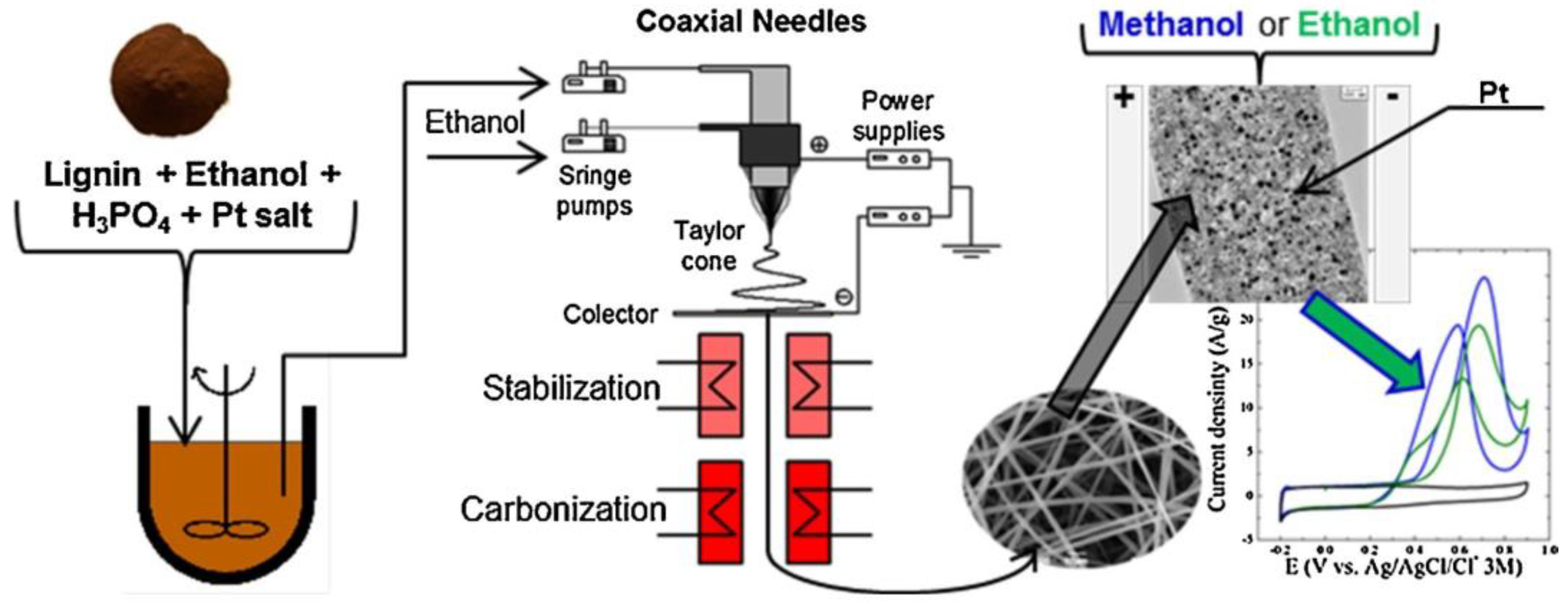
- García-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Lignin-Derived Pt Supported Carbon (Submicron)Fiber Electrocatalysts for Alcohol Electro-Oxidation. Appl. Catal. B 2017, 211, 18–30. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.M. From Waste to Wealth: From Kraft Lignin to Free-Standing Supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Adam, A.A.; Dennis, J.O.; Al-Hadeethi, Y.; Mkawi, E.M.; Abdulkadir, B.A.; Usman, F.; Hassan, Y.M.; Wadi, I.A.; Sani, M. State of the Art and New Directions on Electrospun Lignin/Cellulose Nanofibers for Supercapacitor Application: A Systematic Literature Review. Polymers 2020, 12, 2884. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Abdelwahab, M.A.; Misra, M.; Khalil, H.; Gharabaghi, B.; Mohanty, A.K. Processing, Carbonization, and Characterization of Lignin Based Electrospun Carbon Fibers: A Review. Front. Energy Res. 2020, 8, 208. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Qian, L. Synthesis of Nitrogen-Doped Hierarchical Porous Carbons from Peanut Shell as a Promising Electrode Material for High-Performance Supercapacitors. J. Energy Storage 2020, 30, 101451. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, A.; Yang, A.; Zuo, G.; Dai, J.; Zheng, Y. ZnS, Fe, and P Co-Doped N Enriched Carbon Derived from MOFs as Efficient Electrocatalyst for Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2020, 45, 31863–31870. [Google Scholar] [CrossRef]
- Wang, N.; Li, T.; Song, Y.; Liu, J.; Wang, F. Metal-Free Nitrogen-Doped Porous Carbons Derived from Pomelo Peel Treated by Hypersaline Environments for Oxygen Reduction Reaction. Carbon 2018, 130, 692–700. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Cao, D.; Cheng, D. Facile Preparation of Biomass-Derived Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions. Int. J. Hydrogen Energy 2018, 43, 8611–8622. [Google Scholar] [CrossRef]
- Wu, D.; Shi, Y.; Jing, H.; Wang, X.; Song, X.; Si, D.; Liang, S.; Hao, C. Tea-Leaf-Residual Derived Electrocatalyst: Hierarchical Pore Structure and Self Nitrogen and Fluorine Co-Doping for Efficient Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2018, 43, 19492–19499. [Google Scholar] [CrossRef]
- Tyagi, A.; Banerjee, S.; Singh, S.; Kar, K.K. Biowaste Derived Activated Carbon Electrocatalyst for Oxygen Reduction Reaction: Effect of Chemical Activation. Int. J. Hydrogen Energy 2020, 45, 16930–16943. [Google Scholar] [CrossRef]
- Cozzolino, R.; Lombardi, L.; Tribioli, L. Use of Biogas from Biowaste in a Solid Oxide Fuel Cell Stack: Application to an off-Grid Power Plant. Renew Energy 2017, 111, 781–791. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, N.; Yin, F.; Yang, T.; Wu, P.; Dang, Z.; Liu, M.; Wei, X. Biomass-Derived Heteroatoms-Doped Mesoporous Carbon for Efficient Oxygen Reduction in Microbial Fuel Cells. Biosens. Bioelectron. 2017, 98, 350–356. [Google Scholar] [CrossRef]
- Zan, Y.; Zhang, Z.; Liu, H.; Dou, M.; Wang, F. Nitrogen and Phosphorus Co-Doped Hierarchically Porous Carbons Derived from Cattle Bones as Efficient Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. J. Mater. Chem. A 2017, 5, 24329–24334. [Google Scholar] [CrossRef]
- Liu, Y.; Su, M.; Li, D.; Li, S.; Li, X.; Zhao, J.; Liu, F. Soybean Straw Biomass-Derived Fe-N Co-Doped Porous Carbon as an Efficient Electrocatalyst for Oxygen Reduction in Both Alkaline and Acidic Media. RSC Adv. 2020, 10, 6763–6771. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Liu, S.; Zhang, X.; Wu, T.; Ge, X.; Zang, Y.; Zhao, H.; Wang, G. Shrimp-Shell Derived Carbon Nanodots as Carbon and Nitrogen Sources to Fabricate Three-Dimensional N-Doped Porous Carbon Electrocatalysts for the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2016, 18, 4095–4101. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Ma, N.; Liu, H.; Xia, Y.; Chen, C.; Yang, D.; Yao, X. Scalable and Cost-Effective Synthesis of Highly Efficient Fe2N-Based Oxygen Reduction Catalyst Derived from Seaweed Biomass. Small 2016, 12, 1295–1301. [Google Scholar] [CrossRef]


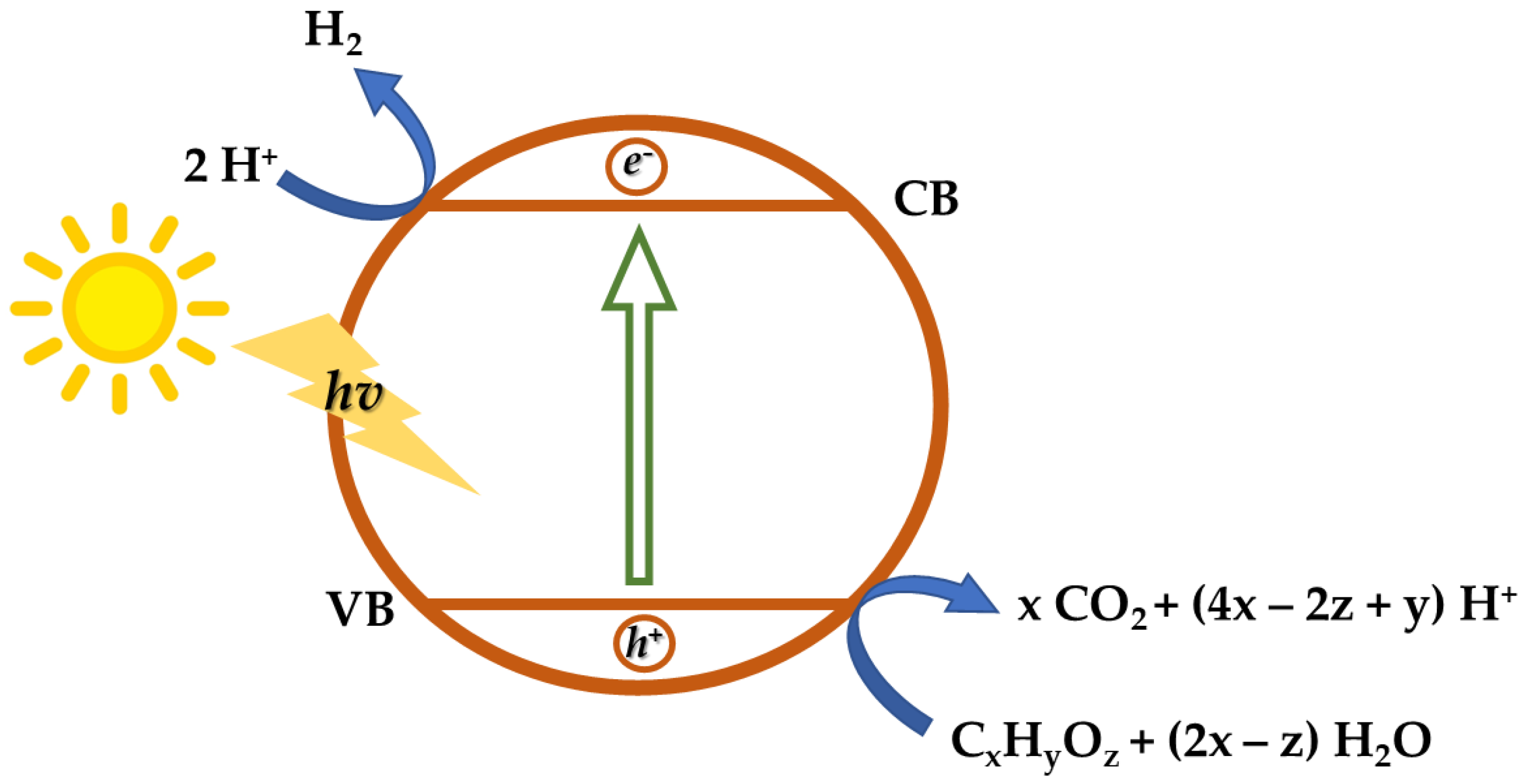

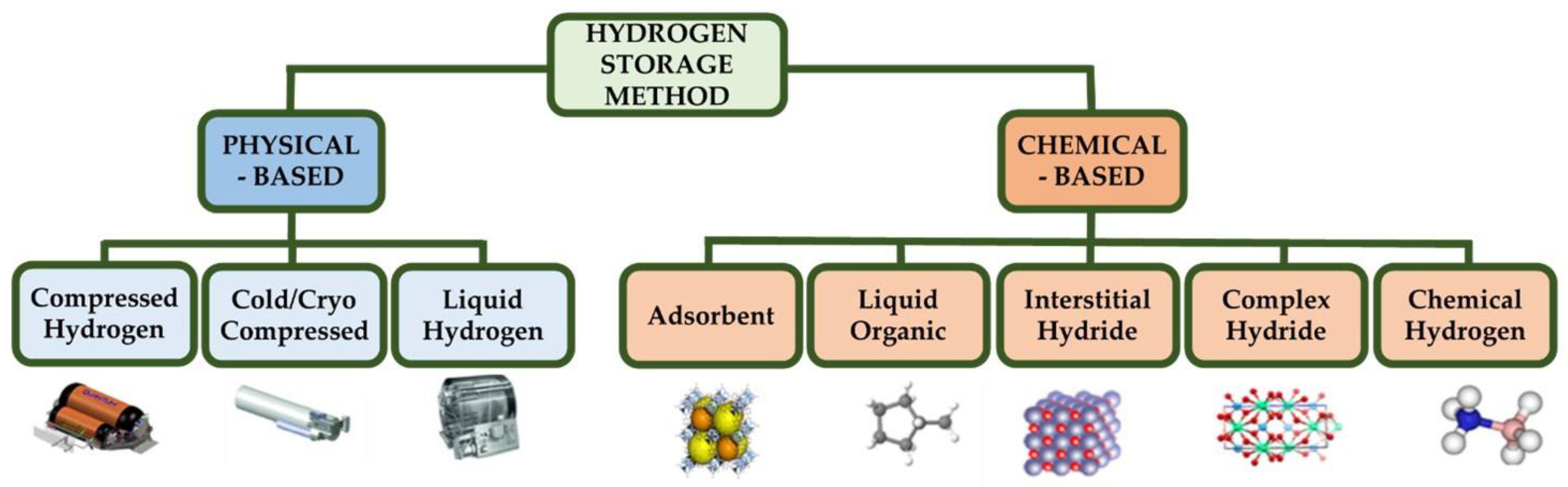
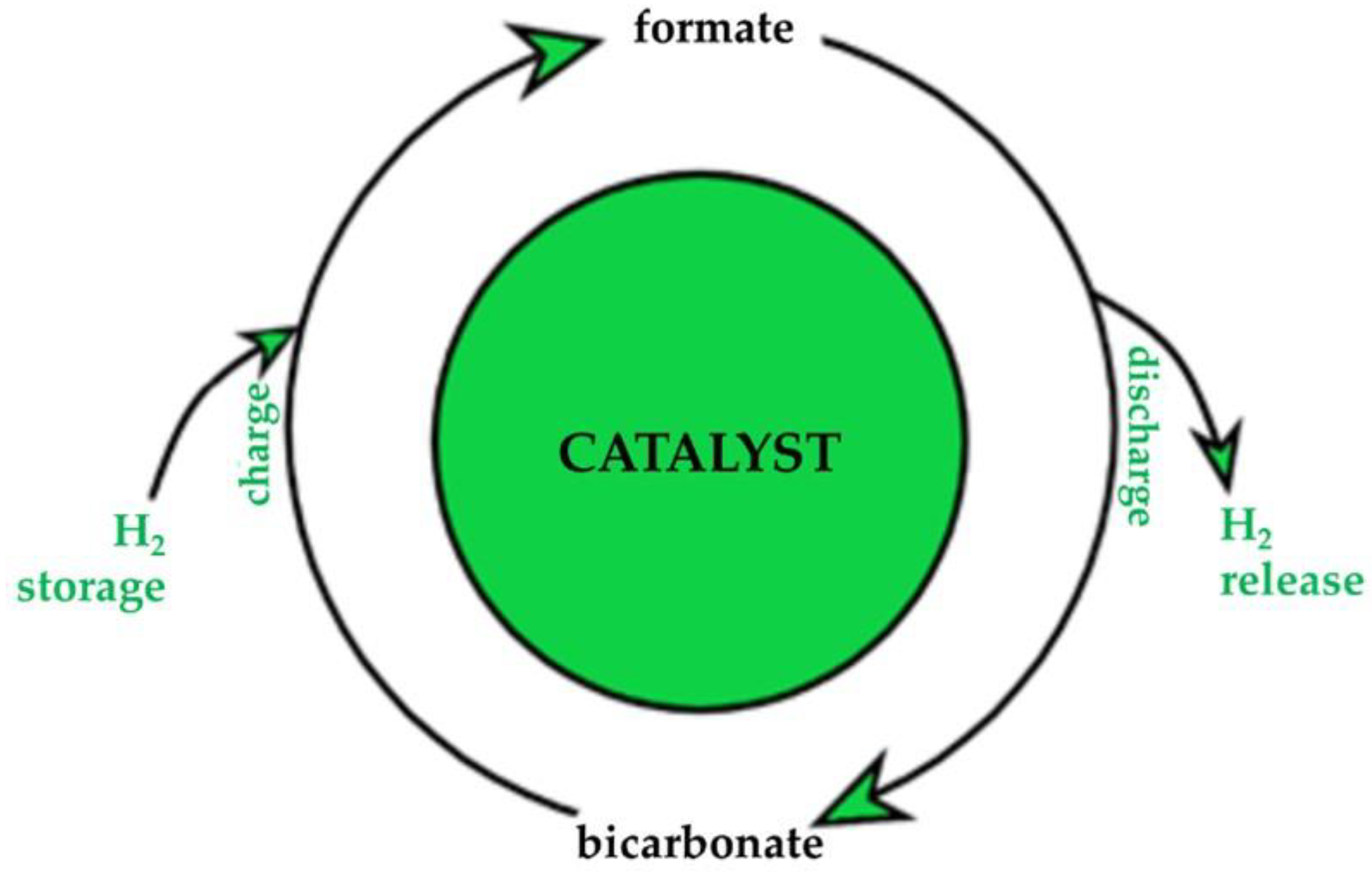

| H2 Production Process from Ethanol | Advantages | Disadvantages | Performances | Ref. |
|---|---|---|---|---|
| Steam Reforming |
|
| H2 Yield: 60–95% EtOH Conversion: 90–100% H2 Selectivity: 70–90% @Ni/Al2O3, 250–500 °C, H2O/EtOH = 3–6 H2 production cost: 1.58–2.6 USD/kgH2 | [19,45,89,90] |
| Partial Oxidation |
|
| H2 Selectivity: 60–97% EtOH Conversion: @Noble metal/Al2O3, 500–600 °C, H2O/O2/EtOH = 3/0.3/1 | [91,92] |
| Autothermal Reforming |
|
| H2 Yield: 47–94% EtOH Conversion: 84–97% @Rh/Al2O3 H2 Selectivity: 40–60% @Pt/Al2O3 400–600 °C, H2O/O2/EtOH = 3/0.3/1 | [93,94] |
| Photocatalysis |
|
| H2 production rate: 30–34 mmol/gh @Pt/TiO2 | [95,96] |
| Electrocatalysis |
|
| H2 volume: 102 mL @Pt/C, 20 °C j = 50 mA/cm−2 | [72] |
| Storage Method | Volumetric Density (KgH2/m3) | Ref. |
|---|---|---|
| High-pressure (350 bar) | ~20 | [117] |
| Liquid H2 | ~20–50 | [104] |
| Formate/Bicarbonate | ~9.5 | [116] |
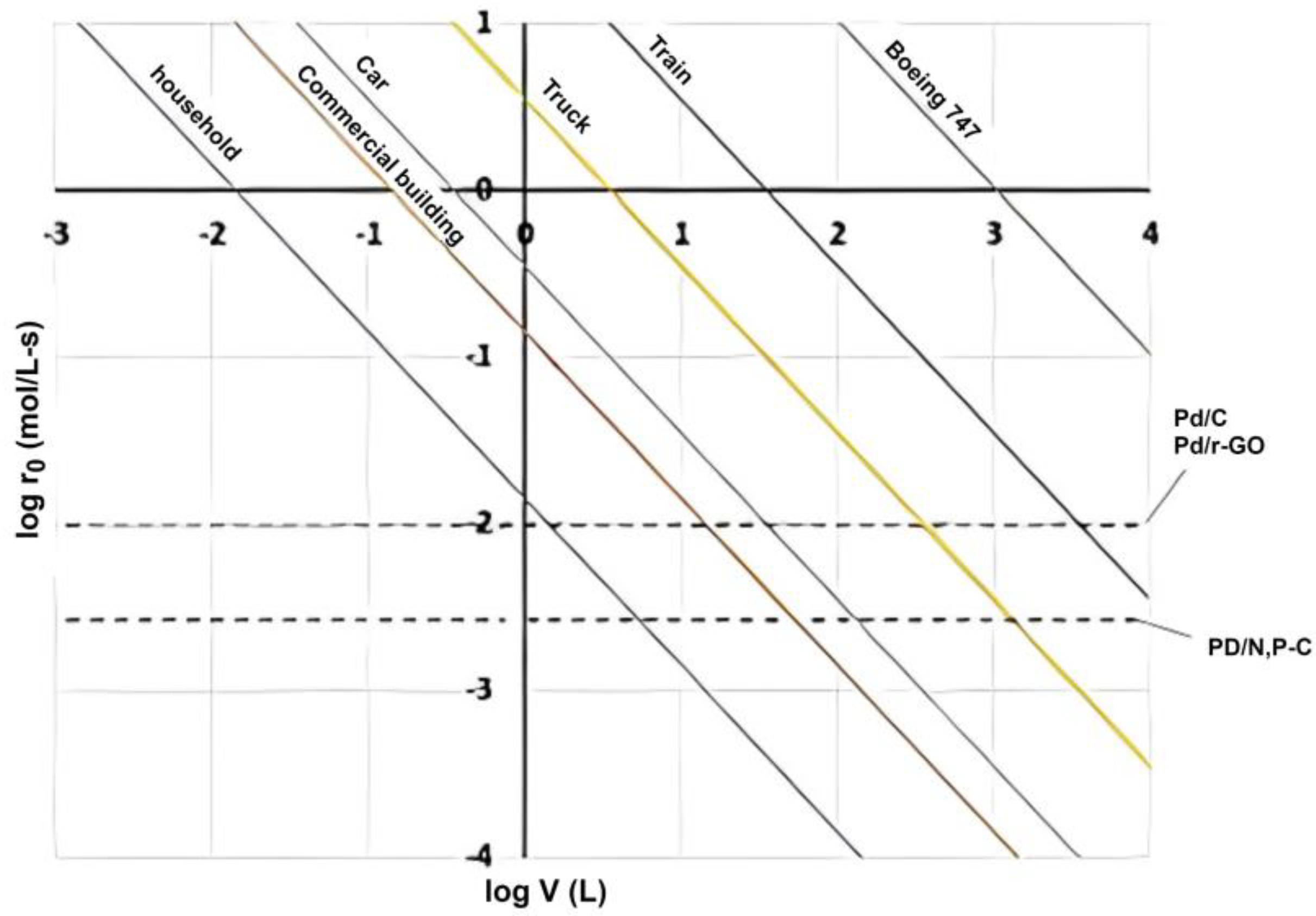
| Catalyst | H2 Yield | TOF (h−1) | Formate Yield | TON | Ref. |
|---|---|---|---|---|---|
| Pd/C (from PANI) | - | 2562 | - | 1625 | [118] |
| Pd/C | - | 5061 | 95.6% | 1769 | [119] |
| Pd/r-GO | 96% | 11,299 | 94.5% | 7088 | [120] |
| Pd/n, P-C | 100% | 3246 | 94.6% | 4027 | [121] |
| Ag/Pd/TiOx/TiO2 | - | 6499 | - | 820 | [122] |
| Fuel Cells | H2 FC | Hydrocarbon FC | Bio-FC | LT-FC <200 °C | HT-FC >600 °C | Anode/ Cathode Catalysts | Electrolyte | Applications |
|---|---|---|---|---|---|---|---|---|
| PEMFCs | Yes | - | - | Yes | - | Platinum [128] | Polymer membrane | Transportation; Portable electronics; Backup power systems; Distributed power generation |
| AFCs | Yes | - | - | Yes | - | Platinum, Silver, Nickel [129] | Alkaline electrolyte | Space missions; Industrial applications |
| PAFCs | Yes | - | - | Yes | - | Platinum [130] | Phosphoric acid | Combined heat and power plants |
| MCFCs | Yes | Yes | Yes | Yes | Nickel [131] | Molten carbonate | Large-scale power plants | |
| SOFCs | Yes | Yes | - | Yes | Nickel [132] | Solid ceramic | Large-scale power generation; Military applications; Combined heat and power plants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, A.; Calabrese, M.; Venezia, V.; Portarapillo, M.; Turco, M.; Di Benedetto, A.; Luciani, G. Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future. Energies 2023, 16, 7908. https://doi.org/10.3390/en16237908
Di Nardo A, Calabrese M, Venezia V, Portarapillo M, Turco M, Di Benedetto A, Luciani G. Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future. Energies. 2023; 16(23):7908. https://doi.org/10.3390/en16237908
Chicago/Turabian StyleDi Nardo, Alessandra, Marcella Calabrese, Virginia Venezia, Maria Portarapillo, Maria Turco, Almerinda Di Benedetto, and Giuseppina Luciani. 2023. "Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future" Energies 16, no. 23: 7908. https://doi.org/10.3390/en16237908
APA StyleDi Nardo, A., Calabrese, M., Venezia, V., Portarapillo, M., Turco, M., Di Benedetto, A., & Luciani, G. (2023). Addressing Environmental Challenges: The Role of Hydrogen Technologies in a Sustainable Future. Energies, 16(23), 7908. https://doi.org/10.3390/en16237908










