A Brief Review of Hydrogen Production Methods and Their Challenges
Abstract
:1. Introduction
2. Methodology
3. Hydrogen Production
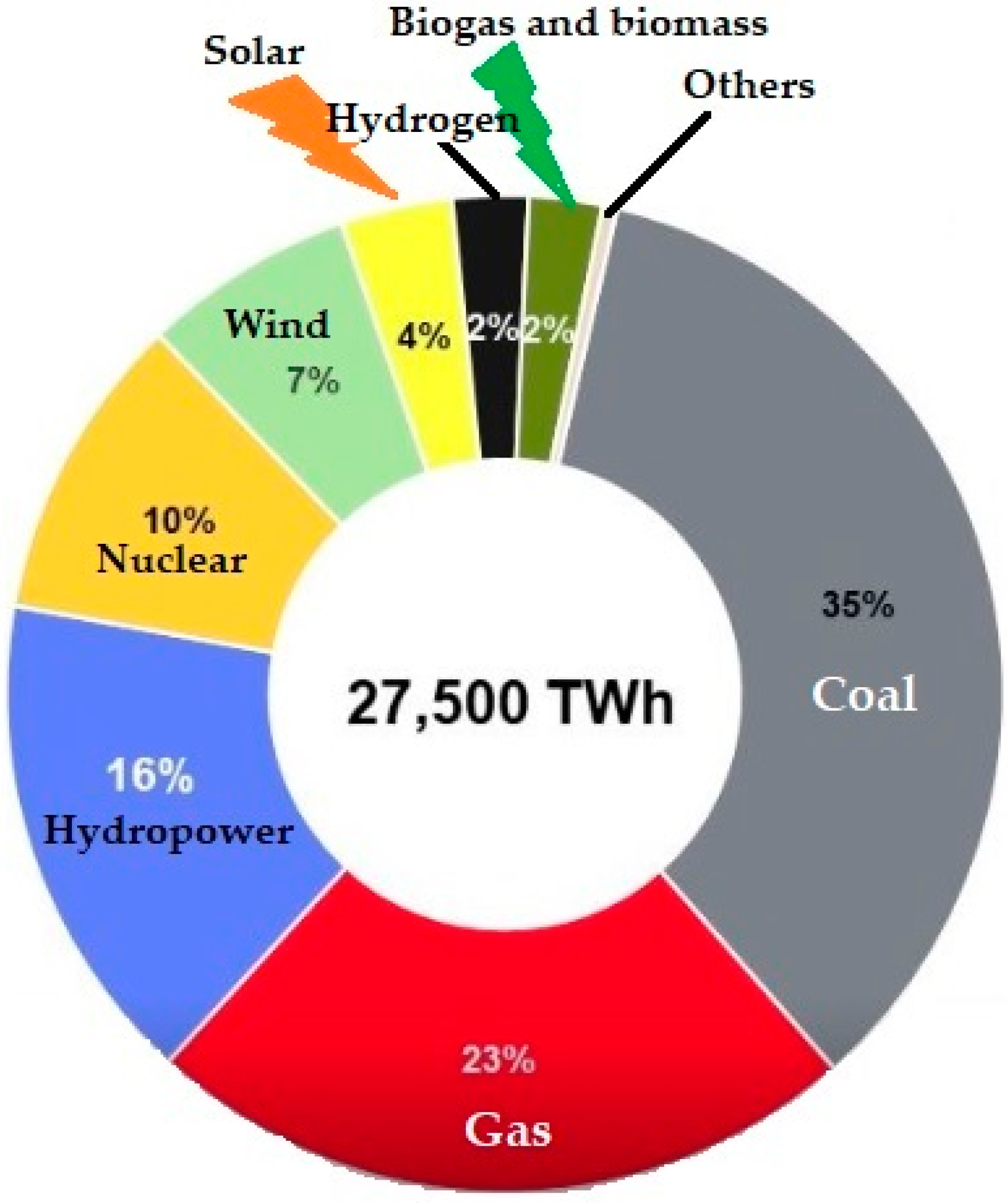
- Different energy sources can be used to produce hydrogen.
- Since hydrogen is the least polluting, using hydrogen in fuel cells or combustion processes results in the production of water.
- All energy needs may be met by hydrogen, which is also utilised in residential applications, hydrogen fuel cell automobiles, energy carriers, and integrated heating and power generation systems. Hydrogen also covers all requirements for energy
3.1. Blue Hydrogen Production Methods
3.2. Purple Hydrogen Production Method
3.3. Turquoise Hydrogen
3.4. Production of Grey Hydrogen
3.5. Green Hydrogen
4. Challenges in Hydrogen Production Methods
5. Recommendation and Conclusions
- Selection of hydrogen energy for various applications can be useful due to its low emissions or zero emissions released into the environment. Therefore, various methods of hydrogen production and utilization based on their sources of production have been analysed.
- The actual production methods for hydrogen and its related challenges have been discussed in this paper.
- The utilization of hydrogen as a source of energy can be helpful to protect the environment from harmful emissions, which has been the main focus of the manuscript.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pour Azarm, E.; Verma, R. Sustainable Energy Solution for Climate Change: Combating Co2 Emissions in Iran. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. The Role of Hydrogen in Global Transition to 100% Renewable Energy. In Lecture Notes in Energy; Springer International Publishing: Cham, Switzerland, 2020; pp. 275–307. ISBN 9783030407377. [Google Scholar]
- Nieto, M.J. Whatever It Takes to Reach Net Zero Emissions around 2050 and Limit Global Warming to 1.5c: The Cases of United States, China, European Union and Japan. SSRN Electron. J. 2022, 2022, 170. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; de Souza, E.G.; de Souza, S.N.M. The Potential of Hydrogen Production from High and Low-Temperature Electrolysis Methods Using Solar and Nuclear Energy Sources: The Transition to a Hydrogen Economy in Brazil. Int. J. Hydrog. Energy 2022, 47, 34727–34738. [Google Scholar] [CrossRef]
- Al-Bassam, A.M.; Conner, J.A.; Manousiouthakis, V.I. Natural-Gas-Derived Hydrogen in the Presence of Carbon Fuel Taxes and Concentrated Solar Power. ACS Sustain. Chem. Eng. 2018, 6, 3029–3038. [Google Scholar] [CrossRef]
- George, J.; Arun, P.; Muraleedharan, C. Stoichiometric Equilibrium Model Based Assessment of Hydrogen Generation through Biomass Gasification. Procedia Technol. 2016, 25, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Ahmed Syed, Z.; Jiu, L.; Bai, J.; Wang, T. Porosity-Enhanced Solar Powered Hydrogen Generation in GaN Photoelectrodes. Appl. Phys. Lett. 2017, 111, 203901. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, X.; Rezazadeh, A. Hydrogen Fuel and Electricity Generation from a New Hybrid Energy System Based on Wind and Solar Energies and Alkaline Fuel Cell. Energy Rep. 2021, 7, 2594–2604. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Senthil Kumar, P.; Rangasamy, G.; Rajendran, S. A Critical Review on Biohydrogen Generation from Biomass. Int. J. Hydrog. Energy, 2022; in press. [Google Scholar] [CrossRef]
- Murray, J.; Clément, A.; Fritz, B.; Schmittbuhl, J.; Bordmann, V.; Fleury, J.M. Abiotic Hydrogen Generation from Biotite-Rich Granite: A Case Study of the Soultz-Sous-Forêts Geothermal Site, France. Appl. Geochem. 2020, 119, 104631. [Google Scholar] [CrossRef]
- Tarnay, D. Hydrogen Production at Hydro-Power Plants. Int. J. Hydrogen Energy 1985, 10, 577–584. [Google Scholar] [CrossRef]
- Ahmadi, P.; Dincer, I.; Rosen, M.A. Multi-Objective Optimization of an Ocean Thermal Energy Conversion System for Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 7601–7608. [Google Scholar] [CrossRef]
- Dash, S.K.; Garg, P.; Mishra, S.; Chakraborty, S.; Elangovan, D. Investigation of Adaptive Intelligent MPPT Algorithm for a Low-cost IoT Enabled Standalone PV System. Aust. J. Electr. Electron. Eng. 2022, 19, 261–269. [Google Scholar] [CrossRef]
- United States General Acco Office (Gao). National Oceanic and Atmospheric Administration: National Weather Service Modernization and Weather Satellite Program; Createspace Independent Publishing Platform: North Charleston, SC, USA, 2018; ISBN 9781720664710. [Google Scholar]
- Air Pollution. Available online: https://www.who.int/health-topics/air-pollution (accessed on 23 December 2022).
- Hausfather, Z. Analysis: Global CO2 Emissions from Fossil Fuels Hit Record High in 2022. Available online: https://www.carbonbrief.org/analysis-global-co2-emissions-from-fossil-fuels-hit-record-high-in-2022/ (accessed on 23 December 2022).
- Apostolou, D.; Xydis, G. A Literature Review on Hydrogen Refuelling Stations and Infrastructure. Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen Economy and Sustainable Development Goals (SDGs): Review and Policy Insight. Sci. Direct. 2021, 31, 100506. [Google Scholar]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, P.; Tsujimura, T. A Fully Renewable and Efficient Backup Power System with a Hydrogen-Biodiesel-Fueled IC Engine. Energy Procedia 2019, 157, 1305–1319. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, D.; Kumar, N. Hydrogen Use in Internal Combustion Engine: A Review. Int. J. Adv. Cult. Technol. 2015, 3, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zheng, D.; Chen, X.; Shi, L.; Dai, X.; Chen, Y.; Jing, X. Standard Thermodynamic Properties for the Energy Grade Evaluation of Fossil Fuels and Renewable Fuels. Renew Energy 2020, 147, 2160–2170. [Google Scholar] [CrossRef]
- Gheorghe, D.; Tutunea, D.; Bică, M.; Gruia, A.; Calbureanu, M. A Review of Hydrogen/Diesel Fuel Blends in Internal Combustion Engines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 595, 012033. [Google Scholar] [CrossRef]
- Research & Development. Available online: https://bioenergyinternational.com/research-development/ (accessed on 23 December 2022).
- Recharge. Available online: https://www.rechargenews.com/ (accessed on 23 December 2022).
- Radowitz Bernd Global News and Intelligence for Energy Transition. Available online: https://www.rechargenews.com/wind/germany-eyes-world-first-tender-for-offshore-windto-hydrogen-pilot-in-2022/2-1-1076562 (accessed on 10 October 2021).
- Bioenergy International. OYSTER Consortium Receives Funding to Investigate Offshore Hydrogen Production. 2021. Available online: https://bioenergyinternational.com/Research-development/oyster-consortium-receives-fundingto-investigate-offshore-hydrogen-production (accessed on 18 December 2022).
- Maersk. Maritime Industry Leaders to Explore Ammonia as Marine Fuel in Singapore. 2021. Available online: https://www.maersk.com/news/articles/2021/03/10/maritime-industryleaders-to-explore-ammonia-as-marine-fuel-in-singapore (accessed on 15 December 2022).
- Brown Trevor Ammonia Energy Association. Available online: https://www.ammoniaenergy.org/articles/maritimeammonia-ready-for-demonstration/ (accessed on 7 May 2020).
- Recharge: Global News and Intelligence for the Energy Transition. Government Policies to Make Green Hydrogen Viable. 2021. Available online: https://www.rechargenews.com/energy-transition/green-hydrogen-is-too-expensive-these38-government-policies-are-needed-to-make-it-viable/2-1-1095533 (accessed on 15 December 2022).
- Ishaq, H.; Dincer, I. Comparative assessment of renewable energy-based hydrogen production methods. Renew. Sustain. Energy Rev. 2020, 135, 110192. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic analysis of hydrogen production from steam reforming process: A literature review. Energy Sources B Energy Econ. Plann 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Agrafiotis, C.; von Storch, H.; Roeb, M.; Sattler, C. Solar thermal reforming of methane feedstocks for hydrogen and syngas productionda review. Renew. Sustain. Energy Rev. 2014, 29, 656–682. [Google Scholar] [CrossRef]
- Pinjari, S.; Bera, T.; Kapur, G.S.; Kjeang, E. The Mechanism and Sorption Kinetic Analysis of Hydrogen Storage at Room Temperature Using Acid Functionalized Carbon Nanotubes. Int. J. Hydrogen Energy 2023, 48, 1930–1942. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Huang, Z.; Zhu, M. Closing the Loop for Hydrogen Storage: Facile Regeneration of NaBH4 from its Hydrolytic Product. Angew Chem. Int. Ed. Engl. 2020, 59, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ouyang, L.; Zhong, H. Converting H from coordinated water into H− enables super facile synthesis of LiBH4. Green Chem. 2019, 21, 4380–4387. [Google Scholar] [CrossRef]
- Dickel, R. Blue Hydrogen as an Enabler of Green Hydrogen: The Case of Germany; OIES Paper; The Oxford Institute for Energy Studies: Oxford, UK, 2020. [Google Scholar]
- Mari, V.; Kristin, J.; Rahul, A. Hydrogen production with CO2 capture. Int. J. Hydrog. Energy 2016, 41, 4969–4992. [Google Scholar]
- Jovan, D.J.; Dolanc, G. Can Green Hydrogen Production Be Economically Viable under Current Market Conditions. Energies 2020, 13, 6599. [Google Scholar] [CrossRef]
- Schiro, F.; Stoppato, A.; Benato, A. Modelling and analyzing the impact of hydrogen enriched natural gas on domestic gas boilers in a decarbonization perspective. Carbon Resour. Convers. 2020, 3, 122–129. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Y.; Xu, H.; Gao, D.; Li, W. Cost-Economic Analysis of Hydrogen for China’s Fuel Cell Transportation Field. Energies 2020, 13, 6522. [Google Scholar] [CrossRef]
- Boretti, A. Production of hydrogen for export from wind and solar energy, natural gas, and coal in Australia. Int. J. Hydrog Energy 2020, 45, 3899–3904. [Google Scholar] [CrossRef]
- Manna, J.; Jha, P.; Sarkhel, R.; Banerjee, C.; Tripathi, A.; Nouni, M. Opportunities for green hydrogen production in petroleum refining and ammonia synthesis industries in India. Int. J. Hydrog. Energy 2021, 46, 38212–38231. [Google Scholar] [CrossRef]
- Rabiee, A.; Keane, A.; Soroudi, A. Green hydrogen: A new flexibility source for security constrained scheduling of power systems with renewable energies. Int. J. Hydrog. Energy 2021, 46, 19270–19284. [Google Scholar] [CrossRef]
- Shiva, S.K.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Julian, H.; Silberhorn, D.; Zill, T.; Bensmann, B.; Hanke-Rauschenbach, R. Hydrogen-powered aviation and its reliance on green hydrogen infrastructure–review and research gaps. Int. J. Hydrog. Energy 2022, 47, 3108–3130. [Google Scholar]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrog. Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- IRENA. Hydrogen from Renewable Power: Technology Outlook for the Energy Transition. 2018. Available online: https://www.irena.org (accessed on 16 December 2022).
- Calise, F.; D’Accadia, M.D.; Santarelli, M.; Lanzini, A.; Ferrero, D. Solar hydrogen production: Processes, systems and technologies. In Solar Hydrogen Production; Academic Press: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Ping, Z.; Laijun, W.; Songzhe, C.; Jingming, X. Progress of nuclear hydrogen production through the iodineesulfur process in China. Renew Sustain. Energy Rev. 2018, 81, 1802–1812. [Google Scholar] [CrossRef]
- Zhiznin, S.Z.; Timokhov, V.M.; Gusev, A.L. Economic aspects of nuclear and hydrogen energy in the world and Russia. Int. J. Hydrog. Energy 2020, 45, 31353–31366. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Ozcan, H.; Zamfirescu, C. Updates on promising thermochemical cycles for clean hydrogen production using nuclear energy. J. Clean Prod. 2020, 262, 121424. [Google Scholar] [CrossRef]
- Scamman, D.; Newborough, M. Using surplus nuclear power for hydrogen mobility and power-to-gas in France. Int. J. Hydrog. Energy 2016, 41, 10080–10089. [Google Scholar] [CrossRef]
- Milewski, J.; Kupecki, J.; Szczęśniak, A.; Uzunow, N. Hydrogen production in solid oxide electrolyzers coupled with nuclear reactors. Int. J. Hydrog. Energy 2021, 46, 35765–35776. [Google Scholar] [CrossRef]
- Wu, N.; Lan, K.; Yao, Y. An Integrated Techno-Economic and Environmental Assessment for Carbon Capture in Hydrogen Production by Biomass Gasification. Resour. Conserv. Recycl. 2023, 188, 106693. [Google Scholar] [CrossRef]
- Corey, P. Bill Gates-Backed Startup to Build ’Turquoisehydrogen’ Pilot by End of 2022. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/latest-newsheadlines/bill-gates-backed-startup-to-build-turquoisehydrogen-pilot-by-end-of-2022-65354106 (accessed on 29 December 2021).
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Schneider, S.; Bajohr, S.; Graf, F.; Kolb, T. State of the art of hydrogen production via pyrolysis of natural gas. Chem. Bio. Eng. Rev. 2020, 7, 150–158. [Google Scholar] [CrossRef]
- Leal Perez, B.J.; Jiménez, J.A.M.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrog. Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Karthikeyan, O.P.; Kumar, G.; Dai-Viet, N.V.; Banu, J.R. Technoeconomic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Roeth, M. The Many Colors of Hydrogen j FleetOwner. Available online: https://www.fleetowner.com/perspectives/ideaxchange/article/21151562/the-many-colors-of-hydrogen (accessed on 22 December 2021).
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrog. Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Woody, A.; Carlson, H. State of Play: Hydrogen in White & Case LLP. 2020. Available online: https://www.whitecase.com/publications/alert/state-playhydrogen-2020 (accessed on 21 December 2021).
- Available online: https://energiforskmedia.blob.core.windows.net/media/23562/5-hydrogen-production-by-electrolysis-ann-cornell-kth.pdf (accessed on 16 December 2022).
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Hu, Y.-W.; Huang, D.; Zhang, J.-N.; Huang, Y.-C.; Balogun, M.; Tong, Y.-X. Dual doping induced interfacial engineering of Fe2N/Fe3N hybrids with favourable d-band towards efficient overall water splitting. ChemCatChem 2019, 11, 6051–6060. [Google Scholar] [CrossRef]
- Wang, G.; Chao, Y.; Jiang, T.; Lin, J.; Peng, H.; Chen, H.; Chen, Z. Analyzing the Effects of Government Policy and Solar Photovoltaic Hydrogen Production on Promoting CO2 Capture and Utilization by Using Evolutionary Game Analysis. Energy Strat. Rev. 2023, 45, 101044. [Google Scholar] [CrossRef]
- Ray, P.; Ray, P.K.; Dash, S.K. Power Quality Enhancement and Power Flow Analysis of a PV Integrated UPQC System in a Distribution Network. IEEE Trans. Ind. Appl. 2022, 58, 201–211. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Liu, J. Life-cycle green-house gas emissions of onshore and offshore wind turbines. J. Clean Prod. 2019, 210, 804–810. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Onshore and offshore wind energy. In 100% Clean, Renewable Energy and Storage for Everything; Cambridge University Press: Cambridge, UK, 2020; pp. 192–247. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. A review on clean energy solutions for better sustainability. Int. J. Energy Res. 2015, 39, 585–606. [Google Scholar] [CrossRef]
- Soltani, M.; Kashkooli, F.M.; Dehghani-Sanij, A.R.; Kazemi, A.R.; Bordbar, N.; Farshchi, M.J.; Elmi, M.; Gharali, K.; Dusseault, M.B. A comprehensive study of geothermal heating and cooling systems. Sustain. Cities Soc. 2019, 44, 793–818. [Google Scholar] [CrossRef]
- Farzanehkhameneh, P.; Soltani, M.; Moradi Kashkooli, F.; Ziabasharhagh, M. Optimization and energy-economic assessment of a geothermal heat pump system. Renew. Sustain. Energy Rev. 2020, 133, 110282. [Google Scholar] [CrossRef]
- Balta, M.T.; Dincer, I.; Hepbasli, A. Geothermal-based hydrogen production using thermochemical and hybrid cycles: A review and analysis. Int. J. Energy Res. 2010, 34, 757–775. [Google Scholar] [CrossRef]
- Bairrão, D.; Soares, J.; Almeida, J.; Franco, J.F.; Vale, Z. Green Hydrogen and Energy Transition: Current State and Prospects in Portugal. Energies 2023, 16, 551. [Google Scholar] [CrossRef]
- Kukharets, V.; Juočiūnienė, D.; Hutsol, T.; Sukmaniuk, O.; Čėsna, J.; Kukharets, S.; Piersa, P.; Szufa, S.; Horetska, I.; Shevtsova, A. An Algorithm for Managerial Actions on the Rational Use of Renewable Sources of Energy: Determination of the Energy Potential of Biomass in Lithuania. Energies 2023, 16, 548. [Google Scholar] [CrossRef]
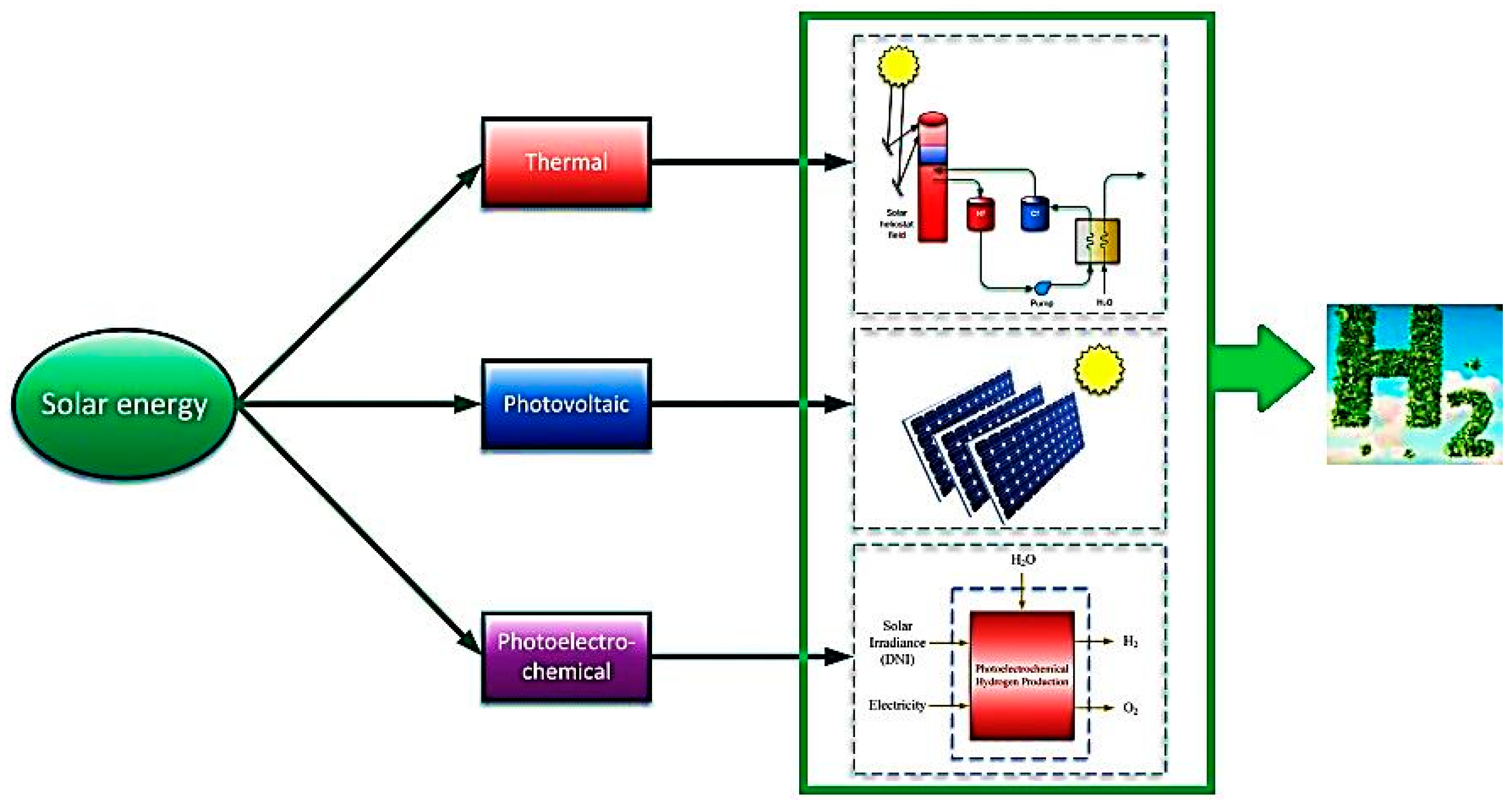
- Dincer, I.; Joshi, A.S. Solar based hydrogen production systems. In New York Heidelberg Dordrecht London; Springer: Cham, Switzerland, 2013. [Google Scholar]
- Coutanceau, C.; Baranton, S.; Audichon, T. Hydrogen production from water electrolysis. Hydrog. Electrochem. Prod. 2018, 2018, 17–62. [Google Scholar] [CrossRef]
- Mazzone, S.; Campbell, A.; Zhang, G.; García-García, F. Ammonia cracking hollow fibre converter for on-board hydrogen production. Int. J. Hydrog. Energy 2021, 46, 37697–37704. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Multi-objective optimization and analysis of a solar energy driven steam and autothermal combined reforming system with natural gas. J. Nat. Gas Sci. Eng. 2019, 69, 102927. [Google Scholar] [CrossRef]
- Ozturk, M.; Dincer, I. A comprehensive review on power-togas with hydrogen options for cleaner applications. Int. J. Hydrog. Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Kovac, A.; Paranos, M.; Marcius, D. Hydrogen in energy transition: A review. Int. J. Hydrog. Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Kanoglu, M.; Bolatturk, A.; Yilmaz, C. Thermodynamic analysis of models used in hydrogen production by geothermal energy. Int. J. Hydrog. Energy 2010, 35, 8783–8791. [Google Scholar] [CrossRef]
- Asaad, S.M.; Inayat, A.; Rocha-Meneses, L.; Jamil, F.; Ghenai, C.; Shanableh, A. Prospective of Response Surface Methodology as an Optimization Tool for Biomass Gasification Process. Energies 2022, 16, 40. [Google Scholar] [CrossRef]
- Mishra, S.; Rajashekaran, S.; Mohan, P.K.; Lokesh, S.M.; Ganiga, H.J.; Dash, S.K.; Roccotelli, M. Implementation of an ADALINE-Based Adaptive Control Strategy for an LCLC-PV-DSTATCOM in Distribution System for Power Quality Improvement. Energies 2023, 16, 323. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical water gasification of biomass for hydrogen production e Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Hydrogen Refuelling Infrastructure. ITM Power. 2017. Available online: https://www.level-network.com/wp-content/uploads/2017/02/ITM-Power.pdf (accessed on 15 December 2022).
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Cerniauskas, S.; Grube, T.; Praktiknjo, A.; Stolten, D.; Robinius, M. Future hydrogen markets for transportation and industry: The impact of CO2 taxes. Energies 2019, 12, 4707. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrog. Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Iribarren, D.; Susmozas, A.; Petrakopoulou, F.; Dufour, J. Environmental and exergetic evaluation of hydrogen production via lignocellulosic biomass gasification. J. Clean Prod. 2014, 69, 165–175. [Google Scholar] [CrossRef]
- Kopp, M.; Coleman, D.; Stiller, C.; Scheffer, K.; Aichinger, J.; Scheppat, B. Energiepark Mainz: Technical and economic analysis of the worldwide largest Power-to-Gas plant with PEM electrolysis. Int. J. Hydrog. Energy 2017, 42, 13311–13320. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jager- € Waldau, A. Green hydrogen in Europe e a regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Minke, C.; Suermann, M.; Bensmann, B.; HankeRauschenbach, R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int. J. Hydrog. Energy 2021, 46, 23581–23590. [Google Scholar] [CrossRef]
- Seyam, S.; Dincer, I.; Agelin-Chaab, M. Analysis of a clean hydrogen liquefaction plant integrated with a geothermal system. J. Clean Prod. 2020, 243, 118562. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrog. Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Ogden, J. Introduction to a future hydrogen infrastructure. Transit. Renew. Energy Syst. 2013, 2013, 795–811. [Google Scholar] [CrossRef]
- Demonstration Advances to Produce Hydrogen Using Molten Salt Reactor Nuclear Technology. Available online: https://www.powermag.com/demonstration-advances-to-produce-hydrogen-usingmolten-salt-reactor-nuclear-technology (accessed on 30 May 2020).
- Boretti, A. White is the color of hydrogen from concentrated solar energy and thermochemical water splitting cycles. Int. J. Hydrog. Energy 2021, 46, 20790–20791. [Google Scholar] [CrossRef]
- Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A.; Khan, Z. Biomass steam gasification for hydrogen production: A systematic review. In Biomass and Bioenergy; Springer: Cham, Switzerland, 2014; pp. 329–343. [Google Scholar]
- Dash, S.K.; Chakraborty, S.; Roccotelli, M.; Sahu, U.K. Hydrogen Fuel for Future Mobility: Challenges and Future Aspects. Sustainability 2022, 14, 8285. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Global warming potential of hydrogen and methane production from renewable electricity via power-to-gas technology. Int. J. Life Cycle Assess 2015, 20, 477–489. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A review on biomass based hydrogen production technologies. Int. J. Hydrog. Energy 2021, 47, 1461–1480. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dash, S.K.; Elavarasan, R.M.; Kaur, A.; Elangovan, D.; Meraj, S.T.; Kasinathan, P.; Said, Z. Hydrogen Energy as Future of Sustainable Mobility. Front. Energy Res. 2022, 10, 893475. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, N.M.; Jayakumar, A.; Dash, S.K.; Elangovan, D. Selected Aspects of Sustainable Mobility Reveals Implementable Approaches and Conceivable Actions. Sustainability 2021, 13, 12918. [Google Scholar] [CrossRef]





| Parameter | Hydrogen | Diesel | Methane |
|---|---|---|---|
| Density at STP (kg/m3) | 0.089 | 830.0 | 0.720 |
| Volumetric energy at STP (MJ/m3) | 1.07 × 10 | 3.5 × 104 | 3.3 × 10 |
| Net Lower Heating value (MJ/kg) | 119.9 | 42.5 | 45.8 |
| Boiling point (K) | 20.0 | 453–633 | 111.0 |
| Auto-ignition temperature (K) | 853 | ~523 | 813 |
| Minimum ignition energy in air at 1 bar & stoichiometry (mJ) | 0.020 | 0.240 | 0.290 |
| Stoichiometry air/fuel mass ratio | 34.4 | 14.5 | 17.2 |
| Quenching distance at NTP (mm) | 0.64 | - | 2.1 |
| Laminar flame speed in air at NTP (m/s) | 1.85 | 0.37–0.43 | 0.38 |
| Diffusion coefficient in air at STP (m2/s) | 85 × 10−7 | - | 19 × 10−7 |
| Flammability limits in air (% vol) | 4–76 | 0.6–5.5 | 5.3–15 |
| Adiabatic flame temperature at NTP (K) | 2480 | ~2300 | 2214 |
| Literature | Significance | Approach | Remarks |
|---|---|---|---|
| [78] | Solar powered hydrogen production | Thermodynamic and sustainable aspect | Environmental impact of solar-based hydrogen production |
| [79,80] | Water electrolysis-based hydrogen production | Thermodynamic aspect | Proton exchange-based electrolysis for hydrogen production |
| [81,82,83] | Analysis and assessment of solar energy powered natural gas reforming system | Cost assessment carbon emission analysis | improved energy and energy efficiencies |
| [84,85,86] | Geothermal energy-based hydrogen production | Thermodynamic modelling | geothermal heat is used to preheat the water and the high-temperature water is used in the electrolyser. It improves the system efficiency |
| [11,87,88] | Production of hydrogen at hydropower plants | Thermodynamic aspect | The hydrogen production efficiency improves |
| [89,90,91] | Production of hydrogen from renewable resources | Comparative assessment of renewable energy resources | hydrogen was found to be produced at high pressure, employing small amount of consumption of energy |
| [92,93,94,95] | biomass gasification-based hydrogen production | biomass gasification-based hydrogen production | The proposed biomass gasification study offered improvement in efficiency |
| [91,96,97] | Analysis and assessment of geothermal energy | Energy and exergy assessment along with study on specific energy consumption | Geothermal energy-powered clean hydrogen liquefaction process |
| [98,99] | future hydrogen infrastructure | Hydrogen infrastructure | energy security issues posed by current fuels are discussed and technical, societal, economic and infrastructure challenges in adopting new technology are analysed in detail |
| [100,101] | Future hydrogen markets for production and industry | Hydrogen Production | In the initial phase, low-cost, long-term storage and better-quality refuelling station utilization makes the best impression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. https://doi.org/10.3390/en16031141
Dash SK, Chakraborty S, Elangovan D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies. 2023; 16(3):1141. https://doi.org/10.3390/en16031141
Chicago/Turabian StyleDash, Santanu Kumar, Suprava Chakraborty, and Devaraj Elangovan. 2023. "A Brief Review of Hydrogen Production Methods and Their Challenges" Energies 16, no. 3: 1141. https://doi.org/10.3390/en16031141








