Thermodynamic Evaluation of the Energy Self-Sufficiency of the Tyre Pyrolysis Process
Abstract
:1. Introduction
2. Materials and Methods
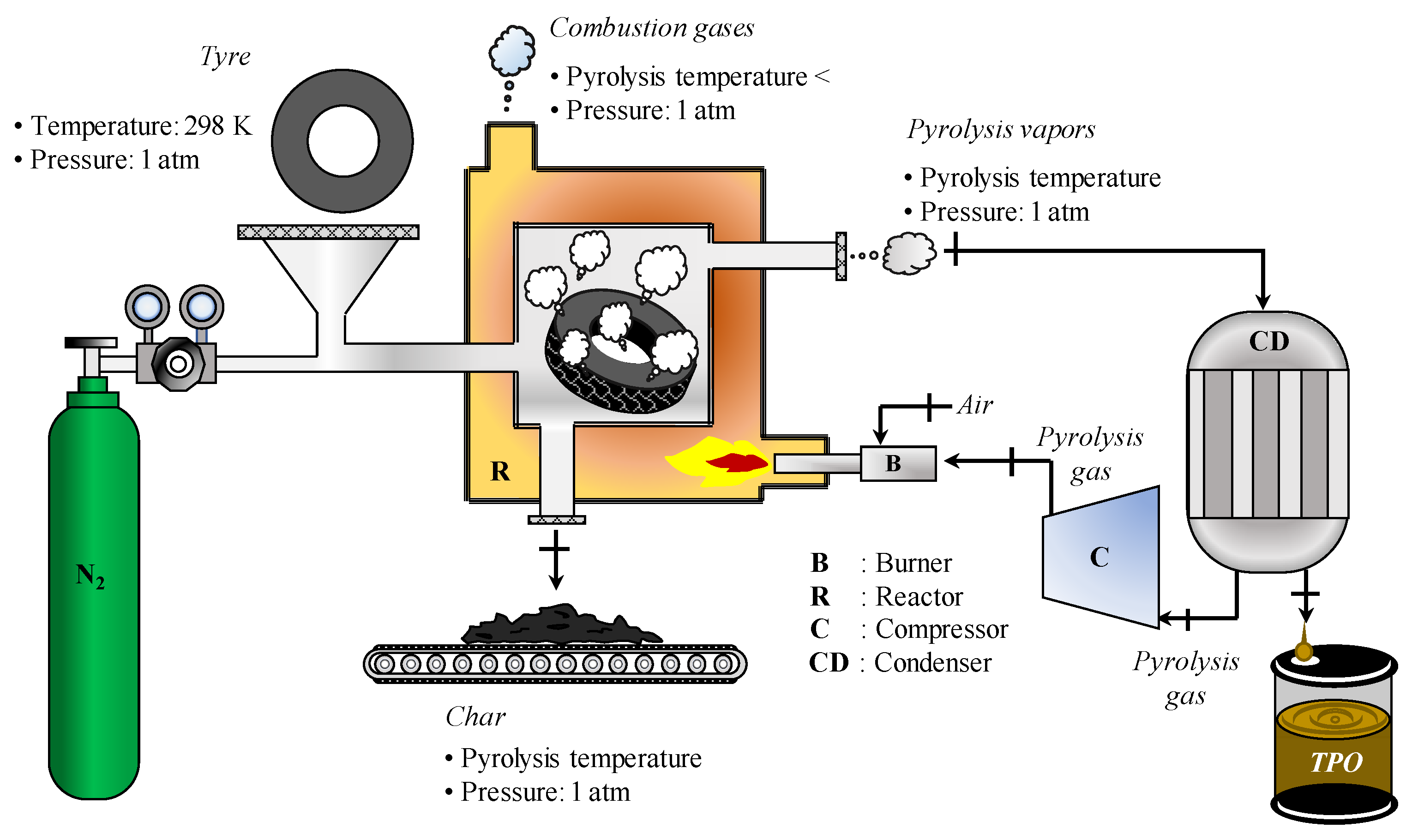
2.1. Idealised Pyrolysis Process
- The reactor operates in a permanent regime at atmospheric pressure (1 atm).
- The temperature inside the reactor is homogeneous (the pyrolysis temperature).
- The tyre material and the nitrogen gas enter the reactor at a temperature of 298 K.
- The pyrolysis of the tyres only produces char and pyrolysis vapours.
- The pyrolysis products and nitrogen gas exit the reactor at the pyrolysis temperature.
- The pyrolysis gas combustion products leave the reactor at a temperature below the pyrolysis temperature.
- The tyre material and its pyrolysis products are homogeneous materials consisting only of C, H, N, S, and O.
- The elemental compositions and mass yields of the pyrolysis products are constant over the operating temperature range of the reactor (the pyrolysis temperature).
- The pyrolysis vapours and the combustion products of the pyrolysis gas behave similarly to an ideal gas.
- The composition of the TPO vapour is invariant during its phase transformation into a liquid, i.e., there are no combinations or decomposition reactions.
- The mass fractions of the pyrolysis gas compounds may change with the pyrolysis temperature.
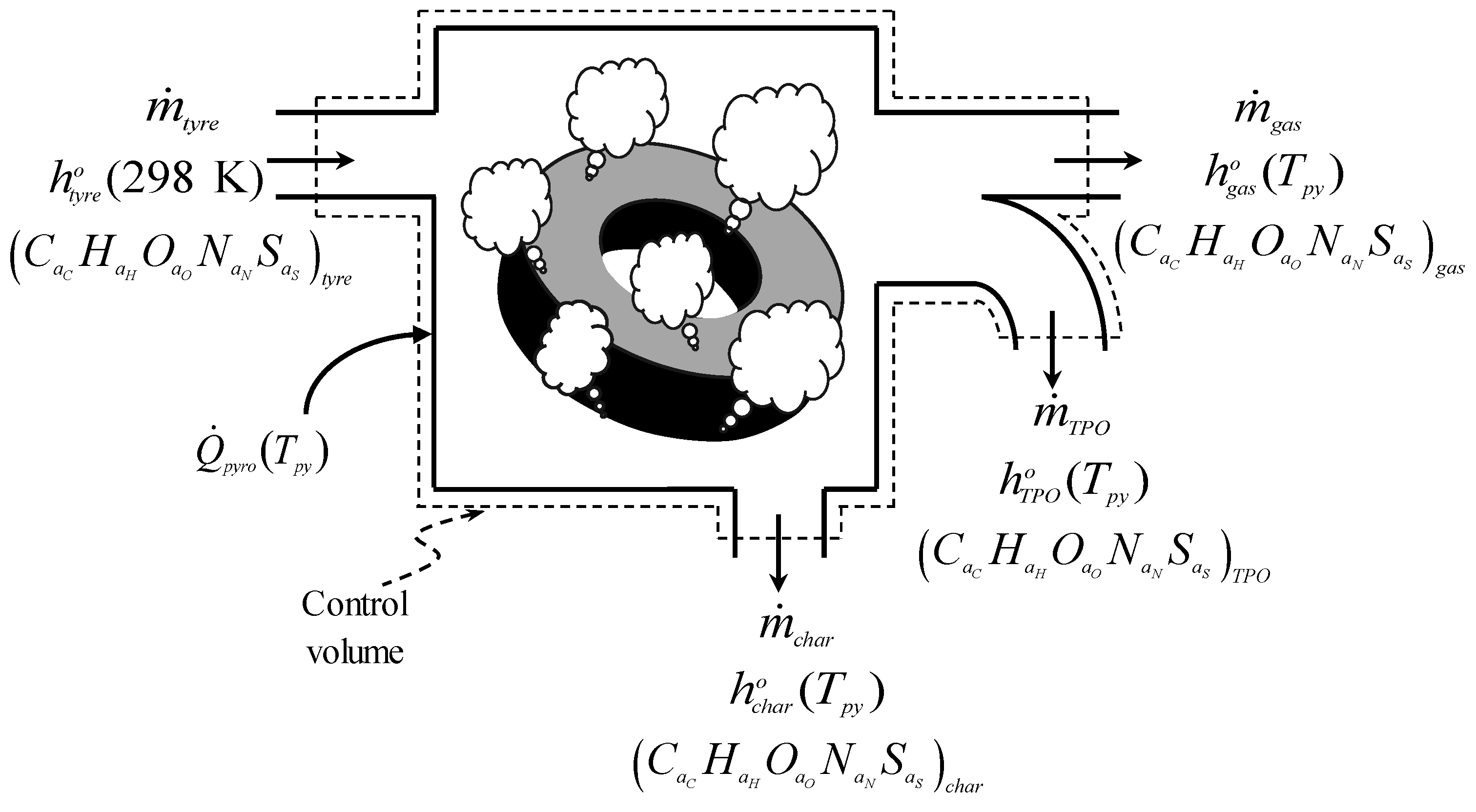
2.1.1. Energy Demand of the Tyre Pyrolysis Process
2.1.2. Energy Self-Sufficiency of the Tyre Pyrolysis Process
- The mass yields of tyre pyrolysis products: , , and ;
- The specific enthalpy of the tyre material at 298 K: ;
- The specific enthalpies of the tyre pyrolysis products: , , and ;
- The specific enthalpy of the pyrolysis gas combustion products: ;
- The heat transferred to nitrogen: ;
- The heat losses due to imperfect insulation of the reactor walls: .
2.2. Experimental Information and Theoretical Foundations
2.2.1. Mass Yields of the Tyre Pyrolysis Products
2.2.2. Specific Enthalpy of the Tyre Material at 298 K
2.2.3. Specific Enthalpies of Tyre Pyrolysis Products
- A. Solid Product: Char
- B. Condensable Fraction of Pyrolysis Vapours: TPO Vapour
- C. Noncondensable Fraction of Pyrolysis Vapours: Pyrolysis Gas
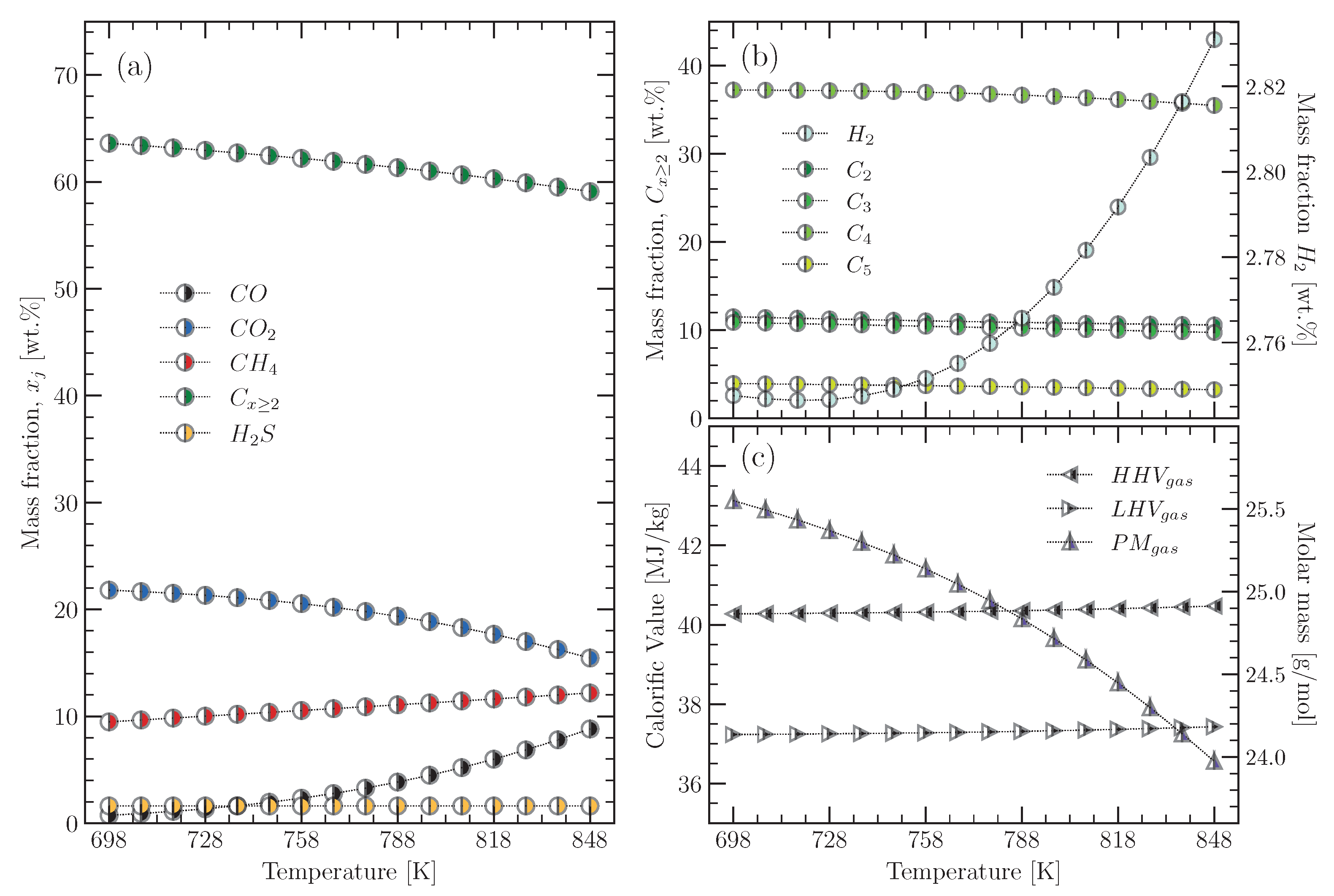
- A range of 0.73% ≤ H (hydrogen) ≤ 20.59%;
- A range of 1.32% ≤ CO (carbon monoxide) ≤ 9.01%;
- A range of 3.29% ≤ CO (carbon dioxide) ≤ 33.02%;
- A range of 1.00% ≤ CH (methane) ≤ 31.19%;
- A range of 1.95% ≤ C: CH (ethylene) + CH (ethane) ≤ 35.21%;
- A range of 3.37% ≤ C3: CH (propene) + CH (prapane) ≤ 39.92%;
- A range of 9.41% ≤ C: CH (1,3-butadiene) + CH (1-butene) + CH (isobutene) ≤ 64.89%;
- A range of 0.36% ≤ C: CH (isoprene) + CH (1-pentane) + CH (n-pentane) ≤ 43.01%;
- A range of 0.06% ≤ HS (hydrogen sulphide) ≤ 4.60%.
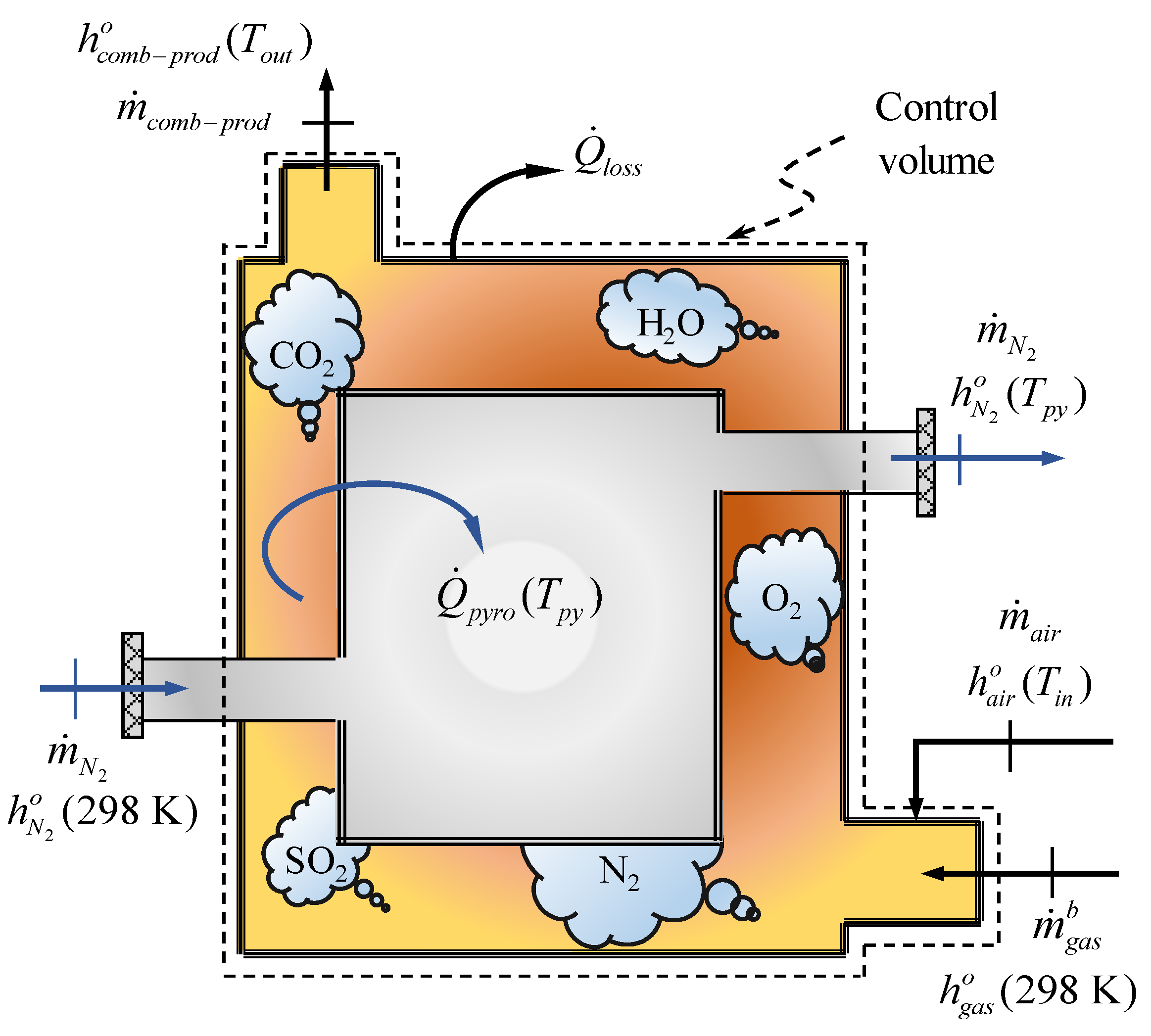
2.2.4. Specific Enthalpy of Pyrolysis Gas Combustion Products
2.2.5. Heat Transferred to Carrier Gas (N)
2.2.6. Heat Losses Due to Imperfect Insulation of the Reactor
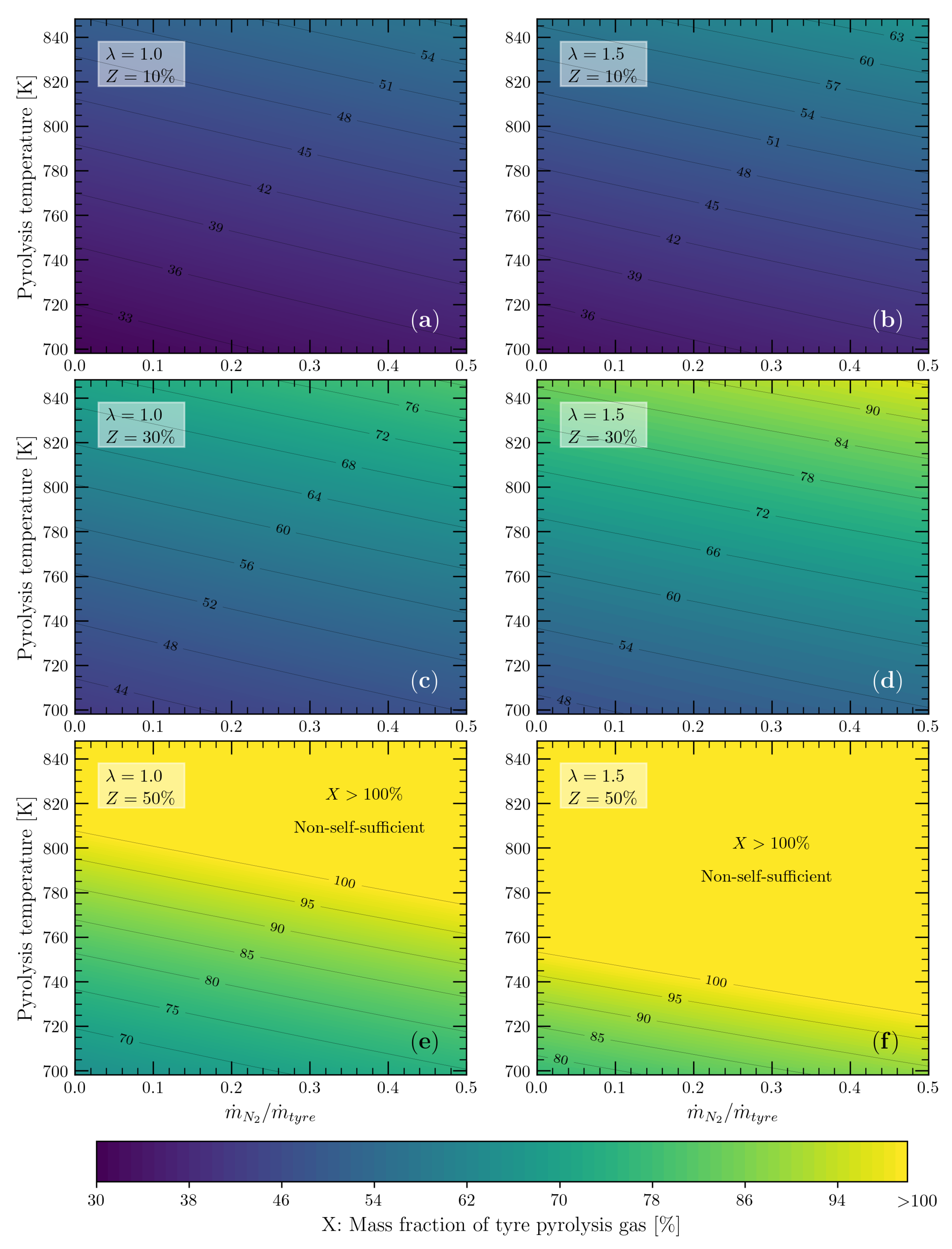
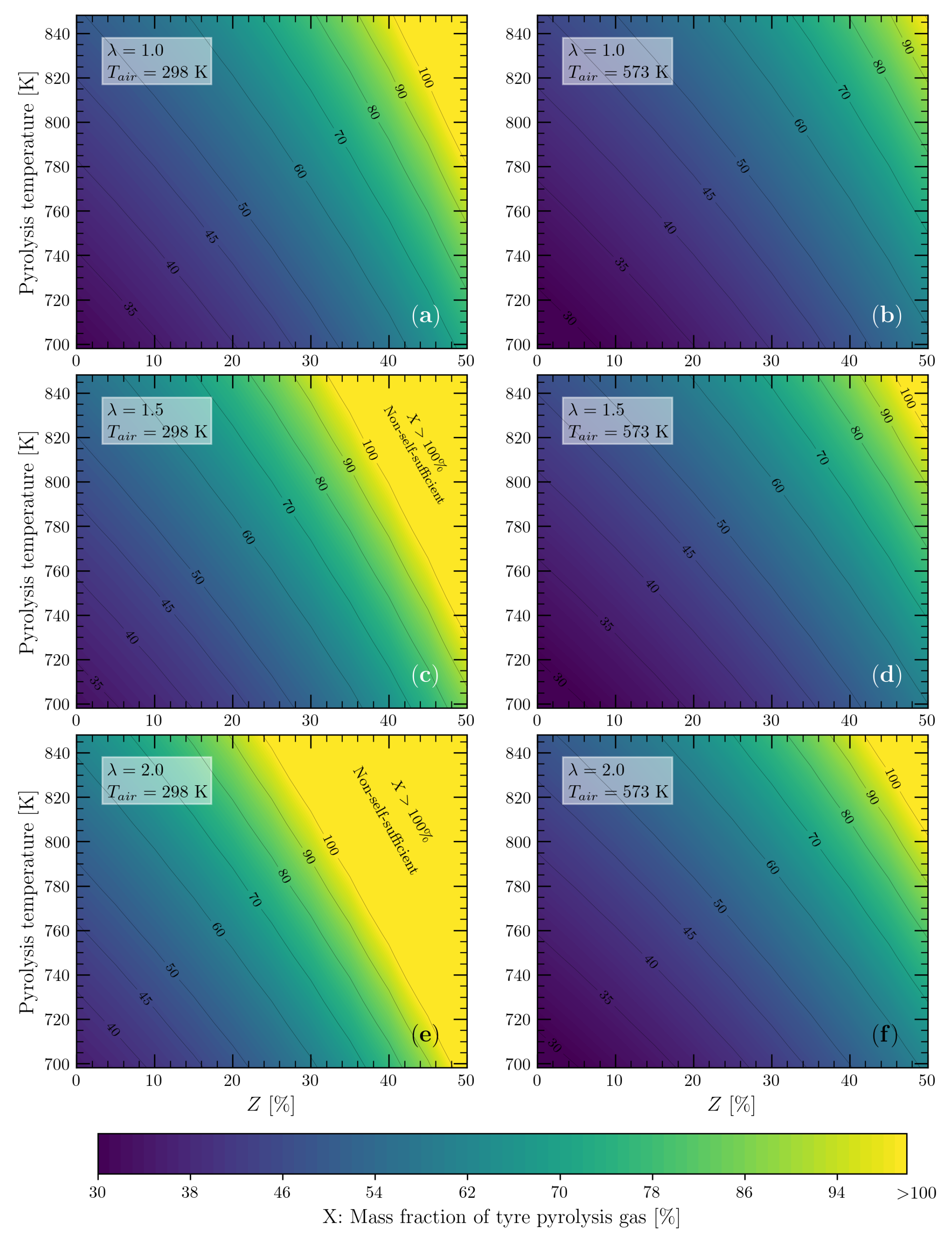
2.3. Mass Fraction of Pyrolysis Gas to Cover the Energy Demand of the Tyre Pyrolysis Process
3. Results and Discussion
3.1. Composition: Tyre Material and Pyrolysis Products
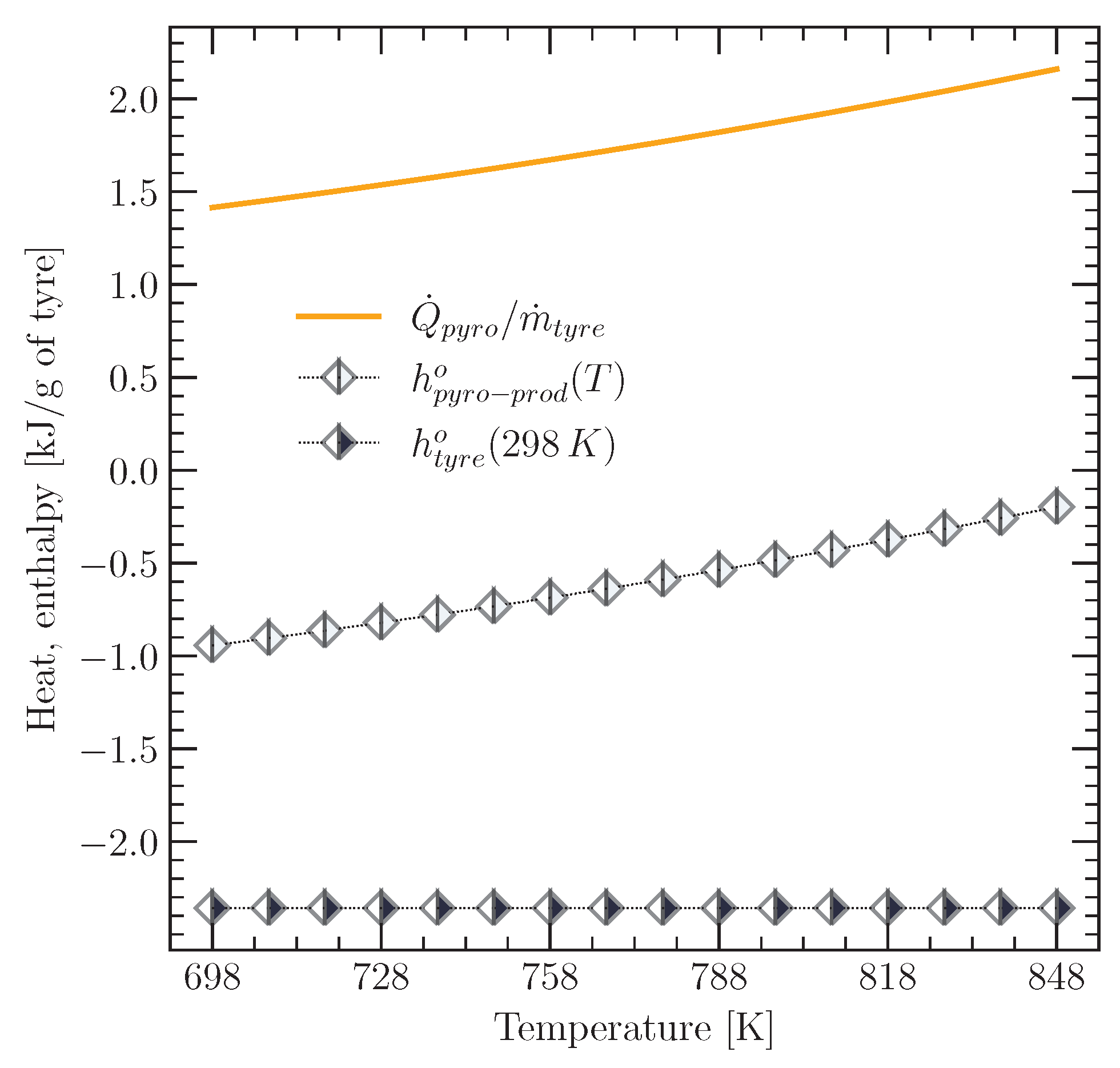
3.2. Enthalpy of the Tyre Material and Its Pyrolysis Products
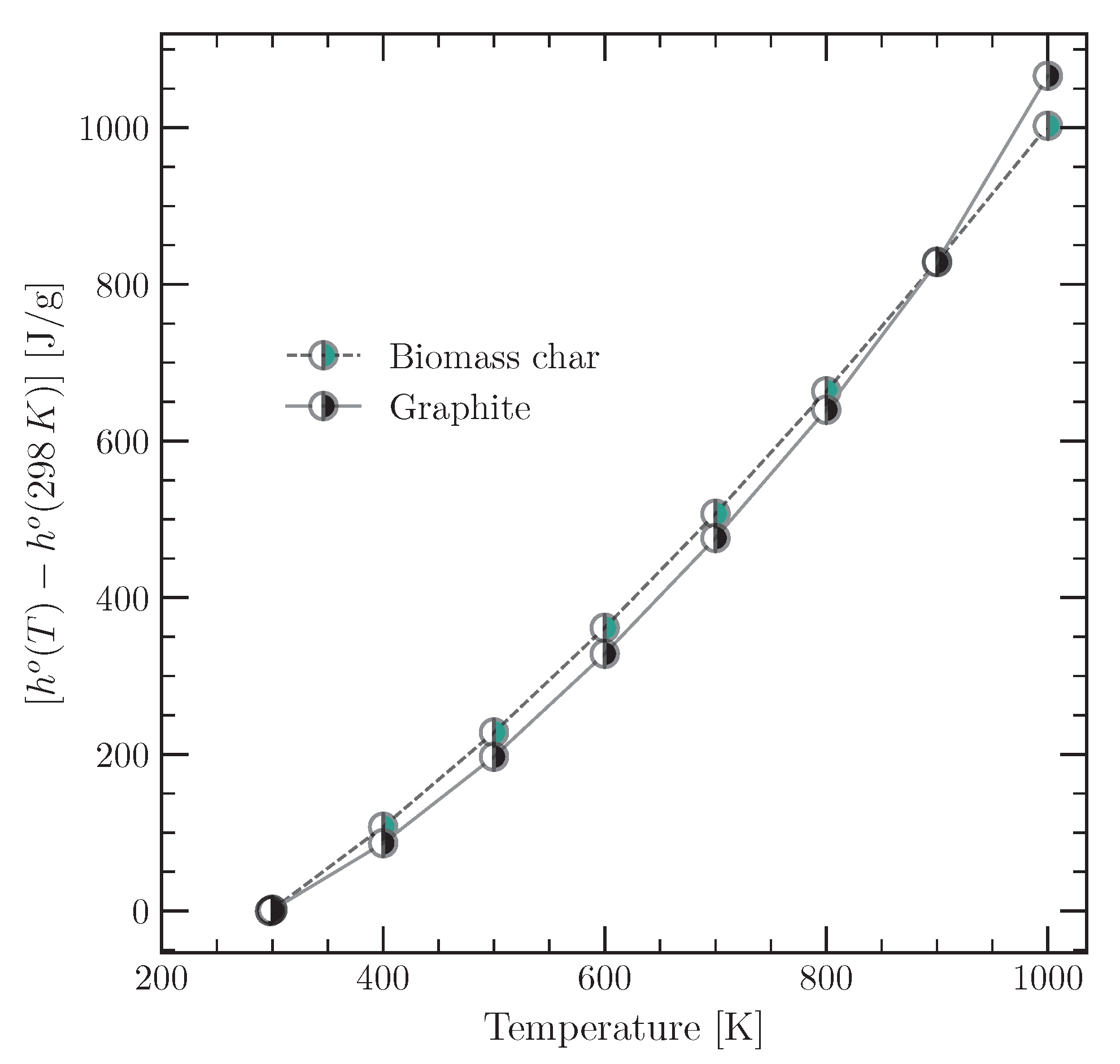
3.2.1. Specific Enthalpy of the Tyre Material and Char
3.2.2. Specific Enthalpy of the TPO Vapour
3.2.3. Specific Enthalpy of the Pyrolysis Gas
3.3. Heat Required by the Tyre Pyrolysis Reaction
3.4. Energy Self-Sufficiency of the Tyre Pyrolysis Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | Number of gram atoms |
| Specific heat at standard pressure, in J/g·K | |
| Molar Gibbs free energy at standard pressure, in J/mol | |
| Gibbs free energy of a mixture of gases | |
| Higher heating value, in J/g | |
| Specific enthalpy at standard pressure, in J/g | |
| Specific enthalpy of formation at standard pressure, in J/g | |
| Molar enthalpy of formation at standard pressure, in J/mol | |
| L | Lagrange function |
| Lower heating value, in J/g | |
| Mass flow rate, in g/s | |
| n | Number of moles |
| Molar mass, g/mol | |
| Heat exchanged per unit of time, in J/s | |
| R | Universal gas constant, in J/mol·K |
| T | Temperature, in K |
| x | Mass fraction |
| Lagrange multiplier | |
| Excess air coefficient | |
| Subscripst | |
| Combustion products | |
| i | Elemental species (C, H, N, S, and O) |
| j | Component of a mixture of gases |
| Pyrolysis | |
| Pyrolysis products | |
| Products | |
| Reagents | |
| r | Molecular group |
Appendix A
References
- Machin, E.B.; Pedroso, D.T.; de Carvalho, J.A. Energetic valorization of waste tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar] [CrossRef]
- World Business Council for Sustainable Development. Global ELT Management—A Global State of Knowledge on Collection Rates, Recovery Routes, and Management Methods. 2019. Available online: https://www.wbcsd.org/Sector-Projects/Tire-Industry-Project/End-of-Life-Tires-ELTs (accessed on 22 October 2023).
- Williams, P.; Bottrill, R.; Cunliffe, A. Combustion of Tyre Pyrolysis Oil. Process Saf. Environ. Prot. 1998, 76, 291–301. [Google Scholar] [CrossRef]
- Gamboa, A.R. Study of Tire Pyrolytic oil Combustion. Master’s Thesis, São Paulo State University, Sao Paulo, Brazil, 2016. (In Portuguese). [Google Scholar]
- García-Contreras, R.; Martínez, J.D.; Armas, O.; Murillo, R.; García, T. Study of a residential boiler under start-transient conditions using a tire pyrolysis liquid (TPL)/diesel fuel blend. Fuel 2015, 158, 744–752. [Google Scholar] [CrossRef]
- Vihar, R.; Žvar Bškovič, U.; Seljak, T.; Katrašnik, T. Combustion and emission formation phenomena of tire pyrolysis oil in a common rail Diesel engine. Energy Convers. Manag. 2017, 149, 706–721. [Google Scholar] [CrossRef]
- Sharma, A.; Murugan, S. Potential for using a tyre pyrolysis oil-biodiesel blend in a diesel engine at different compression ratios. Energy Convers. Manag. 2015, 93, 289–297. [Google Scholar] [CrossRef]
- Doǧan, O.; Çelik, M.B.; Özdalyan, B. The effect of tire derived fuel/diesel fuel blends utilization on diesel engine performance and emissions. Fuel 2012, 95, 340–346. [Google Scholar] [CrossRef]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating rate effects on pyrolytic vapors from scrap truck tires. J. Anal. Appl. Pyrolysis 2017, 123, 419–429. [Google Scholar] [CrossRef]
- Díez, C.; Martínez, O.; Calvo, L.; Cara, J.; Morán, A. Pyrolysis of tyres. Influence of the final temperature of the process on emissions and the calorific value of the products recovered. Waste Manag. 2004, 24, 463–469. [Google Scholar] [CrossRef]
- Martínez, J.D.; Murillo, R.; García, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Aylón, E.; Murillo, R.; Fernández-Colino, A.; Aranda, A.; García, T.; Callén, M.; Mastral, A. Emissions from the combustion of gas-phase products at tyre pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 210–214. [Google Scholar] [CrossRef]
- Frigo, S.; Seggiani, M.; Puccini, M.; Vitolo, S. Liquid fuel production from waste tyre pyrolysis and its utilisation in a Diesel engine. Fuel 2014, 116, 399–408. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Alguacil, F.J.; Lobato, B. Distillation of granulated scrap tires in a pilot plant. J. Hazard. Mater. 2011, 190, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, Y.; Sun, J.; Zhao, X.; Wang, W.; Mao, Y.; Ma, C. Effect of power level on the microwave pyrolysis of tire powder. Energy 2017, 127, 571–580. [Google Scholar] [CrossRef]
- Antoniou, N.; Zabaniotou, A. Features of an efficient and environmentally attractive used tyres pyrolysis with energy and material recovery. Renew. Sustain. Energy Rev. 2013, 20, 539–558. [Google Scholar] [CrossRef]
- CalRecovery, I. Final Report Environmental Factors of Waste Tire Pyrolysis, Gasification, and Liquefaction; CalRecovery report; American Consulting and Commodities, Inc.: Williams, CA, USA, 1995. [Google Scholar]
- Yang, H.; Kudo, S.; Kuo, H.P.; Norinaga, K.; Mori, A.; Mašek, O.; Hayashi, J.i. Estimation of Enthalpy of Bio-Oil Vapor and Heat Required for Pyrolysis of Biomass. Energy Fuels 2013, 27, 2675–2686. [Google Scholar] [CrossRef]
- Daugaard, D.E.; Brown, R.C. Enthalpy for Pyrolysis for Several Types of Biomass. Energy Fuels 2003, 17, 934–939. [Google Scholar] [CrossRef]
- Reed, T.B.; Gaur, S. The High Heat of Fast Pyrolysis for Large Particles. In Developments in Thermochemical Biomass Conversion: Volume 1/Volume 2; Bridgwater, A.V., Boocock, D.G.B., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 97–103. [Google Scholar]
- Gamboa, A.R.; Rocha, A.M.; dos Santos, L.R.; de Carvalho, J.A. Tire pyrolysis oil in Brazil: Potential production and quality of fuel. Renew. Sustain. Energy Rev. 2020, 120, 109614. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.; Danon, B.; van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Waste truck-tyre processing by flash pyrolysis in a conical spouted bed reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Raj, R.E.; Kennedy, Z.R.; Pillai, B. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers. Manag. 2013, 67, 145–151. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Kordoghli, S.; Khiari, B.; Paraschiv, M.; Zagrouba, F.; Tazerout, M. Impact of different catalysis supported by oyster shells on the pyrolysis of tyre wastes in a single and a double fixed bed reactor. Waste Manag. 2017, 67, 288–297. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- De Marco Rodriguez, I.; Laresgoiti, M.; Cabrero, M.; Torres, A.; Chomón, M.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Ucar, S.; Karagoz, S.; Ozkan, A.R.; Yanik, J. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 2005, 84, 1884–1892. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of waste tires in a circulating fluidized-bed reactor. Energy 2001, 26, 385–399. [Google Scholar] [CrossRef]
- Barbooti, M.M. Thermogravimetric and pyrolytic investigations on scrap tires. J. Anal. Appl. Pyrolysis 2014, 110, 419–423. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Laresgoiti, M.; Caballero, B.; de Marco, I.; Torres, A.; Cabrero, M.; Chomón, M. Characterization of the liquid products obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2004, 71, 917–934. [Google Scholar] [CrossRef]
- Díez, C.; Sánchez, M.; Haxaire, P.; Martínez, O.; Morán, A. Pyrolysis of tyres: A comparison of the results from a fixed-bed laboratory reactor and a pilot plant (rotatory reactor). J. Anal. Appl. Pyrolysis 2005, 74, 254–258. [Google Scholar] [CrossRef]
- Lopez, G.; Olazar, M.; Amutio, M.; Aguado, R.; Bilbao, J. Influence of Tire Formulation on the Products of Continuous Pyrolysis in a Conical Spouted Bed Reactor. Energy Fuels 2009, 23, 5423–5431. [Google Scholar] [CrossRef]
- Kyari, M.; Cunliffe, A.; Williams, P.T. Characterization of Oils, Gases, and Char in Relation to the Pyrolysis of Different Brands of Scrap Automotive Tires. Energy Fuels 2005, 19, 1165–1173. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J. Anal. Appl. Pyrolysis 2004, 72, 165–170. [Google Scholar] [CrossRef]
- Jitkarnka, S.; Chusaksri, B.; Supaphol, P.; Magaraphan, R. Influences of thermal aging on properties and pyrolysis products of tire tread compound. J. Anal. Appl. Pyrolysis 2007, 80, 269–276. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Qu, W.; Zhou, Q.; Wang, Y.Z.; Zhang, J.; Lan, W.W.; Wu, Y.H.; Yang, J.W.; Wang, D.Z. Pyrolysis of waste tire on ZSM-5 zeolite with enhanced catalytic activities. Polym. Degrad. Stab. 2006, 91, 2389–2395. [Google Scholar] [CrossRef]
- Choi, G.G.; Jung, S.H.; Oh, S.J.; Kim, J.S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Properties of Chars and Activated Carbons Derived from the Pyrolysis of Used Tyres. Environ. Technol. 1998, 19, 1177–1190. [Google Scholar] [CrossRef]
- Islam, M.R.; Haniu, H.; Beg, M.R.A. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar] [CrossRef]
- Kar, Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011, 31, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Laresgoiti, M.; de Marco, I.; Torres, A.; Caballero, B.; Cabrero, M.A.; Chomón, M. Chromatographic analysis of the gases obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2000, 55, 43–54. [Google Scholar] [CrossRef]
- Leung, D.; Yin, X.; Zhao, Z.; Xu, B.; Chen, Y. Pyrolysis of tire powder: Influence of operation variables on the composition and yields of gaseous product. Fuel Process. Technol. 2002, 79, 141–155. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Catalytic pyrolysis of waste tire using HY/MCM-41 core-shell composite. J. Anal. Appl. Pyrolysis 2016, 121, 297–306. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W. Optimization of fuel recovery through the stepwise co-pyrolysis of palm shell and scrap tire. Energy Convers. Manag. 2015, 99, 334–345. [Google Scholar] [CrossRef]
- Sanahuja-Parejo, O.; Veses, A.; Navarro, M.; López, J.; Murillo, R.; Callén, M.; García, T. Catalytic co-pyrolysis of grape seeds and waste tyres for the production of drop-in biofuels. Energy Convers. Manag. 2018, 171, 1202–1212. [Google Scholar] [CrossRef]
- Wang, W.C.; Bai, C.J.; Lin, C.T.; Prakash, S. Alternative fuel produced from thermal pyrolysis of waste tires and its use in a DI diesel engine. Appl. Therm. Eng. 2016, 93, 330–338. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, D.; Huo, P.; Wang, M.; Han, L.; Chen, G.; Li, H.; Li, X.; Wang, Y.; et al. Derived oil production by catalytic pyrolysis of scrap tires. Chin. J. Catal. 2016, 37, 526–532. [Google Scholar] [CrossRef]
- Aydin, H.; Ilkiliç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel 2012, 102, 605–612. [Google Scholar] [CrossRef]
- McBride, B.J.; Zehe, M.J.; Gordon, S. NASA Glenn Coefficients for Calculating Thermodynamic Properties of Individual Species. Available online: https://ntrs.nasa.gov/citations/20020085330 (accessed on 22 October 2023).
- Alvarez, J.; Lopez, G.; Amutio, M.; Mkhize, N.; Danon, B.; van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor. Energy 2017, 128, 463–474. [Google Scholar] [CrossRef]
- Ayanoğlu, A.; Yumrutaş, R. Rotary kiln and batch pyrolysis of waste tire to produce gasoline and diesel like fuels. Energy Convers. Manag. 2016, 111, 261–270. [Google Scholar] [CrossRef]
- Banar, M.; Akyıldız, V.; Özkan, A.; Çokaygil, Z.; Onay, O. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel). Energy Convers. Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Siva, M.; Onenc, S.; UÇar, S.; Yanik, J. Influence of oily wastes on the pyrolysis of scrap tire. Energy Convers. Manag. 2013, 75, 474–481. [Google Scholar] [CrossRef]
- Choi, G.G.; Oh, S.J.; Kim, J.S. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content. Appl. Energy 2016, 170, 140–147. [Google Scholar] [CrossRef]
- Aslan, D.I.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of Master-Plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Feng, Y. The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers. Manag. 2017, 136, 27–35. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Yang, Y.; Sun, J.; Zhao, X.; Wang, W.; Mao, Y.; Yuan, X.; Wang, Q. Characteristics of limonene formation during microwave pyrolysis of scrap tires and quantitative analysis. Energy 2018, 142, 953–961. [Google Scholar] [CrossRef]
- Hu, H.; Fang, Y.; Liu, H.; Yu, R.; Luo, G.; Liu, W.; Li, A.; Yao, H. The fate of sulfur during rapid pyrolysis of scrap tires. Chemosphere 2014, 97, 102–107. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Zhang, B.; Wang, J.; Min, A.; Ruan, R. Catalytic pyrolysis of waste tire to produce valuable aromatic hydrocarbons: An analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2016, 122, 55–63. [Google Scholar] [CrossRef]
- Uçar, S.; Karagöz, S. Co-pyrolysis of pine nut shells with scrap tires. Fuel 2014, 137, 85–93. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Upgraded fuel from microwave assisted pyrolysis of waste tire. Fuel 2014, 115, 600–608. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.K.; Chun, H.D. Pyrolysis Kinetics of Scrap Tire Rubbers. I: Using DTG and TGA. J. Environ. Eng. 1995, 121, 507–514. [Google Scholar] [CrossRef]
- Helleur, R.; Popovic, N.; Ikura, M.; Stanciulescu, M.; Liu, D. Characterization and potential applications of pyrolytic char from ablative pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 58-59, 813–824. [Google Scholar] [CrossRef]
- Olazar, M.; Aguado, R.; Arabiourrutia, M.; Lopez, G.; Barona, A.; Bilbao, J. Catalyst Effect on the Composition of Tire Pyrolysis Products. Energy Fuels 2008, 22, 2909–2916. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M.; Zagrouba, F. Catalysts’ influence on thermochemical decomposition of waste tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Haseli, Y.; van Oijen, J.; de Goey, L. Modeling biomass particle pyrolysis with temperature-dependent heat of reactions. J. Anal. Appl. Pyrolysis 2011, 90, 140–154. [Google Scholar] [CrossRef]
- Joback, K.; Reid, R. Estimation of pure-component properties from group-contributions. Chem. Eng. Commun. 1987, 57, 233–243. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C.; Zhang, Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J. Anal. Appl. Pyrolysis 2009, 85, 334–337. [Google Scholar] [CrossRef]
- Ayanoǧlu, A.; Yumrutaş, R. Production of gasoline and diesel like fuels from waste tire oil by using catalytic pyrolysis. Energy 2016, 103, 456–468. [Google Scholar] [CrossRef]
- Jarungthammachote, S.; Dutta, A. Thermodynamic equilibrium model and second law analysis of a downdraft waste gasifier. Energy 2007, 32, 1660–1669. [Google Scholar] [CrossRef]
- Vaezi, M.; Passandideh-Fard, M.; Moghiman, M.; Charmchi, M. Gasification of heavy fuel oils: A thermochemical equilibrium approach. Fuel 2011, 90, 878–885. [Google Scholar] [CrossRef]
- Barman, N.S.; Ghosh, S.; De, S. Gasification of biomass in a fixed bed downdraft gasifier—A realistic model including tar. Bioresour. Technol. 2012, 107, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Mendiburu, A.Z.; Carvalho, J.A.; Coronado, C.J. Thermochemical equilibrium modeling of biomass downdraft gasifier: Stoichiometric models. Energy 2014, 66, 189–201. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry Webbook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.; García, T.; Mastral, A. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Navarro, M.V.; Murillo, R.; García, T.; Mastral, A.M. Waste Tire Pyrolysis: Comparison between Fixed Bed Reactor and Moving Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 4029–4033. [Google Scholar] [CrossRef]
- POLIMIX. POLIMIX Ambiental. 2019. Available online: http://www.polimixambiental.com.br (accessed on 22 October 2023).


| Composition | Tyre | Char | TPO | Gas a |
|---|---|---|---|---|
| C [wt.%] | 85.91 | 94.78 | 85.23 | 68.21 |
| H [wt.%] | 7.38 | 1.10 | 10.36 | 13.96 |
| O b [wt.%] | 4.23 | <1.00 | 2.71 | 16.28 |
| N [wt.%] | 0.55 | <1.00 | 0.55 | 0.00 |
| S [wt.%] | 1.93 | 3.05 | 1.15 | 1.52 |
| Yield [wt.%] | 100.00 | 38.02 | 45.78 | 16.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa, A.A.R.; dos Santos, L.R.; Martins, C.A.; Rocha, A.M.A.; Alvarado-Silva, C.A.; de Carvalho, J.A., Jr. Thermodynamic Evaluation of the Energy Self-Sufficiency of the Tyre Pyrolysis Process. Energies 2023, 16, 7932. https://doi.org/10.3390/en16247932
Gamboa AAR, dos Santos LR, Martins CA, Rocha AMA, Alvarado-Silva CA, de Carvalho JA Jr. Thermodynamic Evaluation of the Energy Self-Sufficiency of the Tyre Pyrolysis Process. Energies. 2023; 16(24):7932. https://doi.org/10.3390/en16247932
Chicago/Turabian StyleGamboa, Alexander A. R., Leila R. dos Santos, Cristiane A. Martins, Ana M. A. Rocha, Carlos A. Alvarado-Silva, and João A. de Carvalho, Jr. 2023. "Thermodynamic Evaluation of the Energy Self-Sufficiency of the Tyre Pyrolysis Process" Energies 16, no. 24: 7932. https://doi.org/10.3390/en16247932






