The Effect of Diluents on the Flame Structure and NO Generation Characteristics of H2/CO Micromixing Flames
Abstract
:1. Introduction
2. Methods
2.1. Physical Model
2.2. Numerical Simulation Model and Mechanism
2.3. Boundary Conditions
2.4. Solution
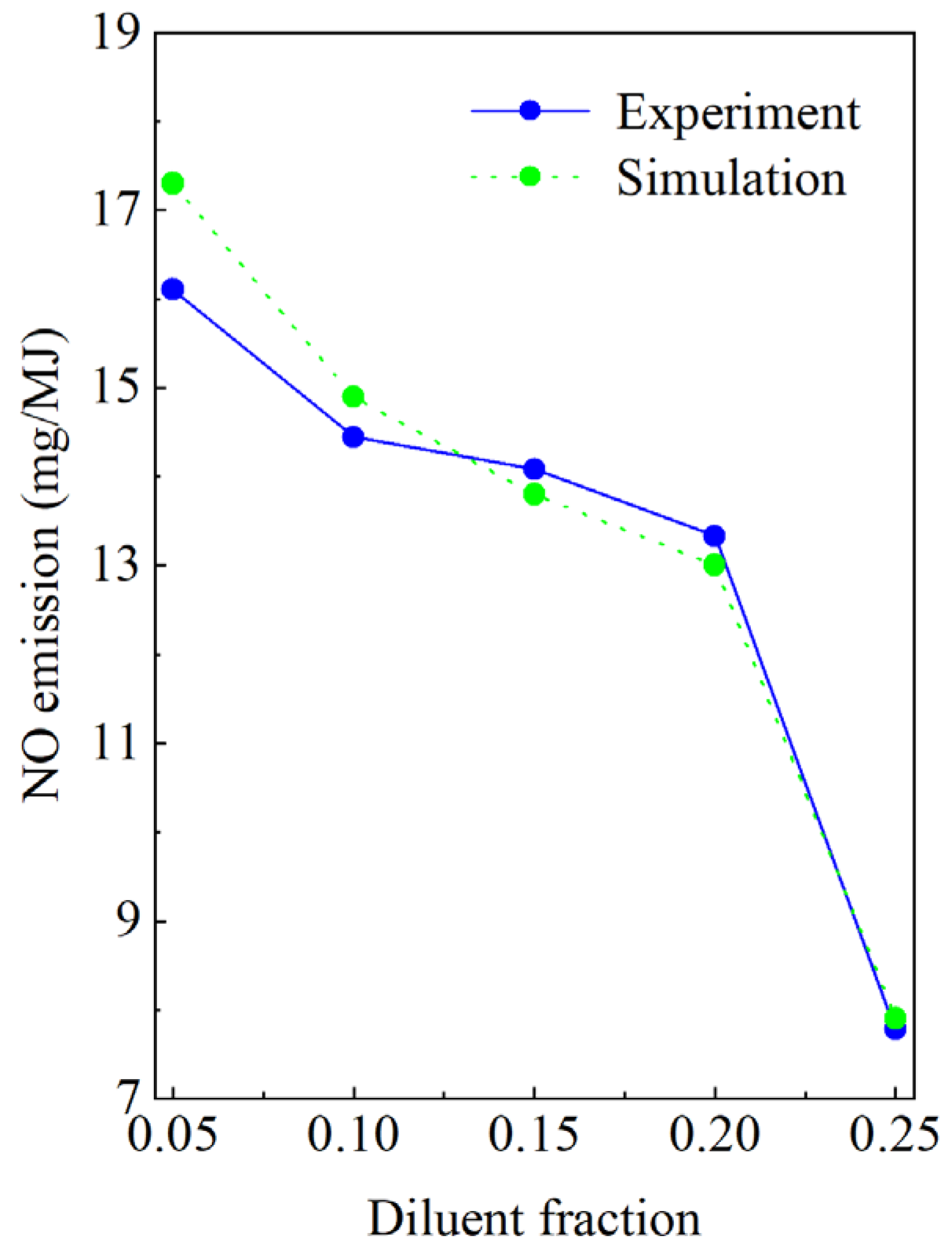
2.5. Model Verification
3. Results and Discussion
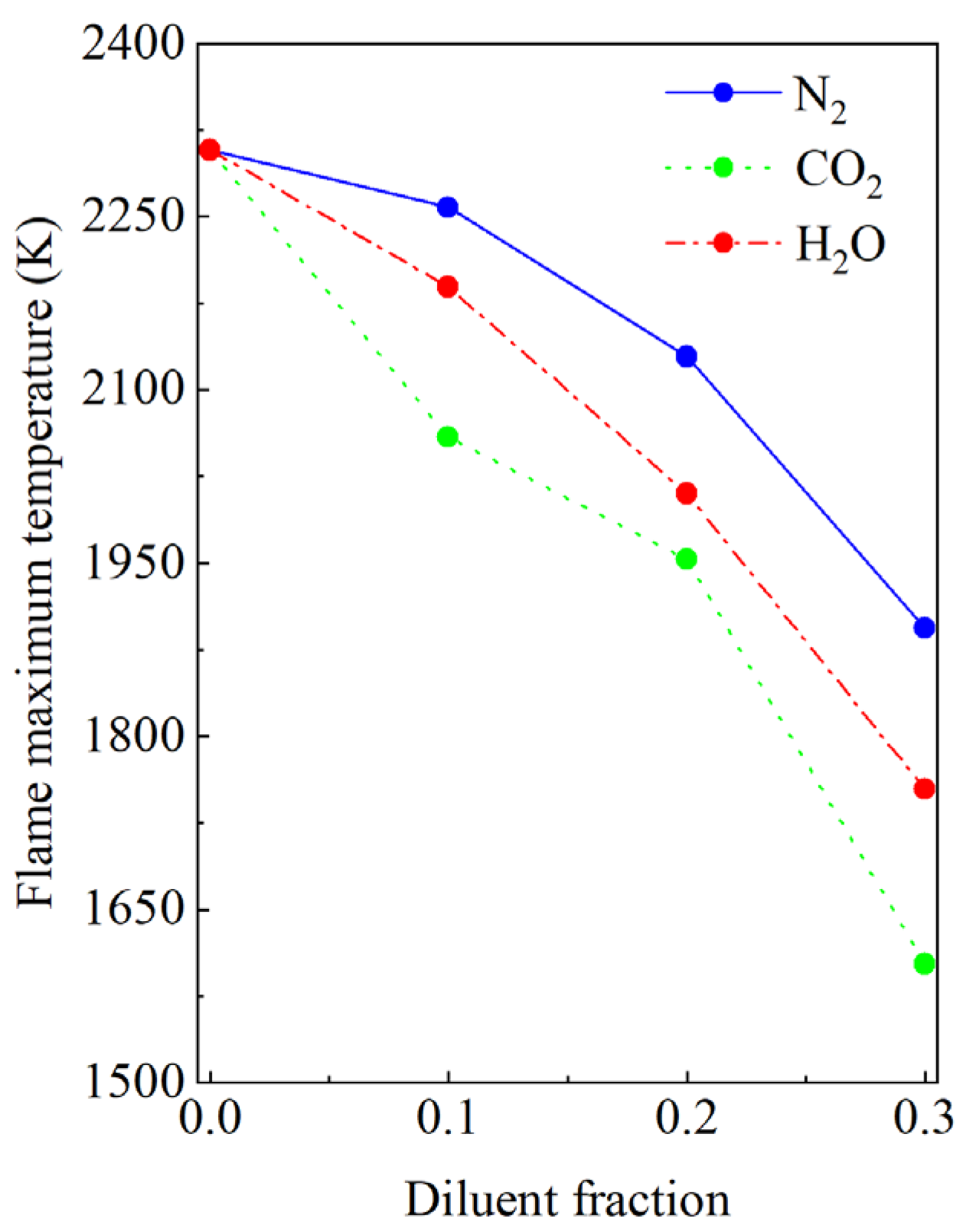
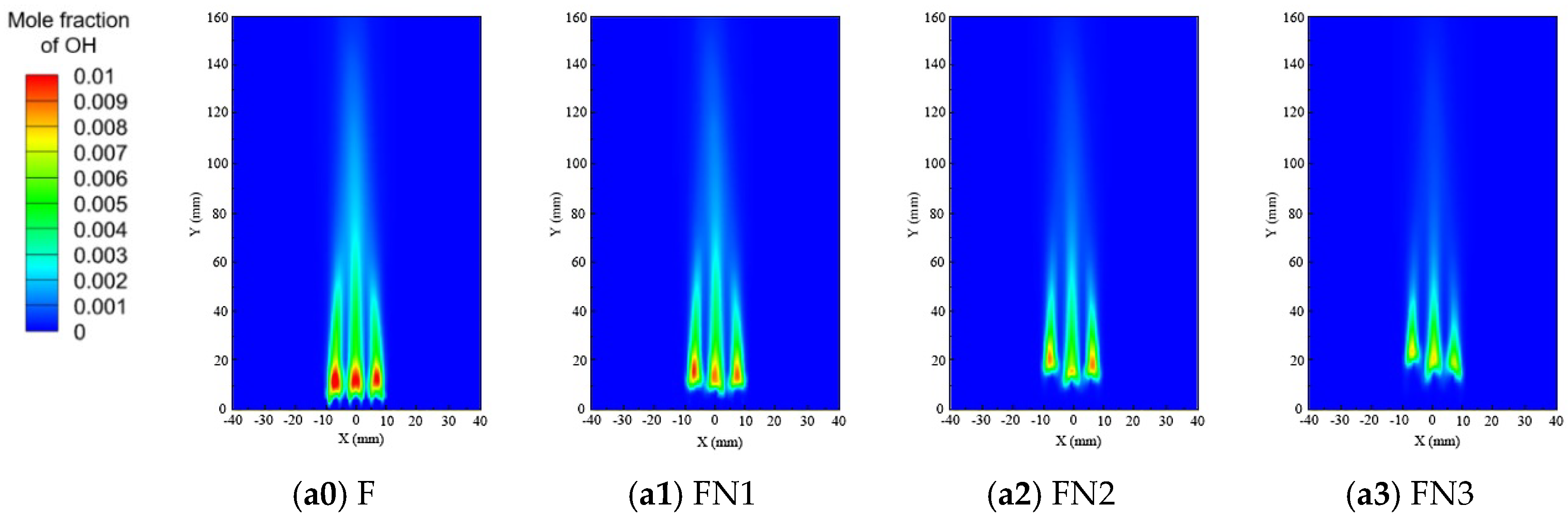
3.1. Effect of the Diluents on the Flame Structure
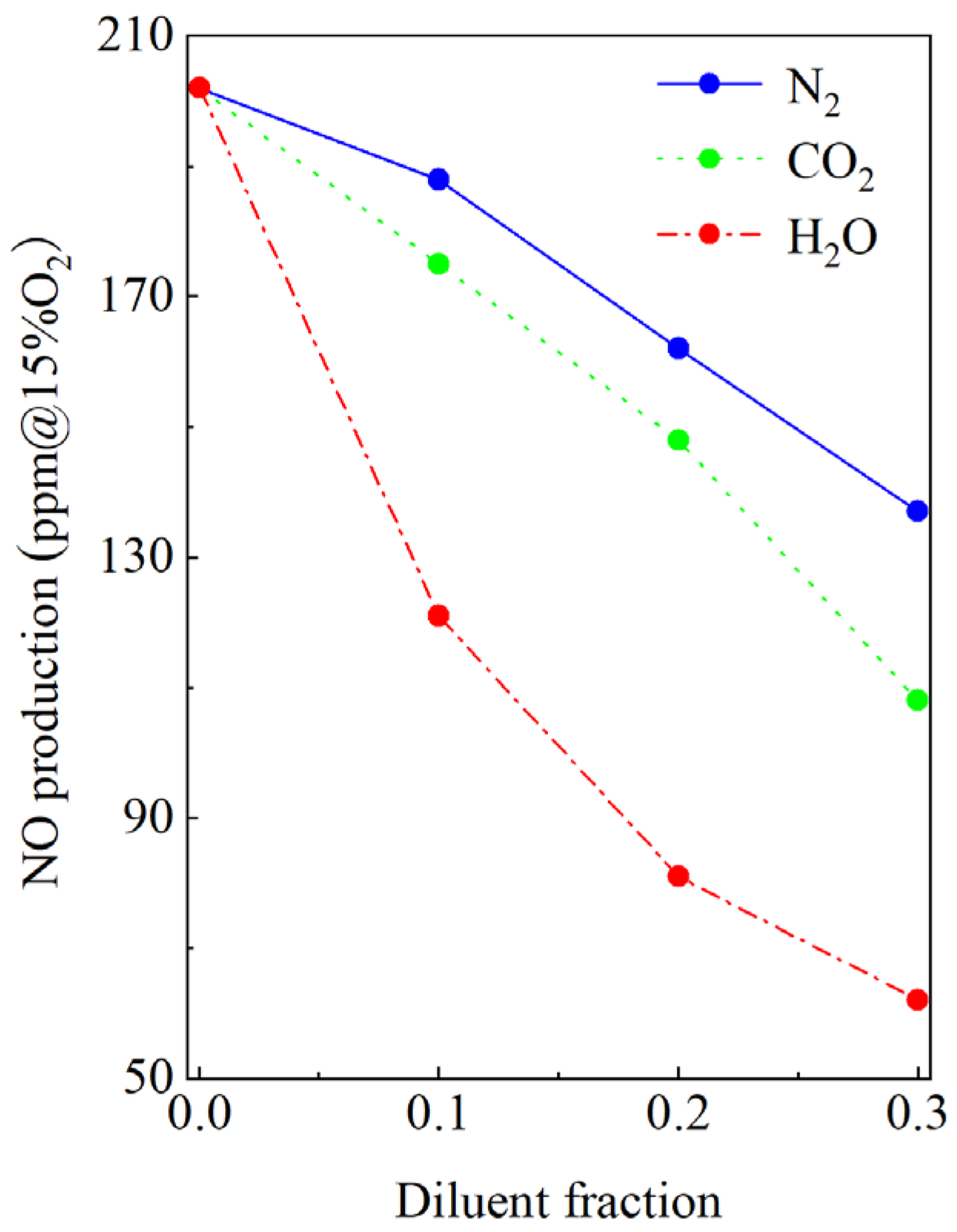
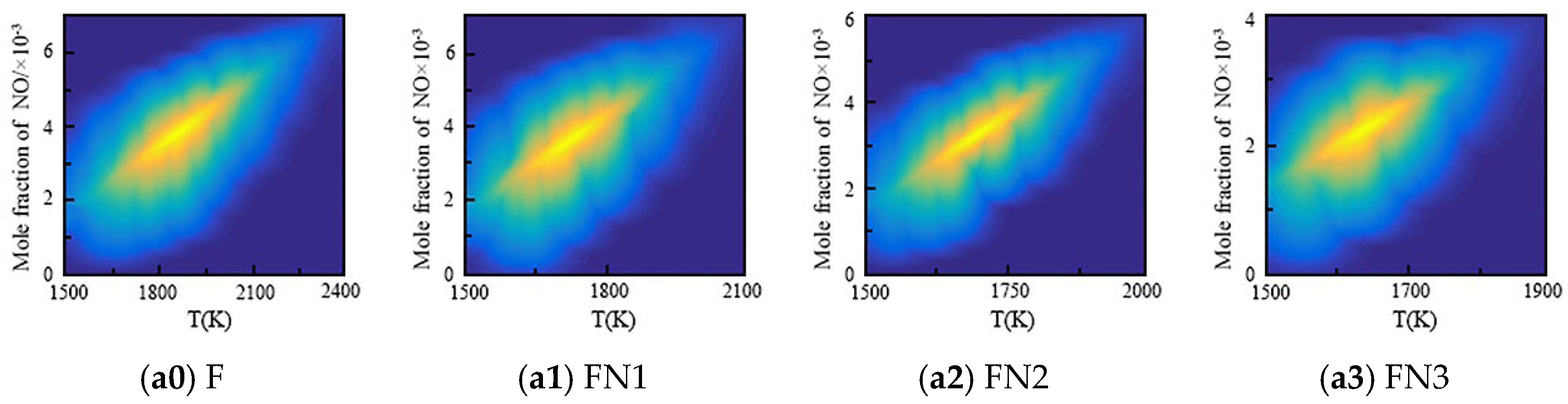
3.2. Effect of the Diluents on the NO Formation Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mu, Y.F.; Li, Z.Y.; Luo, K.; Fan, J.R.; Xing, J.K.; Zhang, B.; Shi, S.P. Analyzing the combustion oscillation of a gas turbine for syngas based on the characteristic time-scale theory. Therm. Sci. Eng. Prog. 2022, 30, 101224. [Google Scholar] [CrossRef]
- Yuan, Y. Experimental and Numerical Study on Dilution Diffusion Combustion of H2/CO Syngas. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 2018. [Google Scholar]
- Xi, J.F.; Gu, Z.C.; Yuan, Y.; Zhang, X.P. Effect of Diluents on Pollutant Emission from Syngas Combustion. J. Chin. Soc. Power Eng. 2018, 38, 394–399. [Google Scholar]
- Wang, F.; Liu, R.; Dou, L.; Liu, D.; Jin, J. A dual timescale model for micro-mixing and its application in LES-TPDF simulations of turbulent nonpremixed flames. Chin. J. Aeronaut. 2019, 32, 875–887. [Google Scholar] [CrossRef]
- Zhang, G.-P.; Li, G.-X.; Li, H.-M.; Lv, J.-C. Effect of diluent gas on propagation and explosion characteristics of hydrogen-rich syngas laminar premixed flame. Energy 2022, 246, 123370. [Google Scholar] [CrossRef]
- Yang, L.; Weng, W.; Zhu, Y.; He, Y.; Wang, Z.; Li, Z. Investigation of Hydrogen Content and Dilution Effect on Syngas/Air Premixed Turbulent Flame Using OH Planar Laser-Induced Fluorescence. Processes 2021, 9, 1894. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.H.; Zhu, Y.Q.; Li, Z.S.; Zhou, J.H.; Huang, Z.Y.; Cen, K.F. Premixed jet flame characteristics of syngas using OH planar laser induced fluorescence. Chin. Sci. Bull. 2011, 56, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- Jithin, E.V.; Raghuram, G.K.S.; Keshavamurthy, T.V.; Velamati, R.K.; Prathap, C.; Varghese, R.J. A review on fundamental combustion characteristics of syngas mixtures and feasibility in combustion devices. Renew. Sustain. Energy Rev. 2021, 146, 111178. [Google Scholar] [CrossRef]
- Zhuo, L.; Jiang, Y.; Qiu, R.; An, J.; Xu, W. Effects of Fuel-Side N2, CO2, H2O Dilution on Combustion Characteristics and NOx Formation of Syngas Turbulent Nonpremixed Jet Flames. J. Eng. Gas Turbines Power 2014, 136, 061505. [Google Scholar] [CrossRef]
- Ding, N.; Arora, R.; Norconk, M.; Lee, S.-Y. Numerical investigation of diluent influence on flame extinction limits and emission characteristic of lean-premixed H2–CO (syngas) flames. Int. J. Hydrogen Energy 2011, 36, 3222–3231. [Google Scholar] [CrossRef]
- Whitty, K.J.; Zhang, H.R.; Eddings, E.G. Emissions from syngas combustion. Combust. Sci. Technol. 2008, 180, 1117–1136. [Google Scholar] [CrossRef]
- He, D.; Yan, W. Influences of different diluents on NO emission characteristics of syngas opposed-flow flame. Int. J. Hydrogen Energy 2018, 43, 2570–2584. [Google Scholar] [CrossRef]
- Yang, K.-H.; Shih, H.-Y. NO formation of opposed-jet syngas diffusion flames: Strain rate and dilution effects. Int. J. Hydrogen Energy 2017, 42, 24517–24531. [Google Scholar] [CrossRef]
- Shi, G.D.; Li, P.F.; Hu, F.; Liu, Z.H. NO mechanisms of syngas MILD combustion diluted with N2, CO2, and H2O. Int. J. Hydrogen Energy 2022, 47, 16649–16664. [Google Scholar] [CrossRef]
- Wang, S.X.; Wang, Z.H.; Han, X.L.; Chen, C.L.; He, Y.; Zhu, Y.Q.; Cen, K.F. Experimental and numerical study of the effect of elevated pressure on laminar burning velocity of lean H2/CO/O2/diluents flames. Fuel 2020, 273, 117753. [Google Scholar] [CrossRef]
- da Silva, C.V.; Deon, D.L.; Centeno, F.R.; França, F.H.R.; Pereira, F.M. Assessment of combustion models for numerical simulations of a turbulent non-premixed natural gas flame inside a cylindrical chamber. Combust. Sci. Technol. 2018, 190, 1528–1556. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, X.; Zhao, T.Z.; Liu, G.; Lu, G.P.; Zheng, H.T. Study on combustion characteristics of swirl premixed combustion chamber. J. Therm. Sci. Technol. 2021, 20, 171–177. [Google Scholar]
- Natarajan, J.; Kochar, Y.; Lieuwen, T.; Seitzman, J. Pressure and preheat dependence of laminar flame speeds of H2/CO/CO2/O2/He mixtures. Proc. Combust. Inst. 2009, 32, 1261–1268. [Google Scholar] [CrossRef]
- Sahu, A.B.; Ravikrishna, R.V. A detailed numerical study of NOx kinetics in low calorific value H2/CO syngas flames. Int. J. Hydrogen Energy 2014, 39, 17358–17370. [Google Scholar] [CrossRef]
- Roache, P. Error Bars for CFD. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 6–9 January 2003. [Google Scholar] [CrossRef]
- Manna, P.; Dharavath, M.; Sinha, P.K.; Chakraborty, D. Optimization of a flight-worthy scramjet combustor through CFD. Aerosp. Sci. Technol. 2013, 27, 138–146. [Google Scholar] [CrossRef]
- Samiran, N.A.; Ng, J.-H.; Jaafar, M.N.M.; Valera-Medina, A.; Chong, C.T. H2-rich syngas strategy to reduce NOx and CO emissions and improve stability limits under premixed swirl combustion mode. Int. J. Hydrogen Energy 2016, 41, 19243–19255. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C. Effects of H2/CO/CH4 syngas composition variation on the NOx and CO emission characteristics in a partially-premixed gas turbine combustor. Sci. China Technol. Sci. 2016, 59, 1804–1813. [Google Scholar] [CrossRef]
- Yu, M.; Zhai, F.; Li, H.; Han, S.; Li, S.; Zheng, K.; Yu, Y. Comparative study on the effect of initial temperatures and pressures on the laminar flame speed of the heavily carbonaceous syngas containing water vapor via reaction kinetics simulation. Int. J. Hydrogen Energy 2022, 47, 32763–32775. [Google Scholar] [CrossRef]
- Nada, Y.; Kidoguchi, Y.; Sakai, H.; Moriyama, Y. Modeling of liftoff heights of nonpremixed turbulent flames in coflows having various temperatures and O2 concentrations. Fuel 2021, 306, 121678. [Google Scholar] [CrossRef]
- Muraleedharan, A.; Jithin, E.V.; Aravind, B.; Kumar, S.; Velamati, R.K. Combustion characteristics of syngas laminar microjet diffusion flames. J. Taiwan Inst. Chem. Eng. 2020, 115, 47–59. [Google Scholar] [CrossRef]
- Xu, P.Y. Study on NOx Generation and Control Strategy of Hydrogen Internal Combustion Engine. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2020. [Google Scholar]






| Diluent | Diluent Fraction | Air Inlet Velocity (m/s) | Fuel Inlet Velocity (m/s) |
|---|---|---|---|
| N2, CO2, H2O | 0 | 5.577 | 1.973 |
| N2, CO2 | 0.1 | 5.577 | 2.192 |
| N2, CO2 | 0.2 | 5.577 | 2.466 |
| N2, CO2 | 0.3 | 5.577 | 2.818 |
| H2O | 0.1 | 5.760 | 1.973 |
| H2O | 0.2 | 5.987 | 1.973 |
| H2O | 0.3 | 6.280 | 1.973 |
| Number | CO2 vol. % | H2 vol. % | CO vol. % |
|---|---|---|---|
| 1 | 5 | 49.5 | 40.5 |
| 2 | 10 | 46.8 | 38.3 |
| 3 | 15 | 44.0 | 36.0 |
| 4 | 20 | 41.3 | 33.8 |
| 5 | 25 | 38.5 | 31.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, D.; Zhu, W.; Sun, R. The Effect of Diluents on the Flame Structure and NO Generation Characteristics of H2/CO Micromixing Flames. Energies 2023, 16, 1252. https://doi.org/10.3390/en16031252
Wang W, Chen D, Zhu W, Sun R. The Effect of Diluents on the Flame Structure and NO Generation Characteristics of H2/CO Micromixing Flames. Energies. 2023; 16(3):1252. https://doi.org/10.3390/en16031252
Chicago/Turabian StyleWang, Wentong, Dengke Chen, Wenkun Zhu, and Rui Sun. 2023. "The Effect of Diluents on the Flame Structure and NO Generation Characteristics of H2/CO Micromixing Flames" Energies 16, no. 3: 1252. https://doi.org/10.3390/en16031252




