Deflagration-to-Detonation Transition in a Semi-Confined Slit Combustor Filled with Nitrogen Diluted Ethylene-Oxygen Mixture
Abstract
:1. Introduction
2. Materials and Methods
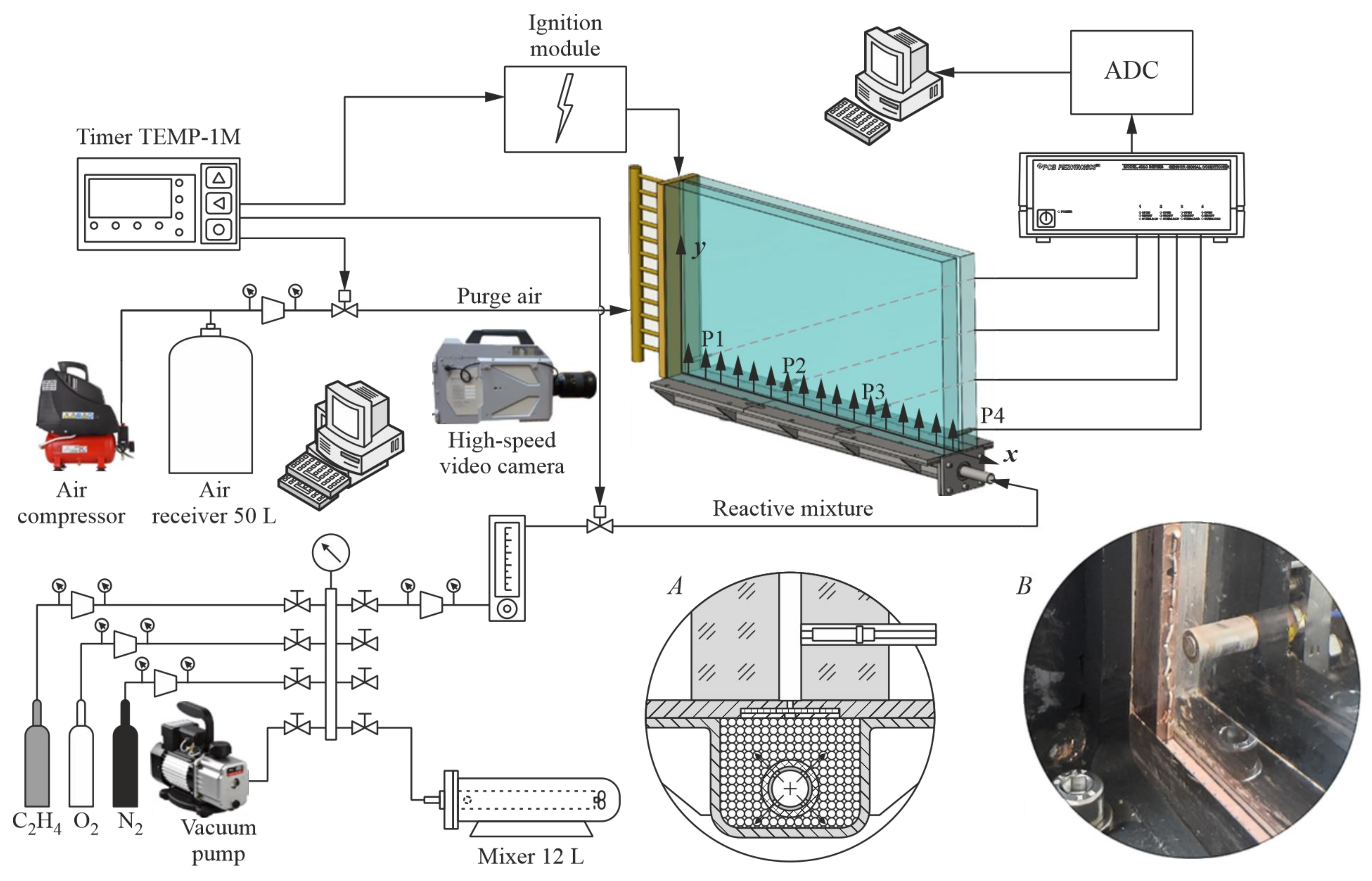
2.1. Experimental Setup
2.2. Methodology
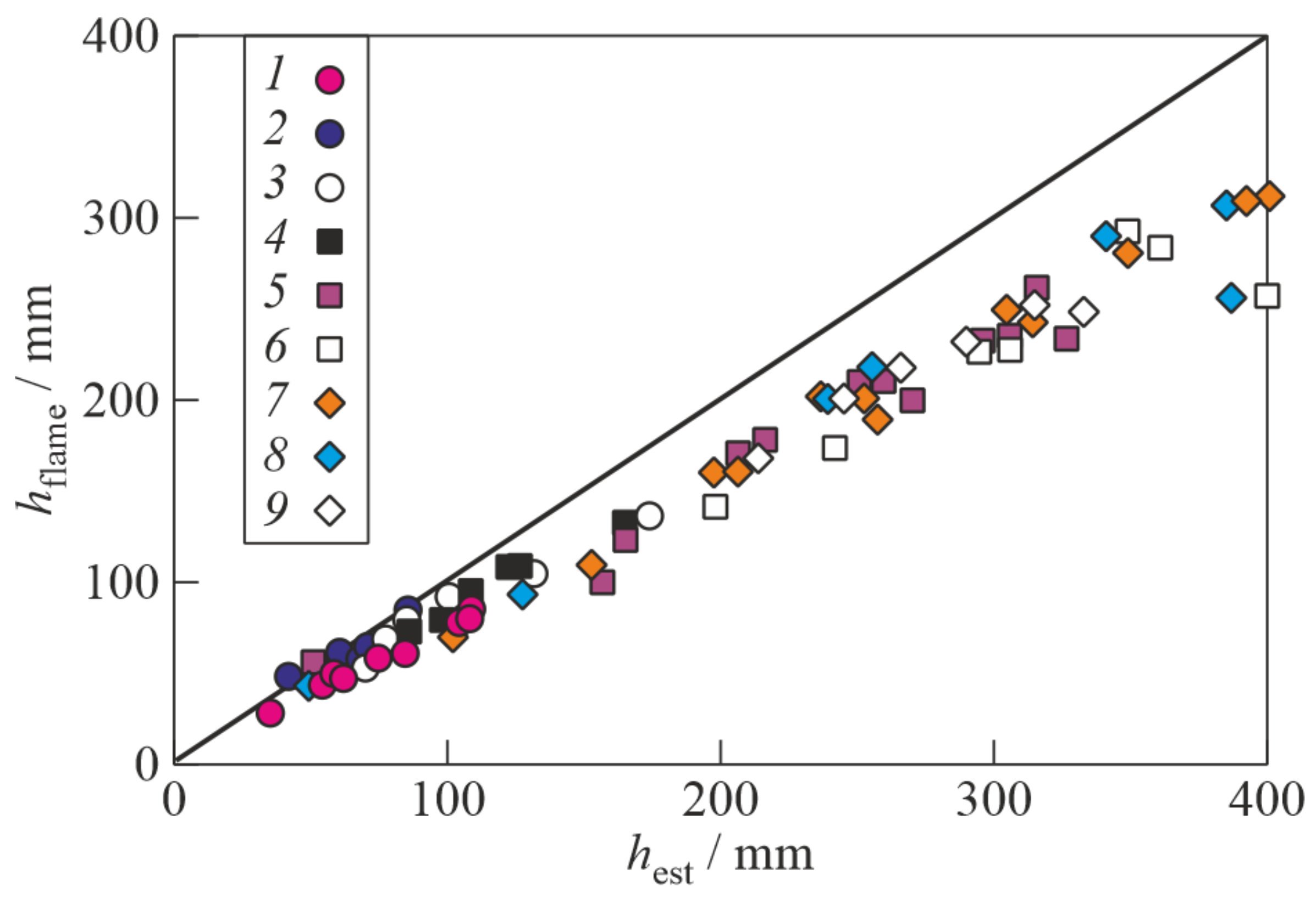
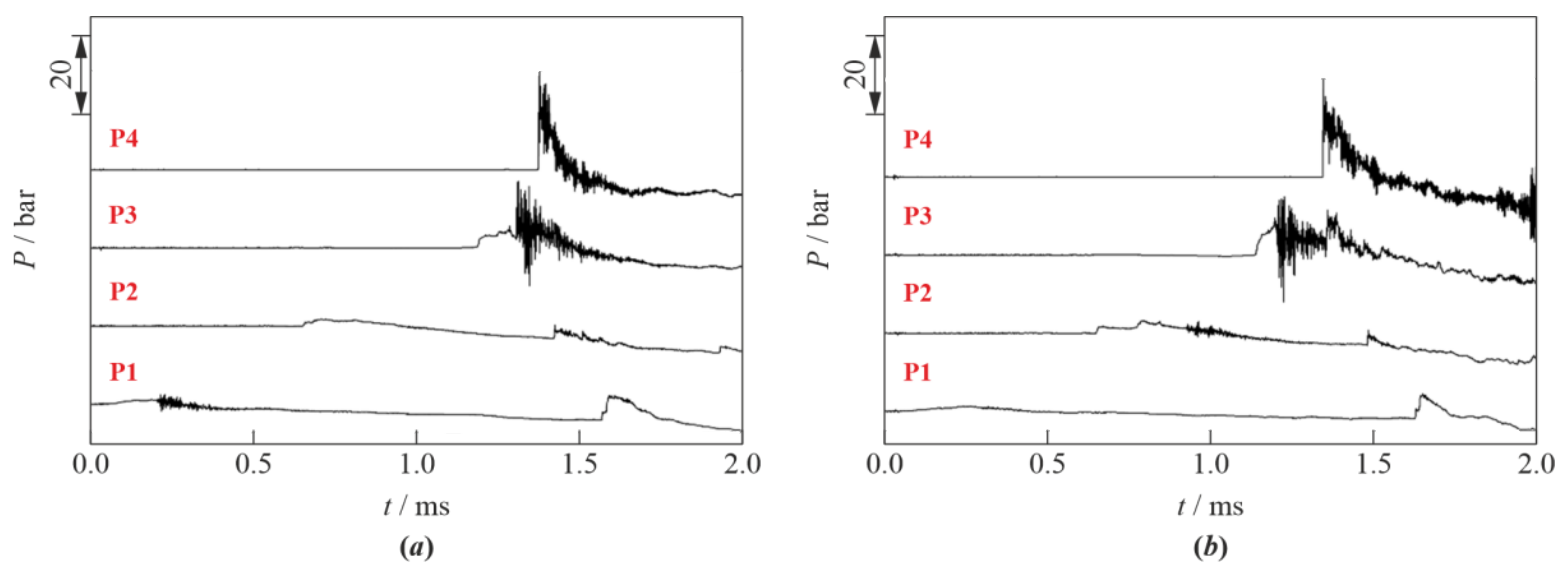
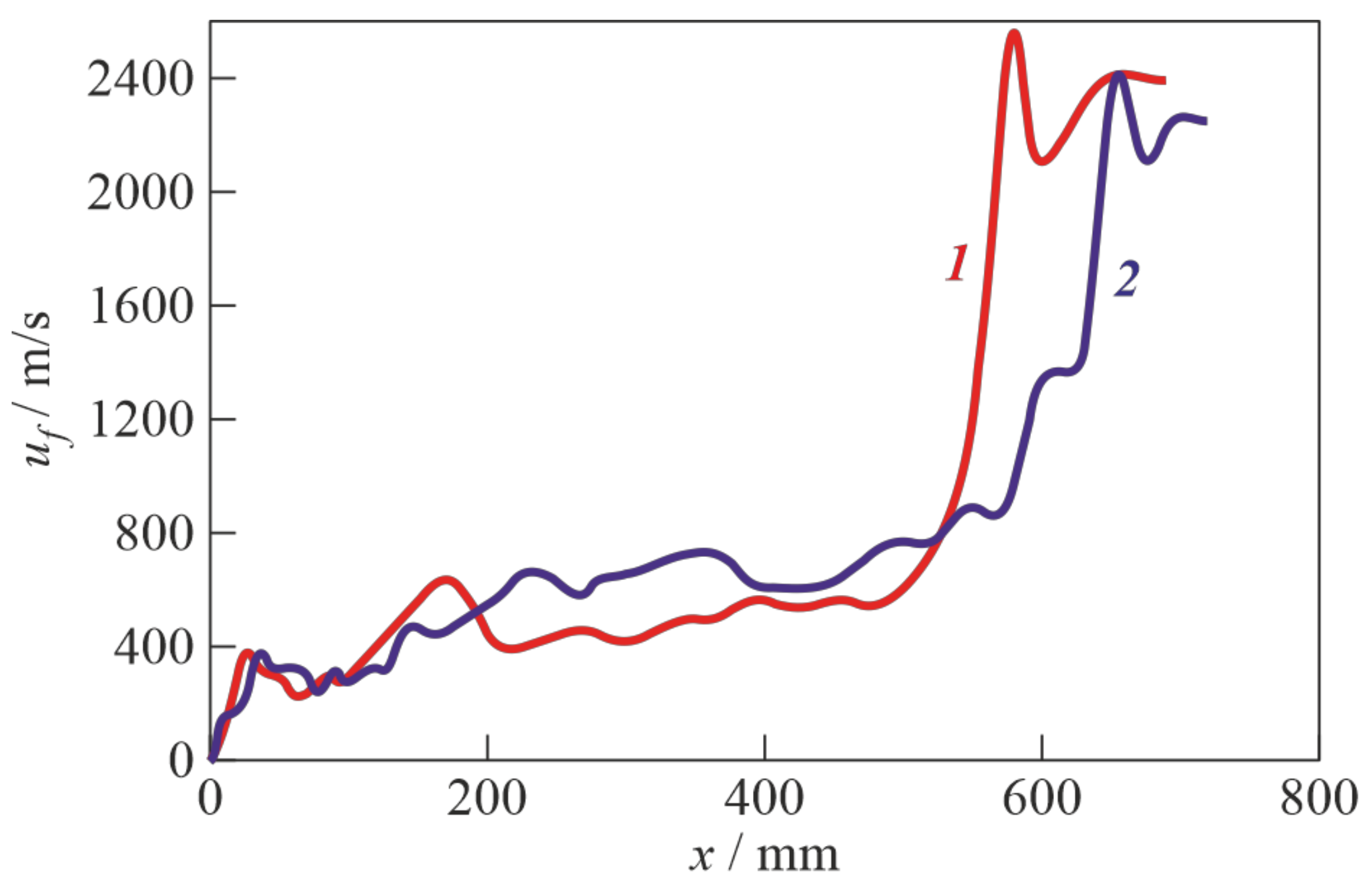
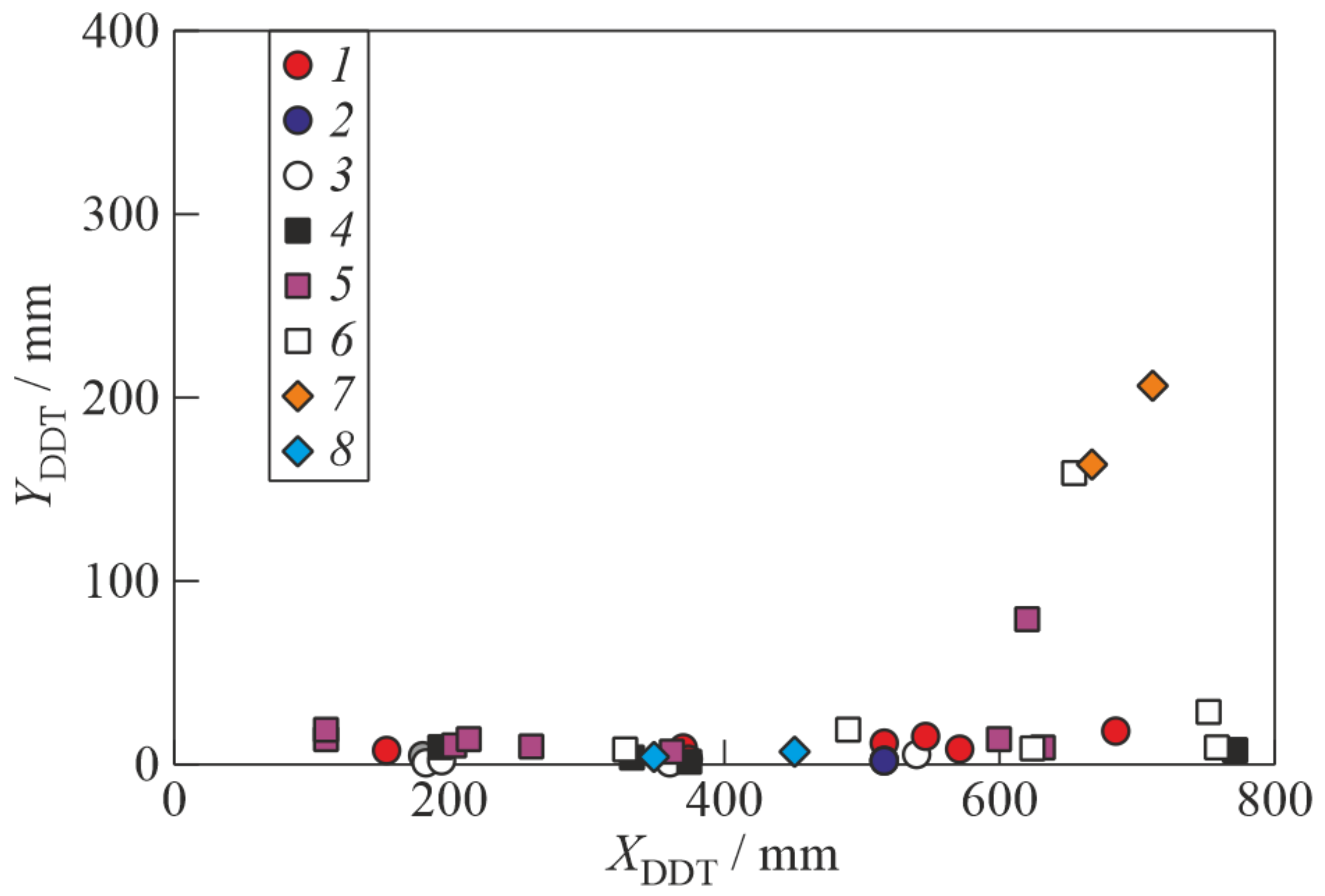
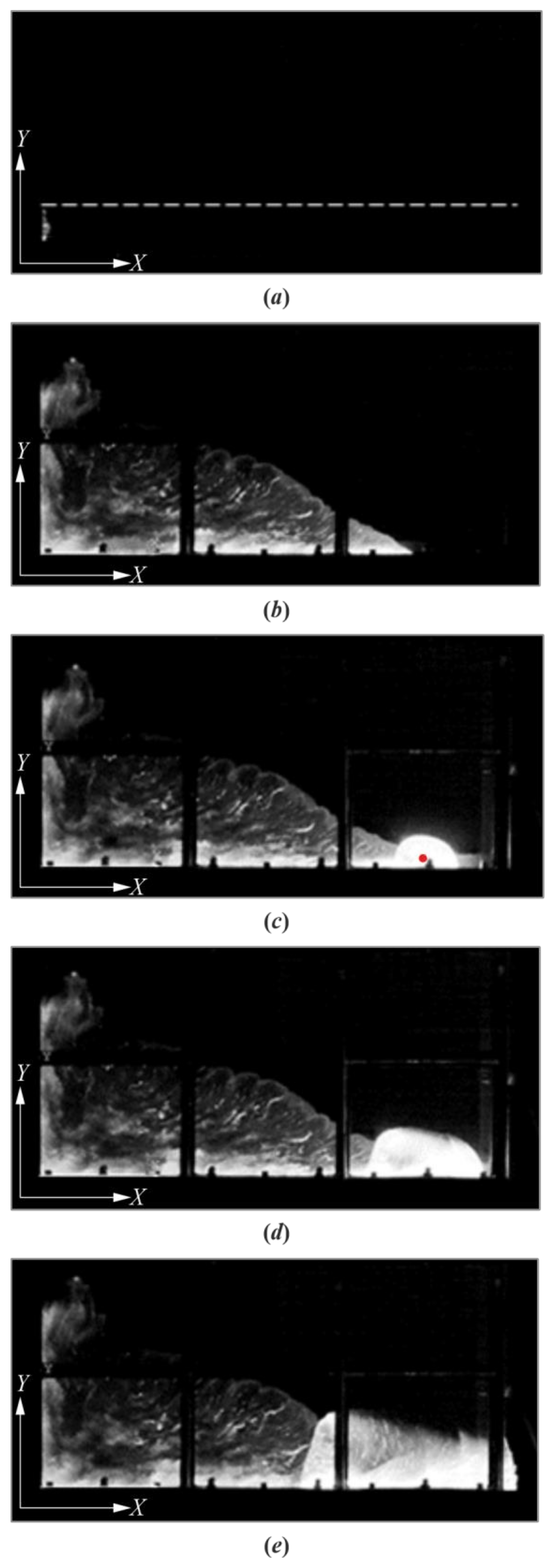
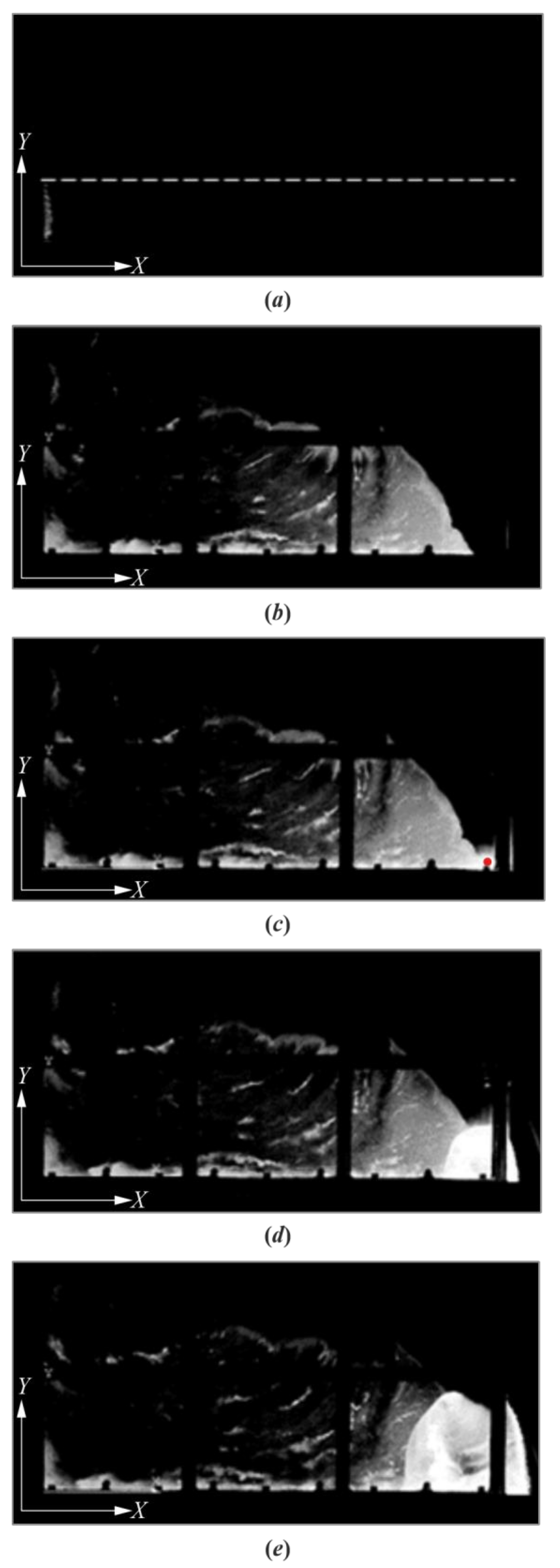
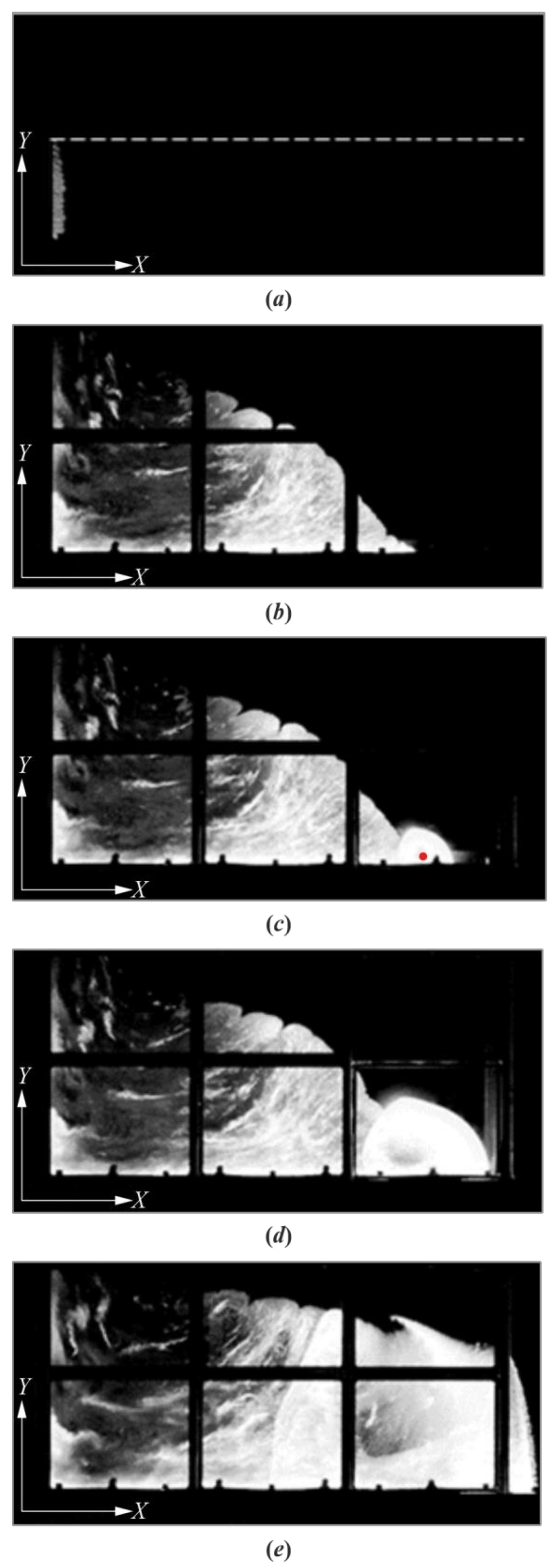
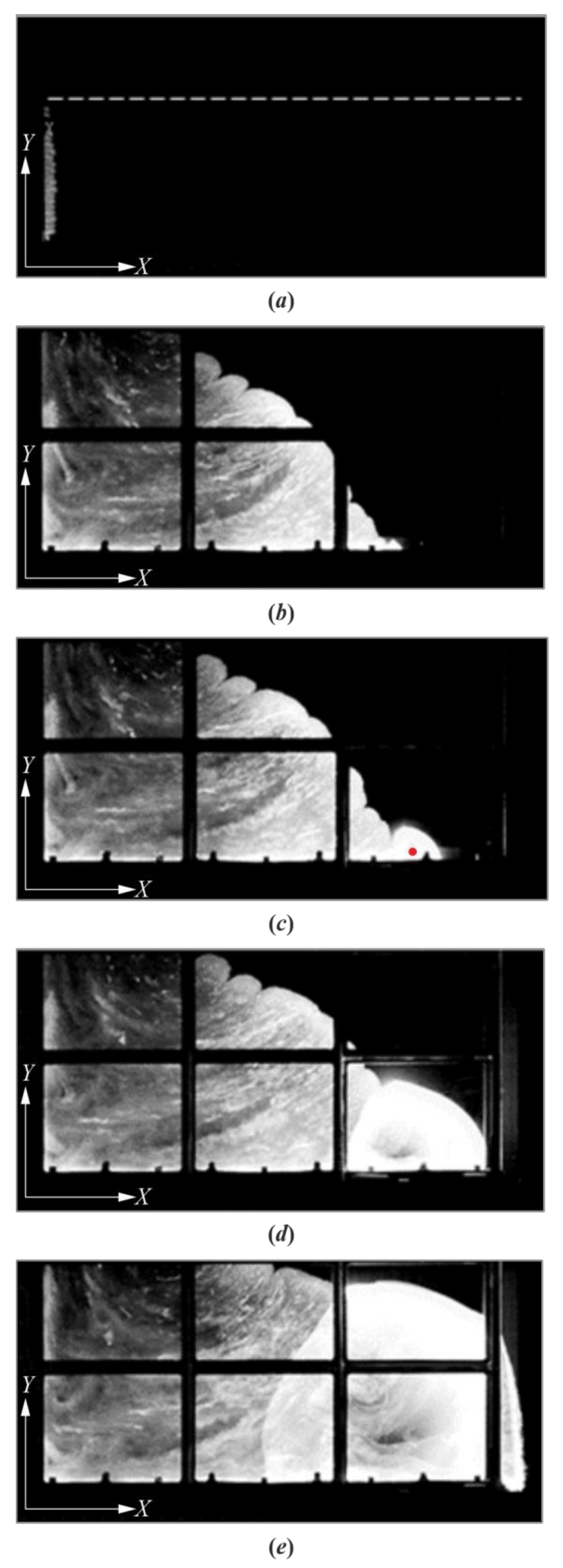
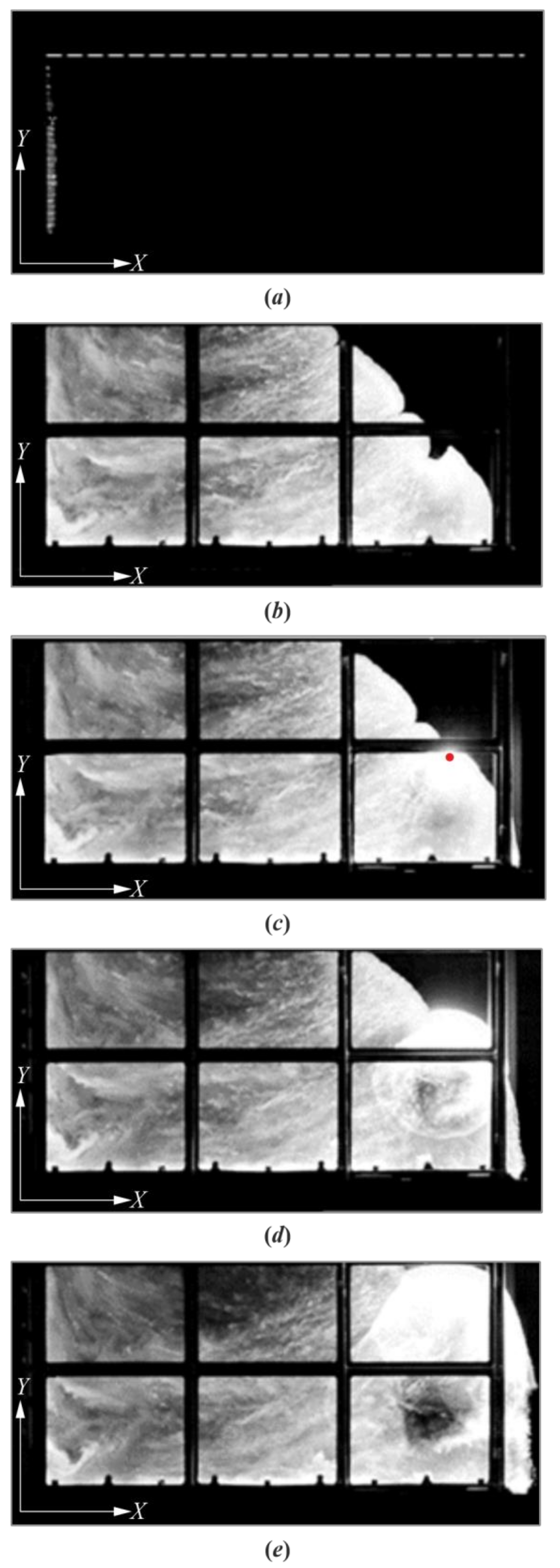
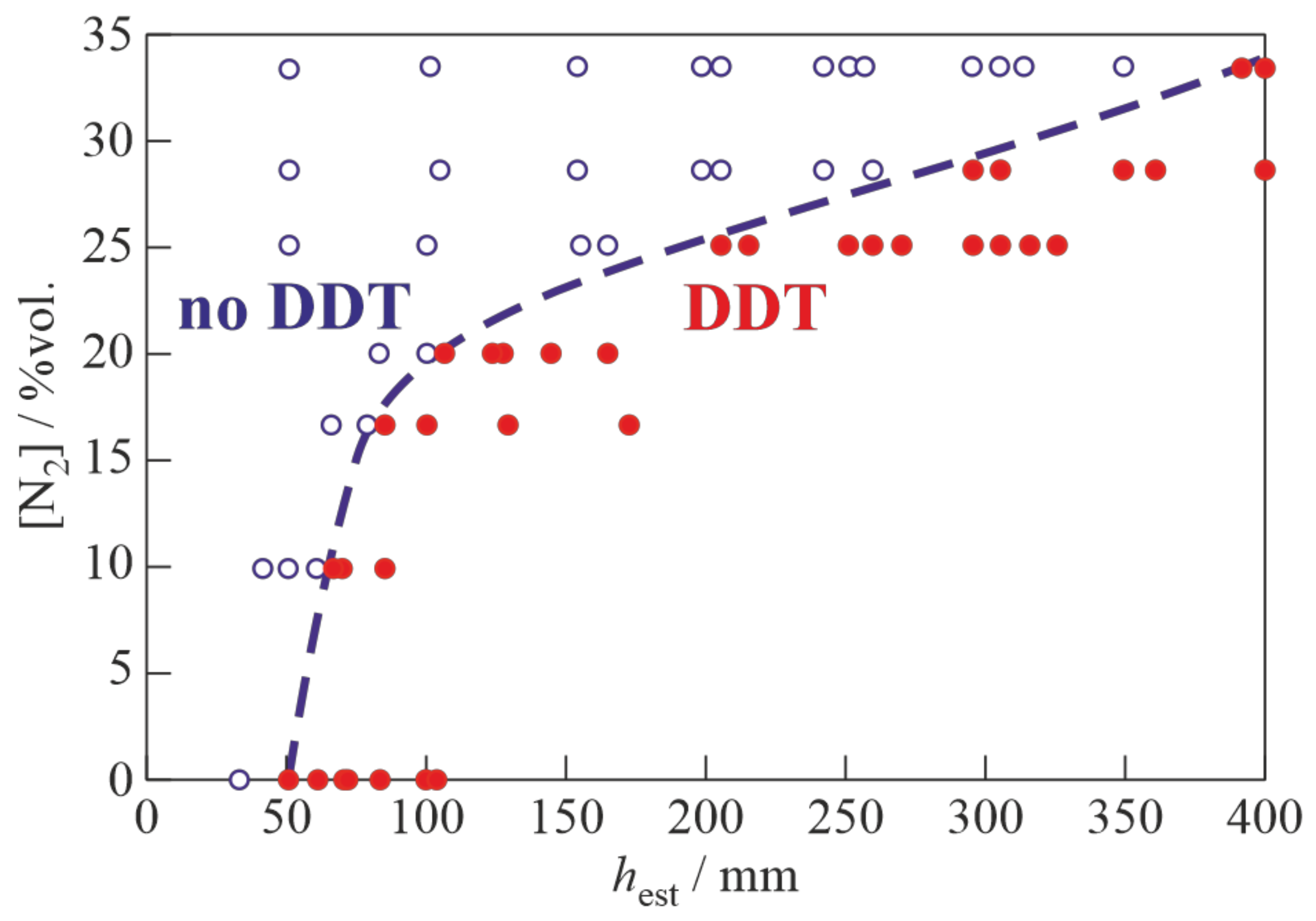
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anand, V.; Gutmark, E. Rotating detonation combustors and their similarities to rocket instabilities. Progr. Energy Combust. Sci. 2019, 73, 182–234. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Song, F.; Xu, S.; Yang, X.; Zheng, Y. Investigation of Rotating Detonation Fueled by Liquid Kerosene. Energies 2022, 15, 4483. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Ivanov, V.S.; Frolov, S.M. Three-dimensional numerical simulation of the operation process in a continuous detonation combustor with separate feeding of hydrogen and air. Russ. J. Phys. Chem. B 2015, 9, 104–119. [Google Scholar] [CrossRef]
- Voitsekhovskii, B.V. Stationary detonation. Dokl. Akad. Nauk SSSR 1959, 129, 1254–1256. [Google Scholar]
- Sommers, W.P.; Morrison, R.B. Simulation of condensed-explosive detonation phenomena with gases. Phys. Fluids 1962, 5, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Dabora, E.K.; Nicholls, J.A.; Morrison, R.B. The influence of a compressible boundary on the propagation of gaseous detonations. Proc. Symp. (Intern.) Combust. 1965, 10, 817–830. [Google Scholar] [CrossRef]
- Adams, T.G. Do weak detonation waves exist? AIAA J. 1978, 16, 1035–1040. [Google Scholar] [CrossRef]
- Ivanov, M.F.; Fortov, V.E.; Borisov, A.A. Numerical simulation of the development of a detonation in gas volumes of finite thickness. Combust. Explo. Shock Waves 1981, 17, 332–338. [Google Scholar] [CrossRef]
- Rudy, W.; Kuznetsov, M.; Porowski, R.; Teodorczyk, A.; Grune, J.; Sempert, K. Critical conditions of hydrogen–air detonation in partially confined geometry. Proc. Combust. Inst. 2013, 34, 1965–1972. [Google Scholar] [CrossRef]
- Kuznetsov, M.; Yanez, J.; Grune, J.; Friedrich, A.; Jordan, T. Hydrogen combustion in a flat semiconfined layer with respect to the Fukushima Daiichi accident. Nucl. Eng. Des. 2015, 286, 36–48. [Google Scholar] [CrossRef]
- Li, J.; Mi, X.; Higgins, A.J. Geometric scaling for a detonation wave governed by a pressure-dependent reaction rate and yielding confinement. Phys. Fluids 2015, 27, 027102. [Google Scholar] [CrossRef]
- Reynaud, M.; Virot, F.; Chinnayya, A. A computational study of the interaction of gaseous detonations with a compressible layer. Phys. Fluids 2017, 29, 056101. [Google Scholar] [CrossRef]
- Bykovskii, F.A.; Zhdan, S.A.; Vedernikov, E.F. Continuous spin detonations. J. Propul. Power 2006, 22, 1204–1216. [Google Scholar] [CrossRef]
- Grune, J.; Sempert, K.; Haberstroh, H.; Kuznetsov, M.; Jordan, T. Experimental investigation of hydrogen–air deflagrations and detonations in semiconfined flat layers. J. Loss Prevent. Proc. Ind. 2013, 26, 317–323. [Google Scholar] [CrossRef]
- Shamshin, I.O.; Ivanov, V.S.; Aksenov, V.S.; Gusev, P.A.; Frolov, S.M. Experimental study of the initial stage of the operation process in detonation rocket and air-breathing engines. In Advances in Detonation Research; Frolov, S.M., Ed.; Torus Press: Moscow, Russia, 2022; pp. 17–20. [Google Scholar] [CrossRef]
- Taguchi, T.; Yamaguchi, M.; Matsuoka, K.; Kawasaki, A.; Watanabe, H.; Itouyama, N.; Kasahara, J.; Matsuo, A. Investigation of reflective shuttling detonation cycle by schlieren and chemiluminescence photography. Combust. Flame 2022, 236, 111826. [Google Scholar] [CrossRef]
- Wu, M.-H.; Kuo, W.-C. Transmission of near-limit detonation wave through a planar sudden expansion in a narrow channel. Combust. Flame 2012, 159, 3414–3422. [Google Scholar] [CrossRef]
- Matsui, H.; Lee, J.H. On the measure of the relative detonation hazards of gaseous fuel–oxygen and air mixtures. Proc. Symp. (Intern.) Combust. 1979, 17, 1269–1280. [Google Scholar] [CrossRef]
- Moen, I.O.; Donato, M.; Knystautas, R.; Lee, J.H. The influence of confinement on the propagation of detonations near the detonability limits. Proc. Symp. (Intern.) Combust. 1981, 18, 1615–1622. [Google Scholar] [CrossRef]
- Kawasaki, A.; Kasahara, J. A novel characteristic length of detonation relevant to supercritical diffraction. Shock Waves 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Gelfand, B.E.; Frolov, S.M.; Nettleton, M.A. Gaseous detonations-a selective review. Prog. Energy Combust. Sci. 1991, 17, 327–371. [Google Scholar] [CrossRef]

| No. | Mixture | %vol. | , m/s | , K | , bar | , mm | |
|---|---|---|---|---|---|---|---|
| 1 | C2H4+3O2 | 0 | 0 | 2376 | 3938 | 33.9 | 50 |
| 2 | N2) | 0.11 | 10.0 | 2331 | 3879 | 32.4 | 65 |
| 3 | N2) | 0.20 | 16.7 | 2299 | 3840 | 31.4 | 80 |
| 4 | N2) | 0.25 | 20.0 | 2283 | 3819 | 30.9 | 110 |
| 5 | N2) | 0.33 | 25.0 | 2259 | 3785 | 30.2 | 200 |
| 6 | N2) | 0.40 | 28.6 | 2240 | 3760 | 29.6 | 290 |
| 7 | N2) | 0.50 | 33.3 | 2215 | 3722 | 28.8 | 390 |
| 8 | N2) | 0.60 | 37.5 | 2191 | 3687 | 28.1 | >400 |
| 9 | N2) | 0.67 | 40.0 | 2177 | 3664 | 27.6 | >400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamshin, I.O.; Ivanov, V.S.; Aksenov, V.S.; Gusev, P.A.; Frolov, S.M. Deflagration-to-Detonation Transition in a Semi-Confined Slit Combustor Filled with Nitrogen Diluted Ethylene-Oxygen Mixture. Energies 2023, 16, 1098. https://doi.org/10.3390/en16031098
Shamshin IO, Ivanov VS, Aksenov VS, Gusev PA, Frolov SM. Deflagration-to-Detonation Transition in a Semi-Confined Slit Combustor Filled with Nitrogen Diluted Ethylene-Oxygen Mixture. Energies. 2023; 16(3):1098. https://doi.org/10.3390/en16031098
Chicago/Turabian StyleShamshin, Igor O., Vladislav S. Ivanov, Viktor S. Aksenov, Pavel A. Gusev, and Sergey M. Frolov. 2023. "Deflagration-to-Detonation Transition in a Semi-Confined Slit Combustor Filled with Nitrogen Diluted Ethylene-Oxygen Mixture" Energies 16, no. 3: 1098. https://doi.org/10.3390/en16031098






