Application of Capacitive Deionization in Water Treatment and Energy Recovery: A Review
(This article belongs to the Section B: Energy and Environment)
Abstract
:1. Introduction

2. Application Progress of CDI Technology in Water Treatment
2.1. Water Desalination
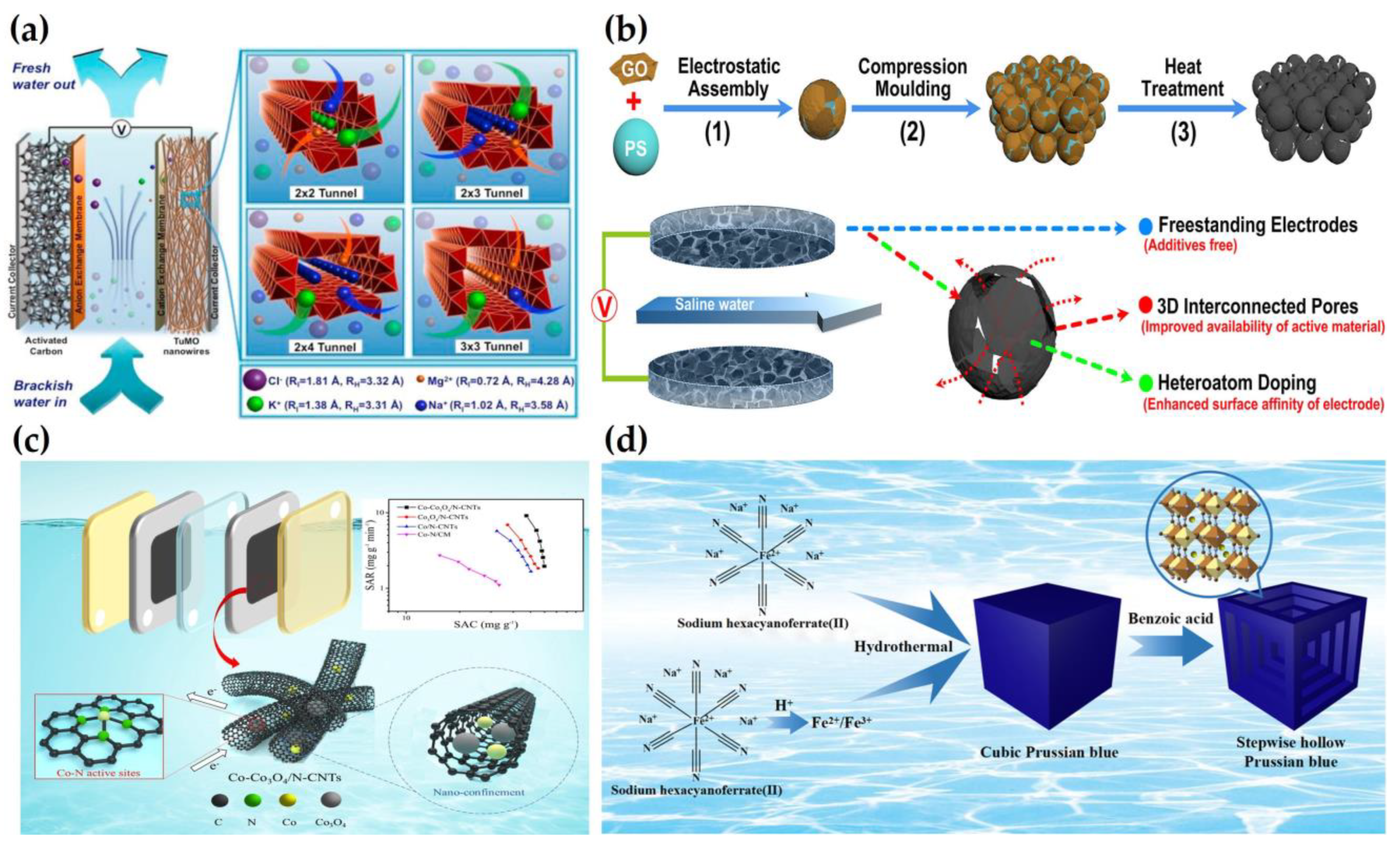
2.2. Water Softening
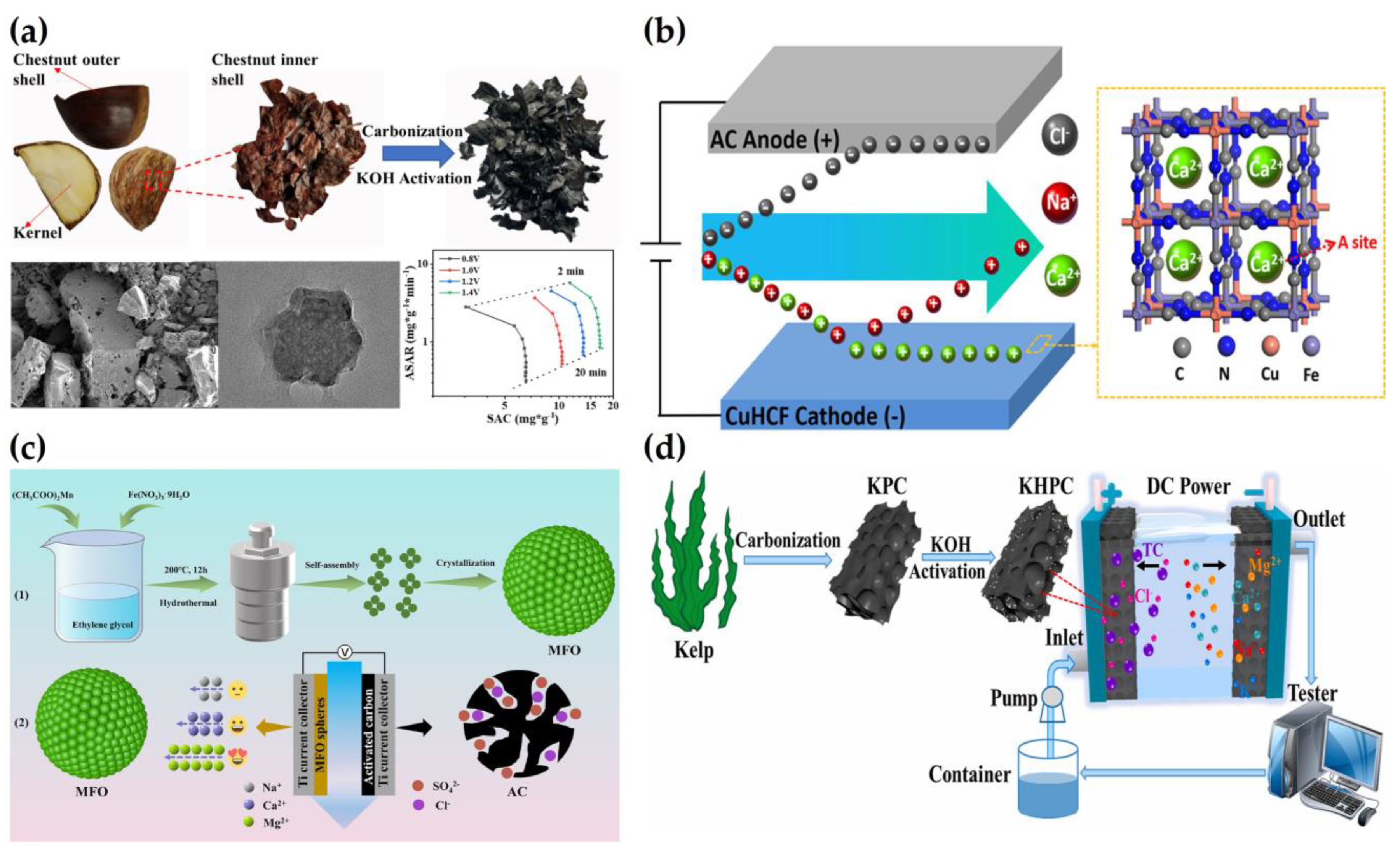
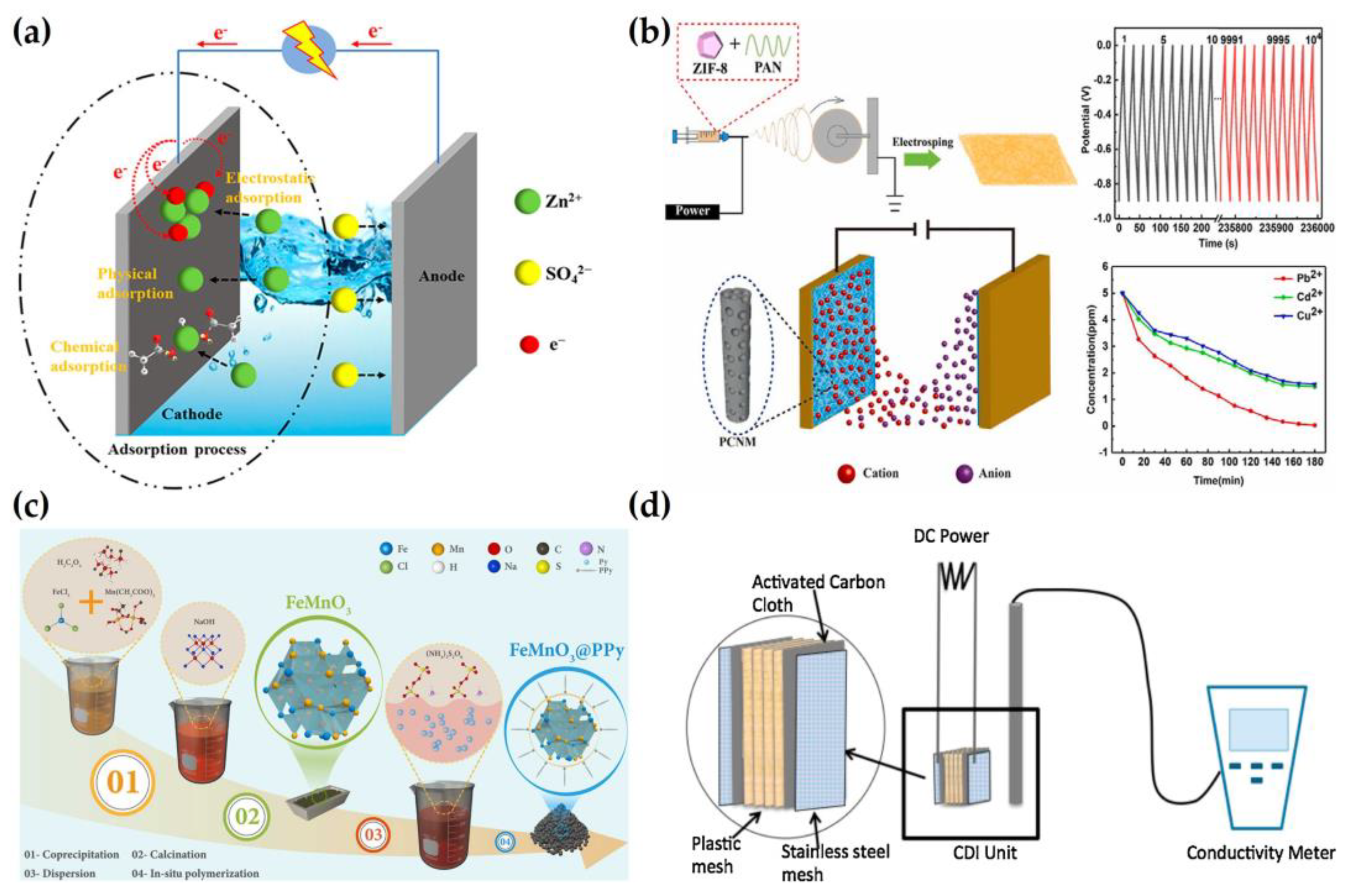
2.3. Removal of Heavy Metals
| Pollutant | Concentration (mg/L) | Electrodes | Flow Rate (mL/min) | Applied Voltage (V) | Removal Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Cu2+ | 96 | Activated carbon | 10 | - | 94.00% | [99] |
| Pb2+ | 50 | self-supporting porous carbon nanofiber membrane | 10 | 1.8 | 99.42% | [98] |
| Cu2+ | 68.46% | |||||
| Cd2+ | 70.36% | |||||
| Zn2+ | 40 | Nitrogen-doped porous carbon | 20 | 1.0 | 33.10 mg/g | [108] |
| As | 0.02–0.1 | Activated carbon | 3000 | 1–1.5 | >80% | [109] |
| Cd2+ | 20 | Perovskite oxide | 20 | 1.2 | 144.60 mg/g | [100] |
| Pb2+ | 100 | Wood-converted Carbon | 5 | 1.2 | 19.52 mg/g | [107] |
| Cr2+ | 50 | 20.00 mg/g | ||||
| V5+ | 1500 | Composite materials based on ion-exchange resin and activated carbon | 30 | - | 106.89 mg/g | [101] |
| V5+ | 750 | Composite materials based on porous carbon derived from ZIF-8 and activated carbon | 30 | 1.2 | 77.27 mg/g | [102] |
| U6+ | 100 | highly porous phosphate-functionalized graphene | 50 | 1.2 | 545.70 mg/g | [110] |
| Ni2+ | 50 | Multi-walled CNTs | 50 | 1.5 | 145.73 mg/g | [111] |
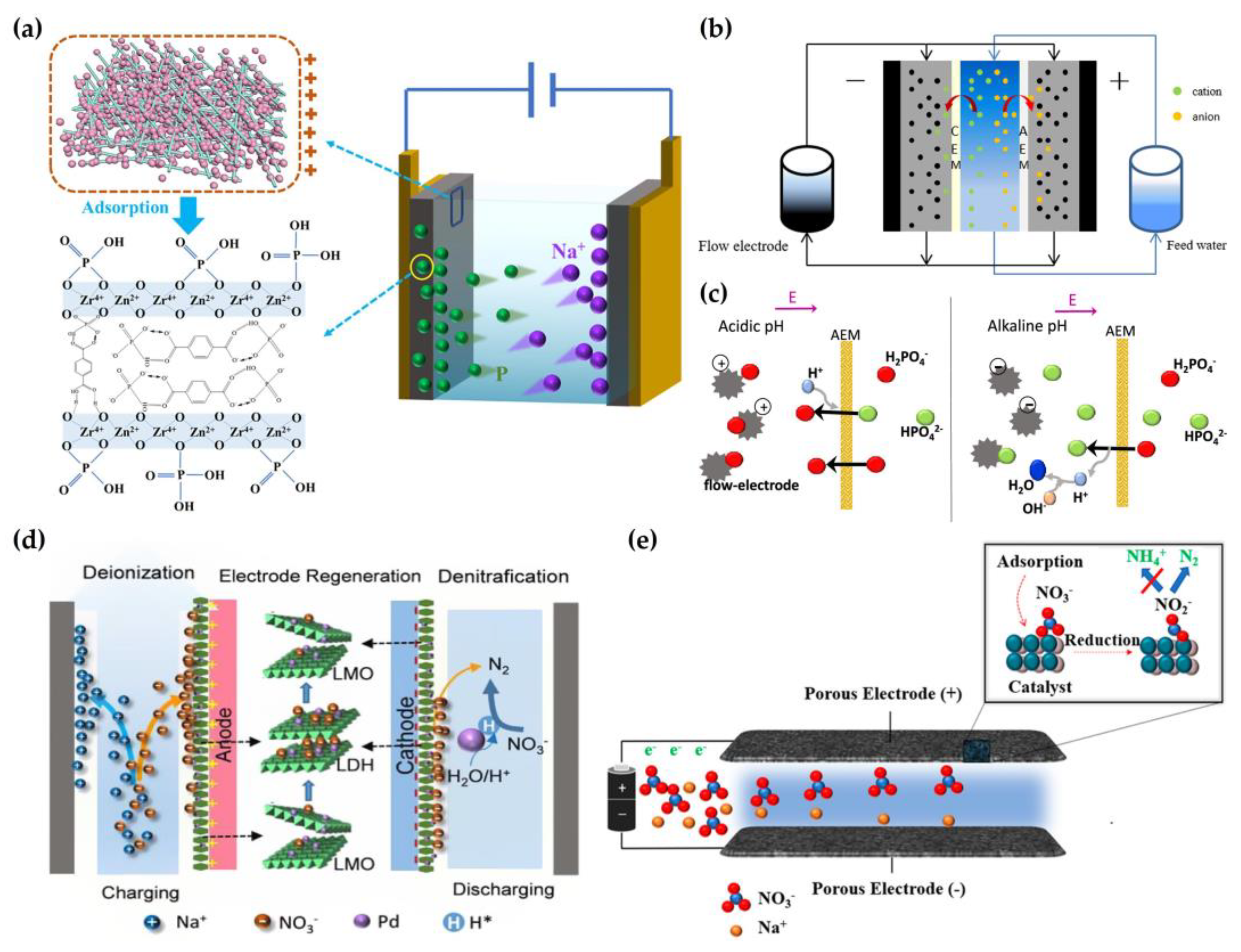
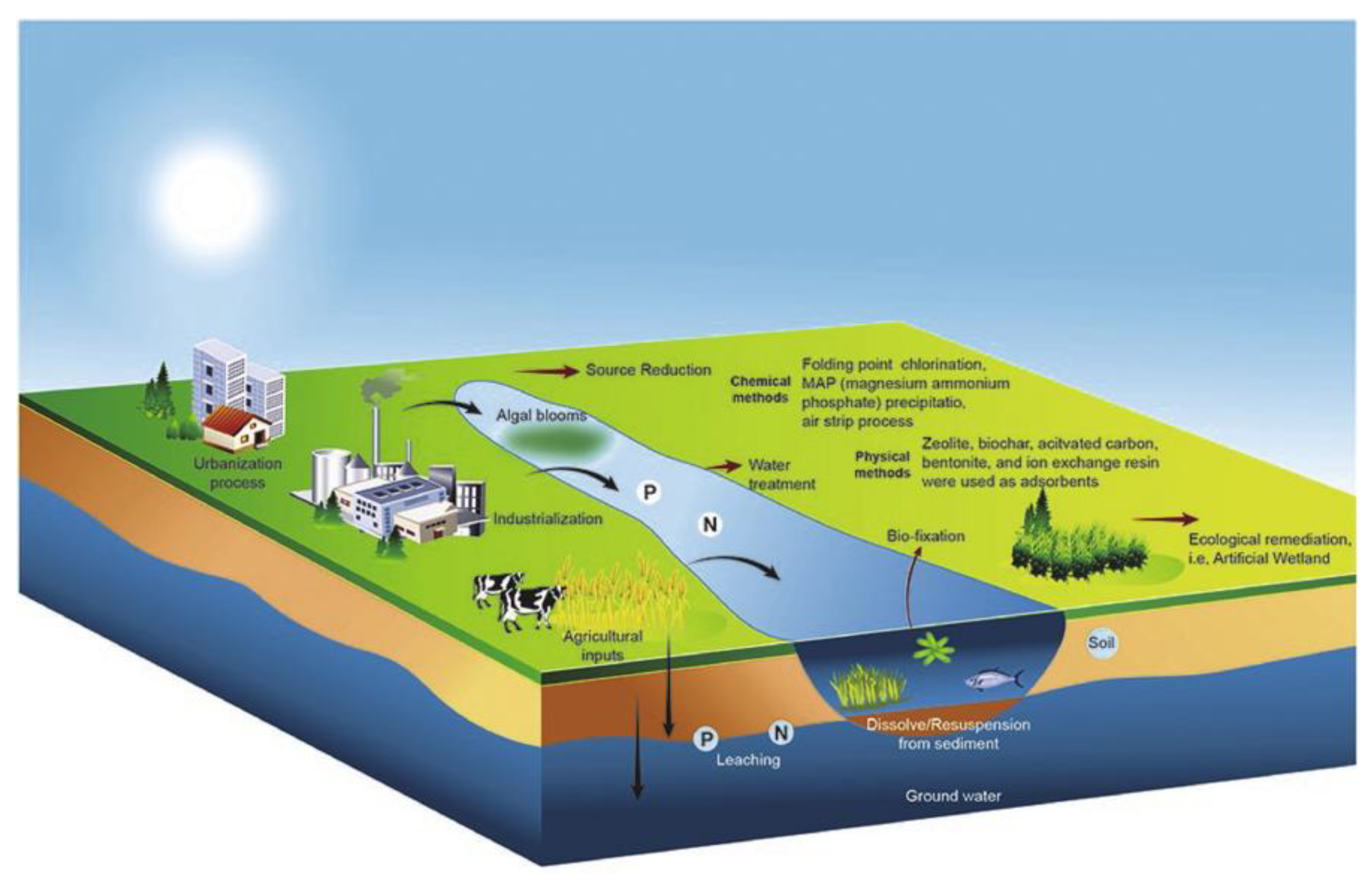
2.4. Removal of Phosphate and Nitrate
| Pollutant | Concentration | CDI type | Applied Voltage (V) | Removal Efficiency | pH | Energy Consumption | Ref. |
|---|---|---|---|---|---|---|---|
| P | 6 mg/L | CDI | 1.2 | 13.65 mg/g | 7 | 0.0075 kWh/g-P | [123] |
| P | 2.5 mM | CDI | 1.2 | 35 mg/g | 6.5 | - | [135] |
| P | 62 mg/L | FCDI | - | 164 mg/L per cycle | 8.3 | 27.8 kWh/kg-P | [136] |
| P | 8 mg/L | FCDI | - | 61.9% | - | 21.8 kWh/kg-P | [137] |
| P | 100 mg/L | FCDI | 1.2 | 97% | 5 | - | [124] |
| P | 500 mg/L | FCDI | 1.2 | 38.3 mg/min | 5 | 0.59 kWh/kg-P and 0.043 kWh/m3-water | [125] |
| N | 0.5 mM | CDI | 1.2 | 2.4 × 10−3 mmol/m2 | 5.6 | 248.8 kJ/mol-NO3− | [138] |
| N | 50 mg/L | CCDI | 1.5 | 91% | - | 13.2 kWh·m−3 order−1 | [134] |
| N | 42 mg/L | CCDI | 1.0 | - | 6.5 | - | [133] |
| N | 200 mg/L | MCDI | 1.8 | 53.3% | 5.32 | - | [139] |
| N | 70 mg/L | CDI | 2.0 | 60.01% | 6 | - | [130] |
| N | 250 mg/L | MCDI | 0.8 | 83.07% | - | - | [131] |
2.5. Removal of Organic Contaminants
2.6. Water Disinfection
3. Energy Recovery and Energy Consumption Assessment
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adam, M.; Othman, M.; Kurniawan, T.; Puteh, M.; Ismail, A.; Khongnakorn, W.; Rahman, M.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Liu, E.; Lee, L.; Ong, S.; Ng, H. Treatment of industrial brine using capacitive deionization (CDI) towards zero liquid discharge—Challenges and optimization. Water Res. 2020, 183, 116059. [Google Scholar] [CrossRef] [PubMed]
- Shahmirzadi, M.; Hosseini, S.; Luo, J.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef] [PubMed]
- Ahualli, S.; Iglesias, G.; Delgado, Á. Principles and theoretical models of CDI: Experimental approaches. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–192. [Google Scholar]
- Khosravi, R.; Moussavi, G.; Ghaneian, M.; Ehrampoush, M.; Barikbin, B.; Ebrahimi, A.; Sharifzadeh, G. Chromium adsorption from aqueous solution using novel green nanocomposite: Adsorbent characterization, isotherm, kinetic and thermodynamic investigation. J. Mol. Liq. 2018, 256, 163–174. [Google Scholar] [CrossRef]
- Zahoran, R.; Kukovecz, A.; Toth, A.; Horvath, D.; Schuszter, G. High-speed tracking of fast chemical precipitations. Phys. Chem. Chem. Phys. 2019, 21, 11345–11350. [Google Scholar] [CrossRef] [Green Version]
- Zha, L.; Li, J.; Zhou, B.; Yuan, Y.; Yuan, H. Research progress on the ion exchange treatment and resource utilization of chromium(Ⅵ) electroplating wastewater. Mater. Prot. 2021, 54, 123–126. [Google Scholar]
- Chen, B.; Bao, S.; Zhang, Y.; Ren, L. A novel and sustainable technique to precipitate vanadium from vanadium-rich solutions via efficient ultrasound irradiation. J. Clean. Prod. 2022, 339, 130755. [Google Scholar] [CrossRef]
- Jarin, M.; Dou, Z.; Gao, H.; Chen, Y.; Xie, X. Salinity exchange between seawater/brackish water and domestic wastewater through electrodialysis for potable water. Front. Environ. Sci. Eng. 2023, 17, 16. [Google Scholar] [CrossRef]
- Xing, W.; Liang, J.; Tang, W.; He, D.; Yan, M.; Wang, X.; Luo, Y.; Tang, N.; Huang, M. Versatile applications of capacitive deionization (CDI)-based technologies. Desalination 2020, 482, 114390. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Bao, S.; Song, S. Desalination by capacitive deionization with carbon-based materials as electrode: A review. Surf. Rev. Lett. 2013, 20, 1330003. [Google Scholar] [CrossRef]
- Suss, M.; Porada, S.; Sun, X.; Biesheuvel, P.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Y.; Wang, R.; Wu, Y.; Xu, S.; Wang, J. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Tang, W.; Liang, J.; He, D.; Gong, J.; Tang, L.; Liu, Z.; Wang, D.; Zeng, G. Various cell architectures of capacitive deionization: Recent advances and future trends. Water Res. 2019, 150, 225–251. [Google Scholar] [CrossRef]
- Suss, M.; Presser, V. Water Desalination with Energy Storage Electrode Materials. Joule 2018, 2, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T. Faradaic reactions in capacitive deionization (CDI)–Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.; Eum, H.; Lee, C. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X.; Ding, Z.; Wang, K.; Sun, X.; Lu, T.; Konarova, M.; Eguchi, M.; Shapter, J.; Pan, L.; et al. Ti3C2 MXenes-derived NaTi2(PO4)3/MXene nanohybrid for fast and efficient hybrid capacitive deionization performance. Chem. Eng. J. 2021, 407, 127148. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Ratajczak, P.; Suss, M.; Kaasik, F.; Beguin, F. Carbon electrodes for capacitive technologies. Energy Storage Mater. 2019, 16, 126–145. [Google Scholar] [CrossRef]
- Jeon, S.; Park, H.; Yeo, J.; Yang, S.; Cho, C.; Han, M.; Kim, D. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Fang, J.; Chu, B.; Li, N.; Shui, L.; Wang, G.; Chen, F. The optimized flow-electrode capacitive deionization (FCDI) performance by ZIF-8 derived nanoporous carbon polyhedron. Sep. Purif. Technol. 2022, 281, 119345. [Google Scholar] [CrossRef]
- Epshtein, A.; Nir, O.; Monat, L.; Gendel, Y. Treatment of acidic wastewater via fluoride ions removal by SiO2 particles followed by phosphate ions recovery using flow-electrode capacitive deionization. Chem. Eng. J. 2020, 400, 125892. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, K.; Yang, S.; Choi, J.; Park, H.; Kim, D. A novel three-dimensional desalination system utilizing honeycomb-shaped lattice structures for flow-electrode capacitive deionization. Energy Environ. Sci. 2017, 10, 1746–1750. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, C.; Yoon, J. Na2FeP2O7 as a Novel Material for Hybrid Capacitive Deionization. Electrochim. Acta 2016, 203, 265–271. [Google Scholar] [CrossRef]
- Divyapriya, G.; Vijayakumar, K.; Nambi, I. Development of a novel graphene/Co3O4 composite for hybrid capacitive deionization system. Desalination 2019, 451, 102–110. [Google Scholar] [CrossRef]
- Jacob, L.; Prasanna, K.; Vengatesan, M.; Santhoshkumar, P.; Lee, C.; Mittal, V. Binary Cu/ZnO decorated graphene nanocomposites as an efficient anode for lithium ion batteries. J. Ind. Eng. Chem. 2018, 59, 108–114. [Google Scholar] [CrossRef]
- Blair, J.; Murphy, G. Electrochemical Demineralization of Water with Porous Electrodes of Large Surface Area; ACS Publications: Washington, DC, USA, 1960. [Google Scholar]
- Ryoo, M.; Kim, J.; Seo, G. Role of titania incorporated on activated carbon cloth for capacitive deionization of NaCl solution. J. Colloid Interface Sci. 2003, 264, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, M.; Seo, G. Improvement in capacitive deionization function of activated carbon cloth by titania modification. Water Res. 2003, 37, 1527–1534. [Google Scholar] [CrossRef]
- Hu, C.; Liu, F.; Lan, H.; Liu, H.; Qu, J. Preparation of a manganese dioxide/carbon fiber electrode for electrosorptive removal of copper ions from water. J. Colloid Interface Sci. 2015, 446, 359–365. [Google Scholar] [CrossRef]
- Chou, T.; Huang, C.; Doong, R.; Hu, C. Architectural design of hierarchically ordered porous carbons for high-rate electrochemical capacitors. J. Mater. Chem. A 2013, 1, 2886–2895. [Google Scholar] [CrossRef]
- Wan, C.; Jiao, Y.; Li, J. Core-shell composite of wood-derived biochar supported MnO2 nanosheets for supercapacitor applications. RSC Adv. 2016, 6, 64811–64817. [Google Scholar] [CrossRef]
- Athouel, L.; Arcidiacono, P.; Ramirez-Castro, C.; Crosnier, O.; Hamel, C.; Dandeville, Y.; Guillemet, P.; Scudeller, Y.; Guay, D.; Belanger, D.; et al. Investigation of cavity microelectrode technique for electrochemical study with manganese dioxides. Electrochim. Acta 2012, 86, 268–276. [Google Scholar] [CrossRef]
- Younes, H.; Zou, L. Asymmetric configuration of pseudocapacitive composite and rGO electrodes for enhanced capacitive deionization. Environ. Sci.-Water Res. Technol. 2020, 6, 392–403. [Google Scholar] [CrossRef]
- Adorna, M., Jr.; Borines, M.; Van Dien, D.; Doong, R. Coconut shell derived activated biochar-manganese dioxide nanocomposites for high performance capacitive deionization. Desalination 2020, 492, 114602. [Google Scholar] [CrossRef]
- Byles, B.; Cullen, D.; More, K.; Pomerantseva, E. Tunnel structured manganese oxide nanowires as redox active electrodes for hybrid capacitive deionization. Nano Energy 2018, 44, 476–488. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Wang, Y.; Huang, Y.; Duan, X. Functionalized graphene hydrogel-based high-performance supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Zhao, Z.; Lin, Z.; Lee, C.; Xu, X.; Wang, C.; Huang, Y.; Shakir, M.; Duan, X. Solution processable holey graphene oxide and its derived macrostructures for high-performance supercapacitors. Nano Lett. 2015, 15, 4605–4610. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef] [Green Version]
- Younes, H.; Lou, D.; Rahman, M.; Choi, D.; Hong, H.; Zou, L. Review on 2D MXene and graphene electrodes in capacitive deionization. Environ. Technol. Innov. 2022, 28, 102858. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Chen, Z.; Long, J.; Xu, C. Freestanding N-doped graphene membrane electrode with interconnected porous architecture for efficient capacitive deionization. Carbon 2022, 187, 86–96. [Google Scholar] [CrossRef]
- Oladunni, J.; Zain, J.; Hai, A.; Banat, F.; Bharath, G.; Alhseinat, E. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 2018, 207, 291–320. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, S.; Ren, L.; Wang, Y. Atomic layer deposition of TiO2 on carbon-nanotubes membrane for capacitive deionization removal of chromium from water. Chin. J. Chem. Eng. 2022, 45, 15–21. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B. A perspective: Carbon nanotube macro-films for energy storage. Energy Environ. Sci. 2013, 6, 3183–3201. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Min, X.; Li, X.; Si, M.; Liu, L.; Zheng, J.; Yang, W.; Zhao, F. Co-Co3O4 encapsulated in nitrogen-doped carbon nanotubes for capacitive desalination: Effects of nano-confinement and cobalt speciation. J. Colloid Interface Sci. 2022, 616, 389–400. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Lu, T.; Pan, L. Electrospun carbon nanofibers reinforced 3D porous carbon polyhedra network derived from metal-organic frameworks for capacitive deionization. Sci. Rep. 2016, 6, 32784. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, M.; Liu, Y.; Lu, T.; Pan, L. Metal-organic framework-engaged formation of a hierarchical hybrid with carbon nanotube inserted porous carbon polyhedra for highly efficient capacitive deionization. J. Mater. Chem. A 2016, 4, 5467–5473. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, F.; Ma, W.; Li, H. Metal-organic-framework derived carbon polyhedron and carbon nanotube hybrids as electrode for electrochemical supercapacitor and capacitive deionization. Electrochim. Acta 2018, 263, 85–93. [Google Scholar] [CrossRef]
- Weng, J.; Wang, S.; Zhang, P.; Li, C.; Wang, G. A review of metal-organic framework-derived carbon electrode materials for capacitive deionization. New Carbon Mater. 2021, 36, 117–129. [Google Scholar] [CrossRef]
- Shi, W.; Ye, C.; Xu, X.; Liu, X.; Ding, M.; Liu, W.; Cao, X.; Shen, J.; Yang, H.; Gao, C. High-performance membrane capacitive deionization based on metal-organic framework-derived hierarchical carbon structures. ACS Omega 2018, 3, 8506–8513. [Google Scholar] [CrossRef]
- Zhou, A.; Cheng, W.; Wang, W.; Zhao, Q.; Xie, J.; Zhang, W.; Gao, H.; Xue, L.; Li, J. Hexacyanoferrate-type prussian blue analogs: Principles and advances toward high-performance sodium and potassium ion batteries. Adv. Energy Mater. 2021, 11, 2000943. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Xiao, D.; Or, T.; Gao, R.; Li, Z.; Feng, M.; Shui, L.; Zhou, G.; Wang, X.; et al. Faradaic electrodes open a new era for capacitive deionization. Adv. Sci. 2020, 7, 2002213. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Zhang, W.; Song, H.; Cheng, Y.; Liu, C.; Wang, C.; Khan, M.; Zhang, H.; Liu, J.; Yu, C.; Wang, L.; et al. Core-shell prussian blue analogs with compositional heterogeneity and open cages for oxygen evolution reaction. Adv. Sci. 2019, 6, 1801901. [Google Scholar] [CrossRef]
- Nai, J.; Lou, X. Hollow structures based on prussian blue and its analogs for electrochemical energy storage and conversion. Adv. Mater. 2019, 31, e1706825. [Google Scholar] [CrossRef] [PubMed]
- Vafakhah, S.; Guo, L.; Sriramulu, D.; Huang, S.; Saeedikhani, M.; Yang, H. Efficient sodium-ion intercalation into the freestanding prussian blue/graphene aerogel anode in a hybrid capacitive deionization system. ACS Appl. Mater. Interfaces 2019, 11, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Zhao, Y.; Liang, B.; Li, K. Stepwise hollow Prussian blue/carbon nanotubes composite as a novel electrode material for high-performance desalination. J. Colloid Interface Sci. 2022, 605, 432–440. [Google Scholar] [CrossRef]
- Younes, H.; Rahman, M.; Hong, H.; AlNahyan, M.; Ravaux, F. Capacitive deionization performance of asymmetric nanoengineered CoFe2O4 carbon nanomaterials composite. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef]
- Gabrielli, C.; Maurin, G.; Francy-Chausson, H.; Thery, P.; Tran, T.; Tlili, M. Electrochemical water softening: Principle and application. Desalination 2006, 201, 150–163. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, K. Hardness removal by a novel electrochemical method. Desalination 2016, 381, 8–14. [Google Scholar] [CrossRef]
- Hazeltine, B.; Bull, C. Field Guide to Appropriate Technology; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Mohammadian, M.; Sahraei, R.; Ghaemy, M. Synthesis and fabrication of antibacterial hydrogel beads based on modified-gum tragacanth/poly(vinyl alcohol)/Ag0 highly efficient sorbent for hard water softening. Chemosphere 2019, 225, 259–269. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Ying, D.; Jia, J.; Shao, J. Dual-layer Janus charged nanofiltration membranes constructed by sequential electrospray polymerization for efficient water softening. Chemosphere 2023, 310, 136929. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, F.; Kianfar, E. Synthesis of isophthalic acid/aluminum nitrate thin film nanocomposite membrane for hard water softening. J. Inorg. Organomet. Polym. Mater. 2019, 29, 2176–2185. [Google Scholar] [CrossRef]
- Oliveira, E.; Alves, C.; Franca, A.; Nascimento, R.; Sasaki, J.; Loiola, A. NaA zeolite synthesized over fiberglass as a strategy for optimizing hard water softening. Quim. Nova 2022, 45, 16–22. [Google Scholar]
- Eleni, G.; Georgios, P.; Konstantina, K.; Alexandros, B. Ca2+ removal from water by the use of Na-palygorskite for potential water softening. Water Supply 2022, 22, 156–169. [Google Scholar]
- Dou, W.; Zhou, Z.; Jiang, L.; Jiang, A.; Huang, R.; Tian, X.; Zhang, W.; Chen, D. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization. J. Environ. Manag. 2017, 196, 518–526. [Google Scholar] [CrossRef]
- Arias-Paic, M.; Cawley, K.; Byg, S.; Rosario-Ortiz, F. Enhanced DOC removal using anion and cation ion exchange resins. Water Res. 2016, 88, 981–989. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Chen, X. Membrane-based electrochemical precipitation for water softening. J. Membr. Sci. 2020, 597, 117639. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, H.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Selective pseudocapacitive deionization of calcium ions in copper hexacyanoferrate. Acs Appl. Mater. Interfaces 2020, 12, 41437–41445. [Google Scholar] [CrossRef]
- Nie, P.; Hu, B.; Shang, X.; Xie, Z.; Huang, M.; Liu, J. Highly efficient water softening by mordenite modified cathode in asymmetric capacitive deionization. Sep. Purif. Technol. 2020, 250, 117240. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.; Kang, J.; Kim, S.; Kim, C.; Yoon, J. Capacitive deionization with Ca-alginate coated-carbon electrode for hardness control. Desalination 2016, 392, 46–53. [Google Scholar] [CrossRef]
- Deng, D.; Luhasile, M.; Li, H.; Pan, Q.; Zheng, F.; Wang, Y. A novel layered activated carbon with rapid ion transport through chemical activation of chestnut inner shell for capacitive deionization. Desalination 2022, 531, 115685. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, S.; Zhou, H.; Wang, G.; Zhang, H.; Zhao, H. Intrinsic pseudocapacitive affinity in manganese spinel ferrite nanospheres for high-performance selective capacitive removal of Ca2+ and Mg2+. ACS Appl. Mater. Interfaces 2021, 13, 38886–38896. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Jeon, H.; Lee, J.; Kim, G.; Park, D.; Nojima, H.; Lee, J.; Moon, S. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Tuan, T.; Chung, S.; Lee, J.; Lee, J. Improvement of water softening efficiency in capacitive deionization by ultra purification process of reduced graphene oxide. Curr. Appl. Phys. 2015, 15, 1397–1401. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Li, Z.; Shao, H.; Qu, L. Frontiers of carbon materials as capacitive deionization electrodes. Dalton Trans. 2020, 49, 5006–5014. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Wu, T.; Li, C.; Wang, Y.; Pan, X.; Zhan, F.; Zhang, Y.; Wang, S.; Qiu, J. Membrane-free hybrid capacitive deionization system based on redox reaction for high-efficiency NaCl removal. Environ. Sci. Technol. 2019, 53, 6292–6301. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, K.; Zhang, X.; Zhang, C.; Liang, P. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review. Environ. Sci.-Water Res. Technol. 2020, 6, 243–257. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Hu, C. Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: Effects of Ca2+ and Mg2+. J. Environ. Sci. 2011, 23, 1634–1639. [Google Scholar] [CrossRef]
- Werner, J.; Arnold, W.; McNeill, K. Water hardness as a photochemical parameter: Tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH. Environ. Sci. Technol. 2006, 40, 7236–7241. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhou, H.; Zhang, H.; Zhang, Y.; Zhao, H.; Wang, G. Synchronous removal of tetracycline and water hardness ions by capacitive deionization. J. Clean. Prod. 2021, 316, 128251. [Google Scholar] [CrossRef]
- Chai, W.; Cheun, J.; Kumar, P.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.; Show, P. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.; Al-Hamadani, Y.; Park, C.; Jang, M.; Kim, D.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Li, P.; Tao, H. Cell surface engineering of microorganisms towards adsorption of heavy metals. Crit. Rev. Microbiol. 2015, 41, 140–149. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Efficient selective extraction of scandium from red mud. Miner. Process. Extr. Metall. Rev. 2022. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022, 183, 107624. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Qu, X.; Li, Z.; Ni, J. Mechanism of combination membrane and electro-winning process on treatment and remediation of Cu2+ polluted water body. J. Environ. Sci. 2009, 21, 764–769. [Google Scholar] [CrossRef]
- Chen, B.; Bao, S.; Zhang, Y. Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale. Int. J. Min. Sci. Technol. 2021, 31, 1095–1106. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bobade, V.; Eshtiagi, N. Heavy metals removal from wastewater by adsorption process: A review. In Proceedings of the Asia Pacific Confederation of Chemical Engineering Congress, Melbourne, Australia, 27 September–1 October 2015. [Google Scholar]
- Chen, R.; Sheehan, T.; Ng, J.; Brucks, M.; Su, X. Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci. Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- Tang, W.; Wang, X.; Zeng, G.; Liang, J.; Li, X.; Xing, W.; He, D.; Tang, L.; Liu, Z. Electro-assisted adsorption of Zn(II) on activated carbon cloth in batch-flow mode: Experimental and theoretical investigations. Environ. Sci. Technol. 2019, 53, 2670–2678. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Qiao, L.; Li, Y.; Wang, D.; Li, D.; Liang, X.; Xu, G.; Gao, M.; Gong, H.; et al. Controlled synthesis of ZnO modified N-doped porous carbon nanofiber membrane for highly efficient removal of heavy metal ions by capacitive deionization. Microporous Mesoporous Mater. 2022, 338, 111889. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Ma, J.; Liang, P. Effective removal and selective capture of copper from salty solution in flow electrode capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 341–350. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, S.; Zhang, X.; Zhou, H.; Zhang, H. High-performance pseudocapacitive removal of cadmium via synergistic valence conversion in perovskite-type FeMnO3. J. Hazard. Mater. 2022, 439, 129575. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Chen, Q.; Zhang, Y.; Tian, X. Optimization of preparation conditions of composite electrodes for selective adsorption of vanadium in CDI by response surface methodology. Chem. Eng. Res. Des. 2021, 168, 37–45. [Google Scholar] [CrossRef]
- Wan, X.; Bao, S.; Zhang, Y. ZIF-8-derived porous carbon: Application in capacitive deionization for vanadium (V) adsorption. J. Appl. Electrochem. 2022, 52, 639–651. [Google Scholar] [CrossRef]
- Gabelich, C.; Tran, T.; Suffet, I. Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ. Sci. Technol. 2002, 36, 3010–3019. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, L.; Li, H.; Zhang, Y.; Zhang, Z.; Chen, Y.; Sun, Z. Electrosorption behavior of cations with carbon nanotubes and carbon nanofibres composite film electrodes. Thin Solid Film. 2009, 517, 1616–1619. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, L.; Cai, Z.; Ren, Z. Individual and competitive removal of heavy metals using capacitive deionization. J. Hazard. Mater. 2016, 302, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zou, L.; Pan, L.; Sun, Z. Using graphene nano-flakes as electrodes to remove ferric ions by capacitive deionization. Sep. Purif. Technol. 2010, 75, 8–14. [Google Scholar] [CrossRef]
- He, R.; Neupane, M.; Zia, A.; Huang, X.; Bowers, C.; Wang, M.; Lu, J.; Yang, Y.; Dong, P. Binder-free wood converted carbon for enhanced water desalination performance. Adv. Funct. Mater. 2022, 32, 2208040. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.; Xiang, S.; Zhou, J.; Liu, F.; Li, Z.; Zhou, H. Selective pseudocapacitive separation of zinc ions via silk cocoon derived N-doped porous carbon. Desalination 2023, 546, 116220. [Google Scholar] [CrossRef]
- Zhang, W.; Mossad, M.; Yazdi, J.; Zou, L. A statistical experimental investigation on arsenic removal using capacitive deionization. Desalination Water Treat. 2016, 57, 3254–3260. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, M.; Chen, D. Electrosorption of uranium(VI) by highly porous phosphate-functionalized graphene hydrogel. Appl. Surf. Sci. 2019, 484, 83–96. [Google Scholar] [CrossRef]
- Iftekhar, S.; Farooq, M.; Sillanpaa, M.; Asif, M.; Habib, R. Removal of Ni(II) using multi-walled carbon nanotubes electrodes: Relation between operating parameters and capacitive deionization performance. Arab. J. Sci. Eng. 2017, 42, 235–240. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.; Huang, L.; Khan, E.; Masek, O.; Parikh, S.; Ok, Y. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.; Vo, D.-V.; Sindhu, J.; Sneka, D.; Subhashini, B. Advanced techniques to remove phosphates and nitrates from waters: A review. Environ. Chem. Lett. 2021, 19, 3165–3180. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.; Preethi, J.; Meenakshi, S.; Park, C. Mechanistic performance of polyaniline-substituted hexagonal boron nitride composite as a highly efficient adsorbent for the removal of phosphate, nitrate, and hexavalent chromium ions from an aqueous environment. Appl. Surf. Sci. 2020, 511, 145543. [Google Scholar] [CrossRef]
- Nodeh, H.; Sereshti, H.; Afsharian, E.; Nouri, N. Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. J. Environ. Manag. 2017, 197, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Berkessa, Y.; Mereta, S.; Feyisa, F. Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory. Appl. Water Sci. 2019, 9, 28. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. AQUA-Water Infrastruct. Ecosyst. Soc. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Tian, J.; Shi, J.; Linhardt, R.; Ye, T.; Chen, S. Recovery of high value-added nutrients from fruit and vegetable industrial wastewater. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1388–1402. [Google Scholar] [CrossRef] [Green Version]
- Meena, V. Role of Rhizospheric Microbes in Soil; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Zhang, H.; Wang, Q.; Zhang, J.; Chen, G.; Wang, Z.; Wu, Z. Development of novel ZnZr-COOH/CNT composite electrode for selectively removing phosphate by capacitive deionization. Chem. Eng. J. 2022, 439, 135527. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Tang, W.; Zhong, Y.; Luo, K.; Duan, M.; Xing, W.; Liang, J. Removal and recovery of phosphorus from low-strength wastewaters by flow-electrode capacitive deionization. Sep. Purif. Technol. 2020, 237, 116322. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, X.; Ren, Z. pH dependence of phosphorus speciation and transport in flow-electrode capacitive deionization. Environ. Sci. Technol. 2020, 54, 9116–9123. [Google Scholar] [CrossRef]
- Benjamin, M.M. Water Chemistry; Waveland Press: Long Grove, IL, USA, 2014. [Google Scholar]
- Tian, J.; Cheng, X.; Deng, S.; Liu, J.; Qu, B.; Dang, Y.; Holmes, D.; Waite, T. Inducing in situ crystallization of vivianite in a UCT-MBR system for enhanced removal and possible recovery of phosphorus from sewage. Environ. Sci. Technol. 2019, 53, 9045–9053. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Zhang, Q.; Aleem, M.; Fang, F.; Xue, Z.; Cao, J. Potentials and challenges of phosphorus recovery as vivianite from wastewater: A review. Chemosphere 2019, 226, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, X.; Wang, M.; Ma, J.; Collins, R.; Kinsela, A.; Zhang, Y.; Waite, T. Phosphate recovery as vivianite using a flow-electrode capacitive desalination (FCDI) and fluidized bed crystallization (FBC) coupled system. Water Res. 2021, 194, 116939. [Google Scholar] [CrossRef]
- Fateminia, R.; Rowshanzamir, S.; Mehri, F. Synergistically enhanced nitrate removal by capacitive deionization with activated carbon/PVDF/polyaniline/ZrO2 composite electrode. Sep. Purif. Technol. 2021, 274, 119108. [Google Scholar] [CrossRef]
- Cetinkaya, A. Life cycle assessment of environmental effects and nitrate removal for membrane capacitive deionization technology. Environ. Monit. Assess. 2020, 192, 543. [Google Scholar] [CrossRef]
- Garcia-Quismondo, E.; Santos, C.; Soria, J.; Palma, J.; Anderson, M. New operational modes to increase energy efficiency in capacitive deionization systems. Environ. Sci. Technol. 2016, 50, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dong, J.; Wang, T.; Liu, R.; Liu, H.; Qu, J. Nitrate electro-sorption/reduction in capacitive deionization using a novel Pd/NiAl-layered metal oxide film electrode. Chem. Eng. J. 2018, 335, 475–482. [Google Scholar] [CrossRef]
- Rogers, T.; Guo, S.; Arrazolo, L.; Garcia-Segura, S.; Wong, M.; Verduzco, R. Catalytic capacitive deionization for adsorption and reduction of aqueous nitrate. ACS EST Water 2021, 1, 2233–2241. [Google Scholar] [CrossRef]
- Gao, F.; Li, X.; Shi, W.; Wang, Z. Highly selective recovery of phosphorus from wastewater via capacitive deionization enabled by ferrocene-polyaniline-functionalized carbon nanotube electrodes. ACS Appl. Mater. Interfaces 2022, 14, 31962–31972. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, C.; Tian, S.; Mao, Y.; Zong, Y.; Zhang, X.; Zhang, B.; Zhang, C.; Wu, D. Selective recovery of phosphorus from synthetic urine using flow-electrode capacitive deionization (FCDI)-based technology. ACS EST Water 2020, 1, 175–184. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Xiao, W.; Ma, J.; Sun, J.; Mo, H.; Waite, T. Phosphate selective recovery by magnetic iron oxide impregnated carbon flow-electrode capacitive deionization (FCDI). Water Res. 2021, 189, 116653. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zuo, K.; Li, Y.; Huang, X.; Hu, J.; Yang, Y.; Wang, W.; Chen, L.; Jain, A.; Verduzco, R.; et al. Eggshell membrane derived nitrogen rich porous carbon for selective electrosorption of nitrate from water. Water Res. 2022, 216, 118351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, F.; Li, F. Denitrification enhancement by electro-adsorption/reduction in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) with copper electrode. Chemosphere 2022, 291, 132732. [Google Scholar] [CrossRef] [PubMed]
- Minhas, M.; Jande, Y.; Kim, W. Hybrid reverse osmosis-capacitive deionization versus two-stage reverse osmosis: A comparative analysis. Chem. Eng. Technol. 2014, 37, 1137–1145. [Google Scholar] [CrossRef]
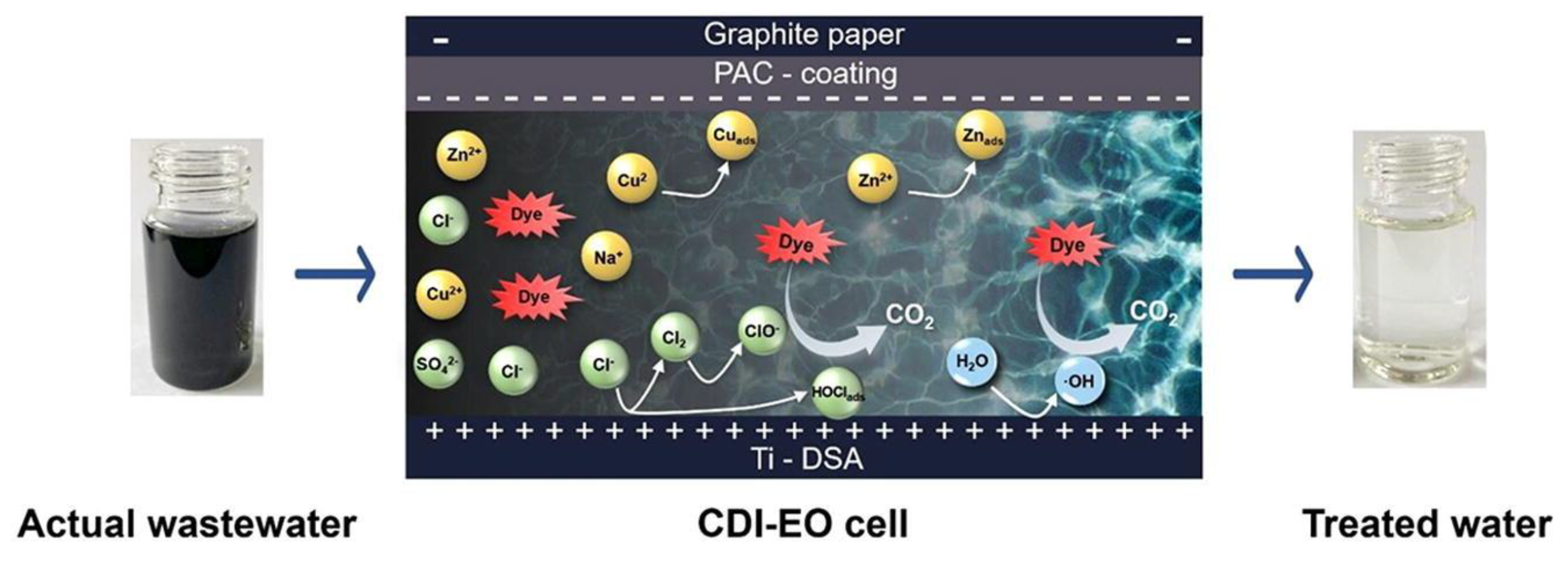
- Chen, W.; He, X.; Jiang, Z.; Li, B.; Li, X.; Lin, L. A capacitive deionization and electro-oxidation hybrid system for simultaneous removal of heavy metals and organics from wastewater. Chem. Eng. J. 2023, 451, 139071. [Google Scholar] [CrossRef]
- Liang, S.; Lia, M.; Cao, J.; Zuo, K.; Bian, Y.; Xiao, K.; Huang, X. Integrated ultrafiltration capacitive-deionization (UCDI) for enhanced antifouling performance and synchronous removal of organic matter and salts. Sep. Purif. Technol. 2019, 226, 146–153. [Google Scholar] [CrossRef]
- Duan, F.; Li, Y.; Cao, H.; Wang, Y.; Crittenden, J.; Zhang, Y. Activated carbon electrodes: Electrochemical oxidation coupled with desalination for wastewater treatment. Chemosphere 2015, 125, 205–211. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Sun, X.; Chen, M.; Wei, D.; Du, Y. Research progress of disinfection and disinfection by-products in China. J. Environ. Sci. 2019, 81, 52–67. [Google Scholar] [CrossRef]
- Krasner, S.W. The formation and control of emerging disinfection by-products of health concern. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2009, 367, 4077–4095. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.; Kimura, S. Emerging environmental contaminants: Challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 2017, 8, 40–56. [Google Scholar] [CrossRef]
- Yang, Y.; Komaki, Y.; Kimura, S.; Hu, H.; Wagner, E.; Marinas, B.; Plewa, M. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Plewa, M.; Wagner, E.; Schoeny, R.; DeMarini, D. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. -Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, D.; Xu, X.; Wang, Z. Formation of known and unknown disinfection by-products from natural organic matter fractions during chlorination, chloramination, and ozonation. Sci. Total Environ. 2017, 587, 177–184. [Google Scholar] [CrossRef]
- Wang, Y.; El-Deen, A.; Li, P.; Oh, B.; Guo, Z.; Khin, M.; Vikhe, Y.; Wang, J.; Hu, R.; Boom, R.; et al. High-performance capacitive deionization disinfection of water with graphene oxide-graft-quaternized chitosan nanohybrid electrode coating. ACS Nano 2015, 9, 10142–10157. [Google Scholar] [CrossRef] [Green Version]
- Laxman, K.; Myint, M.; Al Abri, M.; Sathe, P.; Dobretsov, S.; Dutta, J. Desalination and disinfection of inland brackish ground water in a capacitive deionization cell using nanoporous activated carbon cloth electrodes. Desalination 2015, 362, 126–132. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Wang, D.; Yuan, Y.; Wang, L.; Zhao, Z.; Qiu, J. Contact-active antimicrobial graphene oxide electrodes by covalent grafting of alkoxysilane octadecyldimethyl (3-trimethoxysilylpropyl)-ammonium chloride (ODDMAC) for capacitive deionization disinfection. Mater. Lett. 2020, 272, 127876. [Google Scholar] [CrossRef]
- Cao, C.; Wu, X.; Zheng, Y.; Chen, Y. Three-dimensional cubic ordered mesoporous carbon with chitosan for capacitive deionization disinfection of water. Environ. Sci. Pollut. Res. 2020, 27, 15001–15010. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Jo, K.; Yoon, J. Comparison of salt adsorption capacity and energy consumption between constant current and constant voltage operation in capacitive deionization. Desalination 2014, 352, 52–57. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Lin, C.; Hou, C. Integrating a supercapacitor with capacitive deionization for direct energy recovery from the desalination of brackish water. Appl. Energy 2019, 252, 113417. [Google Scholar] [CrossRef]
- Andres, G.; Yoshihara, Y. A capacitive deionization system with high energy recovery and effective re-use. Energy 2016, 103, 605–617. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.; Zhu, L.; Qiu, Y. Energy recovery and electrode regeneration under different charge/discharge conditions in membrane capacitive deionization. Desalination 2018, 439, 93–101. [Google Scholar] [CrossRef]
- Zhao, R.; Satpradit, O.; Rijnaarts, H.; Biesheuvel, P.; van der Wal, A. Optimization of salt adsorption rate in membrane capacitive deionization. Water Res. 2013, 47, 1941–1952. [Google Scholar] [CrossRef]
- Choi, J.H. Comparison of constant voltage (CV) and constant current (CC) operation in the membrane capacitive deionisation process. Desalination Water Treat. 2015, 56, 921–928. [Google Scholar] [CrossRef]
- Qu, Y.; Campbell, P.; Gu, L.; Knipe, J.; Dzenitis, E.; Santiago, J.; Stadermann, M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionization. Desalination 2016, 400, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jin, Y.; Hong, S. Recent transitions in ultrapure water (UPW) technology: Rising role of reverse osmosis (RO). Desalination 2016, 399, 185–197. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Hong, S. Staged voltage mode in membrane capacitive deionization: Comparison with constant voltage and constant current modes. Desalination 2020, 479, 114327. [Google Scholar] [CrossRef]
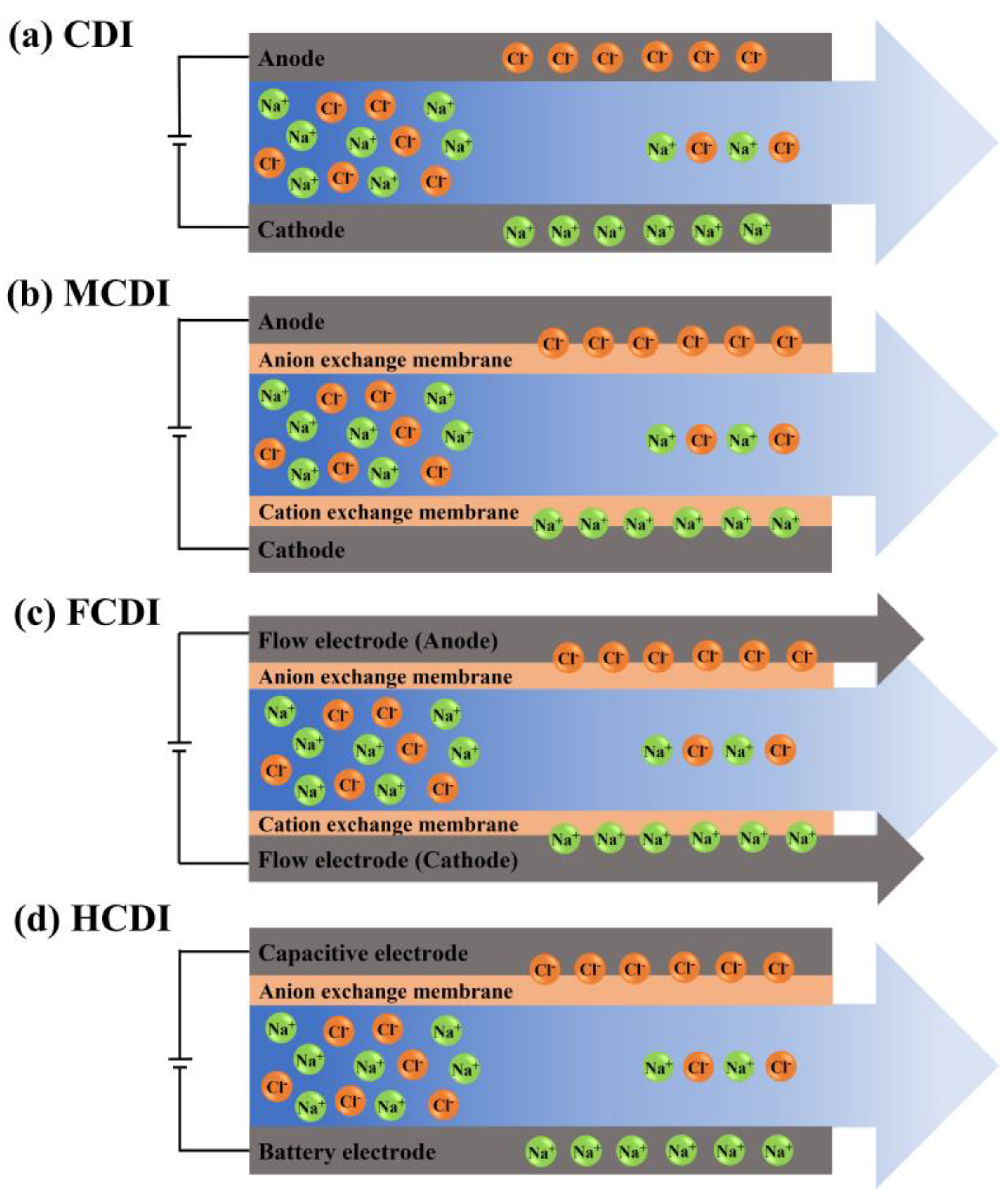


| Hardness Ion/Concentration | Applied Voltage (V) | Flow Rate (mL/min) | Removal Efficiency | CDI Type/Electrode | Ref. |
|---|---|---|---|---|---|
| 100 mg/L CaCl2 | 1.4 | 25 | 42.8 mg/g | HCDI with copper hexacyanoferrate as cathode electrode | [73] |
| 10 mM CaCl2 | 1.2 | 10 | 248 μmol/g | CDI with porous mordenite modified activated carbon electrode | [74] |
| 10 mM CaCl2 | 1.2 | 2 | 14.2 mg/g | Ca-alginate coated on carbon electrode | [75] |
| 0.95 g/L CaCl2 | 1.4 | 25 | 21.0 mg/g | CDI of KOH carbonized and activated carbon electrode | [76] |
| 150 mg/L CaCl2 | 1.2 | 20 | 534.6 μmol/g | PHCI with manganese spinel ferrite as cathode electrode | [77] |
| 75 mg/L MgSO4 | 980.4 μmol/g | ||||
| 350 mg/L CaCO3 | 1.5 | 16 | 80% | CDI with activated carbon cloth electrode | [78] |
| 35 mg/L CaCO3 | 2 | 10 | 3.5 mg/g | CDI with purified reduced graphene oxide electrodes | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, S.; Xin, C.; Zhang, Y.; Chen, B.; Ding, W.; Luo, Y. Application of Capacitive Deionization in Water Treatment and Energy Recovery: A Review. Energies 2023, 16, 1136. https://doi.org/10.3390/en16031136
Bao S, Xin C, Zhang Y, Chen B, Ding W, Luo Y. Application of Capacitive Deionization in Water Treatment and Energy Recovery: A Review. Energies. 2023; 16(3):1136. https://doi.org/10.3390/en16031136
Chicago/Turabian StyleBao, Shenxu, Chunfu Xin, Yimin Zhang, Bo Chen, Wei Ding, and Yongpeng Luo. 2023. "Application of Capacitive Deionization in Water Treatment and Energy Recovery: A Review" Energies 16, no. 3: 1136. https://doi.org/10.3390/en16031136
APA StyleBao, S., Xin, C., Zhang, Y., Chen, B., Ding, W., & Luo, Y. (2023). Application of Capacitive Deionization in Water Treatment and Energy Recovery: A Review. Energies, 16(3), 1136. https://doi.org/10.3390/en16031136








