1. Introduction
The issues of the energy crisis and greenhouse effect continue to be globally paramount concerns. In light of the driving forces behind global energy conservation and emission reduction policies, liquefied natural gas (LNG) has emerged as a pivotal low-carbon and clean energy source, occupying a profoundly significant position within the international energy framework [
1]. In recent years, the rapid development of the shipping industry has brought about imminent challenges such as fuel consumption and greenhouse gas emissions [
2]. While the advent of LNG-powered vessels has met the emission regulations set by the International Maritime Organization (IMO), in making LNG-powered ships the mainstream in the maritime and shipbuilding sectors [
3], there remains an urgent need to address the considerable energy loss resulting from ship operations, notably via the cooling, exhaust, and lubrication systems, which accounts for more than half of the total energy released from fuel combustion [
4,
5]. Moreover, the cold energy released during the LNG vaporization process in LNG-powered ships is ultimately discharged into seawater or the atmosphere, thus not only squandering a substantial amount of high-quality cold energy but also causing significant ecological damage [
6]. Confronting the pressing challenges of energy depletion and environmental degradation, waste heat recovery (WHR) [
7] and LNG vaporization cold energy recovery technologies [
8] have gradually garnered attention as effective means to enhance the energy utilization efficiency and reduce operational costs.
Researchers have proposed various thermodynamic systems to improve the efficiency of WHR technology, including the Rankine cycle [
7,
9], Kalina cycle [
10], Brayton cycle [
11], and combined heat and power systems [
12,
13]. Among these cycles, the Rankine cycle stands out in the field of waste heat recovery due to its advantages of high efficiency, simplicity, and cost-effectiveness. It performs exceptionally well in recovering waste heat of different grades [
14,
15]. To enhance the performance of waste heat recovery systems, researchers have focused on aspects such as the heat source characteristics [
16], working fluids [
17], and cycle configurations [
18]. They have employed interdisciplinary tools such as thermodynamics, economics, and environmental studies to comprehensively evaluate these systems.
The rational selection of working fluids is a crucial factor in determining the efficiency of a Rankine cycle. Yang et al. [
19] analyzed 267 different working fluids, examining the influence of critical temperature, triple point, and other thermophysical properties on cycle performance. They also developed composite indicators to evaluate the optimal combination of critical temperature and boiling point for working fluids. Yan et al. [
20] approached the analysis from a molecular structure perspective, investigating the impact of molecular characteristics on the cycle thermal efficiency and proposing a molecular-structure-based evaluation index for working fluids. Compared to pure working fluids, mixed working fluids exhibit non-isothermal heat transfer characteristics [
21] and temperature glide phenomena [
22] during the heat exchange process. This allows for a better matching of the temperature curves between the heat and cold source, effectively reducing the heat transfer temperature difference, minimizing irreversibility, and improving the cycle efficiency [
23]. Feng et al. [
24] conducted a comparative study on the overall performance of Rankine cycles using R123, R245fa, and their mixtures as working fluids under the same heat source conditions. The experimental results demonstrated that mixed working fluids exhibit a superior thermodynamic performance and moderate economic performance compared to pure working fluids.
Many researchers have focused on improving the overall performance of WHR systems from a structural perspective. Feng et al. [
25] compared the thermal economic performance of basic organic Rankine cycles (BORCs) and regenerative organic Rankine cycles (RORCs). The study found that RORCs had an efficiency advantage of 8.1% compared to BORCs, but their average generation cost was 21.1% higher. Shokati et al. [
26] conducted a comparative analysis of BORCs, dual-pressure organic Rankine cycles (DPORCs), dual-fluid organic Rankine cycles (DFORCs), and Kalina cycles in terms of thermal efficiency, exergy efficiency, and exergy economy performance under the same conditions. The results showed that DPORCs generated the highest amount of electricity, with 15.22%, 35.09%, and 43.48% higher power outputs compared to the optimal conditions of BORCs, DFORCs, and Kalina cycles, respectively. Li et al. [
27] proposed two structures, namely a series two evaporator organic Rankine cycle (STORC) and parallel two evaporator organic Rankine cycle (PTORC), and conducted a thermal economic analysis of both. The results showed that the STORC had a greater improvement compared to the PTORC, with an overall efficiency increase ranging from 0.3% to 5.4% and a maximum reduction in investment cost of 34.2%.
To address the significant waste of cold energy during the LNG regasification process, researchers have made relentless efforts in areas such as power generation [
28,
29], cold storage [
30], desalination [
31], energy storage [
32], air separation [
33], and cryogenic carbon capture [
34]. However, in the face of a deteriorating natural environment, reducing CO
2 emissions is a duty that every country should fulfill [
35]. Although the use of LNG can effectively reduce pollutant emissions compared to traditional fossil fuels, the generation of CO
2 is inevitable, and carbon capture still has great potential for emission reduction [
36]. Currently, carbon capture technologies mainly include oxy-fuel combustion capture, pre-combustion capture, and post-combustion capture. Among them, oxy-fuel combustion technology is relatively easy to achieve CO
2 capture, and the captured CO
2 concentration is high. However, it faces challenges such as investment and high energy consumption in oxygen production technology, as well as difficulties in equipment retrofitting [
37]. Pre-combustion capture achieves a high capture efficiency by converting fossil fuels into a gaseous mixture of H
2 and CO
2. However, it incurs high investment and operating costs for fuel pretreatment [
38]. Post-combustion capture is relatively mature, and some technologies have been deployed in practical projects, mainly including absorption, adsorption, cryogenic, and membrane separation methods [
39]. Among them, the absorption method has advantages and feasibility for large-scale deployment but is hindered by high cost, toxicity, and high energy consumption in the solvent regeneration process [
40]. Currently, reducing energy consumption in the carbon capture process remains a significant challenge for carbon capture technologies. However, utilizing LNG regasification cold energy for low-temperature carbon capture can effectively reduce the energy consumption of capture while achieving green and efficient recovery of LNG regasification cold energy [
41,
42]. In comparison, chemical absorption [
43] and physical adsorption [
44] methods face drawbacks such as a high capture energy consumption, high costs, and complex operations. Additionally, membrane separation [
45,
46] encounters obstacles such as high membrane costs and difficulties in large-scale implementation. Therefore, low-temperature carbon capture is often considered the optimal alternative for high-energy-consumption carbon capture [
47]. Liu et al. [
48] proposed a CCHP system that couples the recovery of LNG cold energy and the utilization of flue gas waste heat with low-temperature carbon capture. Under the optimal conditions, the system achieved a total power generation of 90.65 MW, an exergy efficiency of 41.38%, and a unit electricity cost of 18.05
$/GJ, with a CO
2 capture rate of 7.9236 t/h.
To address the carbon emissions from maritime vessels, the IMO plans to achieve a 70% reduction in the energy efficiency design index via the implementation of carbon capture and storage technology [
49,
50]. Meanwhile, articles discussing ship carbon capture have constantly emerged. Luo et al. [
51] studied the feasibility of ship carbon capture and the limitations of implementing onboard carbon capture using the Wärtsilä 9L46 marine diesel engine as a research subject. Yao et al. [
52] developed an integrated system for a dual-fuel ship engine with oxygen-enriched combustion and waste heat recovery. The results showed a CO
2 capture concentration of 97.09% and a system efficiency of 51.78%. Feenstra et al. [
53] proposed a carbon capture process using MEA and piperazine as absorbents for dual-fuel ships, utilizing flue gas waste heat for rich CO
2 solvent desorption and LNG cold energy for CO
2 liquefaction. However, the chemical absorption process requires a significant amount of space for the absorber and stripping towers, and the limited space on ships becomes another factor hindering the application of this technology [
49]. Oxy-fuel combustion technology also requires modifications to ship engines, and the high investment cost for oxygen production is a limitation, as well as being constrained by limited ship space [
52]. At the same time, adsorption technology has the issue of relatively low capture efficiency [
54]. Current research on ship carbon capture has predominantly focused on LNG-powered ships because LNG fuel allows for extensive heat integration to enhance the process [
53]. LNG-powered ships have the advantage of utilizing cold energy, and if low-temperature carbon capture is employed using LNG regasification cold energy, it can effectively reduce CO
2 emissions while also lowering the cost of carbon capture [
55]. However, the concentration of CO
2 in the exhaust gas from the engine is a key factor limiting the efficiency of low-temperature carbon capture on ships [
56], and measures need to be taken to actively control the CO
2 concentration in the exhaust gas to achieve low-temperature carbon capture on ships. Coupling carbon capture technologies can compensate for the limitations of individual carbon capture technologies, aiming to improve the capture efficiency and reduce energy consumption for capture purposes. Su et al. [
57] proposed an integrated ship system that includes power generation, carbon capture, hydrocarbon adsorption, seawater desalination, and cold storage. The results showed that the integrated system is economically feasible, with an output power, energy efficiency, and exergy efficiency of 270.78 kW, 26.89%, and 54.33%, respectively. Oh et al. [
49] proposed a membrane separation coupled low-temperature carbon capture process system based on LNG-powered ships. The results showed that the integrated system is structurally more compact and has a lower capture energy consumption compared to ammonia absorption carbon capture, but it does not utilize flue gas waste heat.
Based on the aforementioned analysis, the current focus of research regarding waste heat recovery in marine exhaust gases lies in enhancing the overall performance and reducing emissions. In comparison to other waste heat recovery cycles, the DPORC presents a significant reduction in irreversible losses during the heat exchange process, consequently resulting in a substantial improvement in the waste heat conversion efficiency [
58]. The utilization of DPORCs for recovering waste heat from marine engines holds great advantages, with SDPORCs exhibiting a superior thermodynamic performance [
23]. However, in previous studies, the mutual interactions within the high-pressure and low-pressure cycles of SDPORCs have often been overlooked, and further supplementation and refinement are still required concerning the selection of the working fluid mixture. As for carbon capture in LNG-powered ships, considering the limited space onboard and the potential for recovering and utilizing LNG regasification chill energy, post-combustion carbon capture proves to be an ideal method. Among the available options, membrane separation technology stands out due to its low energy consumption, small footprint, easy management, and absence of secondary pollution, offering numerous advantages [
59]. By coupling low-temperature carbon capture with membrane separation, the intake concentration of CO
2 during the capture process can be actively regulated, and the high-pressure, low-temperature residual gas obtained after low-temperature carbon capture can be effectively utilized, achieving a low-energy-consumption and high-efficiency carbon capture process while recovering the LNG regasification chill energy. Currently, the research on membrane separation coupled with low-temperature carbon capture in shipping is limited. Additionally, the limited space available on ships makes membrane separation more advantageous. The combination of membrane separation and low-temperature carbon capture can significantly reduce the energy consumption during the capture process, presenting promising prospects for application on ships.
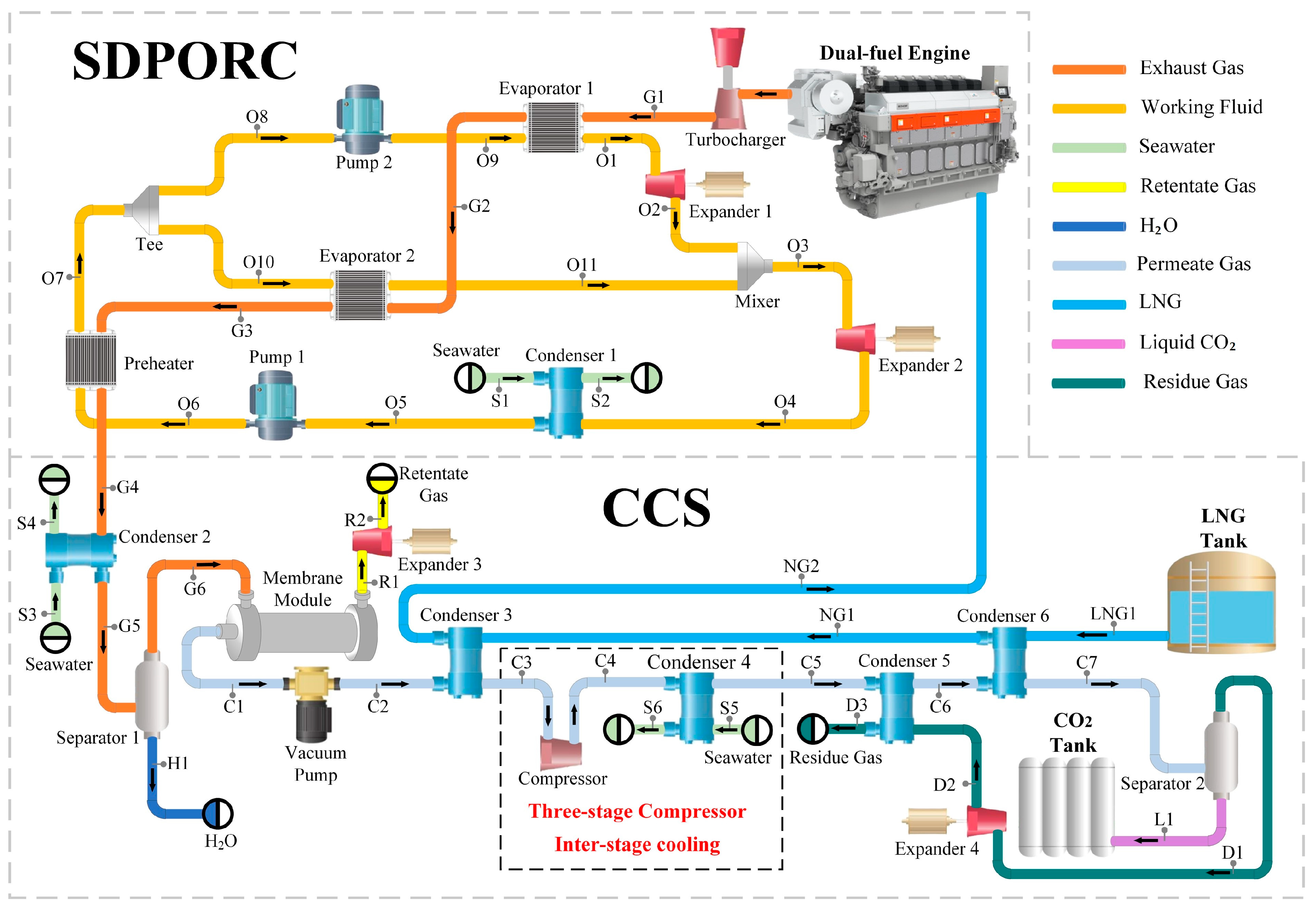
This study proposes an integrated system for low-temperature carbon capture using the coupling of cold and hot energy recovery with membrane separation in LNG-powered ships. The system harnesses the waste heat from LNG-powered ship engines for power generation and utilizes the LNG regasification cold energy for low-temperature CO2 capture, aiming to recover the cold and thermal energy released during the operation of LNG-powered ships. To begin, a mathematical model is established for the integrated system based on thermodynamics. Subsequently, model validation is conducted to ensure accuracy and reliability. The system operating parameters are analyzed comprehensively, while also identifying sensitive parameters. Via the optimization of working fluid and the utilization of genetic algorithms for sensitive parameter optimization, the optimal operating state of the system is determined. Finally, both thermodynamic performance and economic evaluation of the system are conducted. This integrated system offers an economically viable, environmentally friendly, and highly efficient solution, addressing the dual objectives of recovering cold and thermal energy from LNG-powered ships and capturing CO2. Consequently, it meets the requirements for energy conservation and emission reduction and holds the potential to guide on reducing greenhouse gas emissions and improving the energy utilization efficiency.