Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Diagnostics
2.3. Methods
3. Results
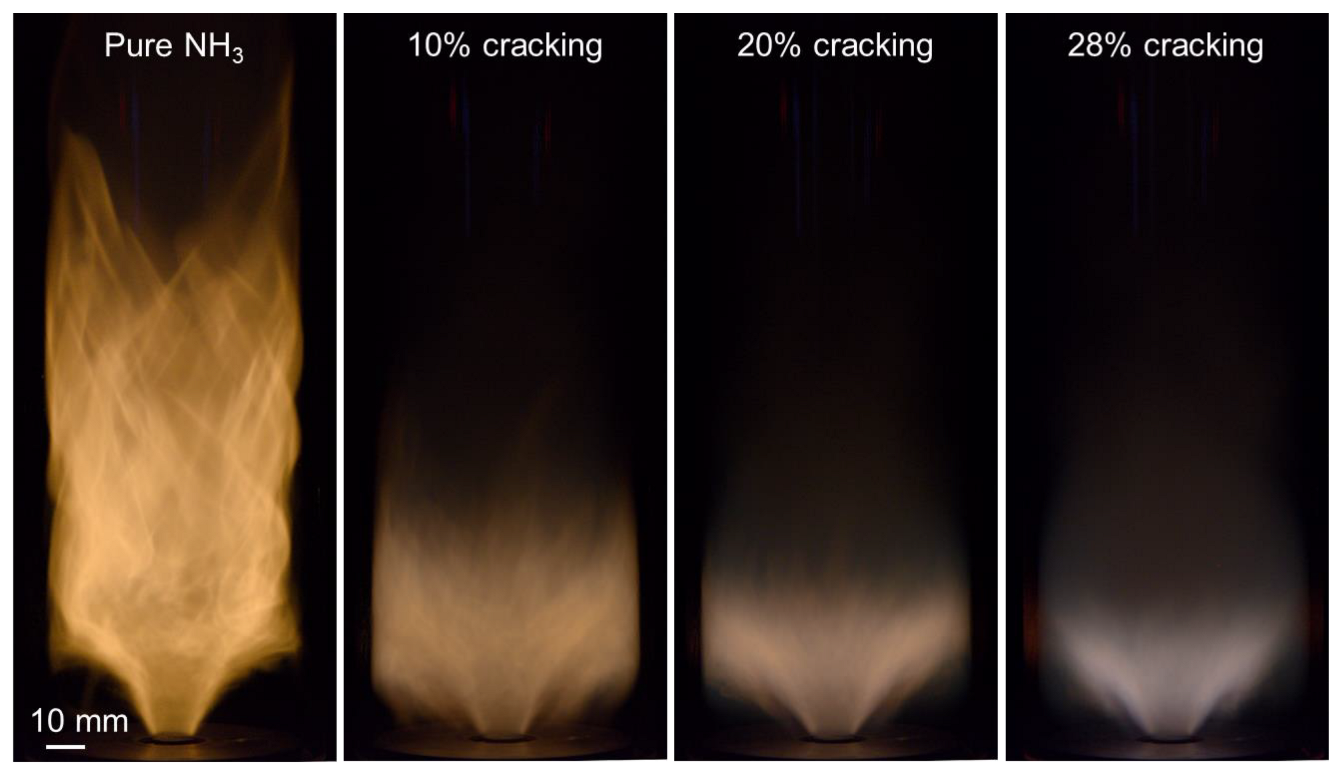
3.1. Effect of Ammonia Cracking on the Overall Burning Velocity
3.2. Lean Blow-Off Limits
3.3. NO and NO2 Emissions
3.4. Flame Transfer Functions
4. Discussion
4.1. Stabilization Mechanisms
4.2. Flame Dynamics
5. Conclusions
- For a swirl flame of about 3.4 kW, with an equivalence ratio of 0.95 and a bulk flow velocity of 7 m/s, cracking of ammonia in the range of 0–28% in mass linearly increased the overall burning velocity.
- For pure ammonia and ammonia at 10% cracking, the equivalence ratio at blow-off did not follow a monotonic trend when the bulk flow velocity was increased from 4 to 13 m/s. A step at about 8 m/s was observed, corresponding to a change in the stabilization mechanism.
- While cracking of ammonia was very efficient in enhancing the lean stability limit, the concentration of NOx in the burned gases was increased by about a factor of two for 28% cracking. In parallel, the response of the flame to acoustic modulation was also enhanced for a large range of frequencies, making these flames more prone to thermoacoustic problems than pure ammonia flames.
- The strong response of ammonia–hydrogen–nitrogen–air flames to acoustic perturbation of the incoming flow can be explained by the strong fluctuation of the top of the flame, i.e., the flame vortex roll-up, which can be explained by the strong resistance of hydrogen flames to strain rate.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability Limits and NO Emissions of Technically-Premixed Ammonia-Hydrogen-Nitrogen-Air Swirl Flames. Int. J. Hydrog. Energy 2020, 45, 22008–22018. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Z.; Wang, D.; Hou, R.; Zhang, T.; Wang, S. Experimental and Numerical Study on Premixed Partially Dissociated Ammonia Mixtures. Part I: Laminar Burning Velocity of NH3/H2/N2/Air Mixtures. Int. J. Hydrog. Energy 2022, 47, 4171–4184. [Google Scholar] [CrossRef]
- Kang, L.; Pan, W.; Zhang, J.; Wang, W.; Tang, C. A Review on Ammonia Blends Combustion for Industrial Applications. Fuel 2023, 332, 126150. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the Recent Advances on Ammonia Combustion from the Fundamentals to the Applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia Combustion Properties and Performance in Gas-Turbine Burners. Symp. Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Wang, G.; Boyette, W.R.; Younes, M.; Jamal, A.; Roberts, W.L. Stability Limits and NO Emissions of Premixed Swirl Ammonia-Air Flames Enriched with Hydrogen or Methane at Elevated Pressures. Int. J. Hydrog. Energy 2021, 46, 11969–11981. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, J.; Shi, X.; Xi, Z.; Li, Y. Enhancement of Ammonia Combustion with Partial Fuel Cracking Strategy: Laminar Flame Propagation and Kinetic Modeling Investigation of NH3/H2/N2/Air Mixtures up to 10 Atm. Combust. Flame 2021, 231, 111472. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, C.; Wang, D.; Hou, R.; Zhang, T.; Wang, S. Experimental and Numerical Study on Premixed Partially Dissociated Ammonia Mixtures. Part II: Numerical Study of Premixed Combustion Characteristics. Fuel 2021, 306, 121660. [Google Scholar] [CrossRef]
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.D.K.A.; Kudo, T.; Kobayashi, H. Experimental Investigation of Stabilization and Emission Characteristics of Ammonia/Air Premixed Flames in a Swirl Combustor. Int. J. Hydrog. Energy 2017, 42, 14010–14018. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Pugh, D.G.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary Study on Lean Premixed Combustion of Ammonia-Hydrogen for Swirling Gas Turbine Combustors. Int. J. Hydrog. Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed Ammonia/Hydrogen Swirl Combustion under Rich Fuel Conditions for Gas Turbines Operation. Int. J. Hydrog. Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Lamioni, R.; Bronzoni, C.; Folli, M.; Tognotti, L.; Galletti, C. Feeding H2-Admixtures to Domestic Condensing Boilers: Numerical Simulations of Combustion and Pollutant Formation in Multi-Hole Burners. Appl. Energy 2022, 309, 118379. [Google Scholar] [CrossRef]
- Zhang, M.; An, Z.; Wang, L.; Wei, X.; Jianayihan, B.; Wang, J.; Huang, Z.; Tan, H. The Regulation Effect of Methane and Hydrogen on the Emission Characteristics of Ammonia/Air Combustion in a Model Combustor. Int. J. Hydrog. Energy 2021, 46, 21013–21025. [Google Scholar] [CrossRef]
- Mashruk, S.; Kovaleva, M.; Alnasif, A.; Chong, C.T.; Hayakawa, A.; Okafor, E.C.; Valera-Medina, A. Nitrogen Oxide Emissions Analyses in Ammonia/Hydrogen/Air Premixed Swirling Flames. Energy 2022, 260, 125183. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the Development of an Efficient Low-NOx Ammonia Combustor for a Micro Gas Turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and Other Emissions in Micro Gas Turbine Combustors Fuelled with Mixtures of Methane and Ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Okafor, E.C.; Nagano, Y.; Kitagawa, T. Experimental and Theoretical Analysis of Cellular Instability in Lean H2-CH4-Air Flames at Elevated Pressures. Int. J. Hydrog. Energy 2016, 41, 6581–6592. [Google Scholar] [CrossRef]
- Dinesh, K.K.J.R.; Shalaby, H.; Luo, K.H.; van Oijen, J.A.; Thévenin, D. High Hydrogen Content Syngas Fuel Burning in Lean Premixed Spherical Flames at Elevated Pressures: Effects of Preferential Diffusion. Int. J. Hydrog. Energy 2016, 41, 18231–18249. [Google Scholar] [CrossRef]
- Lapenna, P.E.; Berger, L.; Attili, A.; Lamioni, R.; Fogla, N.; Pitsch, H.; Creta, F. Data-Driven Subfilter Modelling of Thermo-Diffusively Unstable Hydrogen–Air Premixed Flames. Combust. Theory Model 2021, 25, 1064–1085. [Google Scholar] [CrossRef]
- Candel, S. Combustion Dynamics and Control: Progress and Challenges. Proc. Combust. Inst. 2002, 29, 1–28. [Google Scholar] [CrossRef]
- Lieuwen, T.C.; Yang, V. Combustion Instabilities in Gas Turbine Engines; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2006; Volume 210, ISBN 978-1-56347-669-3. [Google Scholar]
- Palies, P.; Durox, D.; Schuller, T.; Candel, S. The Combined Dynamics of Swirler and Turbulent Premixed Swirling Flames. Combust. Flame 2010, 157, 1698–1717. [Google Scholar] [CrossRef]
- Palies, P.; Durox, D.; Schuller, T.; Candel, S. Experimental Study on the Effect of Swirler Geometry and Swirl Number on Flame Describing Functions. Combust. Sci. Tech. 2011, 183, 704–717. [Google Scholar] [CrossRef]
- Poinsot, T. Prediction and Control of Combustion Instabilities in Real Engines. Proc. Combust. Inst. 2017, 36, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Di Sabatino, F.; Guiberti, T.F.; Boyette, W.R.; Roberts, W.L.; Moeck, J.P.; Lacoste, D.A. Effect of Pressure on the Transfer Functions of Premixed Methane and Propane Swirl Flames. Combust. Flame 2018, 193, 272–282. [Google Scholar] [CrossRef]
- Di Sabatino, F.; Guiberti, T.F.; Moeck, J.P.; Roberts, W.L.; Lacoste, D.A. Fuel and Equivalence Ratio Effects on Transfer Functions of Premixed Swirl Flames. J. Propuls. Power 2020, 36, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, S.; Gruber, A.; Dawson, J. Flame Transfer Functions for Turbulent, Premixed, Ammonia-Hydrogen-Nitrogen-Air Flames. J. Eng. Gas Turbines Power 2022, 145, 031015. [Google Scholar] [CrossRef]
- Lacoste, D.A.; Moeck, J.P.; Durox, D.; Laux, C.O.; Schuller, T. Effect of Nanosecond Repetitively Pulsed Discharges on the Dynamics of a Swirl-Stabilized Lean Premixed Flame. J. Eng. Gas Turbines Power 2013, 135, 101501. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; Ballal, D.R. Gas Turbine Combustion; CRC Press: Amsterdam, The Netherlands, 2010; ISBN 9781420086058. [Google Scholar]
- Price, R.B.; Hurle, I.R.; Sugden, T.M. Optical Studies of the Generation of Noise in Turbulent Flames. Symp. Combust. 1969, 12, 1093–1102. [Google Scholar] [CrossRef]
- Hurle, I.R.; Price, R.B.; Sugden, T.M.; Thomas, A. Sound Emission from Open Turbulent Premixed Flames. Proc. R. Soc. Lond. A 1968, 303, 409–427. [Google Scholar] [CrossRef]
- Baukal, C.E.; Eleazer, P.B. Quantifying NOx for Industrial Combustion Processes. J. Air Waste Manag. Assoc. 1998, 48, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, J.; Sun, W.; Ombrello, T.; Carter, C. Plasma Assisted Ammonia Combustion: Simultaneous NOx Reduction and Flame Enhancement. Combust. Flame 2021, 228, 430–432. [Google Scholar] [CrossRef]
- Skiba, A.W.; Guiberti, T.F.; Boyette, W.R.; Roberts, W.L.; Mastorakos, E. On the Bi-Stable Nature of Turbulent Premixed Bluff-Body Stabilized Flames at Elevated Pressure and near Lean Blow-Off. Proc. Combust. Inst. 2021, 38, 2853–2860. [Google Scholar] [CrossRef]
- Kang, D.M.; Culick, F.E.C.; Ratner, A. Combustion Dynamics of a Low-Swirl Combustor. Combust. Flame 2007, 151, 412–425. [Google Scholar] [CrossRef]
- Külsheimer, C.; Büchner, H. Combustion Dynamics of Turbulent Swirling Flames. Combust. Flame 2002, 131, 70–84. [Google Scholar] [CrossRef]
- Bellows, B.D.; Bobba, M.K.; Forte, A.; Seitzman, J.M.; Lieuwen, T. Flame Transfer Function Saturation Mechanisms in a Swirl-Stabilized Combustor. Proc. Combust. Inst. 2007, 31, 3181–3188. [Google Scholar] [CrossRef]
- Ren, J.Y.; Qin, W.; Egolfopoulos, F.N.; Tsotsis, T.T. Strain-Rate Effects on Hydrogen-Enhanced Lean Premixed Combustion. Combust. Flame 2001, 124, 717–720. [Google Scholar] [CrossRef]









| Condition | Ammonia (SLPM) | Hydrogen (SLPM) | Nitrogen (SLPM) | Air (SLPM) |
|---|---|---|---|---|
| Pure NH3 | 15.53 | 0 | 0 | 58.36 |
| 10% cracking | 13.69 | 2.28 | 0.76 | 57.15 |
| 20% cracking | 11.92 | 4.47 | 1.49 | 56.01 |
| 28% cracking | 10.56 | 6.16 | 2.05 | 55.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shohdy, N.N.; Alicherif, M.; Lacoste, D.A. Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames. Energies 2023, 16, 1323. https://doi.org/10.3390/en16031323
Shohdy NN, Alicherif M, Lacoste DA. Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames. Energies. 2023; 16(3):1323. https://doi.org/10.3390/en16031323
Chicago/Turabian StyleShohdy, Nader N., Mhedine Alicherif, and Deanna A. Lacoste. 2023. "Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames" Energies 16, no. 3: 1323. https://doi.org/10.3390/en16031323
APA StyleShohdy, N. N., Alicherif, M., & Lacoste, D. A. (2023). Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames. Energies, 16(3), 1323. https://doi.org/10.3390/en16031323







