Effects of Alcohol-Blended Waste Plastic Oil on Engine Performance Characteristics and Emissions of a Diesel Engine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Fuels Preparation
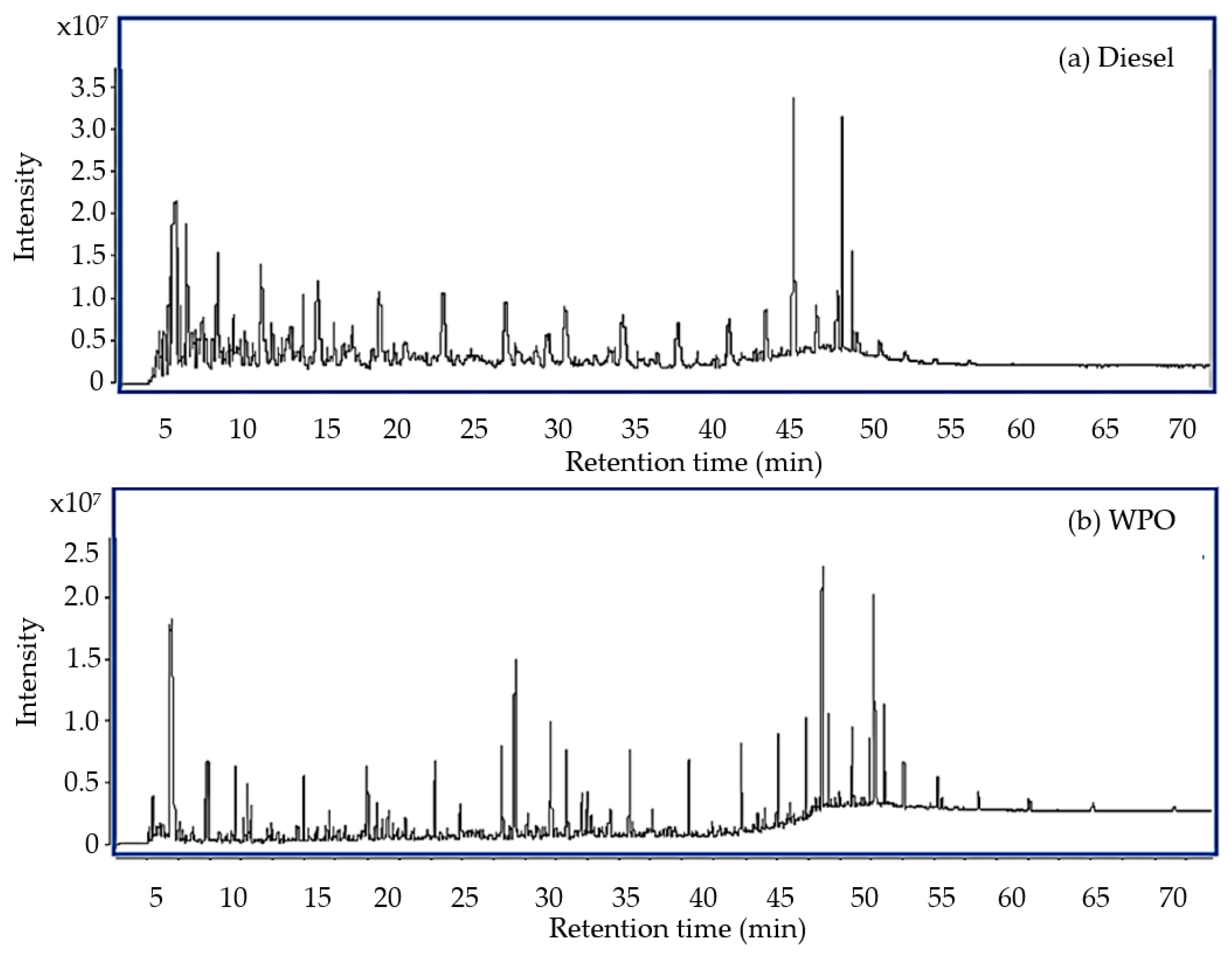
2.1.1. Gas Chromatography–Mass Spectrometry
2.1.2. Fuel Properties Characteristics of Test Fuels
2.2. Experimental Setup and Test Procedure
2.3. Multi-Objective Optimization for the Ratio of n-Butanol and Waste Plastic Oil Blends
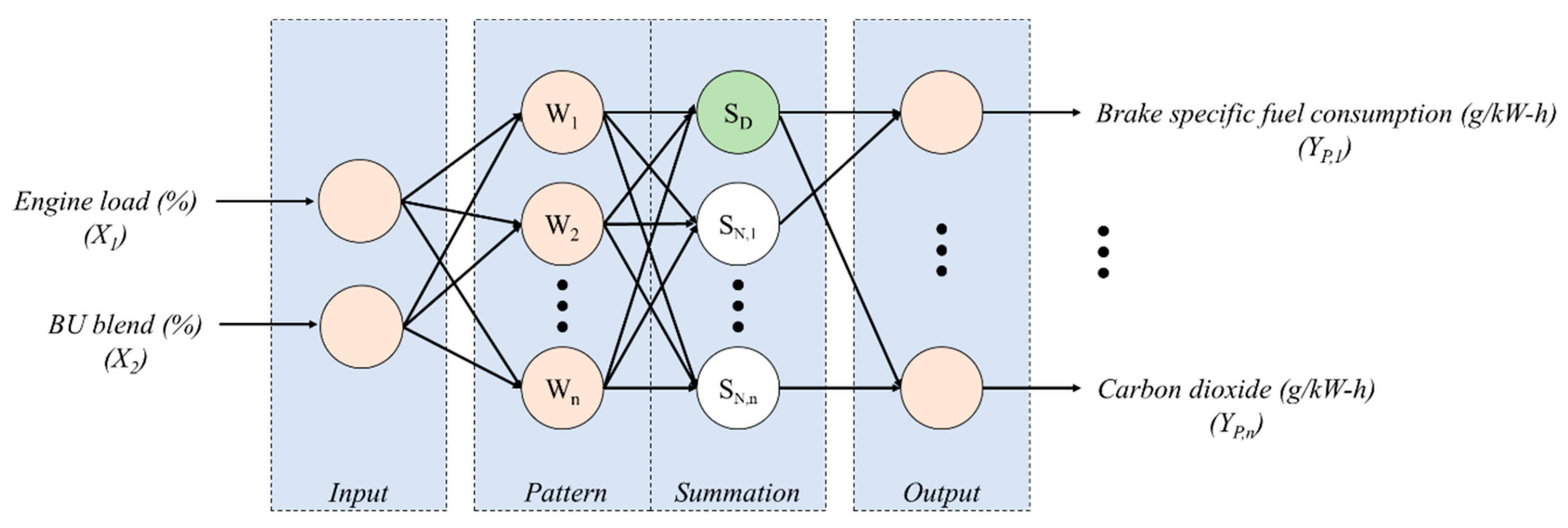
2.3.1. Multi-Objective Function via a General Regression Neural Network (GRNN)
2.3.2. Multi-Objective Optimization via Nondominated Sorting Genetic Algorithm II (NSGA-II)
3. Results
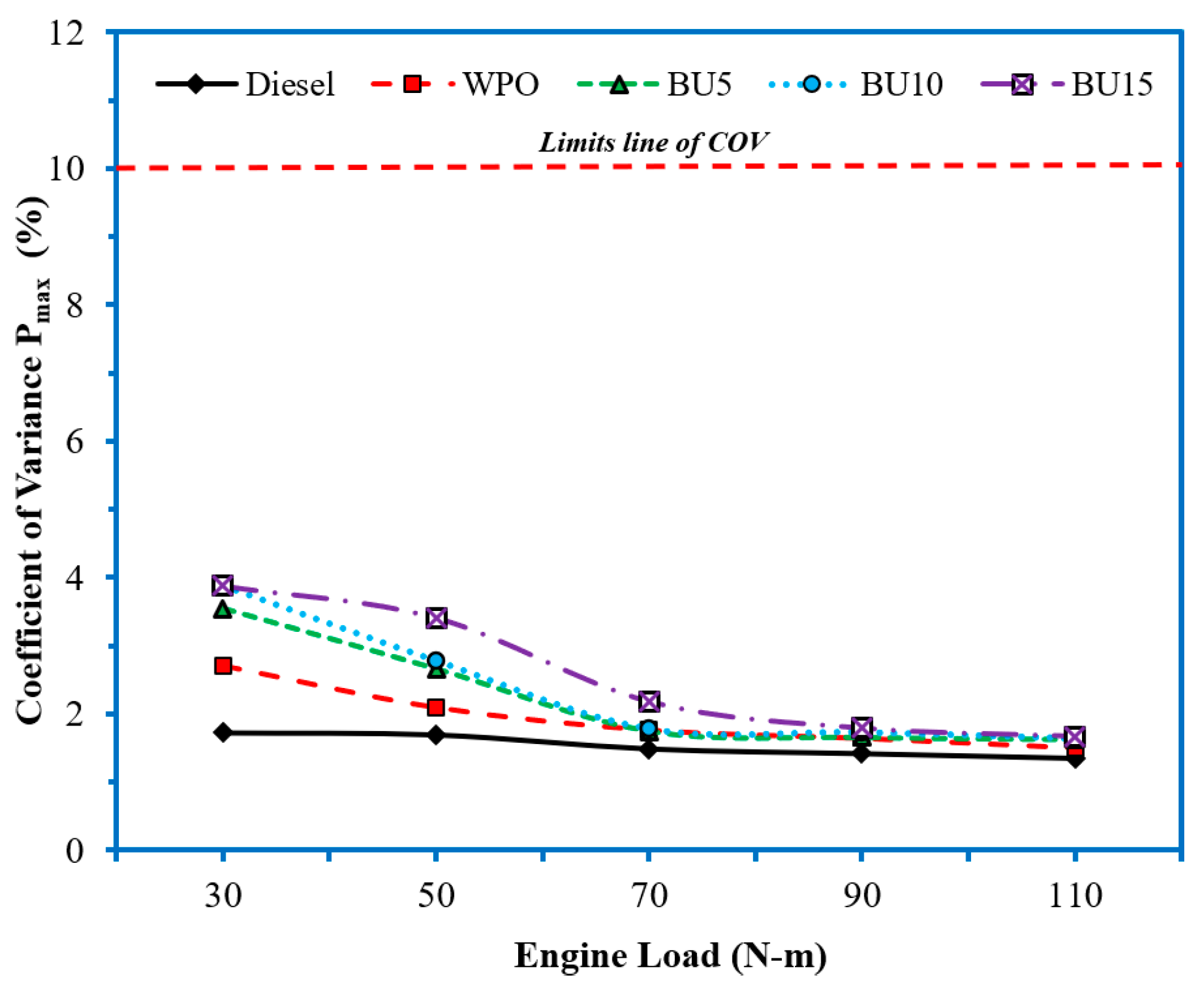
3.1. Analysis of Variance of Results
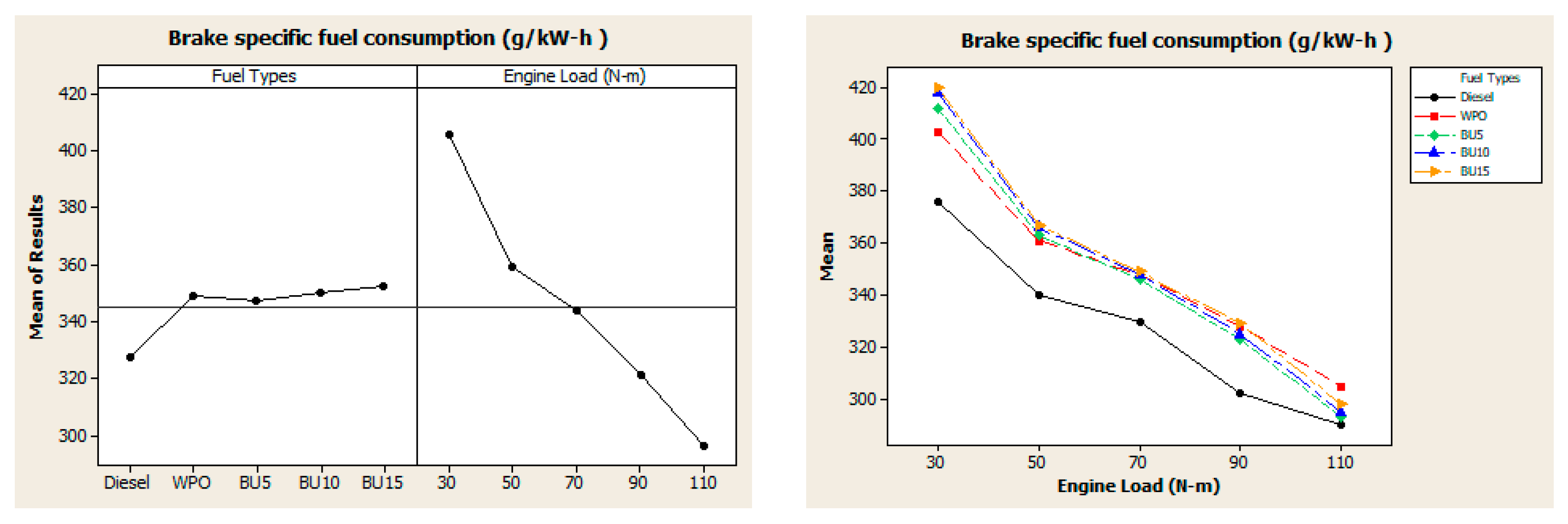
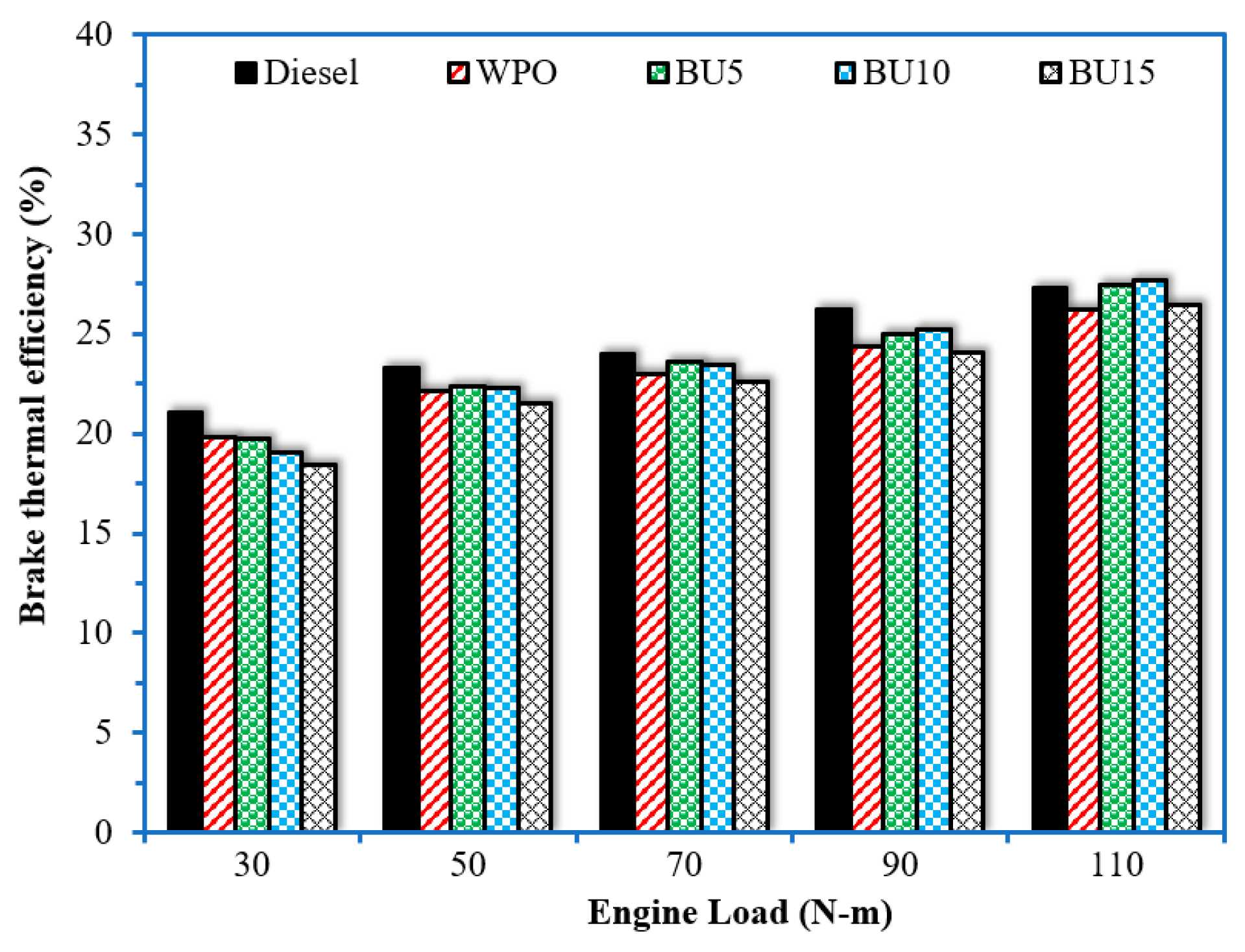
3.2. Engine Performance
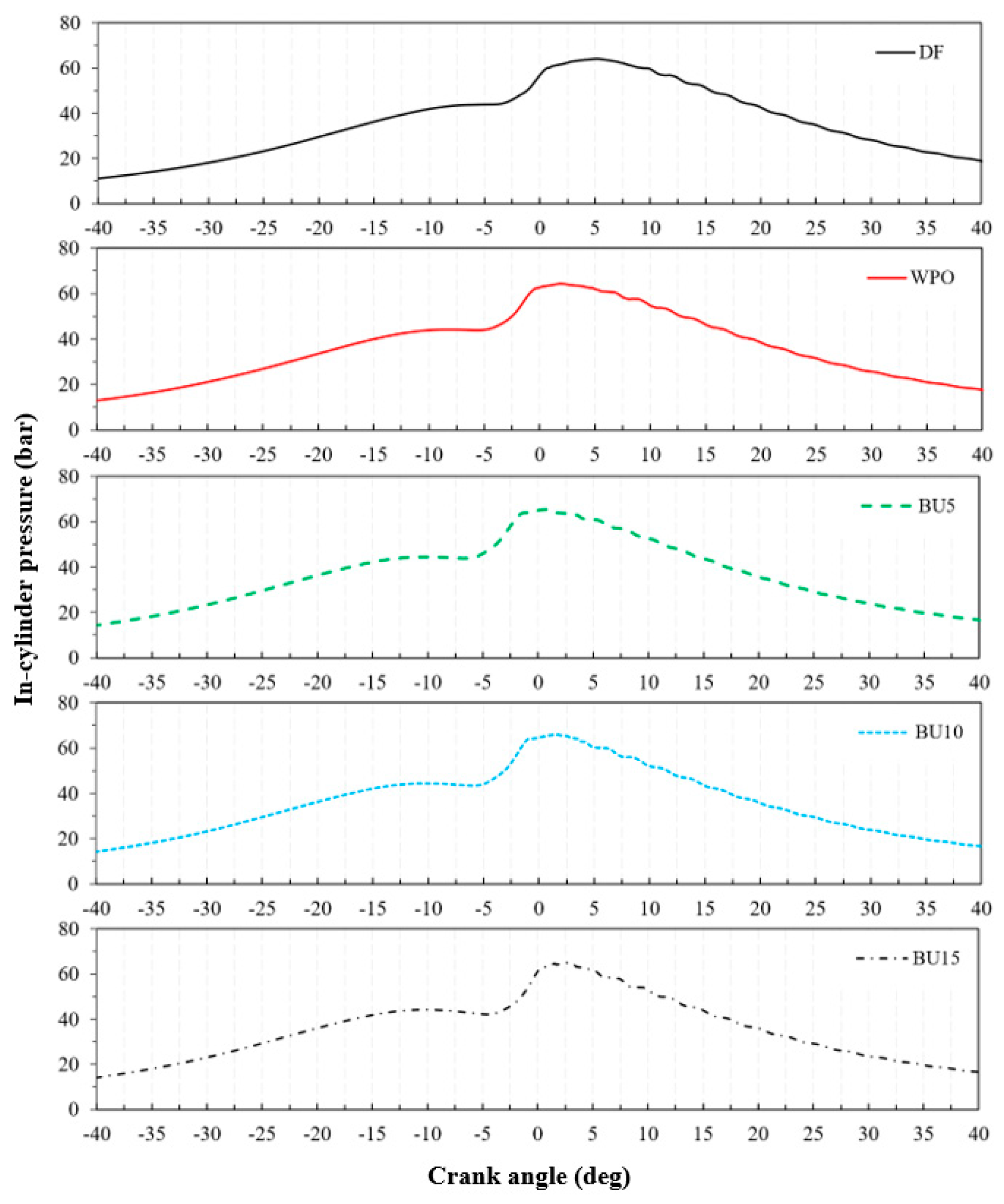
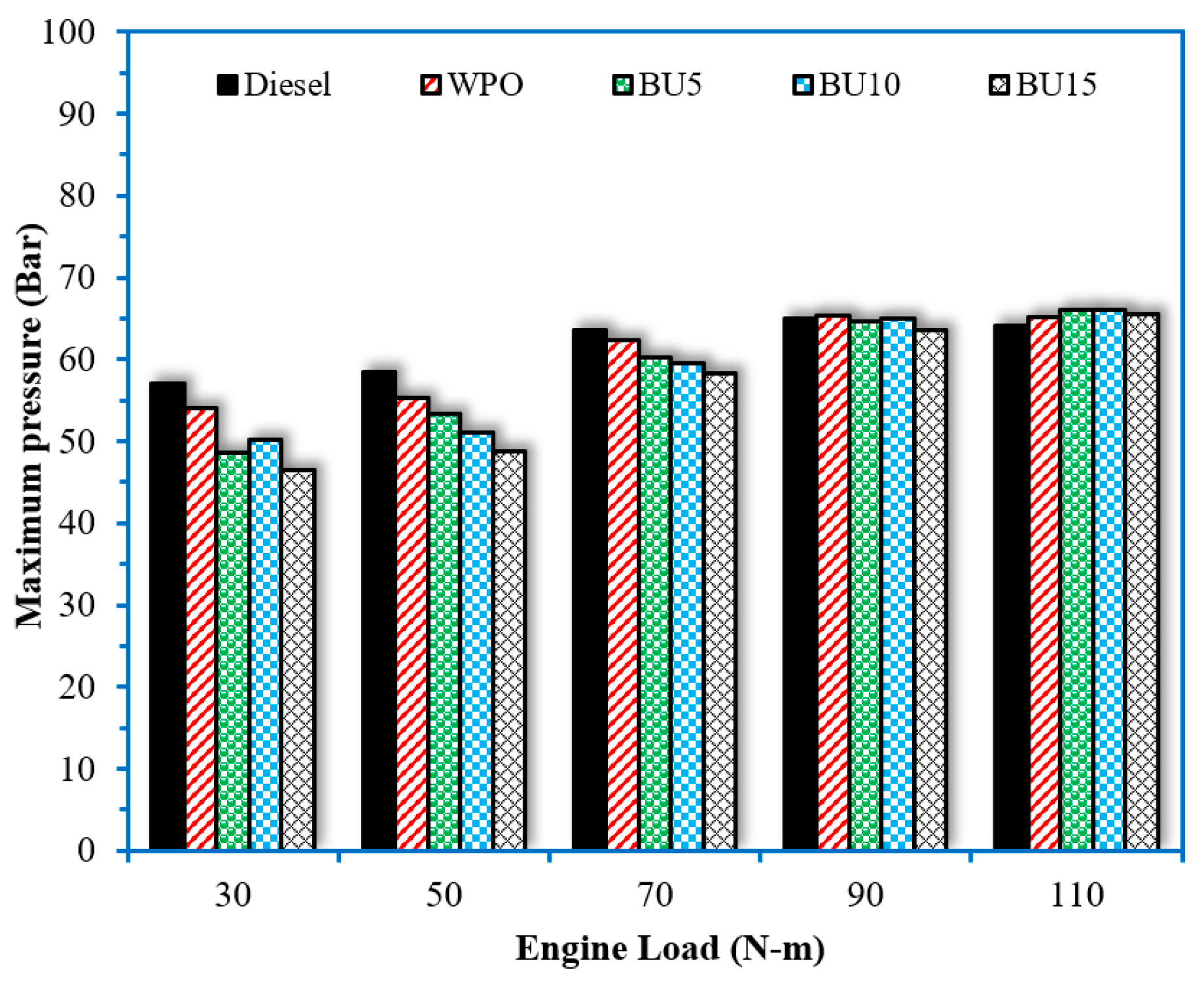
3.3. Combustion Characteristics
3.4. Emissions Characteristics
3.5. Results of Multi-Objective Function via GRNN
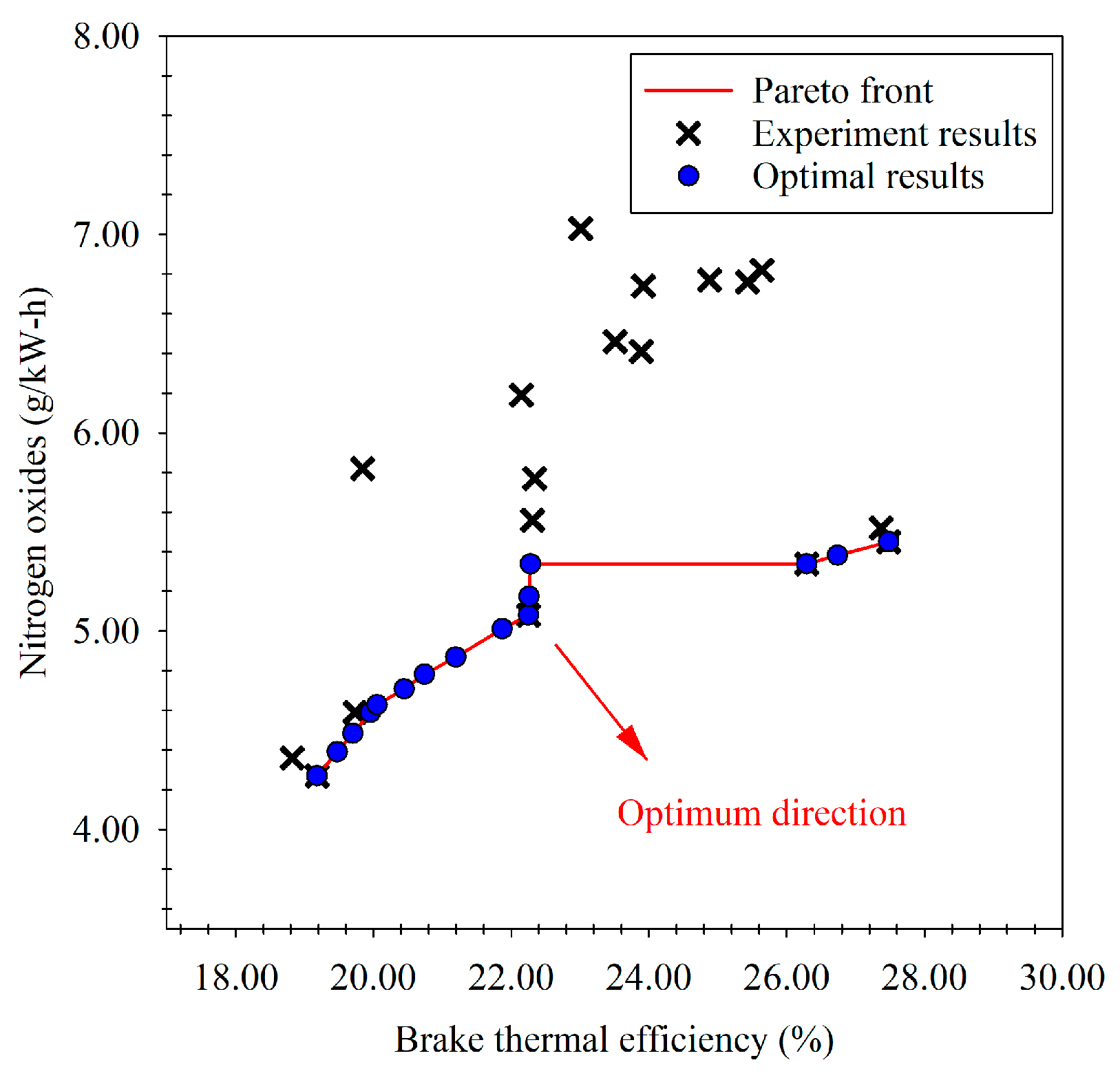
3.6. Optimized Results from NSGA-II Multi-Objective Optimization
4. Conclusions
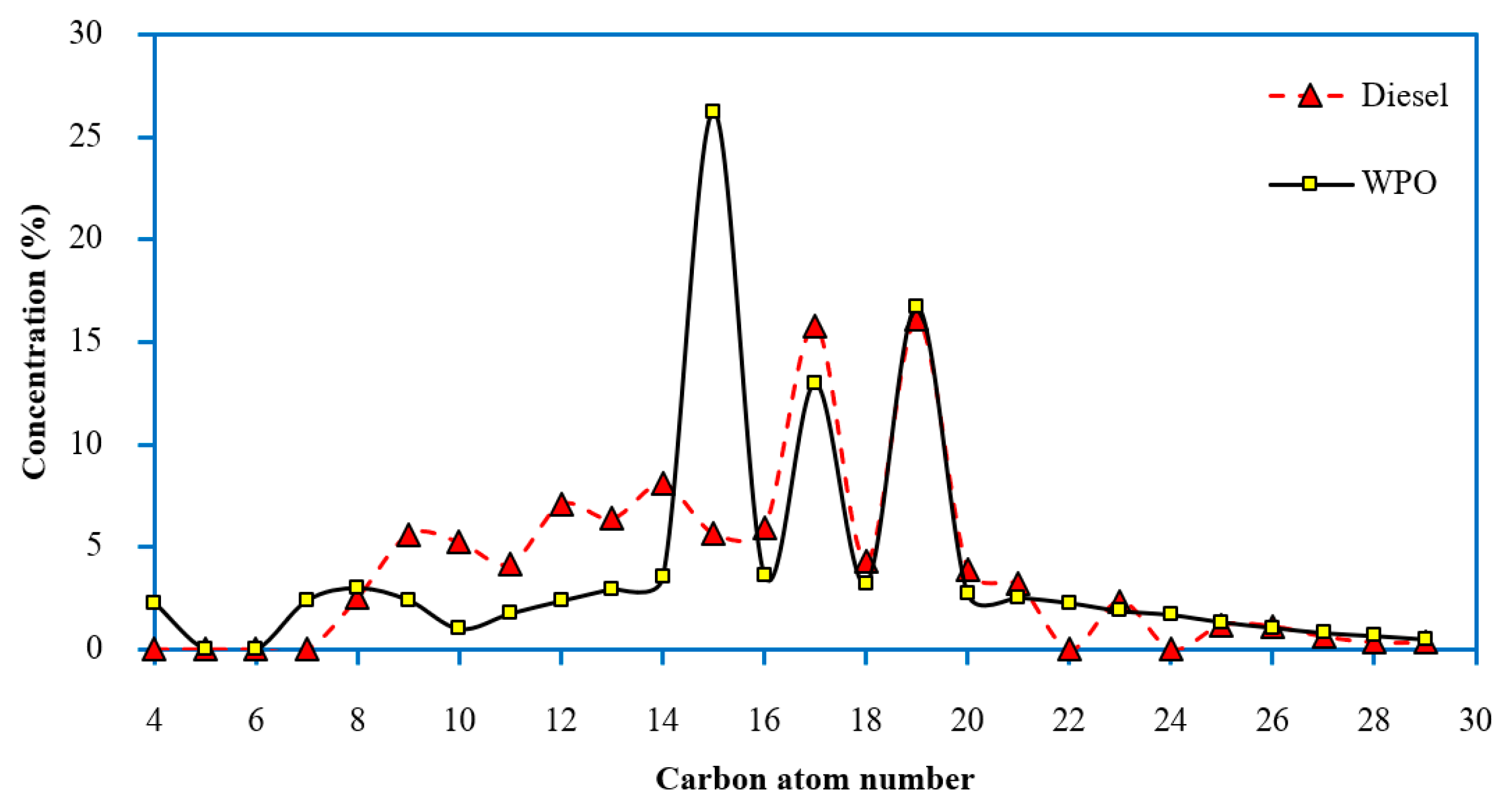
- The main hydrocarbons present in WPO ranged within different diesel fuels (C13–C18) at approximately 74.39%; thus, they can be used as alternative fuels in diesel engines. However, the specific gravity and flash point of WPO were out of the limit prescribed by the diesel fuel specification.
- The addition of n-butanol to WPO was found to reduce engine thermal efficiencies at low loads, while an increase in thermal efficiency was obtained at high loads. The maximum improvement in thermal efficiency was achieved when the engine operated with 10% n-butanol in the WPO at an engine load of 110 N.m.
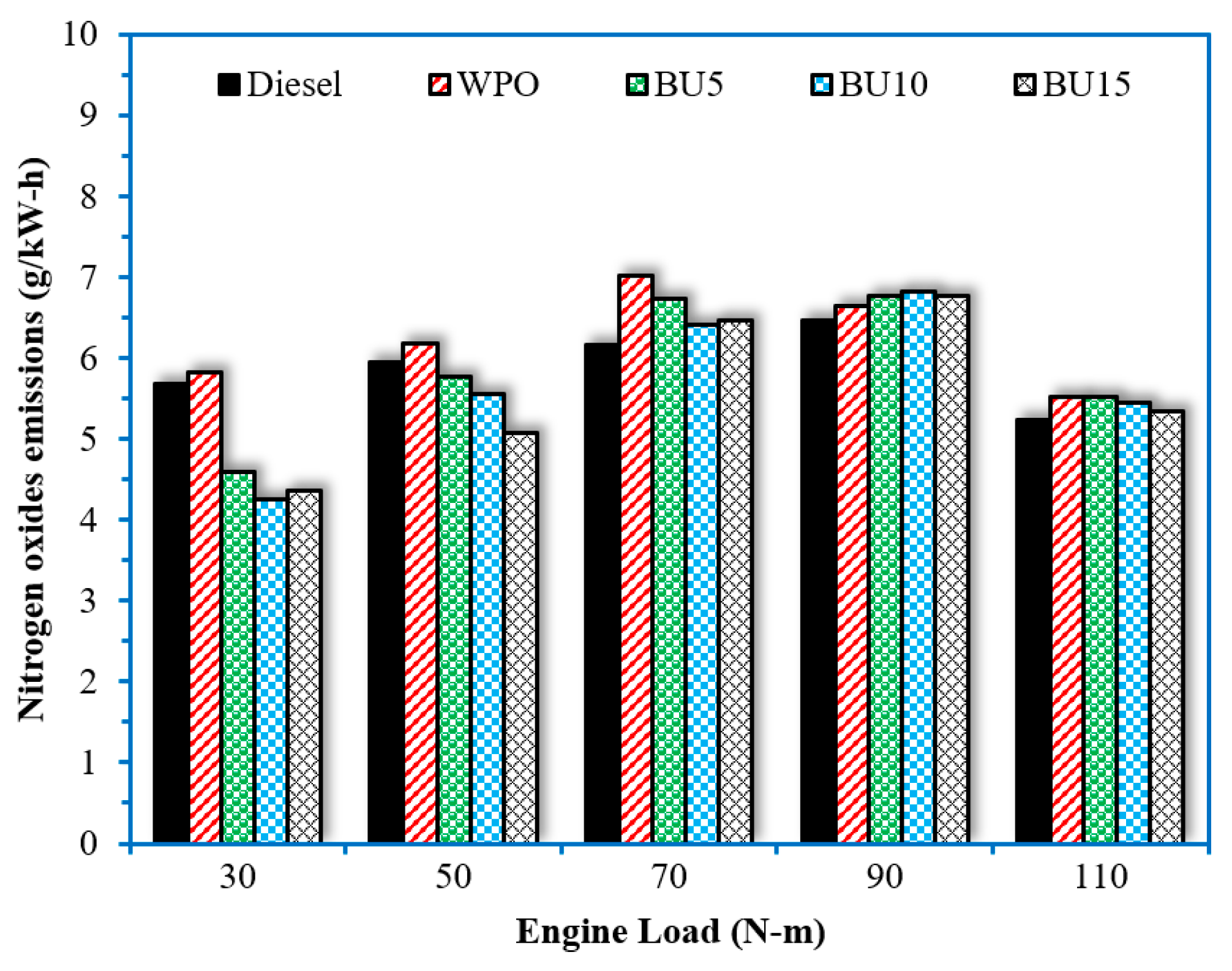
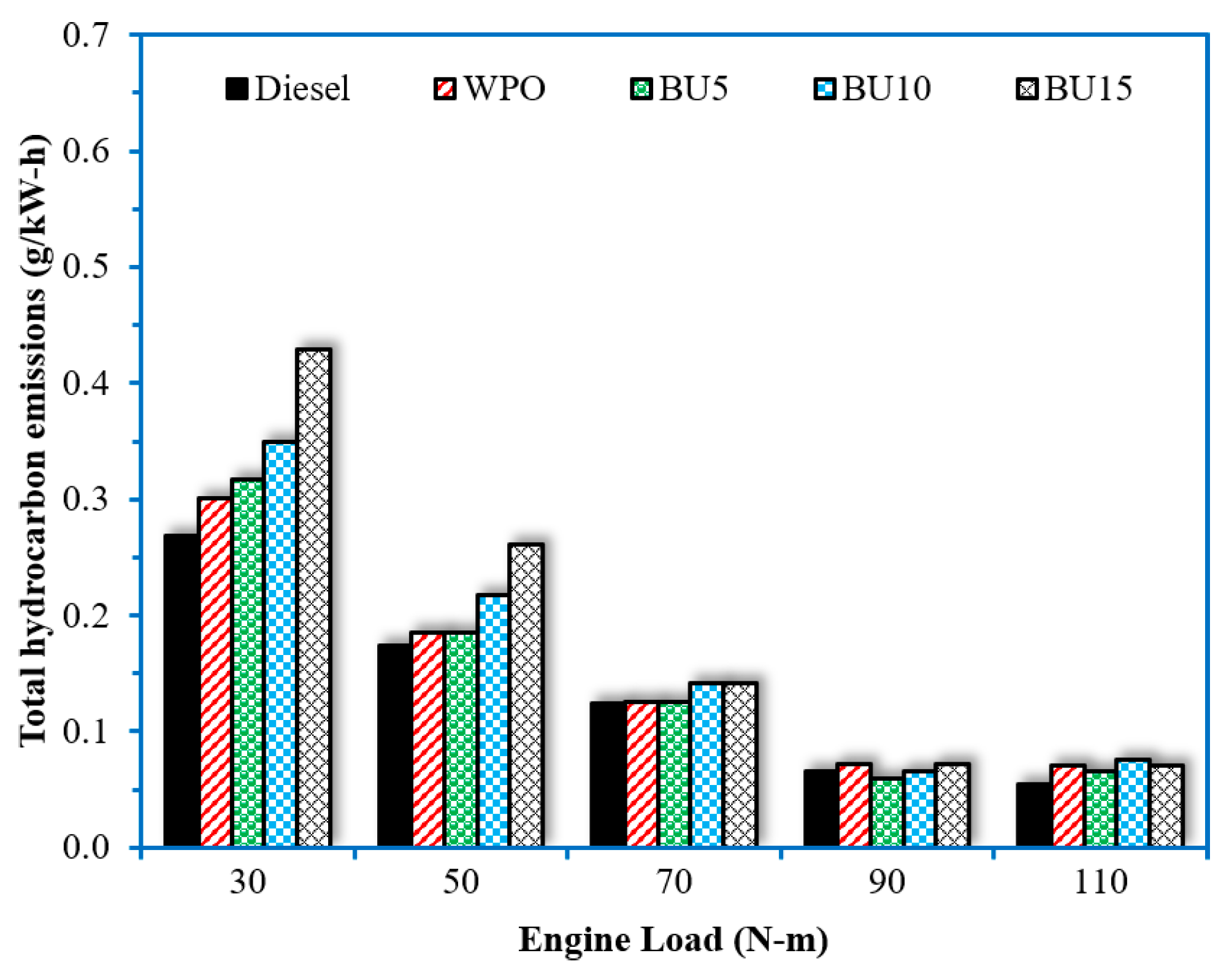
- The addition of n-butanol to WPO tended to increase HC and CO emissions. Higher n-butanol concentrations in the fuel blend promoted higher HC and CO emissions, especially when the engine operated at low-load conditions. The benefit of blending n-butanol with WPO was found with a reduction in NOx emissions, especially at low-load conditions. The average 24% reduction in NOx emissions compared to WPO was achieved when the engine operated at a load of 30 N.m.
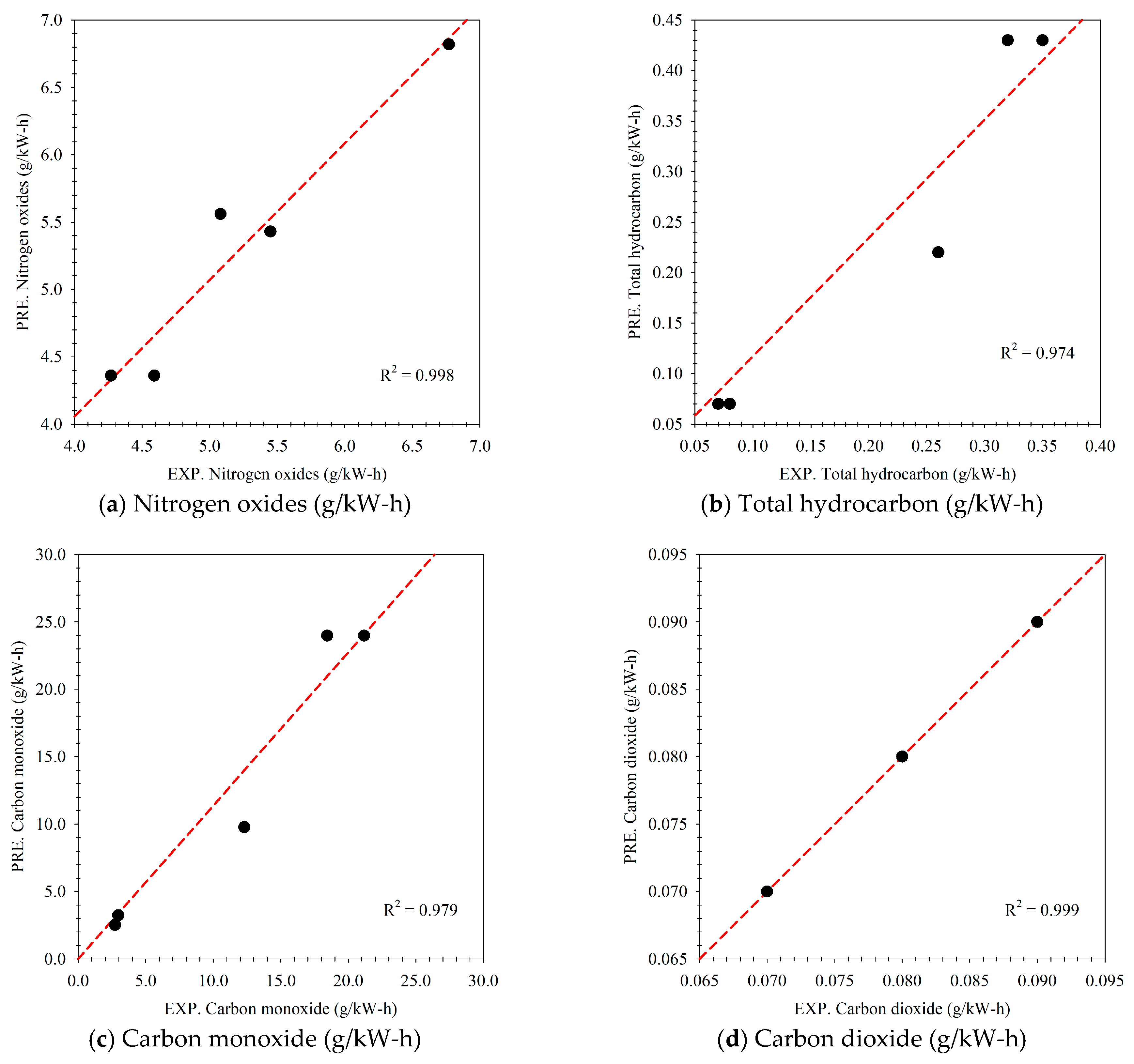
- In the optimization process, it was found that the multi-objective function produced by the general regression neural network (GRNN) can be modeled as the multi-objective function with high predictive performances. The model’s R2, MAPE, and RMSE values were 0.999, 2.606%, and 0.663, respectively, when brake thermal efficiency was considered, while nitrogen oxide values were 0.998, 6.915%, and 0.600, respectively.
- As for the results of the optimization using NSGA-II, a single optimum value cannot be attained with the other methods, but the optimization’s boundary was obtained, which was established by making a trade-off between brake thermal efficiency and nitrogen oxides.
- Following the Pareto frontier, the engine load and the ratio of the n-butanol blend that caused a trade-off between the maximum brake thermal efficiency and minimum nitrogen oxides are within the approximate ranges of 37 N.m to 104 N.m and 9% to 14% according to the input factors, respectively. However, there are many factors that still need to be studied, such as the quality of raw materials, the manufacturing process and products, the cost of economics, and end-user acceptance in the commercialization of waste plastic oil and its blend.
- Future studies on engine modification, such as adjusting fuel injection timing and injection pressure, can be considered for further improvements via the increase in thermal efficiency and the decrease in exhaust emissions by using waste plastic oil and its blends.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BSFC | Brake-specific fuel consumption |
| BTE | Brake thermal efficiency |
| B7 | Commercial diesel fuel with 7% biodiesel |
| BU5 | Blend of 5% n-butanol and 95% waste plastic oil |
| BU10 | Blend of 10% n-butanol and 95% waste plastic oil |
| BU15 | Blend of 15% n-butanol and 95% waste plastic oil |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| COV | Coefficient of variance |
| GC–MS | Gas chromatography–mass spectrometry |
| GRNN | General regression neural network |
| HC | Hydrocarbon |
| MAPE | Mean absolute percentage error |
| NOx | Nitrogen oxides |
| NSGA-II | Nondominated sorting genetic algorithm II |
| RSME | Root mean square error |
| R2 | Coefficient of determination |
| SD | Denominator neuron |
| SN | Numerator neuron |
| SM | Smoothing parameter |
| WPO | Waste plastic oil |
| Wi | Transformed of input of prediction model |
| Xi | Input of prediction model |
| YP,i | Output of prediction model |
| Mean of the actual output |
References
- Siriporn, K.; Narongsak, N. Solid Waste: What is the situation during COVID-19? J. Public Health Nurse 2020, 34, 145–157. [Google Scholar]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisitsankkhakarn, R.; Vassanadumrongdee, S. Enhancing purchase intention in circular economy: An empirical evidence of remanufactured automotive product in Thailand. Resour. Conserv. Recycl. 2020, 156, 104702. [Google Scholar] [CrossRef]
- The Situation of Sustainable Production and Consumption Operations in Thailand in 2021. Thai SCP Network. Available online: https://waa.inter.nstda.or.th/stks/pub/bcg/20210825-bcg-SCP-2564.pdf (accessed on 14 August 2022).
- Hariadi, D.; Saleh, S.M.; Anwar Yamin, R.; Aprilia, S. Utilization of LDPE plastic waste on the quality of pyrolysis oil as an asphalt solvent alternative. Therm. Sci. Eng. Prog. 2021, 23, 100872. [Google Scholar] [CrossRef]
- Doğan, O. The influence of n-butanol/diesel fuel blends utilization on a small diesel engine performance and emissions. Fuel 2011, 90, 2467–2472. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Lv, J.; Wang, S.; Zhong, Y.; Dong, R.; Gao, S.; Cao, C.; Tan, D. Investigation on combustion, performance and emission characteristics of a diesel engine fueled with diesel/alcohol/n-butanol blended fuels. Fuel 2022, 320, 123975. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Dong, R.; Zou, Z.; Gao, S.; Tan, D. Performance, combustion and emission characteristics investigations on a diesel engine fueled with diesel/ethanol/n-butanol blends. Energy 2022, 249, 123733. [Google Scholar] [CrossRef]
- Maithomklang, S.; Sukjit, E.; Srisertpol, J. Experimental investigation of ethanol blended with waste plastic oil as an alternative biofuel in a diesel engine. Suranaree J. Sci. Technol. 2020, 27, 010022. [Google Scholar]
- Chandran, M.; Senthilkumar, T.; Murugesan, C. Characterization studies: Waste plastic oil and its blends. Energy Source Part A 2020, 42, 281–291. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids:(and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Luning Prak, D.J.; Simms, G.R.; Hamilton, M.; Cowart, J.S. Impact of low flash point compounds (hydrocarbons containing eight carbon atoms) on the flash point of jet fuel and n-dodecane. Fuel 2021, 286, 119389. [Google Scholar] [CrossRef]
- Trost, D.; Polcar, A.; Boldor, D.; Nde, D.B.; Wolak, A.; Kumbár, V. Temperature dependence of density and viscosity of biobutanol-gasoline blends. Appl. Sci. 2021, 11, 3172. [Google Scholar] [CrossRef]
- Wathakit, K.; Sukjit, E.; Kaewbuddee, C.; Maithomklang, S.; Klinkaew, N.; Liplap, P.; Arjharn, W.; Srisertpol, J. Characterization and impact of waste plastic oil in a variable compression ratio diesel engine. Energies 2021, 14, 2230. [Google Scholar] [CrossRef]
- Aengchuan, P.; Wiangkham, A.; Klinkaew, N.; Theinnoi, K.; Sukjit, E. Prediction of the influence of castor oil–ethanol–diesel blends on single-cylinder diesel engine characteristics using generalized regression neural networks (GRNNs). Energy Rep. 2022, 8, 38–47. [Google Scholar] [CrossRef]
- Wiangkham, A.; Ariyarit, A.; Aengchuan, P. Prediction of the influence of loading rate and sugarcane leaves concentration on fracture toughness of sugarcane leaves and epoxy composite using artificial intelligence. Theor. Appl. Fract. Mech. 2022, 117, 103188. [Google Scholar] [CrossRef]
- Chong, E.K.; Zak, S.H. An Introduction to Optimization, 4th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Yin, E.; Li, Q. Multi-objective optimization of a concentrated spectrum splitting photovoltaic-thermoelectric hybrid system. Appl. Therm. Eng. 2023, 219, 119518. [Google Scholar] [CrossRef]
- Cepova, L.; Kovacikova, A.; Cep, R.; Klaput, P.; Mizera, O. Measurement system Anal-yses-gauge repeatability and reproducibility methods. Meas. Sci. Rev. 2018, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of waste plastic oil-biodiesel blends as alternative fuels for diesel engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P.; Tigga, V.P. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Olabode, O.S. Production of fuel-blends from waste tyre and plastic by catalytic and integrated pyrolysis for use in compression ignition (CI) engines. Fuel 2021, 297, 120801. [Google Scholar] [CrossRef]
- Bizon, K.; Continillo, G.; Lombardi, S.; Mancaruso, E.; Vaglieco, B.M. ANN-based virtual sensor for on-line prediction of in-cylinder pressure in a diesel engine. Comput. Aided Chem. Eng. 2014, 33, 763–768. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Oh, H.; Jung, J.; Bae, C.; Johansson, B. Combustion process and PM emission characteristics in a stratified DISI engine under low load condition. In Internal Combustion Engines: Performance, Fuel Economy and Emissions; IMechE; Woodhead: Sawston, UK, 2013; pp. 179–192. [Google Scholar]
- Adam, A.; Ramlan, N.A.; Jaharudin, N.F.; Hamzah, H.; Othman, M.F.; Mrwan, A.A.G. Analysis of combustion characteristics, engine performance and exhaust emissions of diesel engine fueled with upgraded waste source fuel. Int. J. Hydrogen Energy 2017, 42, 17993–18004. [Google Scholar] [CrossRef] [Green Version]
- Geng, L.; Bi, L.; Li, Q.; Chen, H.; Xie, Y. Experimental study on spray characteristics, combustion stability, and emission performance of a CRDI diesel engine operated with biodiesel–ethanol blends. Energy Rep. 2021, 7, 904–915. [Google Scholar] [CrossRef]
- Altun, Ş.; Öner, C.; Yaşar, F.; Adin, H. Effect of n-butanol blending with a blend of diesel and biodiesel on performance and exhaust emissions of a diesel engine. Ind. Eng. Chem. Res. 2011, 50, 9425–9430. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Wathakit, K.; Srisertpol, J. The Effect of n-Butanol to Waste Plastic Oil Fuel Blends Utilization on Engine Emissions of a Single Cylinder Diesel Engine. In Proceedings of the IEEE International Conference on Applied System Innovation 2018 IEEE ICASI 2018-Meen, Prior & Lam, Eds, Chiba, Japan, 13–17 April 2018; pp. 1224–1227. [Google Scholar]
- Ramalingam, S.; Rajendran, S. 14-Assessment of performance, combustion, and emission behavior of novel annona biodiesel-operated diesel engine. In Advances in Eco-Fuels for a Sustainable Environment; Azad, K., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 391–405. [Google Scholar]
- Kaimal, V.K.; Vijayabalan, P. An investigation on the effects of using DEE additive in a DI diesel engine fuelled with waste plastic oil. Fuel 2016, 180, 90–96. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Damodharan, D.; Gopal, K.; Rajesh Kumar, B.; De Poures, M.V. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020, 277, 118166. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. The utilisation of oils produced from plastic waste at different pyrolysis temperatures in a DI diesel engine. Energy 2017, 131, 179–185. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Grab-Rogaliński, K. Combustion stability, performance and emission characteristics of a CI engine fueled with diesel/n- butanol blends. Energies 2021, 14, 2817. [Google Scholar] [CrossRef]
- Awang, M.S.N.; Mohd Zulkifli, N.W.; Abbas, M.M.; Amzar Zulkifli, S.; Kalam, M.A.; Ahmad, M.H.; Mohd Yusoff, M.N.A.; Mazlan, M.; Wan Daud, W.M.A. Effect of addition of palm oil biodiesel in waste plastic oil on diesel engine performance, emission, and lubricity. ACS Omega 2021, 6, 21655–21675. [Google Scholar] [CrossRef]
- Lewis, C.D. Industrial and Business Forecasting Methods: A Practical Guide to Exponential Smoothing and Curve Fitting; Butterworth-Heinemann Scientific: Boston, MA, USA; London, UK, 1982. [Google Scholar]







| Test Fuels | Description |
|---|---|
| Diesel | Commercial diesel fuel (B7: containing 7% biodiesel) |
| BU5 | Blend of 5% n-butanol and 95% waste plastic oil |
| BU10 | Blend of 10% n-butanol and 90% waste plastic oil |
| BU15 | Blend of 15% n-butanol and 85% waste plastic oil |
| Retention Time (min) | Chemical Compound | Chemical Formula | Concentration (%) |
|---|---|---|---|
| 4.35 | Octane | C8H18 | 0.94 |
| 4.49 | Cyclohexane, 1,4-dimethyl- | C8H16 | 0.33 |
| 5.05 | Nonane | C9H20 | 0.52 |
| 5.58 | Nonane, 4-methyl- | C10H22 | 1.01 |
| 6.16 | Decane | C10H22 | 2.07 |
| 8.10 | Undecane | C11H24 | 2.28 |
| 9.13 | p-Xylene | C8H10 | 0.57 |
| 9.88 | trans-2-Dodecen-1-ol | C12H24O | 1.76 |
| 10.93 | Dodecane | C12H26 | 1.04 |
| 11.62 | Benzene, 1-ethyl-3-methyl- | C9H12 | 0.45 |
| 13.55 | Benzene, 1,2,3-trimethyl- | C9H12 | 0.54 |
| 14.50 | Tridecane | C13H28 | 2.37 |
| 15.56 | Benzene, 1,2,4-trimethyl- | C9H12 | 0.36 |
| 18.49 | Tetradecane | C14H30 | 0.99 |
| 20.11 | 1-Tetradecene | C14H28 | 0.33 |
| 22.55 | Pentadecane | C15H32 | 0.33 |
| 26.53 | Hexadecane | C16H34 | 2.63 |
| 30.37 | Heptadecane | C17H36 | 3.20 |
| 34.05 | Octadecane | C18H38 | 3.58 |
| 37.55 | Nonadecane | C19H40 | 13.76 |
| 40.80 | Eicosane | C20H42 | 5.26 |
| 43.18 | Heneicosane | C21H44 | 3.64 |
| 44.96 | Hexadecanoic acid, methyl ester | C17H34O2 | 1.57 |
| 46.43 | Tricosane | C23H48 | 1.57 |
| 47.79 | Methyl stearate | C19H38O2 | 3.73 |
| 48.07 | 11-Octadecenoic acid, methyl ester | C19H36O2 | 3.22 |
| 48.68 | Linoleic acid, methyl ester | C19H34O2 | 3.01 |
| 49.03 | Pentacosane | C25H52 | 0.50 |
| 50.44 | Hexacosane | C26H54 | 2.72 |
| 52.10 | Heptacosane | C27H56 | 2.54 |
| 53.97 | Octacosane | C28H58 | 9.26 |
| 56.22 | Nonacosane | C29H60 | 2.27 |
| Retention Time (min) | Chemical Compound | Chemical Formula | Concentration (%) |
|---|---|---|---|
| 4.31 | Pentane, 2,2,4-trimethyl- | C8H18 | 0.94 |
| 4.39 | Heptane | C7H16 | 0.33 |
| 5.95 | Nonane | C9H20 | 0.52 |
| 7.67 | Decane | C10H22 | 1.01 |
| 7.79 | Toluene | C7H8 | 2.07 |
| 9.59 | 1-Butanol | C4H10O | 2.28 |
| 10.12 | Ethylbenzene | C8H10 | 0.57 |
| 10.35 | Undecane | C11H24 | 1.76 |
| 10.55 | Benzene, 1,2-dimethyl- | C8H10 | 1.04 |
| 11.87 | Benzene, 1,4-dimethyl- | C8H10 | 0.45 |
| 13.50 | Benzene, 1-ethyl-4-methyl- | C9H12 | 0.54 |
| 13.89 | Dodecane | C12H26 | 2.37 |
| 14.20 | Mesitylene | C9H12 | 0.36 |
| 15.56 | Benzene, 1,2,3-trimethyl- | C9H12 | 0.99 |
| 15.83 | Dodecane, 4,6-dimethyl- | C14H30 | 0.33 |
| 16.14 | Dodecane, 2-methyl- | C13H28 | 0.33 |
| 17.97 | Tridecane | C13H28 | 2.63 |
| 22.25 | Tetradecane | C14H30 | 3.20 |
| 26.54 | Pentadecane | C15H32 | 3.58 |
| 27.39 | α-Gurjunene | C15H24 | 13.76 |
| 29.63 | β-Gurjunene | C15H24 | 5.26 |
| 30.64 | Hexadecane | C16H34 | 3.64 |
| 31.57 | Alloaromadendrene | C15H24 | 1.57 |
| 31.96 | γ-Gurjunene | C15H24 | 1.57 |
| 34.60 | Heptadecane | C17H36 | 3.73 |
| 38.40 | Octadecane | C18H38 | 3.22 |
| 41.72 | Nonadecane | C19H40 | 3.01 |
| 43.21 | Methyl tetradecanoate | C15H30O2 | 0.50 |
| 44.04 | Eicosane | C20H42 | 2.72 |
| 45.82 | Heneicosane | C21H44 | 2.54 |
| 46.87 | Hexadecanoic acid, methyl ester | C17H34O2 | 9.26 |
| 47.29 | Docosane | C22H46 | 2.27 |
| 48.74 | Tricosane | C23H48 | 1.88 |
| 49.91 | Methyl stearate | C19H38O2 | 1.98 |
| 50.19 | 11-Octadecenoic acid, methyl ester | C19H36O2 | 8.76 |
| 50.30 | Tetracosane | C24H50 | 1.69 |
| 50.80 | Linoleic acid, methyl ester | C19H34O2 | 2.99 |
| 52.07 | Pentacosane | C25H52 | 1.33 |
| 54.19 | Hexacosane | C26H54 | 1.07 |
| 56.78 | Heptacosane | C27H56 | 0.81 |
| 60.01 | Octacosane | C28H58 | 0.67 |
| 64.08 | Nonacosane | C29H60 | 0.50 |
| Fuel Properties | Test Method | Test Fuels | ||||
|---|---|---|---|---|---|---|
| Diesel | WPO | BU5 | BU10 | BU15 | ||
| Kinematic viscosity @ 40 °C (cSt) | ASTM D445 | 3.44 | 3.07 | 2.57 | 2.56 | 2.46 |
| Specific gravity @ 15 °C | ASTM D1298 | 0.828 | 0.800 | 0.798 | 0.803 | 0.805 |
| API gravity | ASTM D1298 | 39.4 | 45.4 | 45.8 | 44.7 | 44.3 |
| Density @ 15 °C (kg/m3) | ASTM D1298 | 827 | 799 | 800 | 802 | 804 |
| Flash point (°C) | ASTM D93 | 78 | 36 | 34 | 32 | 30 |
| Cetane index | ASTM D976 | 60.18 | 68.98 | 67.49 | 64.08 | 61.83 |
| Gross calorific value (MJ/kg) | ASTM D240 | 45.39 | 44.98 | 44.51 | 44.03 | 43.45 |
| Engine Parameters | Specifications |
|---|---|
| Engine model | 4JA1 |
| Engine type | 4-cylinder, 4-cycle, water-cooled |
| Bore × Stroke | 93 mm × 92 mm |
| Compression ratio | 18.4 |
| Displacement | 2449 cc |
| Rated power at 4000 rpm | 64.9 kW |
| Maximum torque at 2000 rpm | 171.5 N.m |
| Fuel injection type | Direct injection system with mechanical fuel injection |
| Number of fuel nozzle | 4 |
| Fuel injection pressure | 18.1 MPa |
| Fuel injection timing | 14° bTDC |
| Source | DF | Seq SS | Adj SS | Adj MS | F | p |
|---|---|---|---|---|---|---|
| Fuel Types | 4 | 6159.6 | 6159.6 | 1539.9 | 543.97 | 0.000 |
| Engine Load (N.m) | 4 | 102,633.9 | 102,633.9 | 25,658.5 | 9063.74 | 0.000 |
| Fuel Types × Engine Load (N.m) | 16 | 1816.8 | 1816.8 | 113.6 | 40.11 | 0.000 |
| Error | 50 | 141.5 | 141.5 | 2.8 | ||
| Total | 74 | 110,751.9 | ||||
| S = 1.68253 R-Sq = 99.87% R-Sq (adj) = 99.81% | ||||||
| Engine Performance or Emission Factors | GR&R (σ2Repeatability) | σ2Total | % of σ2Total |
|---|---|---|---|
| Brake-specific fuel consumption, BSFC | 2.27 | 2056.81 | 0.11 |
| Brake thermal efficiency, BTE | 0.020 | 8.701 | 0.23 |
| Nitrogen oxide, NOx | 0.025 | 0.692 | 3.61 |
| Hydrocarbon, HC | 0.0000095 | 0.0120230 | 0.08 |
| Carbon monoxide, CO | 0.026 | 41.44 | 0.06 |
| Carbon dioxide, CO2 | 0.0000063 | 0.0000560 | 11.25 |
| Average | 2.56 |
| Index | Engine Load (N.m) | BU Blend (%) | Brake Thermal Efficiency (%) | Nitrogen Oxides (g/kW-h) |
|---|---|---|---|---|
| 1 | 43.881 | 12.477 | 22.282 | 5.339 |
| 2 | 37.456 | 10.092 | 19.180 | 4.270 |
| 3 | 103.631 | 9.828 | 27.480 | 5.450 |
| 4 | 40.029 | 12.959 | 21.195 | 4.869 |
| 5 | 39.918 | 9.451 | 19.473 | 4.391 |
| 6 | 37.464 | 10.092 | 19.180 | 4.270 |
| 7 | 40.008 | 12.813 | 20.741 | 4.782 |
| 8 | 103.210 | 12.572 | 26.739 | 5.382 |
| 9 | 103.370 | 11.453 | 27.479 | 5.450 |
| 10 | 44.336 | 13.387 | 22.250 | 5.081 |
| 11 | 101.364 | 13.590 | 26.291 | 5.340 |
| 12 | 39.996 | 12.934 | 20.447 | 4.708 |
| 13 | 103.631 | 9.828 | 27.480 | 5.450 |
| 14 | 39.960 | 9.528 | 19.954 | 4.589 |
| 15 | 42.318 | 12.703 | 22.262 | 5.174 |
| 16 | 39.942 | 11.649 | 19.699 | 4.484 |
| 17 | 39.977 | 12.702 | 20.052 | 4.629 |
| 18 | 40.075 | 13.021 | 21.871 | 5.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewbuddee, C.; Maithomklang, S.; Aengchuan, P.; Wiangkham, A.; Klinkaew, N.; Ariyarit, A.; Sukjit, E. Effects of Alcohol-Blended Waste Plastic Oil on Engine Performance Characteristics and Emissions of a Diesel Engine. Energies 2023, 16, 1281. https://doi.org/10.3390/en16031281
Kaewbuddee C, Maithomklang S, Aengchuan P, Wiangkham A, Klinkaew N, Ariyarit A, Sukjit E. Effects of Alcohol-Blended Waste Plastic Oil on Engine Performance Characteristics and Emissions of a Diesel Engine. Energies. 2023; 16(3):1281. https://doi.org/10.3390/en16031281
Chicago/Turabian StyleKaewbuddee, Chalita, Somkiat Maithomklang, Prasert Aengchuan, Attasit Wiangkham, Niti Klinkaew, Atthaphon Ariyarit, and Ekarong Sukjit. 2023. "Effects of Alcohol-Blended Waste Plastic Oil on Engine Performance Characteristics and Emissions of a Diesel Engine" Energies 16, no. 3: 1281. https://doi.org/10.3390/en16031281
APA StyleKaewbuddee, C., Maithomklang, S., Aengchuan, P., Wiangkham, A., Klinkaew, N., Ariyarit, A., & Sukjit, E. (2023). Effects of Alcohol-Blended Waste Plastic Oil on Engine Performance Characteristics and Emissions of a Diesel Engine. Energies, 16(3), 1281. https://doi.org/10.3390/en16031281







