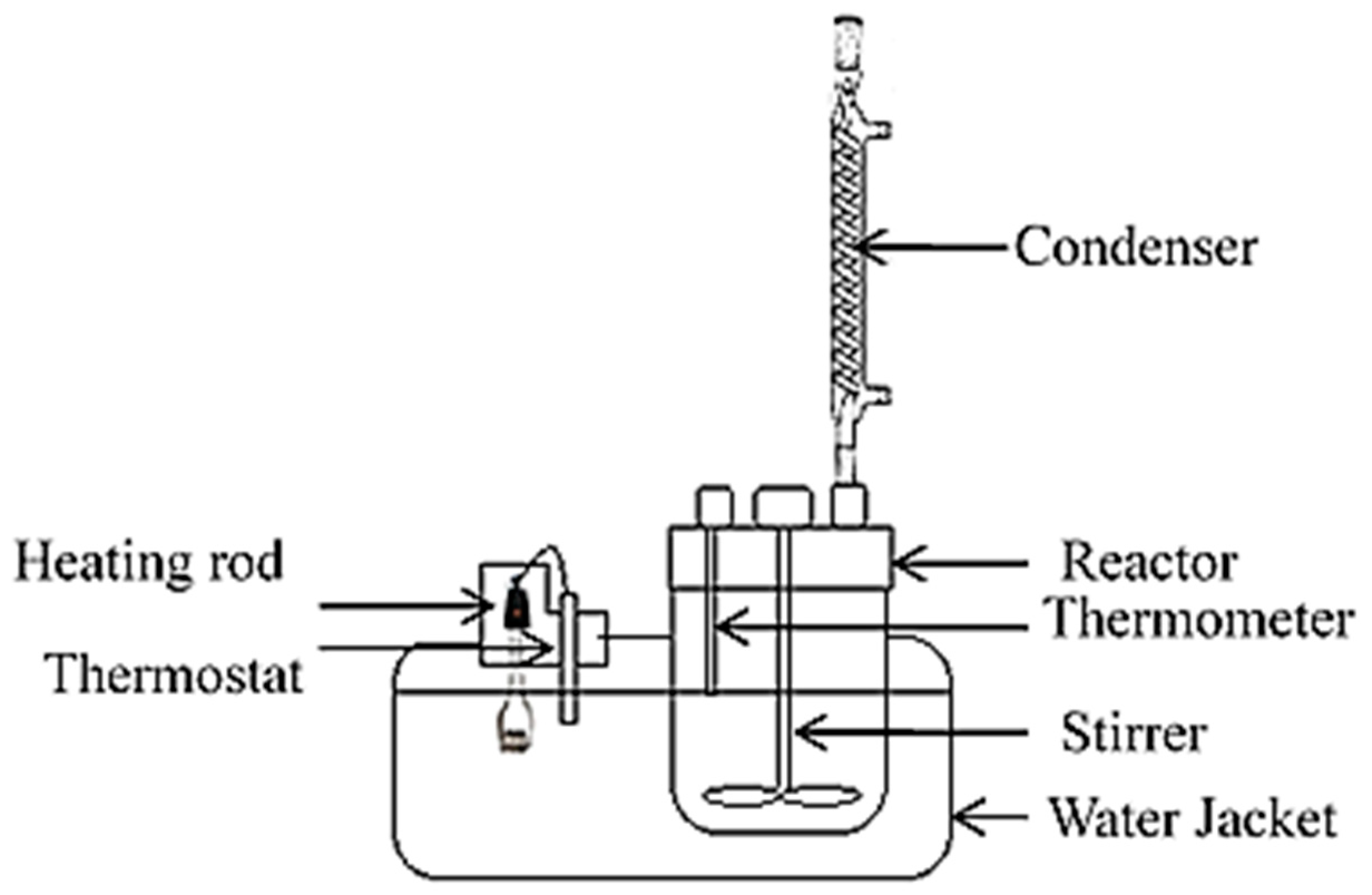
1. Introduction
Industrialization, growing populations, and global economic expansion all contribute to the rapid increase in fossil fuel usage. Alternative energies, including solar, wind, geothermal, and biomass, are available and are being considered [
1,
2,
3,
4]. Hydrocarbon fuel is the primary source of energy for the world economy. Diesel will certainly account for roughly 60% of the energy increase in 2035, which will be over 80% of the entire energy supply. In future years, it is anticipated that the energy landscape will continue to improve [
5,
6,
7,
8]. Rapid depletion of fossil fuels and pollution as a result of the Paris Climate Change Agreement have prompted scientists and researchers to develop green alternative fuels capable of meeting the energy demand in a sustainable manner. In the past few decades, oxygenated liquid fuels have shown an acceptable performance, as well as preventing pollution in the environment [
9]. There are various oxygenated fuels such as alcohols, biodiesel, and bio-oil that are given more emphasis nowadays due to their promising results [
10,
11]. Among the alternatives, biodiesel has been shown to be a good substitute for petrochemical diesel in compression ignition engines [
12]. The primary advantages of biodiesel are that it is recyclable, reusable, carbon neutral, and it emits no dangerous toxins [
13]. Biodiesel is an oxygenated fuel. Triglycerides react with low-molecular alcohols (often methanol) in the presence of a catalyst to produce alkyl ester and glycerol as byproducts, known as transesterification [
14].
The chemical constituents of biofuel, which include saturated and unsaturated fatty acids, have a significant impact on the fuel properties [
15]. High-saturated fatty acid fuels have a strong oxidation stability but poor cold flow qualities, whereas high-unsaturated fatty acid fuels with double or triple carbon–carbon bonds have acceptable cold flow capabilities, but poor oxidation stability [
16,
17]. Oxidation stability refers to the oxidation-resisting tendency of a fuel that occurs during storage. The occurrence of oxidation in biodiesel is caused by the free-radical chain reaction mechanism. The process has three phases in an environment of heat, sunlight, residual metals, and hyper-oxides: activation, spread, and closure. The degree of oxidation varies according to the fatty acid composition. Poor oxidative stability results in gum formation and re-polymerization during storage.
The impact of biofuel on gasoline engine efficiency and emissions has been examined by various researchers by integrating various engine modifications and fuel blends. A substantial reduction in unburnt hydrocarbons (UHC), carbon monoxide (CO), and particulate matter (PM) was reported using biodiesel in IC engines [
18,
19]. Because of the presence of oxygen inside gasoline, the combustion process generates high temperatures, resulting in a modest rise in nitrogen oxides (NOx) when using biodiesel in comparison with conventional diesel fuel [
20,
21,
22].
The differences in the physicochemical properties of biofuel derived from different feedstocks have resulted in different trends in engine characteristics [
23]. Very few studies have reported the effect of oxidatively stabilized and non-stabilized biodiesel on engine performance and emissions. Poorly stabilized fuel results in the formation of aldehydes, ketones, and more soluble polymers, which consequently increase the density, viscosity, and surface tension of the fuel, thus decreasing the calorific value of the fuel [
24]. Non-edible sources that can be obtained from non-agricultural (barren) lands are considered major sources of feedstock for biodiesel production. Jatropha curcas is the most common and promising feedstock to meet the biodiesel demand in India due to because of its abundance [
25]. Liu et al. [
26] reported that, upon increasing the oxidation duration of jatropha biodiesel, CO
2 and NOx emissions increased rapidly in a diesel engine. Fattah et al. [
27] studied the effect of adding antioxidants to a 20% blend of jatropha biodiesel. The results reported that BSFC and BP decreased with the antioxidants when compared with pure 20% jatropha biodiesel. Ryu [
28] conducted a study on the influence of five different antioxidants in biodiesel on engine performance and revealed a drop in BSFC for the fuel containing antioxidants compared with the biodiesel without antioxidants.
Jain et al. [
29] studied the efficiency and exhaust emissions of oxidatively stabilized jatropha biodiesel. Antioxidants were introduced into the jatropha biodiesel. The study reported an increase in BSFC with and without antioxidants as time passed, but less of an increment was found in the case where antioxidants were present. The reason for this is that stabilized biodiesel has less BSFC compared with non-stabilized biodiesel. CO, HC, and NOx decreased with time. Lower traces of metals in the particulate emissions and a decrease in gaseous emissions, except for NOx, were recorded when using jatropha biodiesel in a four-cylinder DI diesel engine [
30]. Rao et al. [
31] treated jatropha biodiesel with Al(OH)
2 nanoparticles and water and found a significant reduction in gaseous emissions, such as NO, CO, UHC, and others. The addition of nanoparticles increased the storage stability, but the agglomeration of particles occurred, which deteriorated the quality of the fuel [
32]. Thus, nanoparticles cannot be used as antioxidants.
From the literature review, it can be found that poor oxidation stability deteriorates the quality of biodiesel. Long-term oxidation results in a poor engine performance and in emissions. Various studies have previously reported that adding antioxidants to biodiesel increased its oxidation stability. However, the use of antioxidants increased the production cost of biodiesel. Jatropha biodiesel consists of 80% unsaturated fatty acids and 20% saturated fatty acids. Sui et al. [
33] reported that in jatropha biodiesel, methyl linoleate (42.21%) is the major unsaturated fatty acid responsible for oxidation. To handle this problem, Jatropha curcas oil was blended with algal oil and the blend was then subjected to low temperature transesterification, and this method was proposed as it is a successful method for handling the high unsaturation and high FFA of Jatropha curcas oil in order to make biodiesel [
7]. In view of this, in the present work, we proposed reducing the unsaturation of Jatropha curcas fats with the addition of highly saturated algae fats. Spirogyra algae have been proven to be a potential third-generation feedstock for biodiesel production. The algae consist of 41% saturated and 59% unsaturated fatty acids.
This study examined the in situ transesterification of Jatropha curcas. It is proposed We combined the dried seed powder of Jatropha curcas with dried Spirogyra algae powder to produce a stabilized biodiesel. As the stability of the algae biodiesel was higher than that of the Jatropha biodiesel, the resulting biodiesel had a higher oxidation stability compared with the pure Jatropha curcas biodiesel. The resulting highly stabilized biodiesel was used in the engine to check the performance and emission characteristics.
5. Conclusions
This study focused on the single-step in situ transesterification of dry spirogyra algae and Jatropha powder, and the engine efficacy and emission analysis of that biofuel prepared using a homogeneous base catalyst. Three operational parameters, including catalyst concentration (0–5 wt.%), methanol to dry algae–Jatropha curcas powder (v/v) (20–60%), and reaction time (60–180 min) at a constant reaction temperature of 50 °C were selected. The maximum biodiesel yield of 88.5% was obtained under the optimized conditions of a catalyst concentration of 3.396% (w/w), methanol/oil ratio of 19.86, and reaction time of 180 min. From the engine tests, the following conclusions were drawn:
It was noted that the BSFC for all of the test blends decreased as the load increased. All of the biodiesel blends reported a higher BSFC compared with diesel fuel. The reason behind this may be that, because of the lower energy content of biodiesel blends per unit mass compared with diesel, more biodiesel fuel is consumed to generate the same power as in the case of diesel.
B5 reported the highest BTE among all of the tested blends, whereas B20 reported the lowest BTE compared with other fuels. This can be accounted for due to an increase in the kinematic viscosity of the fuel with an increase in the blend percentage, which tends to result in poorer atomization and a proper air−fuel mixture, resulting in a poorer combustion process and reduced thermal efficiency.
It can be shown that HC emissions decrease as the load conditions increase. In the case of biodiesel blends, the highest HC emissions were recorded by B5, whereas B20 recorded the lowest amount of HC emissions. This implies that the use of biodiesel significantly reduces HC emissions. This occurs due to the presence of excess oxygen, which maintains adequate oxidation.
All of the biodiesel blends reported lower CO emissions than for diesel fuel at all of the engine loads. At full load, the least amount of emissions was recorded. CO emissions decreased with the increase in the biodiesel blend percentage. As explained earlier, this can be attributed to the higher oxygen content of the blends and proper combustion when compared with diesel.
According to the findings of this investigation, the NOx emissions of biodiesel blends were greater than those of diesel under all load circumstances. B20 emitted more NOx emissions than other blends due to an increase in oxygen concentration as the biodiesel percentage increased.
From the above, it is found that this new approach of making biodiesel will be useful as Jatropha biodiesel can be used to produce biodiesel with algae. On the other hand, the engine performance and emissions are also comparable with diesel.