Bio Oil as Cutter Stock in Fuel Oil Blends for Industrial Applications
Abstract
:1. Introduction
2. Equipment and Methodology
2.1. Sample and Characterization of Recycled Polystyrene
2.2. Pyrolysis Reactor
2.2.1. Pyrolysis Process and Operating Conditions
2.2.2. Characterization of Pyrolysis Products
2.2.3. Preparation and Characterization of Mixtures with Fuel-Oil
3. Results and Discussion
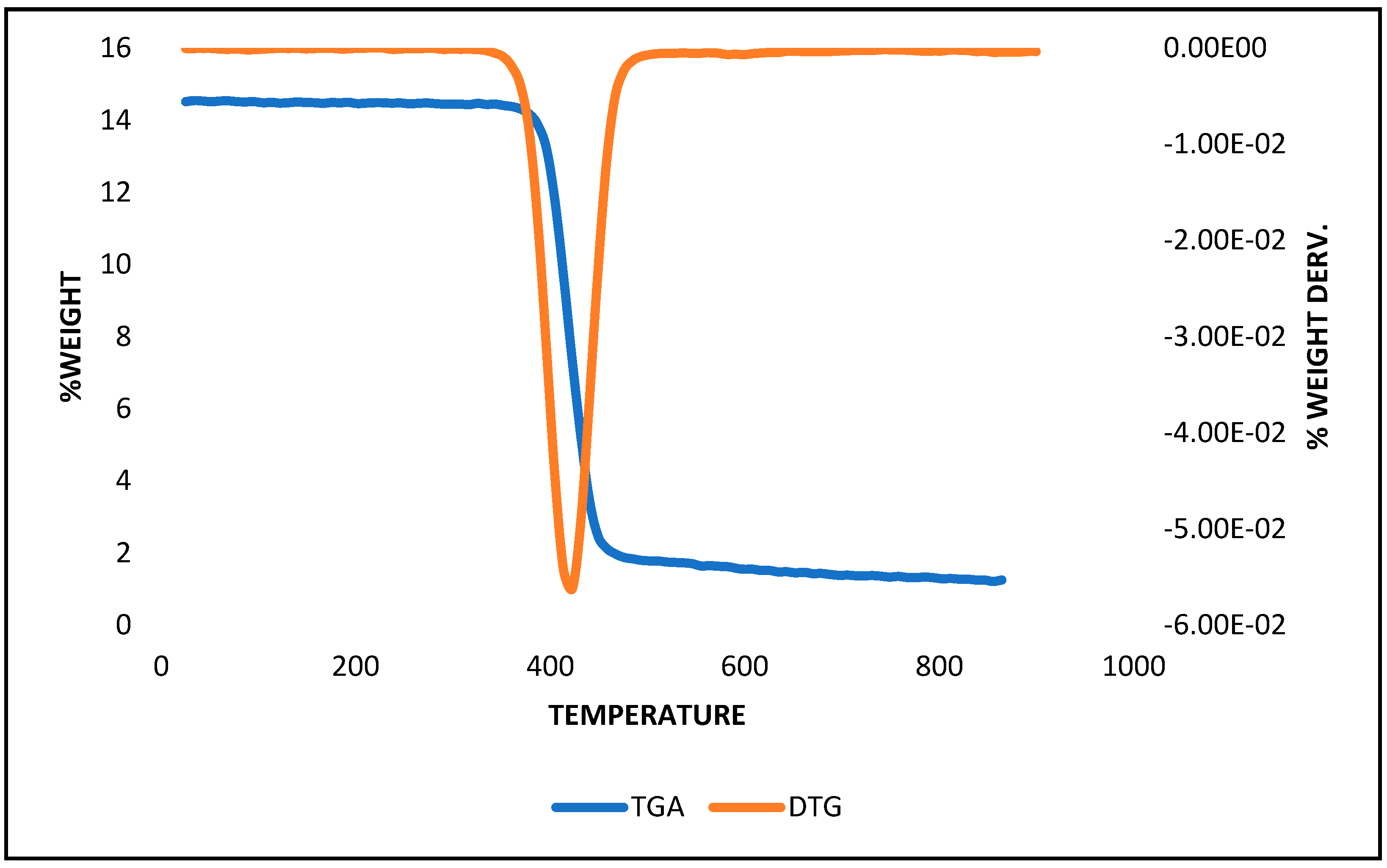
3.1. Characterization of the Recycled Polystyrene
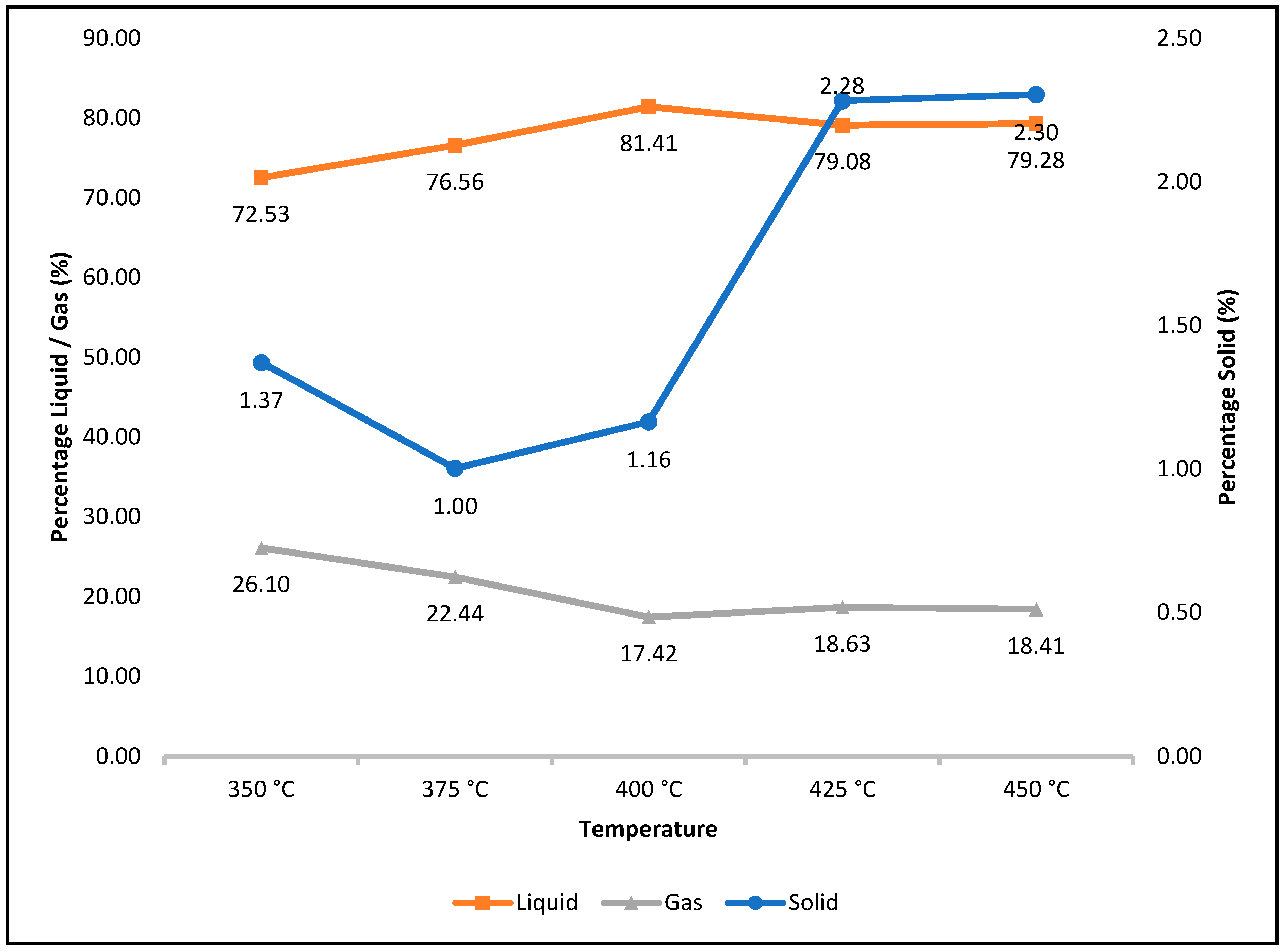
3.2. Process Performance
3.3. Bio-Oil Characteristics
3.4. Bio-Oil/Fuel Oil Mixtures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Compa, M.; Alomar, C.; Wilcox, C.; van Sebille, E.; Lebreton, L.; Hardesty, B.D.; Deudero, S. Risk assessment of plastic pollution on marine diversity in the Mediterranean Sea. Sci. Total Environ. 2019, 678, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ouda, O.K.M.; Raza, S.A.; Nizami, A.S.; Rehan, M.; Al-Waked, R.; Korres, N.E. Waste to energy potential: A case study of Saudi Arabia. Renew. Sustain. Energy Rev. 2016, 61, 328–340. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Rehan, M.; Aburiazaiza, A.; Ismail, I.; Nizami, A. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S.; Shetti, N.P.; Kamali, M.; Walvekar, P.; Aminabhavi, T.M. Waste-to-energy nexus: A sustainable development. Environ. Pollut. 2020, 267, 115501. [Google Scholar] [CrossRef]
- Lee, D.-J.; Lu, J.-S.; Chang, J.-S. Pyrolysis synergy of municipal solid waste (MSW): A review. Bioresour. Technol. 2020, 318, 123912. [Google Scholar] [CrossRef]
- Rathore, D.; Nizami, A.-S.; Singh, A.; Pant, D. Key issues in estimating energy and greenhouse gas savings of biofuels: Challenges and perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
- Colantonio, S.; Cafiero, L.; De Angelis, D.; Ippolito, N.M.; Tuffi, R.; Ciprioti, S.V. Thermal and catalytic pyrolysis of a synthetic mixture representative of packaging plastics residue. Front. Chem. Sci. Eng. 2019, 14, 288–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln. Fuel Process. Technol. 2020, 206, 106455. [Google Scholar] [CrossRef]
- Athapaththu, A.; Thushari, G.; Dias, P.; Abeygunawardena, A.; Egodauyana, K.; Liyanage, N.; Pitawala, H.; Senevirathna, J. Plastics in surface water of southern coastal belt of Sri Lanka (Northern Indian Ocean): Distribution and characterization by FTIR. Mar. Pollut. Bull. 2020, 161, 111750. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martín-Lara, M.Á.; Pérez, A.; Blázquez, G. Production of an Alternative Fuel by Pyrolysis of Plastic Wastes Mixtures. Energy Fuels 2020, 34, 1781–1790. [Google Scholar] [CrossRef]
- Veses, A.; Martínez, J.D.; Callén, M.S.; Murillo, R.; García, T. Application of Upgraded Drop-In Fuel Obtained from Biomass Pyrolysis in a Spark Ignition Engine. Energies 2020, 13, 2089. [Google Scholar] [CrossRef]
- Nisar, J.; Ali, G.; Shah, A.; Iqbal, M.; Khan, R.A.; Sirajuddin; Anwar, F.; Ullah, R.; Akhter, M.S. Fuel production from waste polystyrene via pyrolysis: Kinetics and products distribution. Waste Manag. 2019, 88, 236–247. [Google Scholar] [CrossRef]
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Pyrolysis and in-line catalytic steam reforming of polystyrene through a two-step reaction system. J. Anal. Appl. Pyrolysis 2016, 122, 502–510. [Google Scholar] [CrossRef]
- Park, K.-B.; Jeong, Y.-S.; Guzelciftci, B.; Kim, J.-S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Mohanty, K. Co-pyrolysis of Karanja and Niger seeds with waste polystyrene to produce liquid fuel. Fuel 2015, 153, 492–498. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Barbarias, I.; Arregi, A.; Aguado, R.; Bilbao, J.; Olazar, M. Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor. Waste Manag. 2015, 45, 126–133. [Google Scholar] [CrossRef]
- Baena-González, J.; Santamaria-Echart, A.; Aguirre, J.L.; González, S. Chemical recycling of plastic waste: Bitumen, solvents, and polystyrene from pyrolysis oil. Waste Manag. 2020, 118, 139–149. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.; Nizami, A. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2016, 119, 239–252. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Quesada, L.; Pérez, A.; Godoy, V.; Peula, F.; Calero, M.; Blázquez, G. Optimization of the pyrolysis process of a plastic waste to obtain a liquid fuel using different mathematical models. Energy Convers. Manag. 2019, 188, 19–26. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A.; Rehan, M.; Barakat, M.; Khan, M.; Mustafa, A.; Ismail, I.; Murphy, J. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, Y.; Li, W.; Shi, Y.; Jin, L.; Zhang, Z.; Wang, G. Free radical reaction model for n -pentane pyrolysis. Chin. J. Chem. Eng. 2018, 26, 514–520. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, L.; Wang, Z.; Chen, C.-L.; Tan, G.-Y.A.; Wang, J.-Y. Enhanced styrene recovery from waste polystyrene pyrolysis using response surface methodology coupled with Box–Behnken design. Waste Manag. 2014, 34, 763–769. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Investigation of the Effect of Polystyrene (PS) Waste Washing Process and Pyrolysis Temperature on (PS) Pyrolysis Product Quality. Energy Procedia 2017, 118, 189–194. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Pramanik, H. Pyrolysis of waste expanded polystyrene and reduction of styrene via in-situ multiphase pyrolysis of product oil for the production of fuel range hydrocarbons. Waste Manag. 2020, 120, 330–339. [Google Scholar] [CrossRef]
- Aljabri, N.M.; Lai, Z.; Hadjichristidis, N.; Huang, K.W. Renewable aromatics from the degradation of polysty-rene under mild conditions. J. Saudi Chem. Soc. 2017, 21, 983–989. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Thermal behavior of mixed plastics at different heating rates: I. pyrolysis kinetics. Polymers 2021, 13, 3413. [Google Scholar] [CrossRef]



| Parameter | Specification |
|---|---|
| Material | SUS304 stainless steel |
| Capacity | 5L |
| Working Temperature | 600 °C |
| Working pressure | −0.1 a 8 MPa |
| Heating | Electric heating rod of Power 3.5 kW |
| Mixing speed | 0–750 rpm |
| Motor power | 0.55 kW |
| Parameter | Specification |
|---|---|
| Temperature Range | 350–450 °C |
| Condensation Temperature | 10 °C |
| Mixing | 5 rpm |
| Heating Rate | 15 °C min−1 |
| Purge gas | Nitrogen |
| Waste plastic | PS compact |
| Test | Units | Standard | Results |
|---|---|---|---|
| Gravity API at 60 °F | °API | ASTM D-1298 | 19.800 |
| Relative density | - | ASTM D4052-18a | 0.935 |
| Sulphur | % W | ASTM D-4294-16e1 | 0.112 |
| Cinematic viscosity at 40 °C. | mm2/s | ASTM D445-19a | 1.030 |
| Water content | %V | ASTM D1796-11 | 0.500 |
| Distillation at 90% | °C | ASTM D86-20b | 325.0 |
| Flash point | °C | ASTM D56-16a | 24.1 |
| Cetane number | - | ASTM D4737-10 | 20.06 |
| Gross Heating value | MJ kg−1 | ASTM D240-19 | 42.663 |
| Test | Standard | Fuel-Oil + PS Bio-Oil | Fuel-Oil | |||||
|---|---|---|---|---|---|---|---|---|
| M10 | M20 | M40 | M50 | Min | Max | |||
| Flash Point (°C) | ASTM D56-16ª | 45 | 37 | <18 | <18 | 72 | 60 | - |
| Pour point (°C) | ASTM D97-17b | 12 | 8 | −3 | −5 | 11 | - | 12 |
| Kinematic viscosity at 50 °C (mm2 s−1) | ASTM D445-19ª | 108.4 | 38.6 | 8.8 | 5.4 | 415 | 400 | 510 |
| Water and sediment (%) | ASTM D1796-11 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | - | 0.5 |
| Ashes (%) | ASTM D482-19 | 0.060 | 0.056 | 0.047 | 0.036 | <0.1 | - | 0.1 |
| Sulphur (%W) | ASTM D-4294-16e1 | 1.324 | 1.176 | 0.896 | 0.764 | 1.1 | - | 1.5 |
| Equivalent Toluene (%) | - | 12.5 | 14.3 | 16.7 | 20.0 | 12 | NR | NR |
| Gross heating value (MJ kg−1) | ASTM D240-19 | 39.84 | 40.87 | 42.03 | 42.63 | 40.9 | 39 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmay, P.; Puente, C.; Haro, C.; Bruno, J.C.; Coronas, A. Bio Oil as Cutter Stock in Fuel Oil Blends for Industrial Applications. Energies 2023, 16, 1485. https://doi.org/10.3390/en16031485
Palmay P, Puente C, Haro C, Bruno JC, Coronas A. Bio Oil as Cutter Stock in Fuel Oil Blends for Industrial Applications. Energies. 2023; 16(3):1485. https://doi.org/10.3390/en16031485
Chicago/Turabian StylePalmay, Paul, Cesar Puente, Carla Haro, Joan Carles Bruno, and Alberto Coronas. 2023. "Bio Oil as Cutter Stock in Fuel Oil Blends for Industrial Applications" Energies 16, no. 3: 1485. https://doi.org/10.3390/en16031485





