Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry
Abstract
1. Introduction
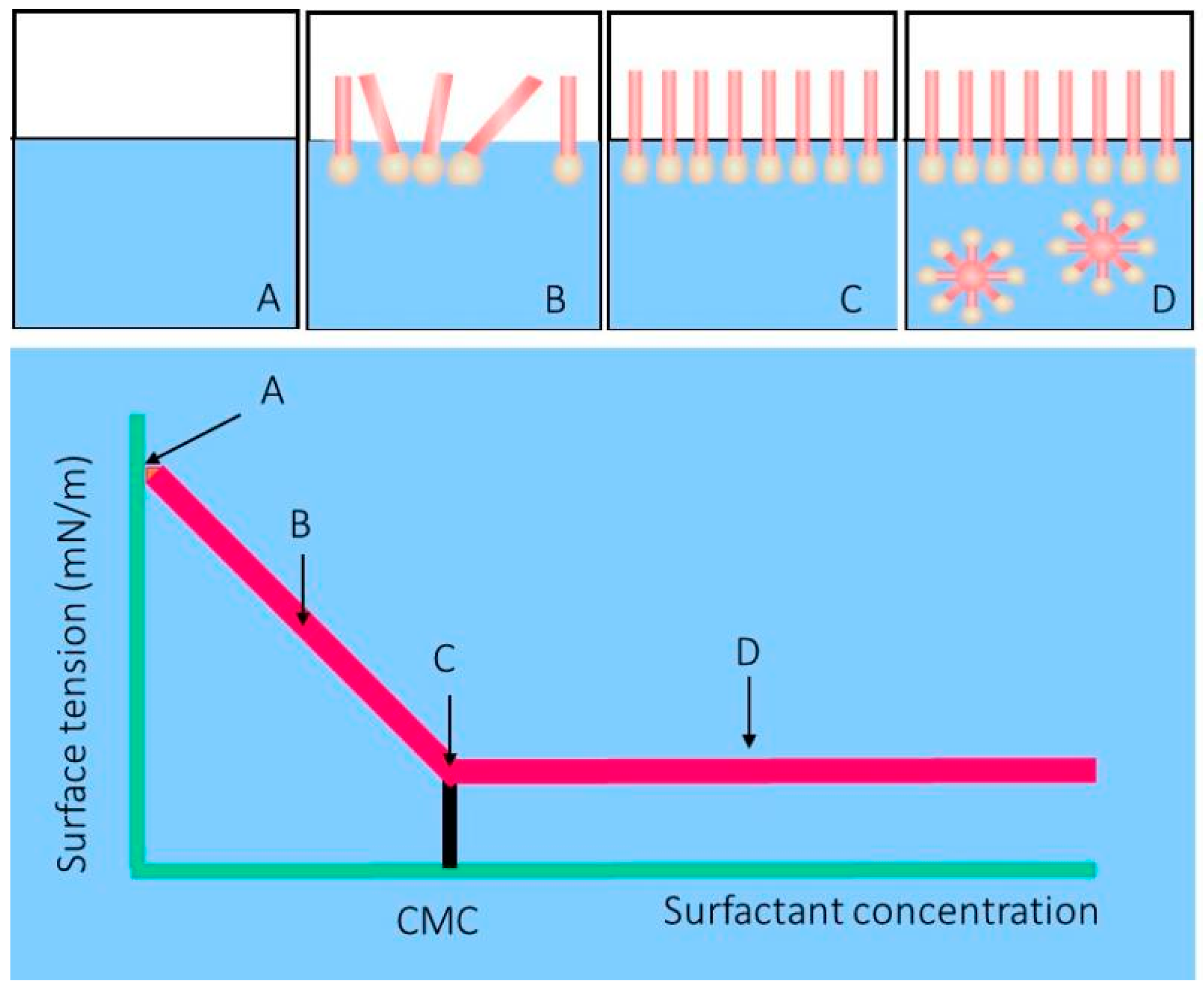
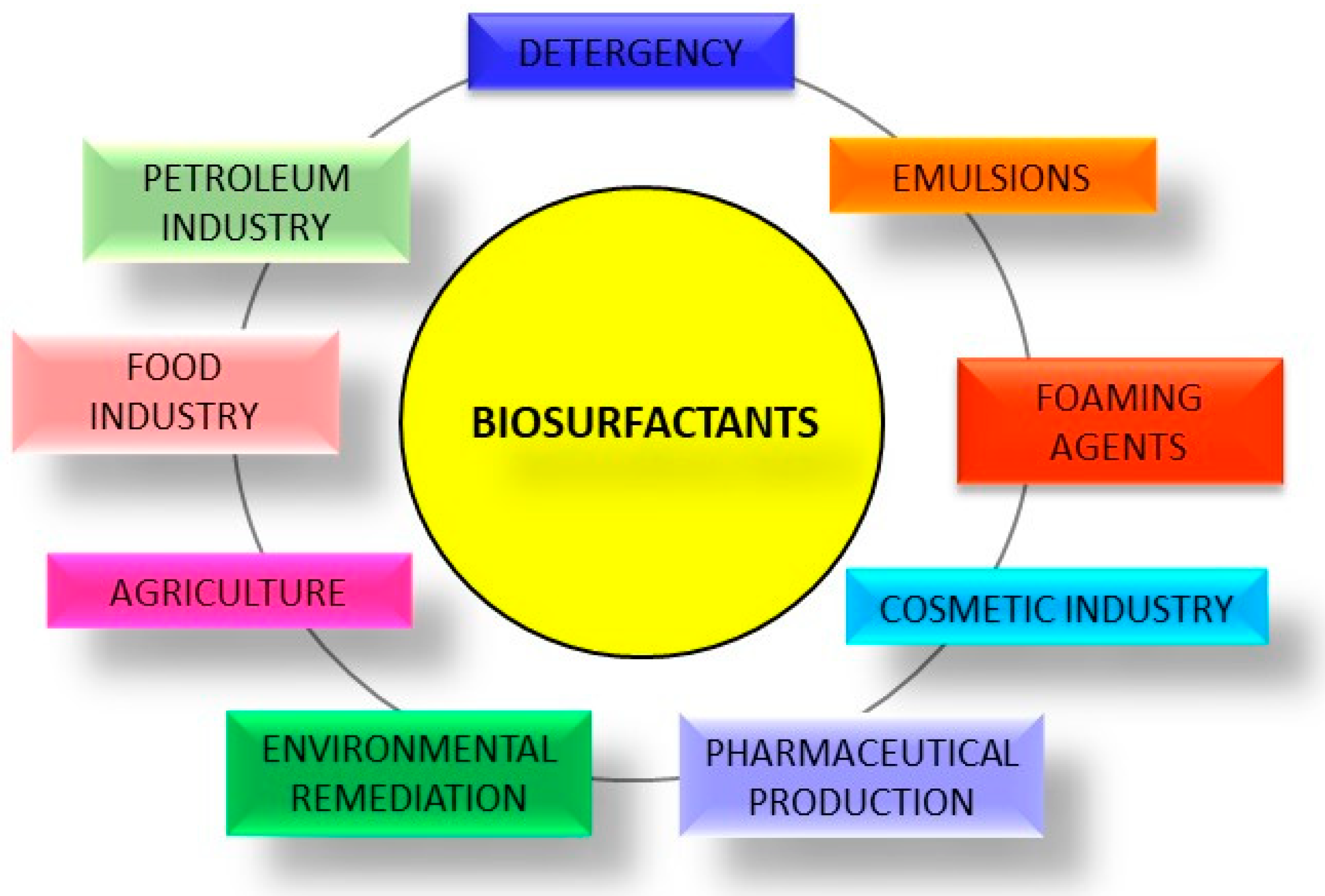
2. Biosurfactants
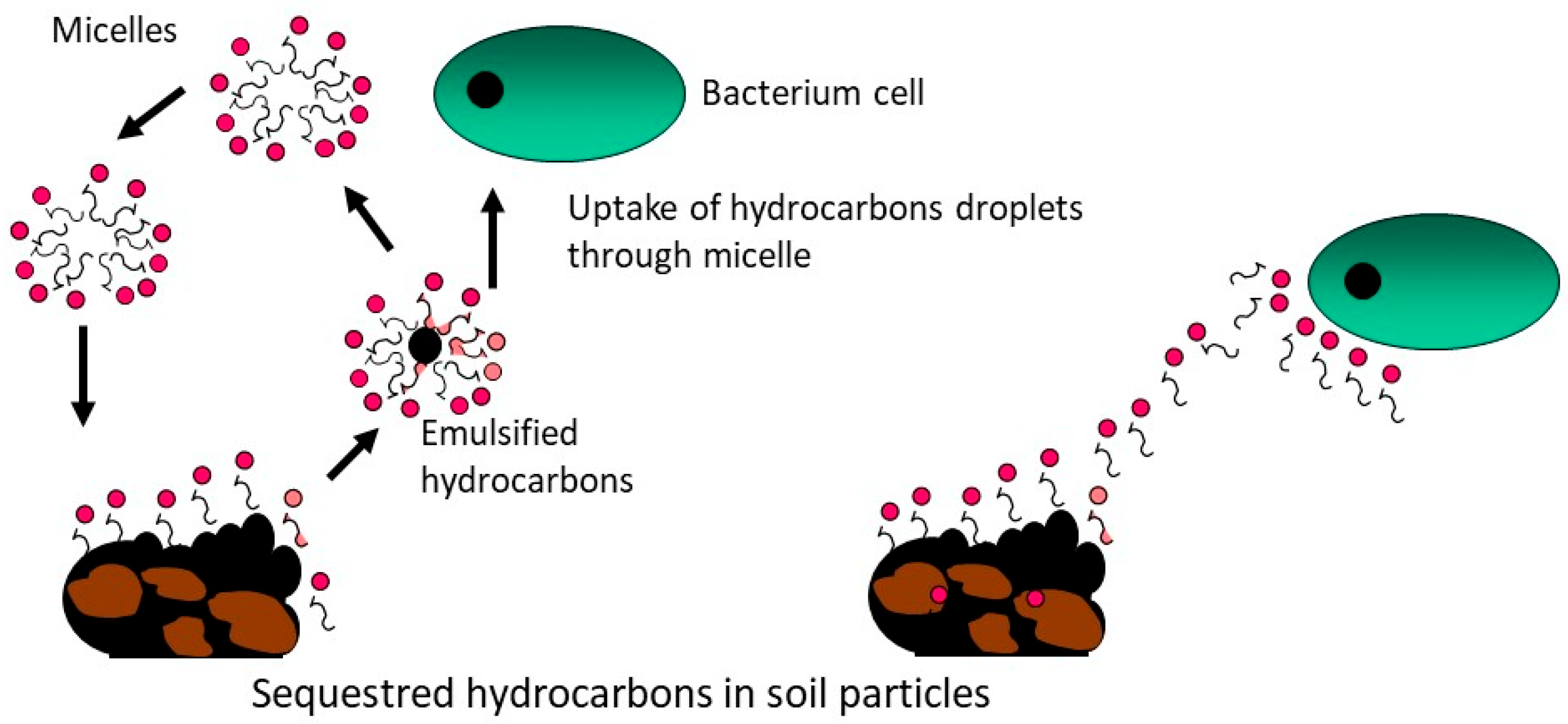
3. Application of Biosurfactants in Soil Remediation
4. Soil Washing
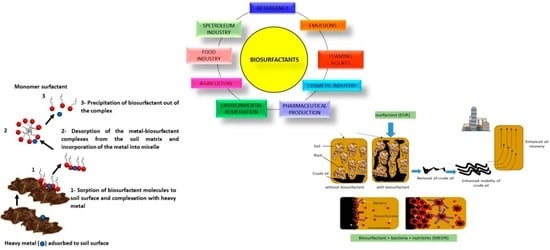
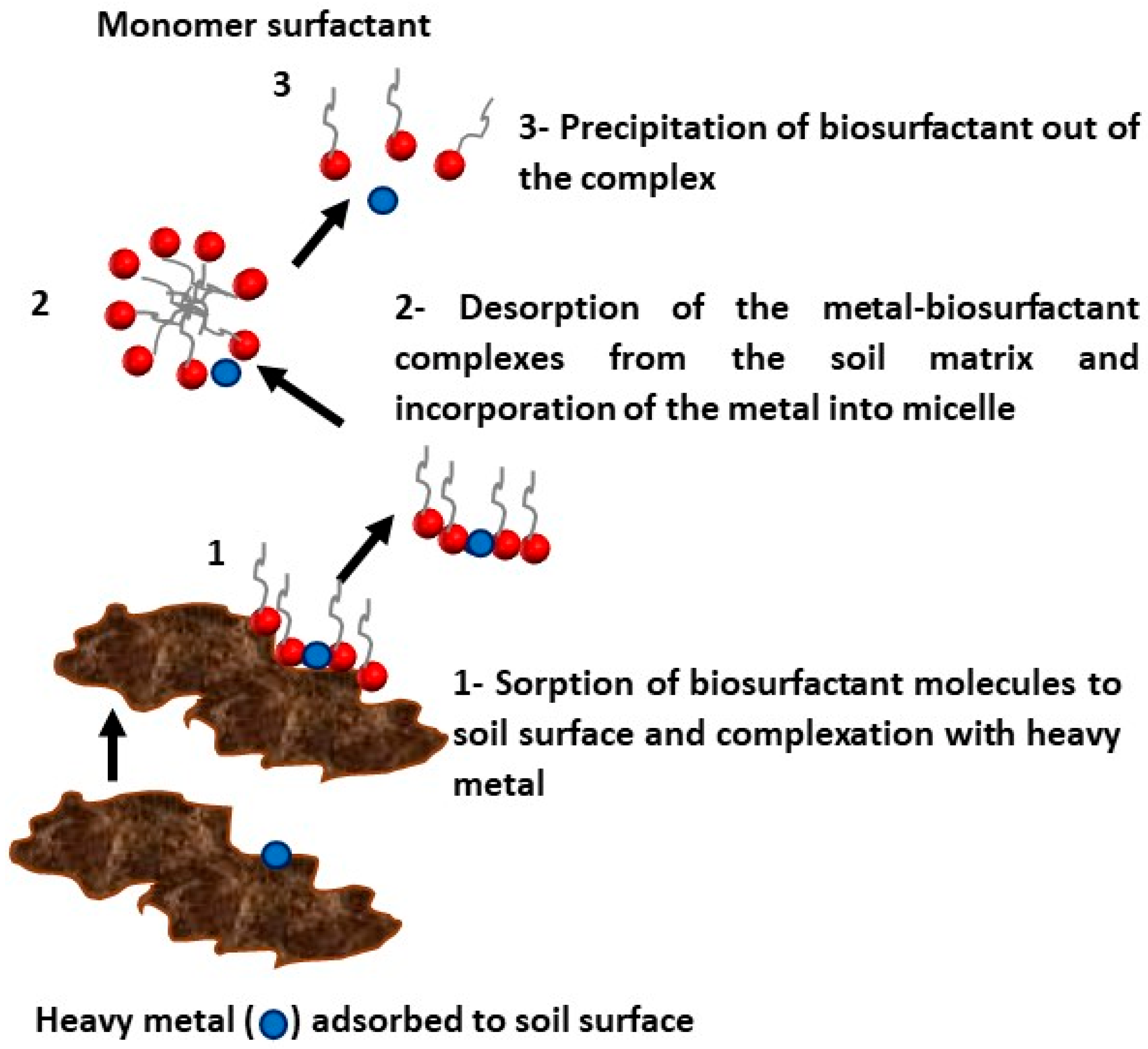
5. Application of Biosurfactants for Removal of Heavy Metals
6. Application of Biosurfactants for Bioremediation of Marine Environment
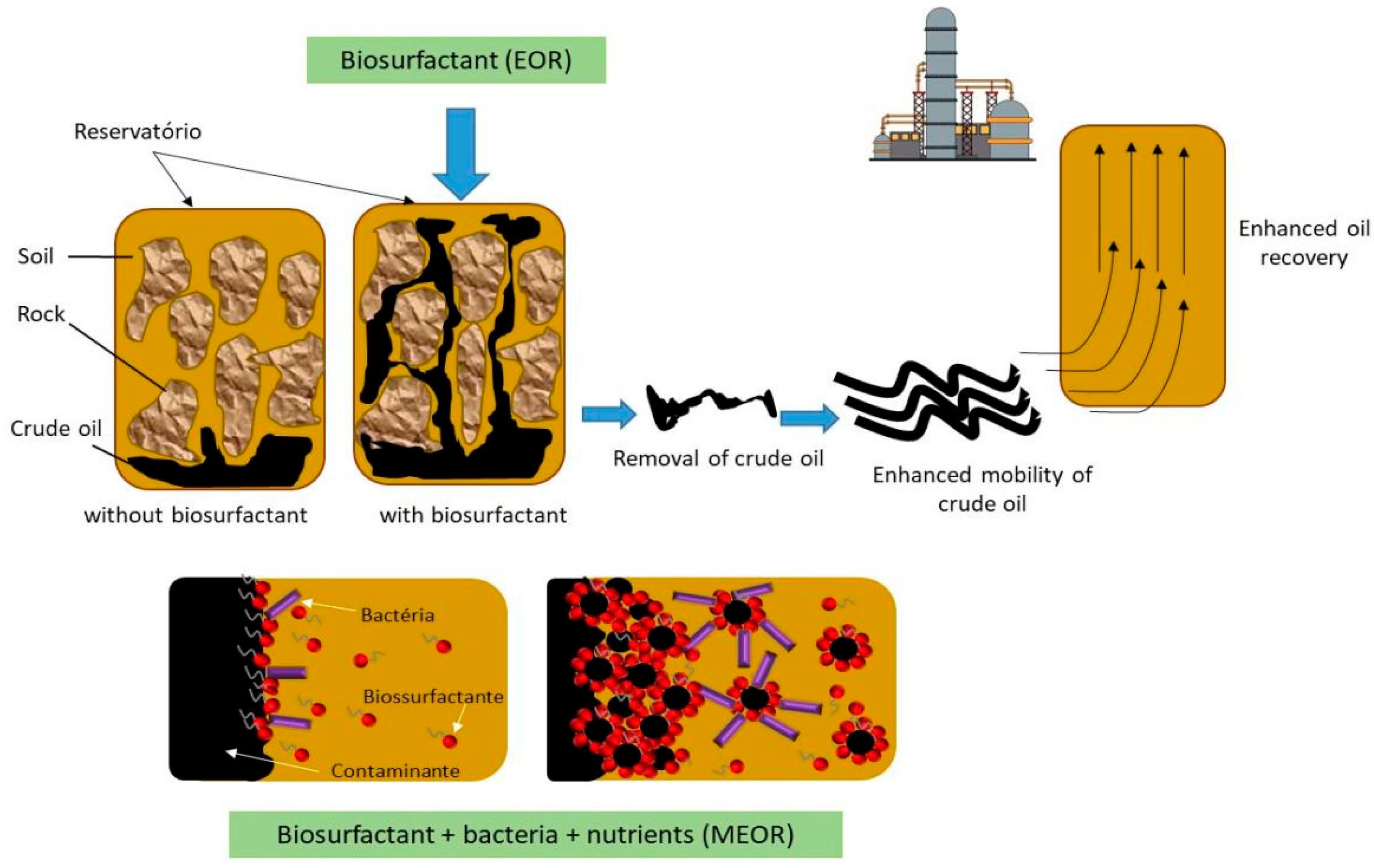
7. Application of Biosurfactants for Oil Recovery from Reservoirs
8. Biosurfactants Applied to Cleaning of Oil Storage Tanks
9. Application of Biosurfactants in Transport of Crude Oil through Pipelines
10. Biosurfactants as Anti-Corrosion Agents
11. Biosurfactants as Demulsifying Agents
12. Application of Biosurfactant in Treatment of Oily Effluents
13. Biosurfactants in Biodesulfurization
14. Application in Formulation of Fuels
15. Application of Biosurfactants in Degreasing Industry
16. Patents with Biosurfactants Applied in Environmental Field and Oil Industry
17. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Silva, M.G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Almeida, F.C.G.; Souza, T.C.; Bezerra, K.G.O.; Durval, I.J.B.; Converti, A.; Sarubbo, L.A. Oil spills: Impacts and perspectives of treatment technologies with focus on the use of green surfactants. Environ. Monit. Assess. 2022, 194, 143. [Google Scholar] [CrossRef] [PubMed]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Green surfactants: Industrial production and market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 2019, 7, 581. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; Bui, X.T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.G.; Soares da Silva, R.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, N.M.P.; Meira, H.M.; Almeida, F.C.G.; Soares da Silva, R.C.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural surfactants and their applications for heavy oil removal in industry. Sep. Purif. Rev. 2019, 48, 267–281. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Invally, K.; Sancheti, A.; Ju, L. A new approach for downstream purification of rhamnolipid biosurfactants. Food Bioprod. Process 2019, 114, 122–131. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Reddy, A.V.B.; Yusup, S.; Goto, M.; Moniruzzaman, M. Ionic liquid-biosurfactant blends as effective dispersants for oil spills: Effect of carbon chain length and degree of saturation. Environ. Pollut. 2021, 284, 117119. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; D’eziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microbiol. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef]
- Silva, R.C.F.S.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef]
- Shakeri, F.; Babavalian, H.; Amoozegar, M.A.; Ahmadzadeh, Z.; Zuhuriyanizadi, S.; Afsharian, M.P. Production and application of biosurfactants in biotechnology. Biointerface Res. Appl. Chem. 2021, 11, 10446–10460. [Google Scholar] [CrossRef]
- Yao, L.; Selmi, A.; Esmaeili, H. A review study on new aspects of biodemulsifiers: Production, features and their application in wastewater treatment. Chemosphere 2021, 284, 131364. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation (DAF) combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: The next generation biomolecules for diverse applications. Environ. Sustain. 2020, 3, 353–369. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.; Zhou, G.W.; Yu, Z.Q.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Ye, R.Q.; Mu, B.Z. Low-toxic and nonirritant biosurfactant surfactin and its performances in detergent formulations. J. Surfactants Deterg. 2020, 23, 109–115. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Carolin, F.; Kumar, C.P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactants: Production and application prospects in the food industry. Biotechnol. Prog. 2020, 36, e3030. [Google Scholar] [CrossRef]
- Selva Filho, A.A.P.; Almeida, F.C.G.; Soares da Silva, R.C.F.; Sarubbo, L.A. Analysis of the surfactant properties of Eichhornia crassipes for application in the remediation of environments impacted by hydrophobic pollutants. Biocatal. Agric. Biotechnol. 2021, 36, 102120. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, X.; Chen, Y.; Lin, Y.; Mohsin, A.; Chu, J. Efficient sophorolipids production via a novel in situ separation technology by Starmerella bombicola. Process Biochem. 2019, 81, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Ricón-Fontán, M.; Vecino, X.; Moldes, A.B.; Cruz, J.M. Biodegradability study of the biosurfactant contained in a crude extract from corn steep water. J. Surfactants Deterg. 2020, 23, 79–90. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M. Bio-surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere 2016, 144, 635–644. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Hegazy, M.M.; Award, M.K.; Shawky, M. Chemical, electrochemical, theoretical (DFT & MEP), thermodynamics and surface morphology studies of carbon steel during gas and oil production using three novel di-cationic amphiphiles as corrosion inhibitors in acidic medium. J. Mol. Liq. 2021, 337, 116541. [Google Scholar] [CrossRef]
- Faccioli, Y.E.S.; Silva, G.O.; Soares da Silva, R.C.F.; Sarubbo, L.A. Application of a biosurfactant from Pseudomonas cepacia CCT 6659 in bioremediation and metallic corrosion inhibition processes. J. Biotechnol. 2022, 351, 109–121. [Google Scholar] [CrossRef]
- Sales da Silva, I.G.; Almeida, F.C.G.; Rocha e Silva, N.M.P.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Wo´zniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial degradation of hydrocarbons—Basic principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed]
- Chaprão, M.J.; Ferreira, I.N.S.; Correa, P.F.; Rufino, R.D.; Luna, J.M.; Silva, E.J.; Sarubbo, L.A. Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand. Electron. J. Biotechnol. 2015, 18, 471–479. [Google Scholar] [CrossRef]
- Bustamante, M.; Duran, N.; Diez, M.C. Biosurfactants are useful tools for the bioremediation of contaminated soil: A review. J. Soil Sci. Plant Nutr. 2012, 12, 667–687. [Google Scholar] [CrossRef]
- Silva, E.J.; Luna, J.M.; Rufino, R.D.; Silva, R.O.; Sarubbo, L.A. Characterization of a biosurfactant produced by Pseudomonas cepacia CCT6659 in the presence of industrial wastes and its application in the biodegradation of hydrophobic compounds in soil. Colloids Surf. B 2014, 117, 36–41. [Google Scholar] [CrossRef]
- Itrich, N.R.; McDonough, K.M.; Van Ginkel, C.G.; Bisinger, E.C.; LePage, J.N.; Schaefer, E.C.; Menzies, J.Z.; Casteel, K.D.; Federle, T.W. Widespread microbial adaptation to L-glutamate-N, N-diacetate (L-GLDA) ollowing its market introduction in a consumer cleaning product. Environ. Sci. Technol. 2015, 49, 13314–13321. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Lai, C.; Huang, Y.; Wei, Y.; Chang, J. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Loza, F.J.; Noordman, W.H.; Jannsen, D.B.; Brusseau, M.L.; Maier, R.M. Effect of clays, metal oxides, and organic matter on rhamnolipid biosurfactant sorption by soil. Chemosphere 2007, 66, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Rocha Junior, R.B.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. [Google Scholar] [CrossRef]
- Halecký, M.; Kozliak, E. Modern Bioremediation Approaches: Use of Biosurfactants, Emulsifiers, Enzymes, Biopesticides, GMOs. In Advanced Nano-Bio Technologies for Water and Soil Treatment. Applied Environmental Science and Engineering for a Sustainable Future, 1st ed.; Filip, J., Cajthaml, T., Najmanová, P., Černík, M., Zbořil, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 495–526. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Ogunkunle, T.; Fadairo, A.; Rasouli, V.; Ling, K.; Oladepo, A.; Chukwuma, O.; Ayoo, J. Microbial-derived bio-surfactant using neem oil as substrate and its suitability for enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2021, 11, 627–638. [Google Scholar] [CrossRef]
- Bandala, E.R.; Aguilar, F.; Torres, L.G. Surfactant-enhanced soil washing for the remediation of sites contaminated with pesticides. Land Contam. Reclamat. 2010, 18, 151–159. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Li, G.; Wu, T.; Sun, D. Efficient remediation of crude oil-contaminated soil using a solvent/surfactant system. RSC Adv. 2019, 9, 2402–2411. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Marinho, P.H.C.; Farias, C.B.B.; Ferreira, S.R.M.; Sarubbo, L.A. Removal of petroleum derivative adsorbed to soil by biosurfactant Rufisan produced by Candida lipolytica. J. Pet. Sci. Eng. 2013, 109, 117–122. [Google Scholar] [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B 2018, 172, 127–135. [Google Scholar] [CrossRef]
- Soares da Silva, R.C.F.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Rufino, R.D.; Sarubbo, L.A. Biosurfactant as an environmental remediation agent: Toxicity, formulation and application in the removal of petroderivate in sand and rock walls. Biointerface Res. Appl. Chem. 2022, 12, 34–48. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.; Zainol, M.R.; Murshed, M.F.; Faris, M.A.; Bayuaji, R. Review on adsorption of heavy metal in wastewater by using geopolymer. MATEC Web Conf. 2017, 97, 01023. [Google Scholar] [CrossRef]
- Rocha Junior, R.B.; Meira, H.M.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation 2019, 30, 215–233. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- Liduino, V.S.; Servulo, E.F.; Oliveira, F.J. Biosurfactant-assisted phytoremediation of multi-contaminated industrial soil using sunflower (Helianthus annuus L.). J. Environ. Sci. Health Part A 2018, 53, 609–616. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process Saf. Environ. Prot. 2016, 102, 558–566. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Brasileiro, P.P.F.; Silveira, G.N.M.; Luna, J.M.; Rufino, R.D.; Santos, V.A. Application of a low cost biosurfactant in the removal of heavy metals in soil. Chem. Eng. Trans. 2018, 64, 433–438. [Google Scholar] [CrossRef]
- Qi, X.; Xu, X.; Zhong, C.; Jiang, T.; Wei, W.; Song, X. Removal of cadmium and lead from contaminated soils using sophorolipids from fermentation culture of Starmerella bombicola CGMCC 1576 fermentation. Int. J. Environ. Res. Public Health 2018, 15, 2334. [Google Scholar] [CrossRef] [PubMed]
- El Gheriany, I.A.; El Saqa, F.A.; Amer, A.A.E.R.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alexandria Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Faksness, L.G.; Brandvik, P.J.; Daling, P.S.; Singsaas, I.; Sorstrom, S.E. The value of offshore field experiments in oil spill techology development for Norwegian waters. Mar. Pollut. Bull. 2016, 111, 402–410. [Google Scholar] [CrossRef]
- Edwards, K.R.; Leop, J.E.; Lewis, M.A. Toxicity comparison of biosurfactants and synthetic surfactants used in oil spill remediation to two estuarine species. Mar. Pollut. Bull. 2003, 46, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Homaei, A.; Patil, S.; Daverey, A. Microbial biosurfactants for oil spill remediation: Pitfalls and potentials. Appl. Microbiol. Biotechnol. 2019, 103, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Z.; Zeng, C.; Wan, X.; Chen, D.; Zhang, J.Y.; Shen, P. Effect of rhamnolipids on degradation of anthracene by two newly isolated strains, Sphingomonas sp. 12A and Pseudomonas sp. 12B. J. Microbiol. Biotechnol. 2008, 18, 63–66. [Google Scholar]
- Liu, Y.; Zeng, G.; Zhong, H.; Wang, Z.; Liu, Z.; Cheng, M.; Liu, G.; Liu, S. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction? J. Hazard. Mater. 2017, 322, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.G.; Brito, J.G.M.; Brasileiro, P.P.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Formulation of a commercial biosurfactant for application as a dispersant of petroleum and by-products spilled in oceans. Front. Microbiol. 2016, 7, 1646. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Resende, A.H.M.; Almeida, D.G.; Soares da Silva, R.C.F.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Evaluation of remediation processes with a biosurfactant from Candida lipolytica UCP0988 and the formulation of a commercial bioremediation agent. Front. Microbiol. 2017, 8, 767. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Mendonça, A.H.R.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Studies on biosurfactant from Bacillus amyloliquefaciens isolated from sea water with biotechnological potential for marine oil spill bioremediation. J. Surfactants Deterg. 2019, 22, 349–363. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Resende, A.H.M.; Rocha, I.V.; Luna, J.M.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Production, characterization, evaluation and toxicity assessment of a Bacillus cereus UCP 1615 biosurfactant for marine oil spills bioremediation. Mar. Pollut. Bull. 2020, 157, 111357. [Google Scholar] [CrossRef]
- Ostendorf, T.A.; Silva, I.A.; Converti, A.; Sarubbo, L.A. Production and formulation of a new low-cost biosurfactant to remediate oil-contaminated seawater. J. Biotechnol. 2019, 295, 71–79. [Google Scholar] [CrossRef]
- Soares da Silva, R.C.F.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Ecotoxicity of the formulated biosurfactant from Pseudomonas cepacia CCT 6659 and application in the bioremediation of terrestrial and aquatic environments impacted by oil spills. Process Saf. Environ. Prot. 2021, 154, 338–347. [Google Scholar] [CrossRef]
- Jin, L.; Garamus, V.M.; Liu, F.; Xiao, J.; Eckerlebe, H.; Willumeit-Römer, R.; Mu, B.; Zou, A. Interaction of a biosurfactant, surfactin with a cationic gemini surfactant in aqueous solution. J. Colloid Interface Sci. 2016, 481, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.U.H.; Moniruzzaman, M.; Sivapragazam, M.; Talukder, M.R.; Yusup, S.B.; Goto, M. A binary mixture of a biosurfactant and an ionic liquid surfactant as a green dispersant for oil spill remediation. J. Mol. Liq. 2019, 280, 111–119. [Google Scholar] [CrossRef]
- Camara, J.M.; Sousa, M.A.; Neto, E.B.; Oliveira, M.C. Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbial-enhanced oil recovery (MEOR). J. Pet. Explor. Prod. Technol. 2019, 1, 2333–2341. [Google Scholar] [CrossRef]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: Production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal. Agric. Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J.; Al-Makhmari, H.S.; Al-Sulaimani, H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013, 81, 141–146. [Google Scholar] [CrossRef]
- Golabi, E.; Sogh, S.R.; Hosseini, S.N.; Gholamzadeh, M.A. Biosurfactant production by microorganism for enhanced oil recovery. Int. J. Sci. Eng. Res. 2012, 3, 1–6. [Google Scholar]
- Sales da Silva, I.G.S.; Rocha e Silva, N.M.P.; Oliveira, J.T.R.; Almeida, F.C.G.; Converti, A.; Sarubbo, L.A. Application of green surfactants in the remediation of soils contaminated by petroderivates. Processes 2021, 9, 1666. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Pet. Sci. Eng. 2020, 195, 107612. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Soares da Silva, R.C.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Production of a biosurfactant from Bacillus methylotrophicus UCP1616 for use in the bioremediation of oil-contaminated environments. Ecotoxicology 2018, 27, 1310–1322. [Google Scholar] [CrossRef]
- Adrion, A.C.; Nakamura, J.; Shea, D.; Aitken, M.D. Screening nonionic surfactants for enhanced biodegradation of polycyclic aromatic hydrocarbons remaining in soil after conventional biological treatment. Environ. Sci. Technol. 2017, 50, 3838–3845. [Google Scholar] [CrossRef]
- Medeiros, A.D.M.D.; Silva Junior, C.J.G.D.; Amorim, J.D.P.D.; Durval, I.J.B.; Costa, A.F.D.S.; Sarubbo, L.A. Oily wastewater treatment: Methods, challenges, and trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Islam, B. Petroleum sludge, its treatment and disposal: A review. Int. J. Chem. Sci. 2015, 13, 1584–1602. [Google Scholar]
- Saeki, H.; Sasaki, M.; Komatsu, K.; Miura, A.; Matsuda, H. Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Biores. Technol. 2009, 100, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Abadshapouri, R.A.; Amani, H.; Hajimohammadi, R.; Soltani, H. Heavy oil storage tanks clean-up using biosurfactants and investigation of the synergistic effect with silica nanoparticles. Tenside Surfact. Det. 2021, 58, 259–264. [Google Scholar] [CrossRef]
- Diab, A.; Din, G.E. Application of the biosurfactants produced by Bacillus sp. (SH 20 and SH 26) and P. aeruginosa SH 29 isolated from the rhizosphere soil of an Egyptian salt marsh plant for the cleaning of oil-contaminated vessels and enhancing the biodegradation. Afr. J. Environ. Sci. Technol. 2013, 7, 671–679. [Google Scholar]
- Ceron-Camacho, R.; Martínez-Palou, R.; Chavez-Gomez, B.; Cuéllar, F.; Bernal-Huicochea, C.; Aburto, J. Synergistic effect of alkyl-O-glucoside and-cellobioside biosurfactants as effective emulsifiers of crude oil in water: A proposal for the transport of heavy crude oil by pipeline. Fuel 2013, 110, 310–317. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Khidr, T.T. Some bio-surfactants used as pour point depressant for waxy egyptian crude oil. Petroleum Sci. Technol. 2016, 34, 1475–1482. [Google Scholar] [CrossRef]
- Mazaheri-Assadi, M.; Tabatabaee, M.S. Biosurfactants and their use in upgrading petroleum vacuum distillation residue: A review. Inter. J. Environ. Res. 2010, 4, 549–572. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Li, J.; Wang, J.; Zhang, L. The use of bio-based surfactant obtained by enzymatic syntheses for wax deposition inhibition and drag reduction in crude oil pipelines. Catalysts 2016, 6, 61. [Google Scholar] [CrossRef]
- Pedeferri, P.; Ormellese, M. General Principles of Corrosion. In Corrosion Science and Engineering, 1st ed.; Pedeferri, P., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- El-din, M.R.N.; Farag, R.K.; Elazbawy, O.E. Utilization of new anionic polymeric surfactants for corrosion inhibition enhancement in petroleum industries. Int. J. Electrochem. Sci. 2016, 11, 815–835. [Google Scholar]
- Abbasov, V.M.; Aliyeva, L.I.; El-lateef, H.M.A.; Ismayilov, I.T. Some surfactants based on the vegetable oils as CO2 corrosion inhibitors for mild steel in oilfield formation water. Int. J. Corros. Scale Inhib. 2015, 4, 162–175. [Google Scholar] [CrossRef]
- Araujo, L.V.; Guimarães, C.R.; Marquita, R.L.S.; Santiago, V.M.J.; Souza, M.P.; Nitscheke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Ali, A.E.; Badr, G.E.; Founda, A.E.S. Citrus sinensis extract as a green inhibitor for the corrosion of carbon steel in sulphuric acid solution. Biointerface Res. Appl. Chem. 2021, 11, 14007–14020. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Roque, B.A.C.; Rocha e Silva, N.M.P.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Yeasts and bacterial biosurfactants as demulsifiers for petroleum derivative in seawater emulsions. AMB Express 2017, 7, 202. [Google Scholar] [CrossRef]
- Silva, E.J.; Silva, I.A.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Use of bacterial biosurfactants as natural collectors in the dissolved air flotation process for the treatment of oily industrial effluent. Bioprocess Biosyst. Eng. 2018, 41, 1599–1610. [Google Scholar] [CrossRef]
- Menezes, C.T.B.; Barros, E.C.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage using the dissolved air flotation technique. Appl. Biochem. Biotechnol. 2011, 163, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.A.; Luna-Finkler, L.C.; Rufino, R.D.; Luna, J.M.; Menezes, C.T.B.; Sarubbo, L.A. Evolution of biosurfactant for removal metal ions from aqueous effluent using flotation techniques. Int. Rev. Chem. Eng. 2012, 4, 156–161. [Google Scholar]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Moura, A.E.; Galdino, R.A.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Effect of biosurfactant addition in a pilot scale dissolved air flotation system. Sep. Sci. Technol. 2015, 50, 618–625. [Google Scholar] [CrossRef]
- Chaprão, M.J.; Soares da Silva, R.C.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Formulation and application of a biosurfactant from Bacillus methylotrophicus as collector in the flotation of oily water in industrial environment. J. Biotechnol. 2018, 285, 15–22. [Google Scholar] [CrossRef]
- Santos, L.B.; Soares da Silva, R.C.F.; Brasileiro, P.P.F.; Baldo, R.D.; Sarubbo, L.A.; Santos, V.A. Oily water treatment in a multistage tower operated under a novel induced pre-saturation process in the presence of a biosurfactant as collector. Biotechnol. Rep. 2021, 30, e00638. [Google Scholar] [CrossRef]
- Nazari, F.; Kefayati, M.E.; Raheb, J. The study of biological technologies for the removal of sulfur compounds. J. Sci. Islam. Repub. Iran 2017, 28, 205–219. [Google Scholar]
- Chafale, A.; Kapley, A. Biosurfactants as microbial bioactive compounds in microbial enhanced oil recovery. J. Biotechnol. 2022, 352, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sai-Chaitanya, P.; Rambabu, V.; Simhadri, K. Investigation on effect of water emulsified with diesel by surfactant addition on performance and emission characteristics of diesel engine. Int. J. Chem. Sci. 2016, 14, 2835–2844. [Google Scholar]
- Kayali, I.; Qamhieh, K.; Olsson, U.; Bemert, L.; Strey, R. Water-diesel microemulsions stabilized by an anionic extended surfactant and a cationic hydrotrope. J. Dispersion Sci. Technol. 2012, 33, 516–520. [Google Scholar] [CrossRef]
- Syafiq, Z.; Fahmi, O.; Awad, O.I.; Adam, A. The study of stability, combustion characteristics and performance of water in diesel emulsion fuel. MATEC Web Conf. 2017, 90, 1–8. [Google Scholar] [CrossRef]
- El-Din, M.N.; El-Hamouly, S.H.; Mohamed, H.M.; Mishrif, M.R.; Ragab, A.M. Water-in-diesel fuel nanoemulsions: Preparation, stability and physical properties. Egyptian J. Petrol. 2013, 22, 517–530. [Google Scholar] [CrossRef]
- Hegde, R.R.; Sharma, P.; Raj, P.; Keny, R.V.; Bhide, P.J.; Kumar, S.; Bhattacharya, S.S.; Lohani, A.; Kumar, A.; Verma, A.; et al. Factors affecting emissions from diesel fuel and water-in-diesel emulsion. Energy Sources Part A 2016, 38, 1771–1778. [Google Scholar] [CrossRef]
- Vellaiyan, S.; Amirthagadeswaran, K.S. Compatibility test in a CI engine using lemon peel oil and water emulsion as fuel. Fuel 2020, 279, 118520. [Google Scholar] [CrossRef]
- Liu, J.; Vipulanandan, C. Effects of Fe, Ni, and Fe/Ni metallic nanoparticles on power production and biosurfactant production from used vegetable oil in the anode chamber of a microbial fuel cell. Waste Manag. 2017, 66, 169–177. [Google Scholar] [CrossRef]
- Gomaa, O.M.; Selim, N.; Fathy, R.; Maghrawy, H.H.; Gamal, M.; Kareem, H.A.E.; Kyazze, G.; Keshavarz, T. Characterization of a biosurfactant producing electroactive Bacillus sp. for enhanced Microbial Fuel Cell dye decolourisation. Enzyme Microb. Technol. 2021, 147, 109767. [Google Scholar] [CrossRef]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. Adv. Exp. Med. Biol. 2010, 672, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, N.M.P.; Almeida, F.C.G.; Rocha e Silva, F.C.P.; Luna, J.M.; Sarubbo, L.A. Formulation of a biodegradable detergent for cleaning oily residues generated during industrial processes. J. Surfactants Deterg. 2020, 23, 1111–1123. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Soares da Silva, R.C.F.; Almeida, F.C.G.; Converti, A.; Santos, V.A.; Sarubbo, L.A. Physicochemical upgrading of a biodetergent for application in the industrial energy sector. Energies 2022, 15, 463. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Soares da Silva, R.C.F.; Almeida, F.C.G.; Santos, V.A.; Sarubbo, L.A. Removal of heavy oil from contaminated surfaces with a detergent formulation containing biosurfactants produced by Pseudomonas spp. PeerJ. 2021, 9, e12518. [Google Scholar] [CrossRef]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int. J. Mol Sci. 2020, 21, 2152. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Hitzman, D.O. Enhanced Oil Recovery Process Using Microorganisms. U.S. Patent 4450908, 29 May 1984. [Google Scholar]
- Sheehy, A. Recovery of Oil from Oil Reservoirs. U.S. Patent 5083610, 28 January 1992. [Google Scholar]
- Clark, J.B.; Jenneman, G.E. Nutrient Injection Method for Subterranean Microbial Processes. U.S. Patent 5083611, 28 January 1992. [Google Scholar]
- Mcnerney, M.J.; Jenneman, G.E.; Knapp, R.M.; Menzie, D.E. Biosurfactant and Enhanced Oil Recovery. U.S. Patent 4522261, 11 June 1985. [Google Scholar]
- Fallon, R.D. Methods for Improved Hydrocarbon and Water Compatibility. U.S. Patent 7992639, 9 August 2011. [Google Scholar]
- Converse, D.R.; Hinton, S.M.; Hieshima, G.B.; Barnum, R.S.; Sowlay, M.R. Process for Stimulating Microbial Activity in a Hydrocarbon-Bearing, Subterranean Formation. U.S. Patent 6543535, 8 April 2003. [Google Scholar]
- Jianjun, L.; Xiaolin, W.; Lulu, B.; Qun, Z.; Yu, L.; Zhaowei, H.; Ying, W. Complex Biological Oil Displacement Agent and Application thereof. CN Patent 102492409, 13 June 2012. [Google Scholar]
- Simaev, J.; Bazekina, L.V.; Tukhteev, R.M.; Tujgunov, M.R.; Kalinskij, B.A. Composition for Increase of Oil Recovery. RU Patent 2143553, 27 December 1999. [Google Scholar]
- Deshpande, M.; Mishra, K.; Bal, A.S.; Khanna, P.; Juwarkar, A.; Babu, P.S. A Process for the Preparation of a Biosurfactant Useful as Microbial Emulsifier for the Recovery of Oil. IN Patent 189459, 22 February 2003. [Google Scholar]
- Lal, B.; Reddy, M.R.V.; Agnihotri, A.; Kumar, A.; Sarbhai, M.P.; Singh, N.; Khurana, R.K.; Khazanchi, S.K.; Misra, T.R. A Process for Enhanced Recovery of Crude Oil from Oil Wells Using Novel Microbial Consortium. WO Patent 2005005773, 24 March 2005. [Google Scholar]
- Bryant, R.S. Microbial Enhanced Oil Recovery and Compositions therefor. U.S. Patent 4905761, 6 March 1990. [Google Scholar]
- Kohr, W.J. Microbial enhanced oil recovery methods. U.S. Patent 8316933, 27 November 2012. [Google Scholar]
- Lora, P.O.; Cortés, G.T.C.; Carrillo, T.G.R.; Peñasco, N.I.Z. Biotechnological Process for Hydrocarbon Recovery in Low Permeability Porous Media. U.S. Patent 8631865, 21 January 2014. [Google Scholar]
- Hendrickson, E.R.; Luckring, A.K.; Keeler, S.J.; Perry, M.P.; Choban, E.R. Steady State Anaerobic Denitrifying Consortium for Application in In-Situ Bioremediation of Hydrocarbon-Contaminated Sites and Enhanced oil Recovery. U.S. Patent 8753865, 17 June 2014. [Google Scholar]
- Carrillo, T.G.R.; Cortés, G.T.C.; Peñasco, N.I.Z.; Ávila, R.J.R.; Berthier, A.M.; Lora, P.O. Heavy Oil Recovery Process Using Extremophile Anaerobic Indigenous Microorganisms. U.S. Patent 8895479, 25 November 2014. [Google Scholar]
- Kohr, W.J.; Zhang, Z.; Galgoczy, D.J. Alkaline Microbial Enhanced Oil Recovery. U.S. Patent 9290688, 22 March 2016. [Google Scholar]
- Wagner, F.; Rapp, P.; Bock, H.; Lindorfer, W.; Schulz, W.; Gebetsberger, W. Method and Installation for Flooding Petroleum Wells and Oil-Sands. C.A. Patent 1119794, 16 March 1982. [Google Scholar]
| Title of Patent | Biosurfactants/Microorganisms | Patent Number | Reference |
|---|---|---|---|
| Enhanced oil recovery process using microorganisms | Mixture of biosurfactant-producing microorganisms | US 4450908 | [119] |
| Recovery of oil from oil reservoirs | Indigenous biosurfactant-producing microorganisms | US 5083610 | [120] |
| Nutrient injection method for subterranean microbial processes | Injection of nutrients to stimulate microbial biosurfactant production | US 5083611 | [121] |
| Biosurfactant and enhanced oil recovery | Lipopeptide | US 4522261 | [122] |
| Methods for improved hydrocarbon and water compatibility | Diverse biosurfactant-producing microbial population | US 7992639 | [123] |
| Process for stimulating microbial activity in a hydrocarbon- bearing, subterranean formation | Microbial consortia | US 6543535 | [124] |
| Complex biological oil displacement agent and application thereof | Combination of biosurfactant (rhamnolipid) and chemical surfactant (fatty acid amide sulpho monoester potassium maleate) | CN 102492409 | [125] |
| Composition for increase of oil recovery | KshAS-M biosurfactant | RU 2143553 | [126] |
| A process for preparation of biosurfactant useful as microbial emulsifier for the recovery of oil | Microbial bioemulsifier | IN 189459 | [127] |
| A process for enhanced recovery of crude oil from oil wells using novel microbial consortium | Three hyperthermophilic, barophilic, acidogenic, anaerobic bacterial strains producing variety of metabolic products, especially CO2, methane, biosurfactant, volatile fatty acids and alcohols | WO 2005005773 | [128] |
| Microbial enhanced oil recovery and compositions therefor | Microbial consortia | US 4905761 | [129] |
| Microbial enhanced oil recovery methods | Exogenous biosurfactant-producing microbes that degrade hydrocarbons | US 8316933 | [130] |
| Biotechnological process for hydrocarbon recovery in low permeability porous media | Biostimulation and bioaugmentation of biosurfactant-producing bacterial consortia | US 8631865 | [131] |
| Steady state anaerobic denitrifying consortium for application in in-situ bioremediation of hydrocarbon-contaminated sites and enhanced oil recovery | Anaerobic denitrifying biosurfactant-producing consortium | US 8753865 | [132] |
| Heavy oil recovery process using extremophile anaerobic indigenous microorganisms | Extremophile anaerobic indigenous biosurfactant-producing microorganisms | US 8895479 | [133] |
| Alkaline microbial enhanced oil recovery | Haloalkaliphilic biosurfactant-producing microbes | US 9290688 | [134] |
| Method and installation for flooding petroleum wells and oil-sands | Glycolipids | CA1 119794 | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selva Filho, A.A.P.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry. Energies 2023, 16, 1209. https://doi.org/10.3390/en16031209
Selva Filho AAP, Converti A, Soares da Silva RdCF, Sarubbo LA. Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry. Energies. 2023; 16(3):1209. https://doi.org/10.3390/en16031209
Chicago/Turabian StyleSelva Filho, Alexandre Augusto P., Attilio Converti, Rita de Cássia F. Soares da Silva, and Leonie A. Sarubbo. 2023. "Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry" Energies 16, no. 3: 1209. https://doi.org/10.3390/en16031209
APA StyleSelva Filho, A. A. P., Converti, A., Soares da Silva, R. d. C. F., & Sarubbo, L. A. (2023). Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry. Energies, 16(3), 1209. https://doi.org/10.3390/en16031209