Abstract
The paper describes a new, proprietary method of HS extraction from digested sewage sludge. HS was extracted using the processes of dissolving organic substances and precipitating mineral micropollutants. The obtained substances were tested by determining their IR spectrum, elemental composition, and content of micropollutants and comparing them with HS obtained using the classical method. Using Phytotestkit, it was shown that HSs isolated from digested sewage sludge contain relatively large amounts of the nutrients necessary for plants (including N and P) and are a good component of a biostimulating substance. The obtained HS contains more ammonium and phosphorus ions. During extraction, inorganic impurities (including heavy metals) are reduced, and the humic product does not contain pathogens, parasites, and their spores. The method is simple, economically justified, and can be used on an industrial scale.
1. Introduction
Currently, there is interest in the search for renewable sources of biostimulants that can be used for agricultural purposes, including land reclamation and remediation. Environmental and economic concerns are important aspects of the research. For producers, an important element is the availability of the substrate simplicity of the final product manufacturing process and the prospect of using the method on an industrial scale. For this reason, research is being conducted on the possibility of obtaining various substances from bio-waste, including humic substances. Municipal waste, especially fermented sewage sludge, is a very cheap source of humic substances. The production of HS from this source simultaneously eliminates the recirculation of the digested sludge filtrate to the sewage treatment plant. In this way, the load of impurities resistant to biodegradation is reduced, as well as the probability of struvite precipitation, which increases the efficiency of the wastewater treatment process and the reliability of the treatment plant equipment. The lightest fractions of humic substances (fulvic acid (FA)) will not be discharged with treated sewage to surface waters, which will reduce their migration in the aquatic environment and of other substances with which they form labile and passive complexes.
So far, HS has been obtained from various sources. Xu and Geelen in the review presented examples of HS derived from organic waste and by-products, where they pointed to the composting of sewage sludge as a process that increases the maturity of organic matter and thus the HS content. HS extracted from vermicompost could modify plant physiology and metabolism [1]. Canellas et al. (2020) showed that the addition of humic acid (HA) isolated from vermicompost reduces the negative impact of abiotic stress factors in treated maize seedlings [2]. Jindo et al. (2012) researched HS obtained from urban waste as a plant growth promoter; the materials were composted and non-composted sewage sludge and municipal solid waste. The HA samples showed a root-growth-promoting effect and proton pump activity in maize vesicles. In addition, acids isolated from composted samples were characterized by a higher content of carboxyl groups and hydrophobicity than non-composted samples [3]. According to Palumbo et al. (2018), humic acids (FA + HA) extracted from an amendment obtained by a combination of composted olive mill wastewater and pre-processed organic material from municipal solid waste can be used as valuable biostimulators in maize cultivation [4]. Landfill leachate is another urban waste source of HS, and the characterization of humic acids obtained from leachate is presented in the works of Qi et al. (2012), Xiaoli et al. (2012), and Orliński and Anielak (2021) [5,6,7]. HS can be isolated from landfill leachate via nanofiltration or the tight ultrafiltration process [8,9,10]. Research by Ye et al. (2019) presents that HS extracted from landfill leachate significantly stimulated the seed germination and growth of the green mung bean plants with no obvious phytotoxicity, demonstrating HS as a valuable fertilizer component [10]. The results obtained by Kulikowska et al. (2020) showed that there are high concentrations of HS in the stabilized organic remaining fraction after the autoclaving process [11]. Researchers indicate the possibility of using HS obtained from stabilized waste and sewage sludge compost as a washing agent in the remediation of soils contaminated with heavy metals, due to the amphiphilic nature of HS and their presence in the structure of carboxyl and phenolic functional groups involved in the formation of metal-humus complexes [10,12]. The results obtained by Angelova et al. (2013) show that the application of compost and vermicompost reduces the extractable levels of heavy metals in the soil, which indicates their immobilization by humic substances [13]. Ayuso et al. (1996) used aerobic sewage sludge from sewage treatment plants and compost made of aerobic sewage sludge mixed with the organic fraction of municipal solid waste and total organic carbon in a ratio of 1:1. HS extracted from municipal waste increased the yield of barley plants (Hordeum vulgare) in calcareous soil and N and P uptake compared to HS isolated from peat, leonardite, and commercial HA [14].
In the study by Li et al. (2014a), HA and FA were recovered from mixed excess and primary sludge before the anaerobic digestion process. The obtained retentate contained 4083 mg/L of humus acids, and the possibility of further processing (concentration) of the product to obtain humic fertilizer was indicated. The recovery of humus acids before the fermentation process improved the efficiency of biogas production and the removal rate of volatile solids from the sludge [15].
In the work of Cristina et al. (2020), HA was extracted from the anaerobic sewage sludge according to the method of alkaline extraction of HS from soil using KOH and K4P2O7 described by Stevenson 1994 [16]. Then samples were encapsulated in alginate beds to allow for their controlled release in the soil environment. EDX (Energy Dispersive X-ray Spectroscopy) analysis showed that a heavy metal-free product was obtained. In a pot experiment for Lactuca sativa L. (lettuce plant), it was shown that immobilized HA molecules positively affected the hypogean dry biomass (+63% over the untreated control), but there was no effect on epigean parts of the plant [16]. Li et al. 2014 extracted humus acids from digested sludge via alkaline treatment and the ultrafiltration process. The raw sludge was composed of 80% primary sludge and 20% biofilm sludge from a local full-scale municipal sewage treatment plant. The raw sludge was fermented in a laboratory-scale anaerobic digester. The total concentration of humus acids in the retention solution was 4239 mg/L, which could be a promising source of liquid organic fertilizer [17].
It should be emphasized that extracting humic substances from waste and recirculating them to the soil is a more beneficial action than using artificial fertilizers. These substances positively influence the soil environment. The effect of humic substances on plants can be indirect and direct. The indirect effect consists of modifying the properties of the soil, including increasing water retention, buffering the pH of the soil, affecting the rhizosphere, changing the interaction between the soil, plant, and microorganisms, and complexing (chelation) nutrients. On the other hand, the direct effect of HS on plants is manifested by affecting the physical and metabolic processes of plants, including increasing the activity of growth hormones, the production of antioxidants reducing free radicals, cell membrane permeability (effective transport of mineral compounds to metabolically active sites), and improving the synthesis of chlorophyll [18,19]. The formation of stable soil aggregates with the participation of HS and the improvement of the solubility of elements results in the reduction of erosion and prevents the leaching of elements from the soil, which positively affects seed germination and the development of the root system [20,21]. The addition of HS has a positive effect on the production of chlorophyll A and B and the content of carotenoids in plants, causing the activation of H+-ATPase and increasing the accumulation of auxins in the roots, which results in root elongation and the development of lateral roots and hairs [22]. The phenolic and carboxyl groups present in the HS structure accelerate the process of seed germination and root development [23]. It has been shown that as a result of the application of HS to plants, the effects of abiotic and biotic stress (including drought, excessive heat, and salinity) were mitigated through interaction with hormones (auxin, jasmonic, abscisic, and gibberellic acids) and due to to the action of organic functional groups (phenolic and carboxylic acid) [2,24,25]. HS is characterized by the ability to ion exchange and can sorb or desorb selected cations, and thus, they are responsible for their release or removal from the environment, minimizing the toxicity of heavy metals present in the soil and increasing the bioavailability of nutrients for soil and plants [19]. Depending on the concentration of cations and pH, cations can be retained or released into the soil solution [26]. The described features make HS an agricultural and ecological product. In addition, by reducing the consumption of water and fertilizers and extraction of HS from biowaste and renewable sources, it is possible to obtain a prospective and economically justified source of organic biostimulator for soil and plants.
However, there are limited works considering the recovery of HS from digested sewage sludge, especially derived from a full-scale separated fermentation chamber. HS is characterized by properties that support the recovery of this material from renewable sources, such as bio-wastes. This is especially important in the case of municipal sources of HS because these substances are resistant to biodegradation, therefore they are not completely removed in wastewater treatment processes and are discharged into surface waters. In turn, during water treatment processes, HS becomes a precursor of halogen derivatives and other compounds with mutagenic, carcinogenic, and teratogenic properties.
Therefore, the paper presents its own, innovative method of extracting humic substances from digested sewage sludge, their qualitative characteristics, and an assessment of the impact of isolated HS on the growth of selected plants. The described method is characterized by the ease of use, economic and environmental benefits, and high efficiency of the extracted substances.
2. Materials and Methods
2.1. Materials
The material for the study was digested sewage sludge from two municipal wastewater treatment plants (WWTP), Kujawy and Płaszów in Krakow, the characteristics of which are presented in Table 1. Kujawy and Płaszów are mechanical and biological wastewater treatment plants with increased nutrient removal, operating in a 3-phase Bardenpho system. Thickened primary sludge, excess sludge, and fats are directed to separate fermentation chambers. The produced biogas is desulphurized, stored, and used to produce heat and energy. The digested sludge is a residue from the process of anaerobic methane fermentation carried out at 37 °C.

Table 1.
Content of metals in digested sludge; arithmetic mean of three measurements.
2.2. Procedure of HS Extraction and Analytics
Various technical and technological methods of producing humic substances are used. There is a known method based on the concentration and adsorption of humic substances (soluble fraction of the so-called fulvic acids) from a mixture of solutions on a resin (adsorbent), followed by the desorption of fulvic acids from the resin. To obtain a pure product, the obtained desorbate is subjected to fractionation in the ultrafiltration process [27]. Natural humic substances can be a substrate for obtaining high-purity products, as HS is mixed with an aqueous NaOH solution, the undissolved suspension is removed, and the obtained r-r is acidified to pH = 1.5 and subjected to centrifugation [28]. The humic acids obtained this way are characterized by a significant NaCl content. The substrate can be peat, sea diatoms, plankton, seaweed, etc., which are acidified to pH < 2, the temperature is increased to 90–100 °C, and various reagents are added, e.g., iron sulfate and hydrogen peroxide [29]. The process is energy-intensive and requires the use of a large number of reagents. Humic substances are obtained from tea trees [30], coal powder, which is first treated with HCl and then the separation of solids with NaOH [31], natural humic substances, which are alkalized with NaOH to pH 10 and acidified with strong acid HCl to pH 1 [32], percolate from municipal solid waste landfill [33,34], sewage sludge [35], natural substances contained in coal, peat, soil, and water [36], kitchen waste [37], a plant containing lignin [38], and increased reaction pressure [39].
Humic substances extracted from digested sludge using two methods, the author’s method and the classical method according to the International Humic Substances Society (IHSS), were used for the study. The author’s method has been described in patent application No. P. 441242. It consists of the alkalization of digested sludge with NH4OH solution to pH > 9, then in order for the base to react with humic substances, the mixture is left to react. Next, the dissolved humic substances are centrifuged in a sedimentation centrifuge and neutralized with orthophosphoric acid H3PO4. The resulting effluent is a solution of humic substances that can be used in liquid or solid form (after water evaporation), e.g., in powder or granular form. As a result of the extraction process, an aqueous suspension solution of HS was obtained (a mixture of fulvic and humic acids and insoluble humins).
Elemental composition (C, H, N, and O) analysis was performed using the chromatographic detection combustion technique in a Flash 2000 from Thermo Fisher Scientific (Waltham, MA, USA). The content of the basic elements is provided in the form of an ash-free mass. The content of inorganic micropollutants in the samples of the extracted material was determined by atomic emission spectrometry with excitation in inductively coupled plasma ICP-OES (PN-EN ISO 11885:2009, 15309:2010) and XRF X-ray fluorescence (PN-EN 15309:2010) in Bruker’s S8 Tiger spectrometer (Bruker AXS, Billerica, MA, USA). Total Organic Carbon (TOC) was measured with the Shimadzu TOC Analyzer (Kyoto, Japan). The SDT Q600 thermogravimeter from TA Instruments was used to determine the ash content. The absorption of radiation in the ultraviolet and visible light (UV-VIS) ranges was tested using the WTW photoLab 7600 spectrophotometer (Xylem Analytics, Weilheim, Germany), while absorption in the Fourier Transform Infrared (FTIR) was measured in the Spectrum Two spectrophotometer by Perkin Elmer (Waltham, MA, USA).
2.3. Phytotestkit Procedure
Phytotestkit tests for the liquid phase were used to determine the direct effect of fertilizing agents on seed germination and plant growth in the early development phase. The test contains three plant species: Sorghum saccharatum (sorghum), Lepidium sativum (cress), and Sinapis alba (white mustard). The test compared the effects of three fertilizing agents: Humic substances extracted from digested sewage sludge and two commercial mineral fertilizers, phosphoric (enriched superphosphate from Ampol-Merol: P2O5 40%, CaO 10%), and nitrogen (ammonium sulphate N34 from Anwil: Total nitrogen content 34%; N-NO3 17% w/w, N-NH4+ 17% w/w). Tests were performed in two series, set up in a completely randomized block design.
Preparation of the test plates included placing foam pads, a sheet of parafilm, and one thick white filter on the lower part of the test plates. Next, the selected substance (fertilizing agent or deionized water) in a volume of 20 cm3 was spread evenly over the entire surface of the white filter. After the white filter was completely saturated, the black filter was placed on top of it. When the black filter was completely wet, 10 plant seeds were placed on the surface of the filter in one row and at equal distances from each other, as marked on the test plate. The next step was to turn the closed test plate upside down for a few hours to facilitate contact between the seeds and the fertilizing agents. Then the test plates were placed vertically in the stabilizer and incubated for 3 days at 25 °C (±1 °C). After the test, both root and shoot lengths were measured, and the percentage effect of the testing agents on plant growth was calculated compared to values of untreated controls. Main effects and interactions were calculated based on the Yates method [40]. The research was planned according to the multifactor matrix, and in the 2 × 2 × 2 factorial experiment system, the parameters were assumed at two levels of values. The following doses of the tested agents were used: Nitrogen fertilizer, 50 kg/ha; phosphorus fertilizer, 50 kg/ha; and humic substances, 4.9 kg/ha. Deionized water was used as a non-treated control (combination I).
3. Results and Discussion
3.1. Characteristics of Digested Sludge
The digested sludge samples collected from the sewage treatment plants in Kujawy and Płaszów were subjected to qualitative analysis for the content of metals and organic matter. The obtained test results are presented in Table 1 and Table 2. The analysis of the results shows a high convergence of the qualitative composition of the tested digested sludge, both in terms of the content of metals and organic substances, determined with the use of total organic carbon (TOC) and loss on ignition in sludge at 550 °C. These sediments were characterized by a relatively high concentration of potassium (Kujawy 10,349 mg/kg DM, Płaszów 13,183 mg/kg DM) and zinc (Kujawy 1182 mg/kg DM, Płaszów 1279 mg/kg DM). The average amount of organic matter for the sludge from Kujawy, determined by TOC, was 58.719 mg/kg DM. The average content of organic substances determined on the basis of loss on ignition in sludge at 550 °C ranged from 57.2–58.2% of the total dry mass of the solid phase. These values indicate that the digested sludge has large amounts of inorganic substances, so-called ash (from 41.8–42.8% of the dry mass of the solid phase). Therefore, this sludge should not be used directly as organic material.

Table 2.
Content of total organic carbon (TOC) in digested sludge from Kujawy, dry residue (DR), residue on ignition (ROI), and loss on ignition (LOI); arithmetic mean of n = 51 measurements over 2 years.
Table 3 presents the qualitative analysis of effluents obtained in industrial conditions as a result of the centrifugation of digested sludge in a sedimentation centrifuge. These characteristics indicate that the effluents have significantly less metal than digested sludge and increased amounts of TOC, potassium, and calcium. K and Ca are components beneficial to the soil. Thus, effluents from digested sludge are a good source for obtaining HS.

Table 3.
Content of metals in digested sludge effluents; arithmetic mean of three measurements.
3.2. Characteristics of HS Extracted by an Innovative Method Developed by the Authors
Digested sludge collected from separate fermentation chambers in the Płaszów and Kujawy wastewater treatment plants was subjected to centrifugation in laboratory conditions for the purpose of HS extraction. Centrifugation speeds equal to 8000 rpm and 4000 rpm (rotate per minute) were used, and the resulting HS solution was called the HS extract. The centrifugation speed is important for the cost of running the process. To compare the quality of the HS extract using the author’s method with the quality of effluent obtained from digested sludge without adding reagents, laboratory tests were carried out using the same digested sludge and the conditions for obtaining the filtrate. The test results are presented in Table 4, Table 5, Table 6 and Table 7. The analysis of the HS extract and the effluent without extraction showed that their dry residues are characterized by a similar elemental composition (Table 4 and Table 5), but there is a significant difference in the ash content. Fulvic acids cFA1 and cFA2 and humic cHA extracted by the IHSS method had even less ash, ranging from 14.09 to 15.64%, which is the result of the extraction method consisting of precipitation, sorption, and ion exchange.

Table 4.
Elemental composition and its atomic ratios for HS extracted from digested sludge according to the classical (IHSS) and author’s method.

Table 5.
Elemental composition for digested sludge.

Table 6.
Inorganic micropollutants in the HS extract centrifuged at 4000 and 8000 rpm.

Table 7.
Inorganic micropollutants in the digested sludge effluents centrifuged at 4000 rpm and at 8000 rpm.
For example, the digested sludge effluent from Płaszów WWTP obtained by centrifugation at 8000 rpm contained DS1 = 50.74% ash in the dry residue (Table 5), more than twice as much as the extract HS1 = 24.33% (Table 4). Similar relationships were obtained using 4000 rpm centrifugation for Płaszów WWTP HS2 = 22.83% < DS2 = 41.00% and for Kujawy WWTP HS3 = 27.31% < DS3 = 46.15%. The elemental composition of organic substances was largely convergent. The carbon content was slightly higher for the author’s method extract (eg HS1 = 49.28% > DS1 = 46.85%). Larger differences were observed in the case of nitrogen content (HS1=10.18% > DS1 = 8.47%, HS3 = 8.53% < DS3 = 8.54%). Therefore, the C/N ratios (Table 4) for HS1, HS2, and HS3 were lower than for cFA1, cFA2, and cHA. The O/C ratio gives us information about the degree of oxidation of HS molecules, and values > 0.6 for FA and < 0.6 for HA are significant. The O/C ratios for HS1, HS2, and HS3 are almost equal in the range of 0.50–0.55, which means that the extracted HS mainly contains fractions with an aromatic structure typical for humic acids. The H/C ratio informs us about stabilization, maturity, and the degree of condensation. This quotient is higher for HS1, HS2, and HS3 than for cFA1, cFA2, and cHA because HS is a mixture of different humic substances. Small values of O/C and high values for H/C indicate the low polarity of aliphatic groups.
Samples HS1, HS2, and HS3 (Table 4) have more nitrogen (8.53–10.18%) than IHSS extracted samples (4.71–8.08%) and digested sludge effluents (8.47–8.69%), which is due to the extraction method and is good for the soil. Similar results were obtained in [41].
Summing up, the analysis of the elemental composition of HS extracted by the author’s method is largely consistent with the composition characteristic for humic substances obtained by the IHSS method, which confirms that the HS extract contains humic substances.
Comparing the data presented in Table 6 and Table 7, we can analyze in detail how the HS extraction from digested sludge affects the composition and which micropollutants are easily removed in a given process and which remain in the final product. HS extracted from the sludge of Płaszów and Kujawy WWTPs have lower amounts of micropollutants than digested sludge effluents. The potassium content in the HS extract from Kujawy WWTP is relatively high (36,559 mg/kg DM), but lower than in the effluent (57,709 mg/kg DM), and the amount of phosphorus also decreased from 42,078 to 29,222 mg/kg DM. The Sr content decreased in each sample, e.g., for centrifugation at 8000 rpm, from 28.0 mg/kg DM to 14.0 mg/kg DM (Płaszów WWTP). At the same time, the extraction conditions favor the leaching of iron from the sludge. For Płaszów WWTP, the amount of Fe increased from 1145 to 2163 mg/kg DM at the centrifuge speed of 8000 rpm and from 2449 to 3049 mg/kg DM, and for the Kujawy WWTP, from 5436 to 6761 mg/kg DM at the sample centrifugation speed of 4000 rpm. Leaching of Al and Zn from the sludge also occurred.
In the case of elements such as Ca, Mg, and B, a reduction in their quantity is also observed—higher content in the digested sludge effluent and much lower in the extracted HS. For Ba, values are similar for effluents and HS, ranging from 13.0 (DS1) to 16.0 mg/kg DM (HS1), from 36.0 (HS2) to 37.0 mg/kg DM (DS2), and from 25.0 (HS3) to 30.0 mg /kg DM (DS3). The amounts of Cr, Mn, and Pb are similar or the same in the extracted HS and in the digested sludge effluents.
In relation to the content of Ca in the digested sludge effluent (18,656 mg/kg DM) and in the extracted HS, there is a reduction of the Ca amount by 72% in HS3. In the case of Mg (3560 mg/kg DM), the reduction of the amount of HS3 is equal to 85%. It was not possible to reduce the concentration of Zn, Ni, and Cu. The content of Ba, Cr, and Mn remained at a similar level both for the initial sludge DS3 and for the extracted HS1, HS2, and HS3.
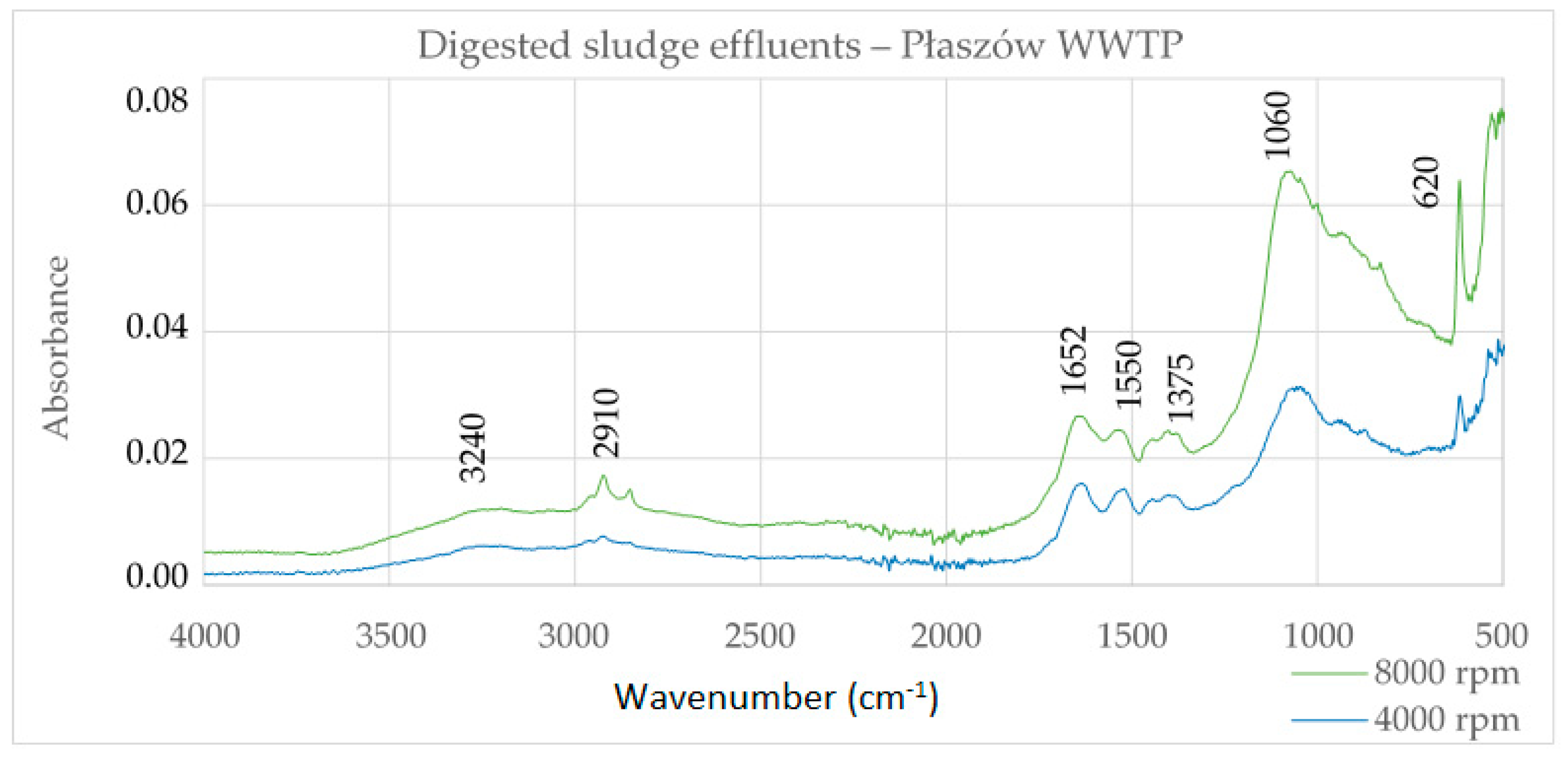
Figure 1 shows the FTIR spectrum for digested sludge effluents from Płaszów WWTP. The effluent sample DS2 centrifuged at 4000 rpm contains less ash (41.00%) than the DS1 sample (50.74%) centrifuged at 8000 rpm. The more inorganic impurities there are, the greater the intensity of the spectral bands. The nature of the spectra is consistent with the spectra in Figure 2, HS and cHA, which proves that the main substances present in the digested sludge effluents are humic substances, which contain a significant load of inorganic pollutants.
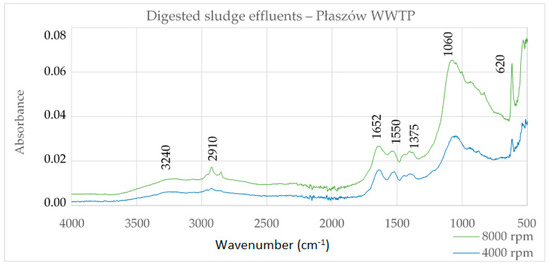
Figure 1.
Infrared (FTIR) spectra of digested sludge effluents of the Płaszów WWTP.
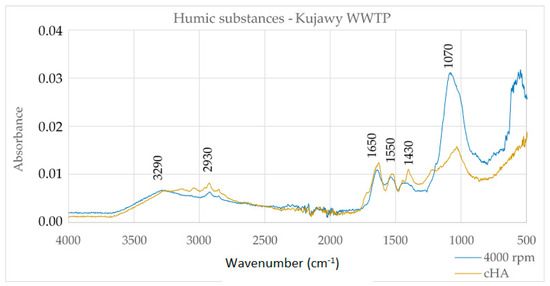
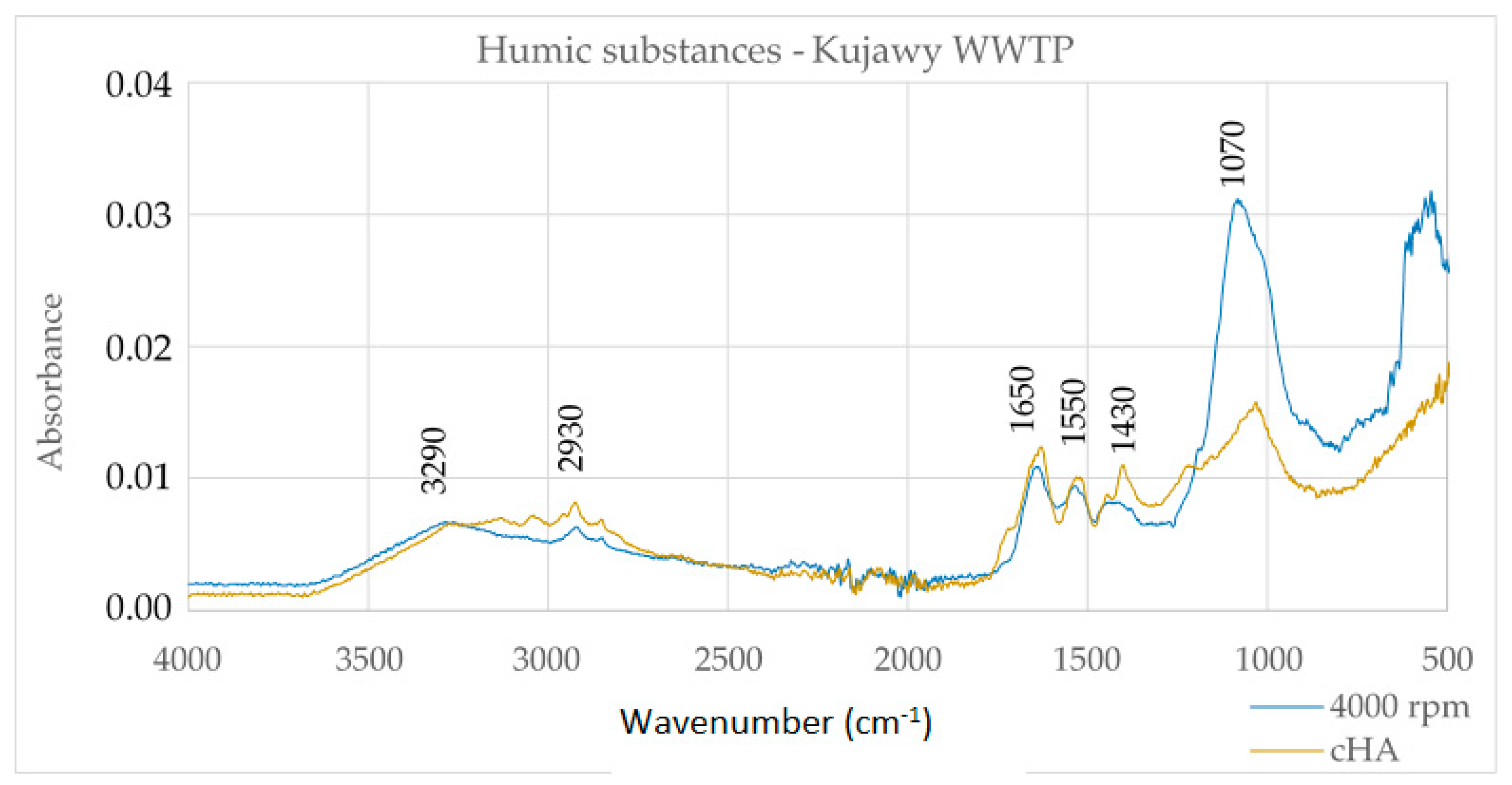
Figure 2.
Infrared (FTIR) spectra of HS extracted from digested sludge of the Kujawy WWTP (author’s method) and cHA (classical method by IHSS).
The spectra of the HS and cHA extracts in Figure 2 are largely convergent in the wavenumber range from 1500–4000 cm–1. There are broad bands with peaks of 2930–3290 cm–1, which, together, with the band of 1650 cm–1 and 1070 cm–1, indicate the presence of carboxyl groups. In addition, the 1650 cm–1 band characterizes aromatic acids. It can therefore be concluded that the extracted humic substances contain mainly humic acids, which is confirmed by the O/C and H/C ratios, the values of which are significant for humic acids.
3.3. Microbiological Testing
Central Laboratory of Krakow Water S.A. performed microbiological tests for the presence of pathogens and parasites in the HS extract obtained from the digested sludge of Kujawy WWTP. Test results showed no live ATT and Salmonella sp.
3.4. Effect of Extracted HS on the Growth of Selected Plants—Phytotestkit
The assessment of the effect of HS on the growth of Lepidium sativum, Sinapis alba, and Sorghum saccharatum in the early development phase was carried out using the Phytotestkit test for the liquid phase. As one of the fertilizing agents, an HS extract isolated from digested sludge using an author’s method was used. HS3 extracted from the DS3 was used for the Phytotestkit test. The concentration of HS in the solution was 67.5 g/L. The results of the research are presented in Table 8, Table 9, Table 10, Table 11 and Table 12 and in Figure 3, Figure 4 and Figure 5. Table 8 presents the experiment planning matrix, information on the presence of individual factors, and their dose depending on the combination. Combinations are listed using the Yates method. In this experiment, eight combinations were planned: I (deionized water), a (humic substances—HS), b (nitrogen fertilizer—N), ab (HS + N), c (phosphate fertilizer—P), ac (HS + P), bc (N + P) and abc (HS + N + P). The HS dose was 0.27 g/L, while the N and P doses were 2.75 g/L.

Table 8.
Trial planning matrix with doses of fertilizing agents depending on the combination.

Table 9.
Percentage effect of testing agents on root and shoot growth.

Table 10.
Mean main factorial effects, average interactions, and their significance for Lepidium sativum.

Table 11.
Mean main factorial effects, average interactions, and their significance for Sinapis alba.

Table 12.
Mean main factorial effects, average interactions, and their significance for Sorghum saccharatum.
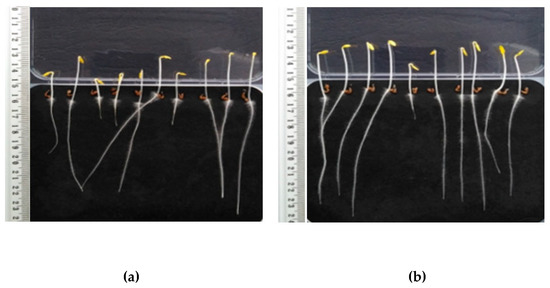
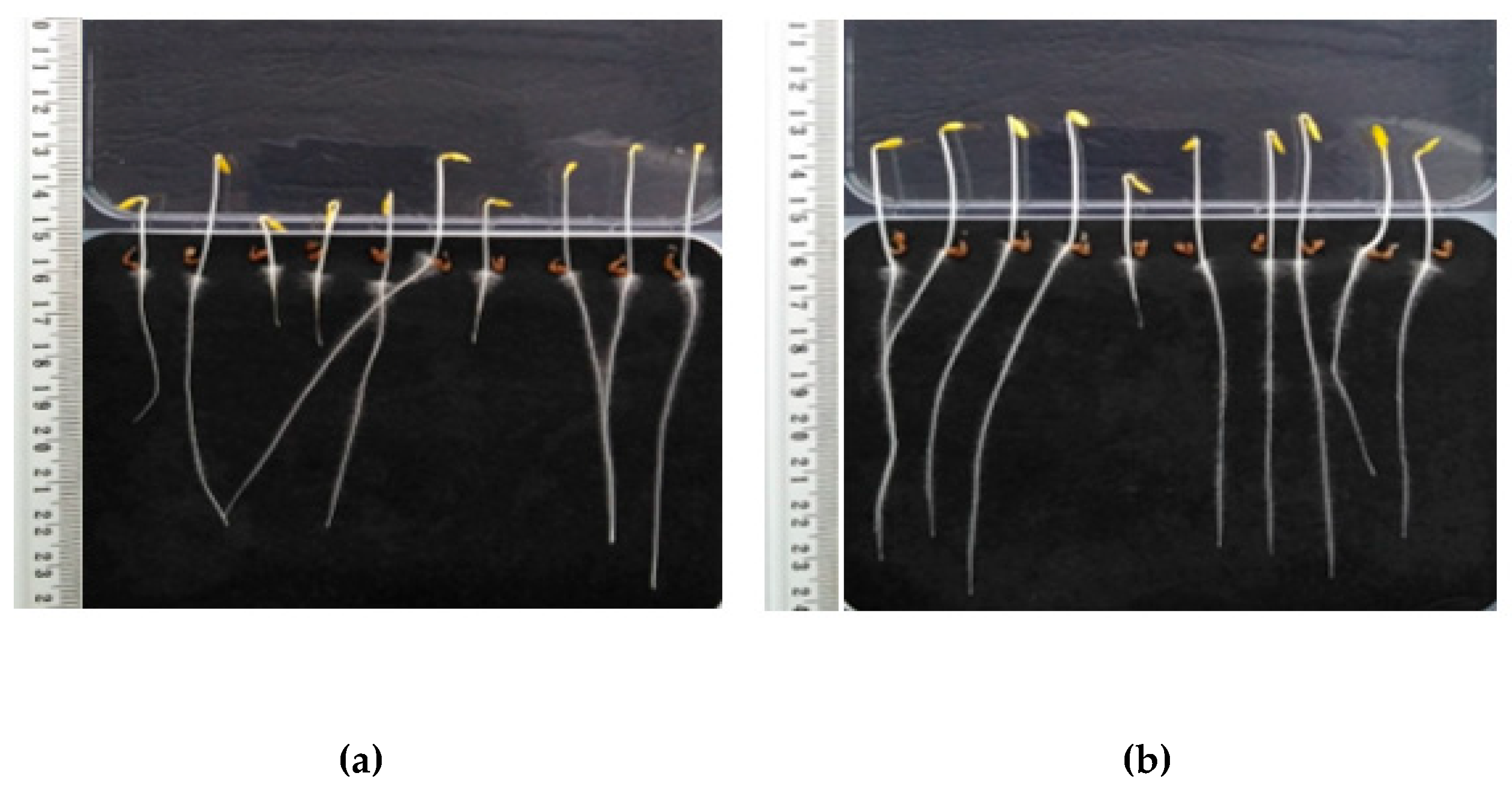
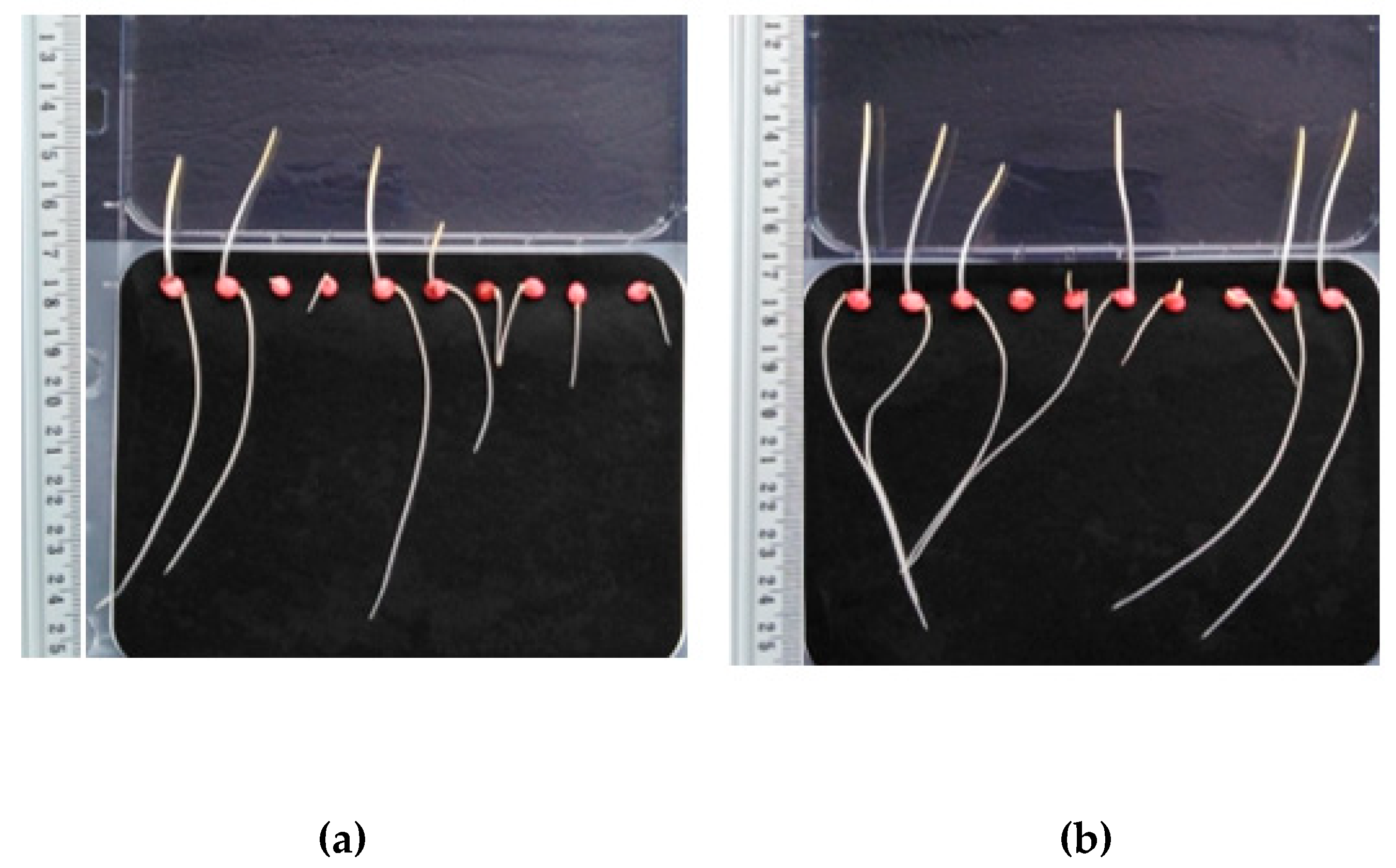
Figure 3.
Lepidium sativum after three days of incubation: (a) Untreated sample, (b) HS fertilization.
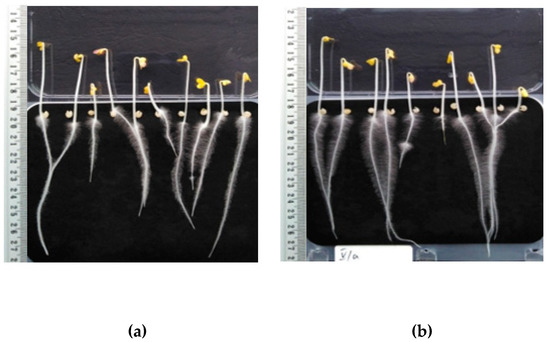
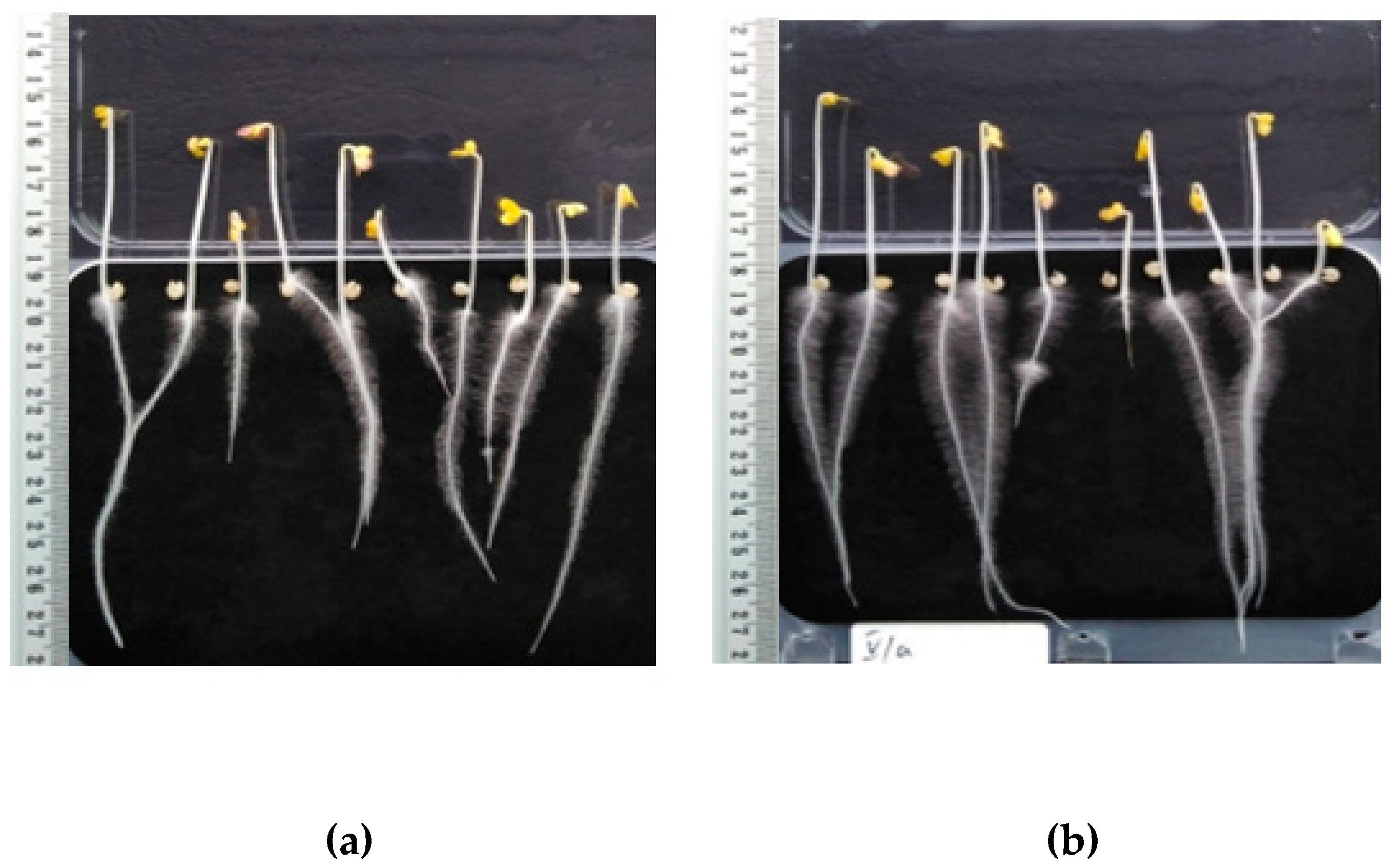
Figure 4.
Sinapis alba after three days of incubation: (a) Untreated sample, (b) HS fertilization.
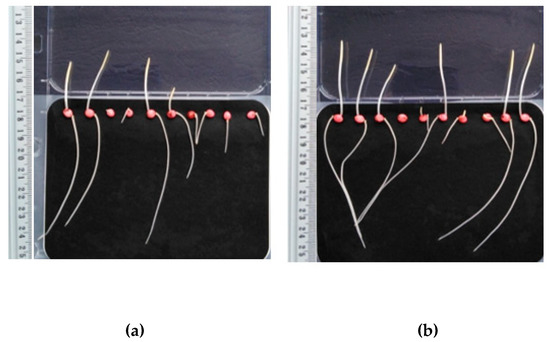
Figure 5.
Sorghum saccharatum after three days of incubation: (a) Untreated sample, (b) HS fertilization.
Table 9 shows the percentage effects of the applied fertilizing agents on the growth of the root part and shoots of the tested plants. The results regarding the influence of individual factors are expressed in comparison with a sample of deionized water. For the growth of the root parts of Lepidium sativum, a positive effect is observed with all combinations, with the highest value attributed to the use of HS (51.79%) and HS + P (49.45%). In the case of Sinapis alba, the largest percentage effect can be attributed to P (35.05%), HS (32.83%), and HS + P (32.32%), while the negative effect on root growth is noted for N (–0.81%). When analyzing the effects of fertilizing factors for Sorghum saccharatum, it is noted that HS has the best effect (60.68%) on the development of the root part of the plant in comparison with other factors used individually. A clear positive effect for Sorghum saccharatum is also observed for the combinations HS + P (42.71) and HS + N (41.53%). The percentage effects of the applied fertilizing agents on the shoot growth of the tested plants are lower than in the case of the root parts. For Lepidium sativum, the highest value was obtained when using HS (12.0%). For Sinapis alba, the best shoot growth effects were obtained for the combinations HS + N (24.62%) and HS + P (18.46%). In the case of Sorghum saccharatum, the combination of HS + N (42.55%) and N + P (38.30%) turned out to be the most effective for shoot development. HS extracted from digested sewage sludge using the author’s method, for all tested plants, showed clearly positive effects on the growth of roots and shoots, both used individually and in combination with N or P.
Below, in Table 10, Table 11 and Table 12, the results of an in-depth analysis of the influence of the tested fertilizing agents on Lepidium sativum are presented. The results in the second column refer to the average length of the root part for the individually used factors a (HS), b (N), and c (P) and their use in combination. The highest average root length values are observed for fertilization with HS (69 mm), HS+P (68 mm), and N and N + P (64 mm). The fourth column lists the main effects and interactions whose positive value is observed for P (= 7.66), HS ( = 5.63), and HS + N + P interactions ( = 1.84). For Lepidium sativum, a positive and statistically significant main effect of humic substances was found at the level of 0.05 (factor a), phosphate fertilizer at the level of 0.01 (factor c), and a positive but statistically insignificant combination of HS+N+P (ABC interaction).
Negative values of main effects and interactions occur for HS + N ( = −8.13), HS + P ( = −7.97), N ( = −5.00), and N + P = −1.09). A negative and statistically significant effect is observed for the combination of HS + N (interaction AB) and HS + P (interaction AC), and the significance level is 0.01. A negative but statistically insignificant effect on the tested plants was observed for nitrogen fertilizer (factor b) and the N + P combination (BC interaction). The negative effects of using HS in combination with N or P may result from the fact that HS extracted from sewage sludge characterized by a significant content of N and P. An excess concentration of fertilizing factors may inhibit or even negatively affect the growth of the tested plants, in this case, Lepidium sativum.
Figure 3 shows the tested Lepidium sativum plants to which deionized water (a) and HS (b) were dosed. It can be seen that plants fertilized with HS are characterized by longer lengths of root and shoot parts than unfertilized samples.
The mean root lengths, main effects, and interactions calculated for the results of studies conducted with Sinapis alba plants are presented in Table 11. Plants with the longest roots were fertilized with P (84 mm), HS (82 mm), and HS + P (82 mm). As in the case of Lepidium sativum, a positive effect of HS (= 8.02), P (= 4.98), and HS + N + P ( 3.67) is also observed for Sinapis alba. For mustard, positive and statistically significant main effects of humic substances (factor a) and phosphate fertilizer (factor c) were found at the level of 0.01. The HS + N + P interaction (ABC interaction) is also positive, and the significance level here is 0.05. A negative and statistically significant effect at the level of 0.01 is observed for nitrogen fertilizer (factor b), the combination of HS + P (interaction AC), and N + P (interaction BC). For the HS + N combination (interaction AB), a negative effect is also observed, but it is statistically insignificant.
Figure 4 shows Sinapis alba; specifically, on the left (a) are plants with deionized water dosing, and on the right (b) are plants treated with HS obtained from digested sludge. Plant samples fertilized with HS have longer roots, but it is worth noting that they also have a much more developed system of lateral roots and hairs.
The results of an in-depth analysis regarding the determination of the effect of fertilizing factors on the early growth of Sorghum saccharum are presented in Table 12. The longest average values of the length of the root part were obtained using HS (59 mm). Positive main effect values for Sorghum saccharatum were obtained for individual HS dosing (= 362) and N + P = 92). For sorghum, a positive main effect was found for humic substances (factor a), for which the significance level is 0.01. The combination of N + P (interaction BC) is positive, but not statistically significant.
In the remaining cases, the values of main effects and interactions were negative and statistically significant at the level of 0.01—HS + N combination (AB interaction), HS + P combination (AC interaction), and HS + N + P combination (ABC interaction). For nitrogen fertilizer (factor b) and phosphate fertilizer (factor c), negative main effects are observed for sorghum, but they are statistically insignificant. The negative impact of fertilizing factors on the tested plants (except for HS and HS + N + P) may indicate an inhibitory effect associated with too high a content of nutrients supplied to the plants. HS extracted from digested sludge are characterized by a relatively high concentration of N and P, which may be an appropriate dose during individual fertilization of Sorghum saccharatum plants.
Figure 5 shows the tested plants of Sorghum saccharatum not fertilized (a) and fertilized with HS isolated from digested sludge (b). For plants with HS dosing, much longer root parts and a greater number of developed shoots of the Sorghum saccharatum plant are observed in comparison to unfertilized plants.
The methods of HS extraction used so far (alkalization with NaOH and acidification with HCl) may lead to soil salinization (the permissible concentration of NaCl is 103 g/L). With an excessive amount of NaCl, plant leaves turn bluish-green, then turn brown and dry up, are brittle, and fall off easily. Sodium chloride damage occurs at the content of 0.25% Na and 0.5% Cl in the dry matter of the leaves. In addition, the neutralization of the solution with orthophosphoric acid is very advantageous. Phosphorus plays a special role in the plant. Phosphate bonds accumulate a great deal of energy used in the processes taking place in cells.
3.5. Advantages of the Author’s Method
Numerous organic compounds can only be metabolized when a phosphorus ion is attached to them. Plants take up phosphorus primarily in the form of orthophosphate solution, at soil pH 6–7. The presented author’s method of obtaining humic substances mainly consists of enriching the substrate with the necessary substances (ammonium, phosphorus ions) and the additionally extracted HS independently remove heavy metals, pathogens, parasites, and their spores without the use of sorption and ion exchange resins requiring regeneration, used in many patents. The substrate is sewage sludge (a waste product), which does not require additional treatment processes, is not exploited (coal, peat), and does not require tree felling (tea trees, beech trees, etc.).
Humic substances are carriers of heavy metals and organic compounds of anthropogenic origin, with which, for example, fulvic acids form soluble complexes (with high mobility). Therefore, recirculated digested sludge effluents hinder the wastewater treatment process, and at the same time, they easily react with organic substances (e.g., pharmaceuticals) and metals, including heavy metals contained in the treated wastewater. Humic substances are partially adsorbed on activated sludge. On the other hand, the lightest fractions (fulvic acids) remain in treated wastewater and are discharged with them into surface waters. The extraction of humic substances from digested sludge will reduce the migration of humic substances and other substances with which they form labile and passive complexes in the aquatic environment. The elimination of digested sludge effluent currently recirculated to treated wastewater in a biological reactor will reduce the load of impurities resistant to biodegradation and the probability of struvite precipitation, which will increase the efficiency of the wastewater treatment process and the reliability of the treatment plant equipment. The production of cheap humic substances will enable the remediation of degraded soils and wastelands.
4. Conclusions
- The presented author’s method of obtaining HS from digested sewage sludge is a simple and economically justified extraction method that can be used on an industrial scale. A centrifugation speed of 4000 rpm can be used in the extraction process.
- As a result of extraction using the author’s method, the number of inorganic micropollutants in HS was reduced in relation to the initial digested sewage sludge.
- HS isolated from digested sewage sludge does not contain Salmonella Sp. and live ATT parasites.
- For the tested plants, positive effects of using HS extracted from digested sewage sludge were obtained in comparison with traditional NP fertilization.
- The results of the Phytotestkit studies may indicate that HS isolated from digested sewage sludge, containing relatively high amounts of nutrients necessary for plants (including N and P), are a good component of a fertilizing/biostimulating substance. Positive effects may also be influenced by the property of HS, which increases the bioavailability of nutrients for plants.
- The use of biostimulants derived from biowaste, especially HS, can contribute to reducing the use of chemical synthetic fertilizers.
- There are no legal grounds for the use of the described HS in agriculture (possible organic contamination, e.g., pharmaceuticals), but the prospect of using the obtained HS in soil reclamation is promising.
Author Contributions
A.M.A., conceptualization, methodology, investigation, writing—original draft preparation, supervision, funding acquisition, and project administration; A.K., investigation, writing, and visualization; B.Ł., investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Center for Research and Development in Poland from EU funds, grant number POIR.04.01.04-00-0039/17.
Data Availability Statement
Cracow University of Technology Faculty of EEE Project report number POIR.04.01.04-00-0039/17.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Canellas, N.O.; da Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. [Google Scholar] [CrossRef]
- Palumbo, G.; Schiavon, M.; Nardi, S.; Ertani, A.; Celano, G.; Colombo, C.M. Biostimulant potential of humic acids extracted from an amendment obtained via combination of olive mill wastewaters (OMW) and a pre-treated organic material derived from municipal solid waste (MSW). Front. Plant Sci. 2018, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yue, D.; Nie, Y. Characterization of humic substances in bio-treated municipal solid waste landfill leachate. Front. Environ. Sci. Eng. 2012, 6, 711–716. [Google Scholar] [CrossRef]
- Xiaoli, C.; Guixiang, L.; Xin, Z.; Yongxia, H.; Youcai, Z. Fluorescence excitation–emission matrix combined with regional integration analysis to characterize the composition and transformation of humic and fulvic acids from landfill at different stabilization stages. Waste Manag. 2012, 32, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Orliński, T.; Anielak, A.M. Characteristics of fulvic acids generated in communes waste landfills. Arch. Environ. Prot. 2021, 47, 41–52. [Google Scholar]
- Gu, N.; Liu, J.; Ye, J.; Chang, N.; Li, Y.Y. Bioenergy, ammonia and humic substances recovery from municipal solid waste leachate: A review and process integration. Bioresour. Technol. 2019, 293, 122159. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Li, X.; Lin, J.; Liao, Y.; Jin, Z. Recovery of humic substances from leachate nanofiltration concentrate by a two-stage process of tight ultrafiltration membrane. J. Clean. Prod. 2017, 161, 84–94. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Jiang, M.; Lin, J.; Ye, K.; Fang, S.; He, Z. Sustainable management of landfill leachate concentrate through recovering humic substance as liquid fertilizer by loose nanofiltration. Water Res. 2019, 157, 555–563. [Google Scholar] [CrossRef]
- Kulikowska, D.; Bernat, K.; Wojnowska-Baryła, I.; Klik, B.; Michałowska, S.; Kasiński, S. Stabilizate from autoclaved municipal solid waste as a source of valuable humic substances in a waste circular economy. Waste Biomass Valorization 2020, 11, 6147–6157. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.R.; Akova, V.I.; Artinova, N.S.; Ivanov, K.I. The effect of organic amendments on soil chemical characteristics. Bulg. J. Agric. Sci. 2013, 19, 958–971. [Google Scholar]
- Ayuso, M.; Hernández, T.; García, C.; Pascual, J.A. A comparative study of the effect on barley growth of humic substances extracted from municipal wastes and from traditional organic materials. J. Sci. Food Agric. 1996, 72, 493–500. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jin, Y.; Zou, S.; Li, C. Recovery of sludge humic acids with alkaline pretreatment and its impact on subsequent anaerobic digestion. J. Chem. Technol. Biotechnol. 2014, 89, 707–713. [Google Scholar] [CrossRef]
- Cristina, G.; Camelin, E.; Ottone, C.; Garofalo, S.F.; Jorquera, L.; Castro, M.; Tommasi, T. Recovery of humic acids from anaerobic sewage sludge: Extraction, characterization and encapsulation in alginate beads. Int. J. Biol. Macromol. 2020, 164, 277–285. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zou, S.; Li, C. Extracting humic acids from digested sludge by alkaline treatment and ultrafiltration. J. Mater. Cycles Waste Manag. 2014, 16, 93–100. [Google Scholar] [CrossRef]
- Chen, Y.O.N.A.; Clapp, C.E.; Magen, H. Mechanisms of plant growth stimulation by humic substances: The role of organo-iron complexes. Soil Sci. Plant Nutr. 2004, 50, 1089–1095. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.R.; Melo, C.A.; Ferreira, O.P.; Moreira, A.B.; Mounier, S.; Piccolo, A.; Spaccini, R.; Bisinoti, M.C. Humic extracts of hydrochar and Amazonian Dark Earth: Molecular characteristics and effects on maize seed germination. Sci. Total Environ. 2020, 708, 135000. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Yue, W. Exogenous jasmonic acid and humic acid increased salinity tolerance of sorghum. Agron. J. 2020, 112, 871–884. [Google Scholar] [CrossRef]
- Anielak, A.M.; Świderska, R. The influence of the adsorbents’ electrokinetic potential on the adsorption process of humic substances. Environ. Prot. Eng. 2001, 27, 23–34. [Google Scholar]
- Tsinghua University. Method for Extracting Humic Acid Substances from Methane Fluid. CN102146101, 10 August 2011. [Google Scholar]
- Gyo, H. Extacting Method for Humic Acid and Fulvic Acid from Humic Substances. KR20120108816, 5 October 2012. [Google Scholar]
- Dong, S.H. Manufacturing Method of Liqufied Activated Humic Substances. KR20040008104, 28 January 2004. [Google Scholar]
- Qingdao Laoxiang Tea Product, Mountain tea tree biological fertilizer. CN103708941, 9 April 2014.
- Bowen, Y. Preparation method of antibacterial and corrosion-free type air conditioner cleaning agent. CN106753829, 31 May 2017. [Google Scholar]
- Gyo, H. Bacterial Enrichment Method of Using Bacterial Enrichment Composition Comprising Humic Acid. KR20120108795, 5 October 2012. [Google Scholar]
- Depuracque. Process for recovering humic substances from percolate originating from urban solid refuse dumps or the like, and humic substance obtained by the process. IT2012VE0013, 11 October 2013. [Google Scholar]
- Depuraque. Process for Recovering Humic Substances from Percolate Originating from Urban Solid Refuse Dumps or the Like and Humic Substances Obtained by Said Process. RO-130328, 30 June 2015. [Google Scholar]
- Luo, I. Sewage Factory Sludge Produced Organic Acid Fertilizer and its Product. CN1966474, 23 May 2007. [Google Scholar]
- Gyo, H. Bacterial Reduction Compostion Comprising Fulvic Acid. KR20120108820, 5 October 2012. [Google Scholar]
- Beijing Goldenway Bio Tech. Biochemical Humic Acid Product Prepared from Kitchen Waste and the Method of Preparing the Same. CN101941851, 12 January 2011. [Google Scholar]
- Xingyi, F. Hua Humic Acid. CN104072378, 1 October 2014. [Google Scholar]
- Xi An Lanxin Organic Fertilizer. High-Activity Composite Humic Acid and Preparation Process Thereof. CN106396762, 15 February 2017. [Google Scholar]
- Oktaba, W. Elementy Statystyki Matematycznej i Metodyka Doświadczalnictwa, 4th ed.; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1976. [Google Scholar]
- Anielak, A.M.; Kłeczek, A. Humic acids in the digested sludge and their properties. Materials 2022, 15, 1475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).