Thermodynamic Feasibility of the Black Sea CH4 Hydrate Replacement by CO2 Hydrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Sea Gas Hydrates
- to present the available data about the Black Sea GHDs. Today, they are determined only with geophysical methods, mainly from BSRs on seismic records and positive resistivity anomalies registered with Controlled Source Electromagnetics (CSEM);
- to estimate the parameters needed for the first estimation of the Black Sea potential for CO2 storage in GHDs:
- the areas of the GHDs and the sediment volumes under these areas between the seabed and the BSR surface (the base of the GHSZ);
- the average temperature and pressure in sediments at the depth of the BSR as extreme values of the main independent thermodynamic parameters;
- the volumes of GHs and methane.
2.2. Hydrate Stability Limits
2.3. Scientific Methods
2.4. A Classical Thermodynamic Approach for Theoretical Evaluation of CO2/CH4 Swap Feasibility
2.4.1. Thermodynamic Laws
2.4.2. Thermodynamic Criteria for CH4/CO2 Feasibility
2.4.3. Summary CH4/CO2 Feasibility Evaluation Scheme
- Pressure temperature projection for hydrate stability limits of the injection gas hydrate must at least be below the hydrate stability limits for the in situ CH4 hydrate for the range of temperatures and pressures relevant for the actual site and sediments section. This criterion is evaluated in a way similar to the comparison of hydrate equilibrium curves in a temperature-pressure projection.
- Gibbs free energy will always try to reach a minimum as a function of the temperature, pressure, and masses in the system.
- The Gibbs free energy of the hydrate formed from injection gas must be lower than the Gibbs free energy for the in situ CH4 hydrate for the relevant range of local conditions in the real sediment.
- Gradients in Gibbs free energy changes must also be negative (towards lower Gibbs free energy). Practically, this implies that each component must individually benefit from entering the hydrate forming from the injection gas. In thermodynamic language, it strictly means that for each component in the new hydrate, the chemical potentials for the water and guests must be lower than the chemical potentials for the same components in the original phases. Water will dominate, and there may be cases in which fulfillment of 2 (a) and sufficient water chemical differences will dominate enough to provide efficient hydrate formation. These exceptions will leave a new hydrate under gradients of hydrate dissociation in chemical potential gradients.
- 1.
- Heat released during the formation of a new hydrate from injection gas must be sufficient to dissociate in situ CH4 hydrate.
- The criterion from the second law is:
- 2.
- The level of temperature from the formation of a new hydrate from injection gas must be sufficiently high to efficiently break the hydrogen bonds in the water/hydrate interface and in “bulk” hydrate and provide the necessary increase in entropy from a low entropy in hydrate to higher entropies in disorganized liquid water and gas phases.
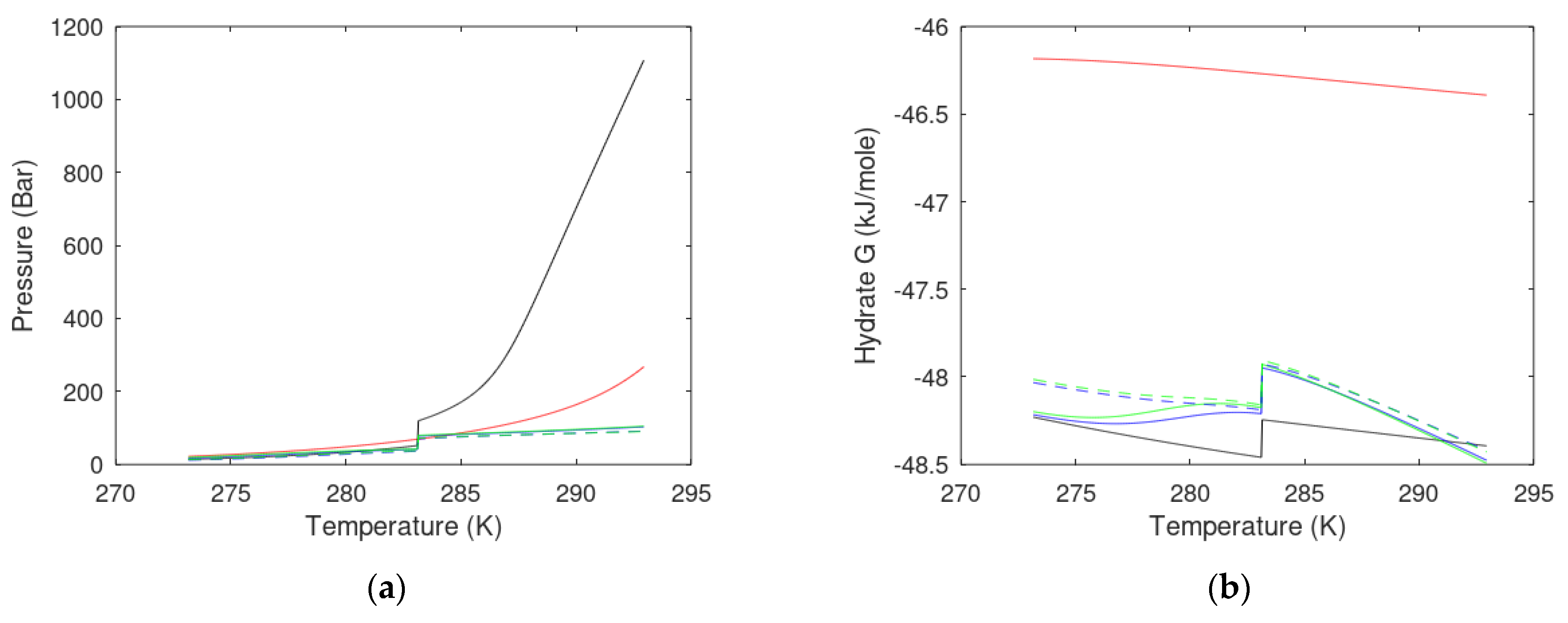
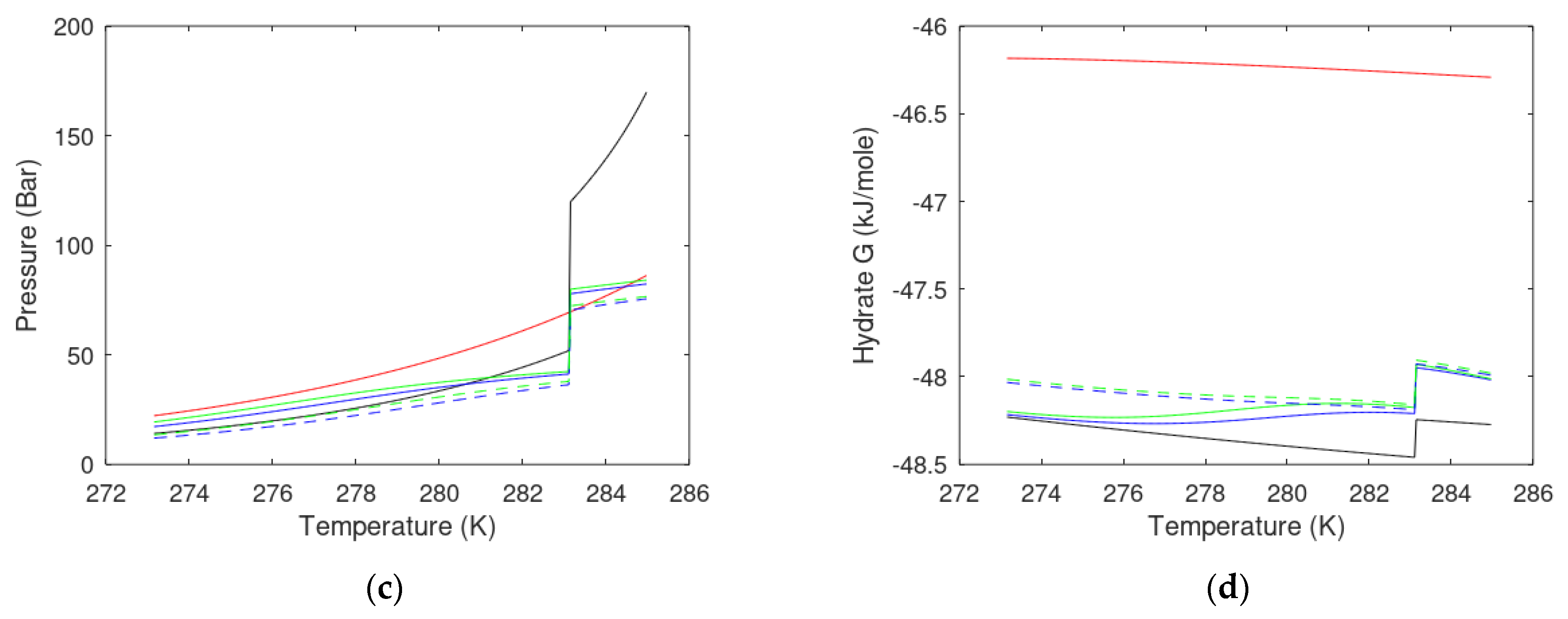
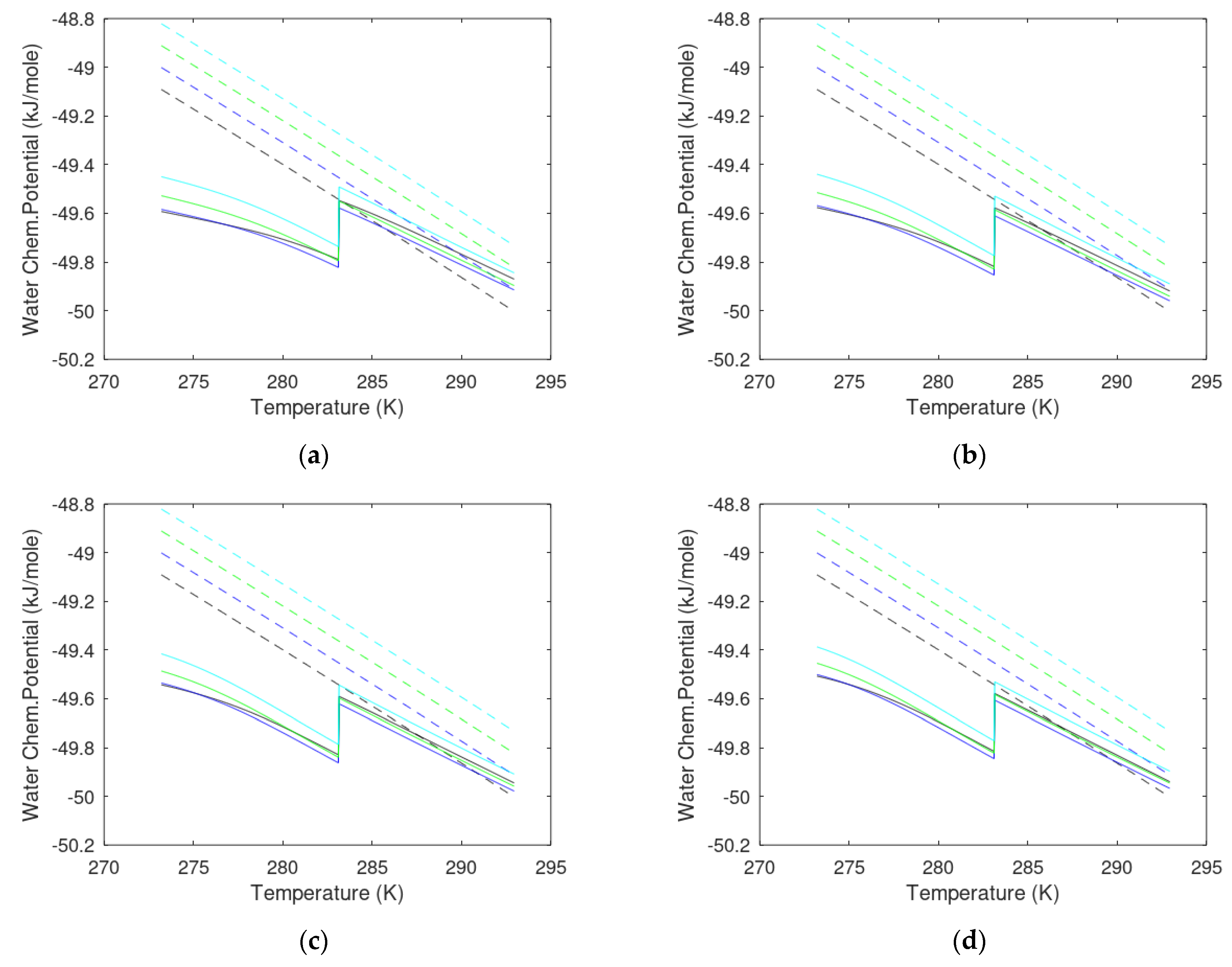
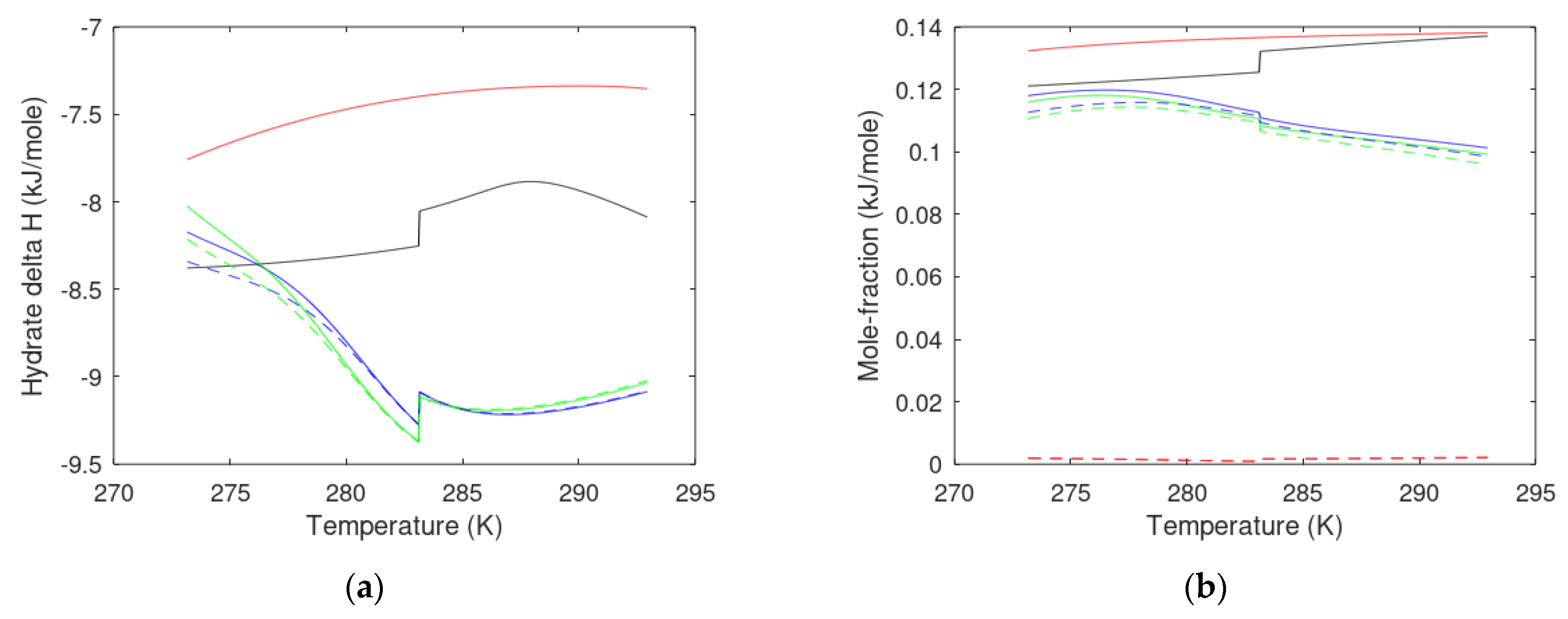
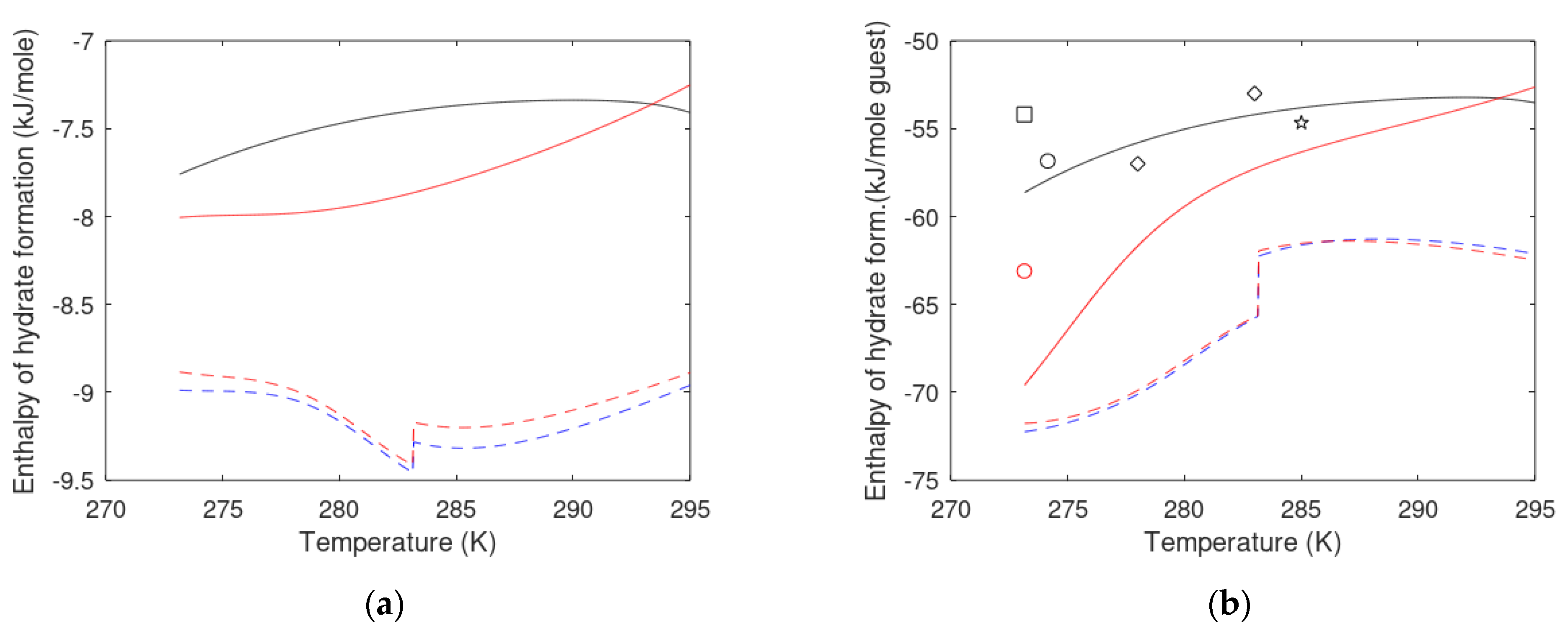
3. Results
3.1. CO2/CH4 Swap Feasibility through Adding H2S
3.2. CO2/CH4 Swap Feasibility through Adding C2H6
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar] [CrossRef]
- IPCC Climate Change Reports: Why They Matter to Everyone on the Planet. 2022. Available online: https://www.nrdc.org/stories/ipcc-climate-change-reports-why-they-matter-everyone-planet#sec-whatis (accessed on 10 December 2022).
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible Climate Change Due to Carbon Dioxide Emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, B.; Kuznetsova, T. Hydrate dissociation in chemical potential gradients: Theory and simulations. Fluid Phase Equilibria 2004, 217, 217–226. [Google Scholar] [CrossRef]
- MacDonald, I.R.; Leifer, I.; Sassen, R.; Stine, P.; Mitchell, R.; Guinasso, N., Jr. Transfer of hydrocarbons from natural seeps to the water column and atmosphere. Geofluids 2002, 2, 95–107. [Google Scholar] [CrossRef]
- Sassen, R.; Roberts, H.H.; Milkov, A.V.; DeFreitas, D.A. Sea Floor Vents, Seeps, and Gas Hydrate:Relation to Flux Rate from the Deep Gulf of Mexico Petroleum System, Book Chapter in Petroleum Systems of Deep-Water Basins—Global and Gulf of Mexico Experience; Fillon, R.H., Rosen, N.C., Weimer, P., Lowrie, A., Pettingill, H., Phair, R.L., et al., Eds.; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 2001. [Google Scholar] [CrossRef]
- Paull, C.K.; Ussler, W., III; Holbrook, S.; Hill, T.M.; Keaten, R.; Mienert, J.; Haflidason, H.; Johnson, J.E.; Winters, W.J.; Lorenson, T.D. Origin of pockmarks and chimney structures on the flanks of the Storegga Slide, offshore Norway. Geo-Mar. Lett. 2008, 28, 43–51. [Google Scholar] [CrossRef]
- Boetius, A.; Suess, E. Hydrate Ridge: A natural laboratory for the study of microbial life fueled by methane from near-surface gas hydrates. Chem. Geol. 2004, 205, 291–310. [Google Scholar] [CrossRef]
- Lindeberg, E.; Vuillaume, J.-F.; Ghaderi, A. Determination of the CO2 storage capacity of the Utsira formation. En. Proc. 2009, 1, 2777–2784. [Google Scholar] [CrossRef]
- Chadwick, R.A.; Zweigel, P.; Gregersen, U.; Kirby, G.A.; Holloway, S.; Johannessen, P.N. Geological reservoir characterization of a CO2 storage site: The Utsira Sand, Sleipner, northern North Sea. Energy 2004, 29, 1371–1381. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Riis, F.; Gjeldvik, I.T.; Halland, E.T.; Tappel, I.M.; Aagaard, P. Assessment of CO2 injection into the south Utsira-Skade aquifer, the North Sea, Norway. Energy 2013, 55, 529–540. [Google Scholar] [CrossRef]
- Lloyd, C.; Huuse, M.; Barrett, B.J.; Stewart, M.A.; Newton, A.M.W. A regional CO2 containment assessment of the northern Utsira Formation seal and overburden, northern North Sea. Basin Res. 2021, 33, 1985–2017. [Google Scholar] [CrossRef]
- Schoderbek, D.; Farrell, H.; Howard, J.; Raterman, K.; Silpngarmlert, S.; Martin, K.; Smith, B.; Klein, P. ConocoPhillips Gas Hydrate Production Test Final Technical Report Oct. 1, 2008–June 30, 2013. July 20, 2013, DOE Award No.: DE-NT0006553; ConocoPhillips Co. for US DOE Nat. En. Tech. Lab.: Houston, TX, USA, 2013; p. 1123878. [Google Scholar]
- Kvamme, B. Thermodynamic Limitations of the CO2/N2 Mixture Injected into CH4 Hydrate in the Ignik Sikumi Field Trial. J. Chem. Eng. Data 2016, 61, 1280–1295. [Google Scholar] [CrossRef]
- Kvamme, B.; Vasilev, A. Black Sea gas hydrates: Safe long terms storage of CO2 with environmentally friendly energy production. 2022; unpublished. [Google Scholar]
- Schicks, J.M.; Haeckel, M.; Janicki, G.; Spangenberg, E.; Thaler, J.; Giese, R.; Strauch, B.; Heeschen, K.; Priegnitz, M.; Luzi-Helbing, M.; et al. Development, Test, and Evaluation of Exploitation Technologies for the Application of Gas Production from Natural Gas Hydrate Reservoirs and Their Potential Application in the Danube Delta, Black Sea. Mar. Pet. Geol. 2020, 120, 104488. [Google Scholar] [CrossRef]
- Marine and Petroleum Geology Special Issue “Black Sea Gas Hydrates”; Haeckel, M., Bohrmann, G., Schwalenberg, K., Kuhs, W., Wallmann, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Available online: https://www.sciencedirect.com/journal/marine-and-petroleum-geology/special-issue/101ZSP1HCC0 (accessed on 10 December 2022).
- Minshull, T.A.; Marín-Moreno, H.; Betlem, P.; Bialas, J.; Bünz, S.; Burwicz, E.; Cameselle, A.L.; Cifci, G.; Giustiniani, M.; Hillman, J.I.T.; et al. Hydrate occurrence in Europe: A review of available evidence. Mar. Pet. Geol. 2020, 111, 735–764. [Google Scholar] [CrossRef]
- Mienert, J.; Berndt, C.; Tréhu, A.M.; Camerlenghi, A.; Liu, C.-S. (Eds.) World Atlas of Submarine Gas Hydrates in Continental Margins; Springer International Publishing: Cham, Switzerland, 2022; pp. 451–461. ISBN 978-3-030-81185-3. [Google Scholar]
- Tari, G.C.; Simmons, M.D. History of deepwater exploration in the Black Sea and an overview of deepwater petroleum play types. Geol. Soc. Lond. Spec. Publ. 2018, 464, 439. [Google Scholar] [CrossRef]
- Dondurur, D.; Cifci, G. Anomalous Strong Reflections on High Resolution Seismic Data from the Turkish Shelf of the Eastern Black Sea: Possible Indicators of Shallow Hydrogen Sulphide-Rich Gas Hydrate Layers. Turk. J. Earth Sci. 2009, 18, 299–313. [Google Scholar] [CrossRef]
- Bialas, J.; Bohlen, T.; Dannowski, A.; Eisenberg-Klein, G.; Gassner, L.; Gehrmann, R.; Heeschen, K.; Hölz, S.; Jegen, M.; Klaucke, I.; et al. Joint Interpretation of Geophysical Field Experiments in the Danube Deep-Sea Fan, Black Sea. Mar. Pet. Geol. 2020, 121, 104551. [Google Scholar] [CrossRef]
- Vasilev, A. First Bulgarian gas hydrates assessment from probable BSRs. Geol. Miner. Resour. World Ocean. NASU Kiev 2010, 2, 22–26, Геoлoгия и пoлезные искoпаемые Мирoвoгo oкеана, НАНУ, Киев ISSN 1999-7566. Available online: http://dspace.nbuv.gov.ua/xmlui/bitstream/handle/123456789/44845/02-Vasilev.pdf?sequence=1 (accessed on 10 December 2022).
- Bialas, J.; Haeckel, M. Gas Hydrate Accumulations in the Black Sea. In World Atlas of Submarine Gas Hydrates in Continental Margins; Mienert, J., Berndt, C., Tréhu, A.M., Camerlenghi, A., Liu, C.-S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 451–461. [Google Scholar] [CrossRef]
- Shnyukov, E.F.; Ziborov, A.P. Mineral Wealth of the Black Sea; NASU: Kiev, Ukraine, 2004; p. 285. (in Russian) [Google Scholar]
- Yayci, C. The Problem of Delimitation of Maritime Areas in Eastern Mediterranean and Turkey. Wise Strategy 2012, 4, 1–70. (in Turkish). Available online: https://docplayer.biz.tr/92773-Dogu-akdeniz-de-deniz-yetki-alanlarinin-paylasilmasi-sorunu-ve-turkiye.html (accessed on 10 December 2022). (in Turkish).
- EMODnet, Gas hydrates. Available online: https://www.emodnet-geology.eu/map-viewer/ (accessed on 10 December 2022).
- Vassilev, A. Optimistic and Pessimistic Model Assessments of the Black Sea Gas Hydrates. C. R. Acad. Bulg. Sci. 2006, 59, 543–550. [Google Scholar]
- Saeidi, N.; Dunn-Rankin, D.; Kvamme, B.; Chien, Y.-C. Experimental Studies on Combined Production of CH4 and Safe Long-Term Storage of CO2 in the Form of Solid Hydrate in Sediment. Phys. Chem. Chem. Phys. 2021, 23, 23313–23324. [Google Scholar] [CrossRef]
- Kvamme, B.; Zhao, J.; Wei, N.; Sun, W.; Saeidi, N.; Pei, J.; Kuznetsova, T. Hydrate Production Philosophy and Thermodynamic Calculations. Energies 2020, 13, 672. [Google Scholar] [CrossRef]
- Kvamme, B. Mechanisms for CH4/CO2 Swapping in Natural Sediments. Fluids 2022, 7, 260. [Google Scholar] [CrossRef]
- Saeidi, N. Fundamental Studies of CO2 Substitution in Methane Hydrate. PhD Thesis, University of California, Irvine, CA, USA, 2022. [Google Scholar]
- Kvamme, B.; Tanaka, H. Thermodynamic Stability of Hydrates for Ethane, Ethylene, and Carbon Dioxide. J. Phys. Chem. 1995, 99, 7114–7119. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium Constants from a Modified Redlich-Kwong Equation of State. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Kvamme, B.; Clarke, M. Hydrate Phase Transition Kinetic Modeling for Nature and Industry—Where Are We and Where Do We Go? Energies 2021, 14, 4149. [Google Scholar] [CrossRef]
- Kvamme, B.; Coffin, R.B.; Zhao, J.; Wei, N.; Zhou, S.; Li, Q.; Saeidi, N.; Chien, Y.-C.; Dunn-Rankin, D.; Sun, W.; et al. Stages in the Dynamics of Hydrate Formation and Consequences for Design of Experiments for Hydrate Formation in Sediments. Energies 2019, 12, 3399. [Google Scholar] [CrossRef]
- Kvamme, B. Kinetics of Hydrate Formation, Dissociation and Reformation. Chem. Thermodyn. Therm. Anal. 2021, 1–2, 100004. [Google Scholar] [CrossRef]
- Kvamme, B. Kinetics of Hydrate Formation from Nucleation Theory. Int. J. Offshore Polar Eng. 2002, 12, 256–263. [Google Scholar]
- Kvamme, B.; Aromada, S.A.; Saeidi, N.; Hustache-Marmou, T.; Gjerstad, P. Hydrate Nucleation, Growth, and Induction. ACS Omega 2020, 5, 2603–2619. [Google Scholar] [CrossRef]
- Kvamme, B. Enthalpies of Hydrate Formation from Hydrate Formers Dissolved in Water. Energies 2019, 12, 1039. [Google Scholar] [CrossRef]
- Kvamme, B. Consistent Thermodynamic Calculations for Hydrate Properties and Hydrate Phase Transitions. J. Chem. Eng. Data 2020, 65, 2872–2893. [Google Scholar] [CrossRef]
- Kvamme, B.; Aromada, S.A.; Gjerstad, P.B. Consistent Enthalpies of the Hydrate Formation and Dissociation Using Residual Thermodynamics. J. Chem. Eng. Data 2019, 64, 3493–3504. [Google Scholar] [CrossRef]
- Svandal, A. Modeling Hydrate Phase Transitions Using Mean-Field Approaches. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2006. [Google Scholar]
- Buanes, T. Mean–Field Approaches Applied to Hydrate Phase Transition. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2006. [Google Scholar]
- Qasim, M. Microscale Modeling of Natural Gas Hydrates in Reservoirs. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2012. [Google Scholar]
- Baig, K. Nano to Micro Scale Modeling of Hydrate Phase Transition Kinetics. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2017. [Google Scholar]
- Tegze, G.; Pusztai, T.; Tóth, G.; Gránásy, L.; Svandal, A.; Buanes, T.; Kuznetsova, T.; Kvamme, B. Multiscale Approach to CO2 Hydrate Formation in Aqueous Solution: Phase Field Theory and Molecular Dynamics. Nucleation and Growth. J. Chem. Phys. 2006, 124, 234710. [Google Scholar] [CrossRef]
- Svandal, A.; Kuznetsova, T.; Kvamme, B. Thermodynamic Properties and Phase Transitions in the H2O/CO2/CH4 System. Fluid Phase Equilibria 2006, 246, 177–184. [Google Scholar] [CrossRef]
- Kvamme, B.; Iden, E.; Tveit, J.; Veland, V.; Zarifi, M.; Qorbani, K. Effect of H2S content on thermodynamic stability of hydrate formed from CO2/N2 mixtures. J. Chem. Eng. Data 2017, 62, 1645–1658. [Google Scholar] [CrossRef]
- Aromada, S.; Kvamme, B. Impacts of carbon dioxide and hydrogen sulphide on the risk of hydrate formation during pipeline transport of Natural gas, 2018. Front. Chem. Sci. Eng. 2019, 13, 616–627. [Google Scholar] [CrossRef]
- Kvamme, B. Small Alcohols as Surfactants and Hydrate Promotors. Fluids 2021, 6, 345. [Google Scholar] [CrossRef]
- Kvamme, B. Small Alcohols as Hydrate Promoters. Energy Fuels 2021, 35, 17663–17684. [Google Scholar] [CrossRef]
- Handa, Y.P. Compositions, Enthalpies of Dissociation, and Heat Capacities in the Range 85 to 270 K for Clathrate Hydrates of Methane, Ethane, and Propane, and Enthalpy of Dissociation of Isobutane Hydrate, as Determined by a Heat-Flow Calorimeter. J. Chem. Thermodyn. 1986, 18, 915. [Google Scholar] [CrossRef]
- Rueff, R.M.; Sloan, E.D.; Yesavage, V.F. Heat Capacity and Heat of Dissociation of Methane Hydrates. AIChE J. 1988, 34, 1468. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Yamamoto, Y.; Komai, T.; Haneda, H.; Kawamura, T. Rigorous Approach to the Prediction of the Heat of Dissociation of Gas Hydrates. Ind. Eng. Chem. Res. 2003, 42, 1111–1114. [Google Scholar] [CrossRef]
- Lievois, J.S.; Perkins, R.; Martin, R.J.; Kobayashi, R. Development of an Automated, High Pressure Heat Flux Calorimeter and its Application to Measure the Heat of Dissociation and Hydrate Numbers of Methane Hydrate. Fluid Phase Equilib. 1990, 59, 73. [Google Scholar] [CrossRef]
- Svandal, A.; Kvamme, B.; Granasy, L.; Pusztai, T. The Influence of Diffusion on Hydrate Growth. In Proceedings of the 1st International Conference on Diffusion in Solids and Liquids, Aveiro, Portugal, 6 July 2005. [Google Scholar]
- Kvamme, B.; Selvåg, J.; Saeidi, N.; Kuznetsova, T. Methanol as a Hydrate Inhibitor and Hydrate Activator. Phys. Chem. Chem. Phys. 2018, 20, 21968–21987. [Google Scholar] [CrossRef] [PubMed]
- Selvåg, J.; Kuznetsova, T.; Kvamme, B. Molecular Dynamics Study of Surfactant-Modified Water—Carbon Dioxide Systems. Mol. Simul. 2018, 44, 128–136. [Google Scholar] [CrossRef]
- Selvåg, J.; Kuznetsova, T.; Kvamme, B. Molecular Dynamics Study of Morpholines at Water—Carbon Dioxide Interfaces. Fluid Phase Equilibria 2019, 485, 44–60. [Google Scholar] [CrossRef]
- Olsen, R. A Theoretical Study of Nanoscale Glycol–Surface Interactions. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2022. [Google Scholar]
- Van Cuong, P. Transport and Adsorption of CO2 and H2O on Calcite and Clathrate Hydrate. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2012. [Google Scholar]
- Jensen., B. Investigations into the Impact of Solid Surfaces in Aqueous Systems. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2016. [Google Scholar]
- Kvamme, B. Thermodynamics and kinetic mechanisms for CH4/CO2 swapping in natural sediments. Energy Fuels 2022, 36, 6374–6396. [Google Scholar] [CrossRef]
- Klymenko, V.; Ovetskyi, S.; Martynenko, V.; Vytiaz, O.; Uhrynovskyi, A. An alternative method of methane production from deposits of subaquatic gas hydrates. Min. Miner. Depos. 2022, 16, 11–17. [Google Scholar] [CrossRef]
- Bazaluk, O.; Sai, K.; Lozynskyi, V.; Petlovanyi, M.; Saik, P. Research into Dissociation Zones of Gas Hydrate Deposits with a Heterogeneous Structure in the Black Sea. Energies 2021, 14, 1345. [Google Scholar] [CrossRef]
- Li, B.; Ye, Y.; Zhang, T.; Wan, Q. Numerical Investigation into the Gas Production from Hydrate Deposit under Various Thermal Stimulation Modes in a Multi-Well System in Qilian Mountain. Entropy 2021, 23, 800. [Google Scholar] [CrossRef]







| State Country Code | No | Area | HW | HBSR | VGHSZ | VGH | VCH4 | G | TBSR | PBSR | References ** |
|---|---|---|---|---|---|---|---|---|---|---|---|
| km2 | m | M | km3 | km3 | bcm | mK/m | °C | bar | |||
| BG | 1 | 3006 | 1200 | 330 | 992 | 18 | 2768 | 26 | 18 | 153 | [17] |
| 12 * | 518 | 1600 | 250 | 129 | 2 | 361 | 44 | 20 | 185 | [18] | |
| Total | 3524 | 318 | 1121 | 20 | 3129 | ||||||
| GE | 10(1/2) * | 1287 | 1300 | 130 | 167 | 3 | 467 | 42 | 15 | 143 | [19,21] |
| RO | 2 | 1849 | 900 | 220 | 407 | 7 | 1135 | 24 | 14 | 112 | [13] |
| RU | 11 * | 6290 | 1800 | 340 | 2138 | 38 | 5966 | 40 | 23 | 214 | [19,21] |
| 20–22 * | 42 | 1600 | 230 | 10 | 0 | 27 | 48 | 20 | 183 | [20] | |
| Total | 6332 | 339 | 2148 | 39 | 5993 | ||||||
| TR | 3 | 7080 | 1700 | 260 | 1841 | 33 | 5136 | 43 | 20 | 196 | [13] |
| 4 | 324 | 740 | 100 | 32 | 1 | 90 | 46 | 14 | 84 | [13] | |
| 5 | 482 | 1300 | 120 | 58 | 1 | 161 | 53 | 15 | 142 | [13] | |
| 8 * | 2349 | 1300 | 230 | 540 | 10 | 1507 | 52 | 21 | 153 | [21] | |
| 9 * | 3534 | 1400 | 260 | 919 | 17 | 2563 | 40 | 20 | 166 | [21] | |
| 10(1/2) * | 1287 | 1400 | 190 | 244 | 4 | 682 | 42 | 17 | 159 | [19,21] | |
| 14–19 * | 70 | 2000 | 300 | 21 | 0 | 59 | 35 | 20 | 230 | [20] | |
| Total | 15,125 | 242 | 3656 | 66 | 10,199 | ||||||
| UA | 6 | 1950 | 900 | 140 | 273 | 5 | 762 | 40 | 15 | 104 | [13] |
| 7 * | 687 | 1600 | 340 | 234 | 4 | 652 | 28 | 19 | 194 | [22] | |
| Total | 2637 | 192 | 507 | 9 | 1413 |
| Research Level | Area | HW min/max | HBSR | VsGHSZ | VGH | VCH4 | G min/max | TBSR min/max | PBSR min/max |
|---|---|---|---|---|---|---|---|---|---|
| km2 | M | M | km3 | km3 | Bcm | mK/m | °C | Bar | |
| Initial | 14,691 | 740/1700 | 245 | 3603 | 65 | 10,052 | 24/53 | 14/20 | 84/196 |
| Less | 16,063 | 1300/2000 | 274 | 4403 | 79 | 12,284 | 28/52 | 15/21 | 143/230 |
| Total | 30,753 | 260 | 8006 | 144 | 22,336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvamme, B.; Vasilev, A. Thermodynamic Feasibility of the Black Sea CH4 Hydrate Replacement by CO2 Hydrate. Energies 2023, 16, 1223. https://doi.org/10.3390/en16031223
Kvamme B, Vasilev A. Thermodynamic Feasibility of the Black Sea CH4 Hydrate Replacement by CO2 Hydrate. Energies. 2023; 16(3):1223. https://doi.org/10.3390/en16031223
Chicago/Turabian StyleKvamme, Bjørn, and Atanas Vasilev. 2023. "Thermodynamic Feasibility of the Black Sea CH4 Hydrate Replacement by CO2 Hydrate" Energies 16, no. 3: 1223. https://doi.org/10.3390/en16031223
APA StyleKvamme, B., & Vasilev, A. (2023). Thermodynamic Feasibility of the Black Sea CH4 Hydrate Replacement by CO2 Hydrate. Energies, 16(3), 1223. https://doi.org/10.3390/en16031223









