Catalysts for ORR Based on Silver-Modified Graphene Oxide and Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Catalysts
2.3. Preparation of Catalytic Ink
2.4. Characteristics of Catalysts
2.5. Electrochemical Experiment
3. Results and Discussion
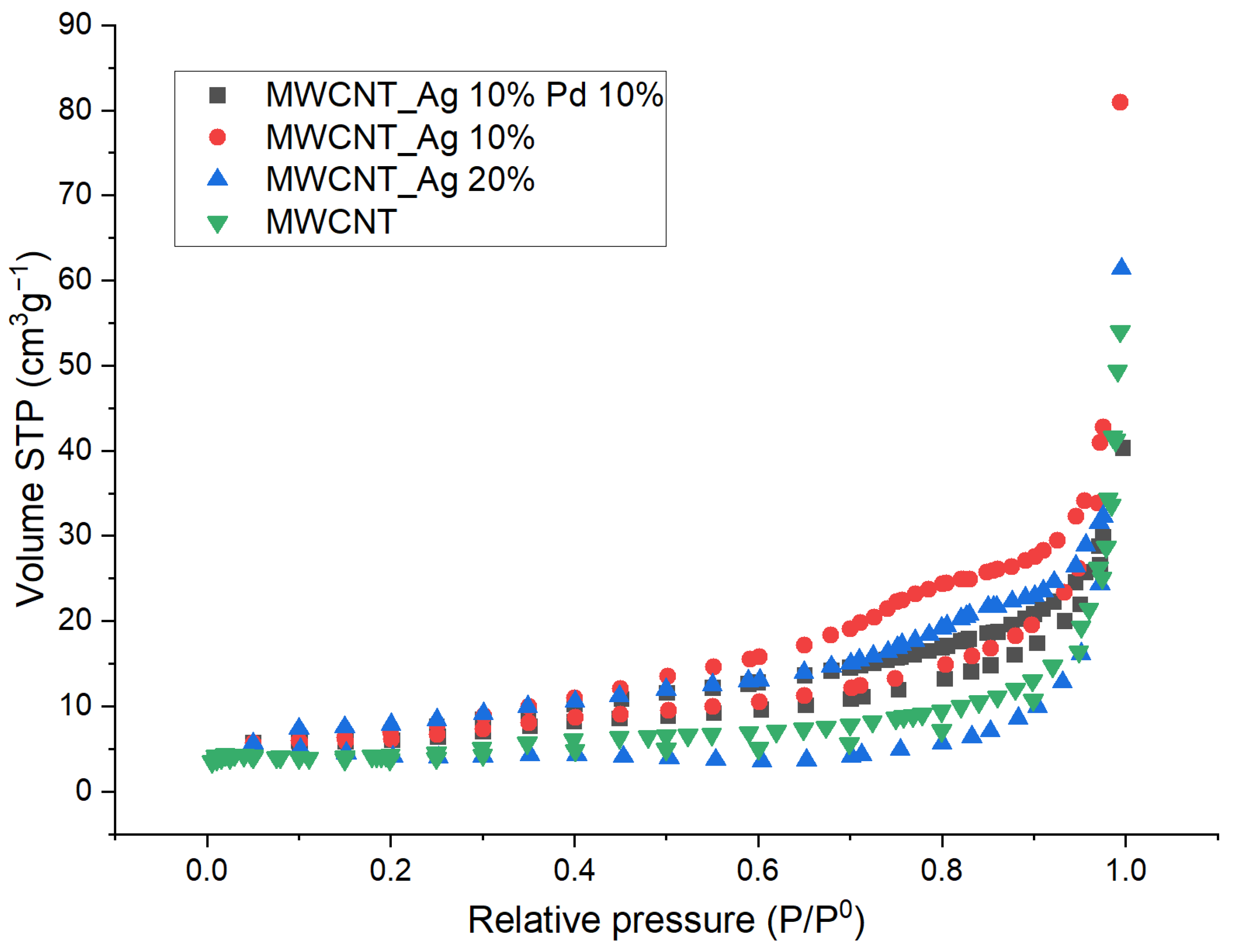
| Sample | Surface Area, SBET, m2/g | Pore Volume, VP, сm3/g | Pore Diameter, Dv(d),nm |
|---|---|---|---|
| MWCNT | 14.9 | 0.08 | 3.4 |
| GO_Ag 10% 1 | - | - | - |
| GO_Ag 20% 1 | - | - | - |
| MWCNT_Ag 10% | 21.8 | 0.12 | 3.8 |
| MWCNT_Ag 20% | 11.5 | 0.09 | 8.6 |
| MWCNT_Ag 10% Pd 10% | 20.8 | 0.05 | 3.4 |
- The presence of surface oxygen-containing functional groups;
- Specific surface area of the catalyst;
- Porosity.
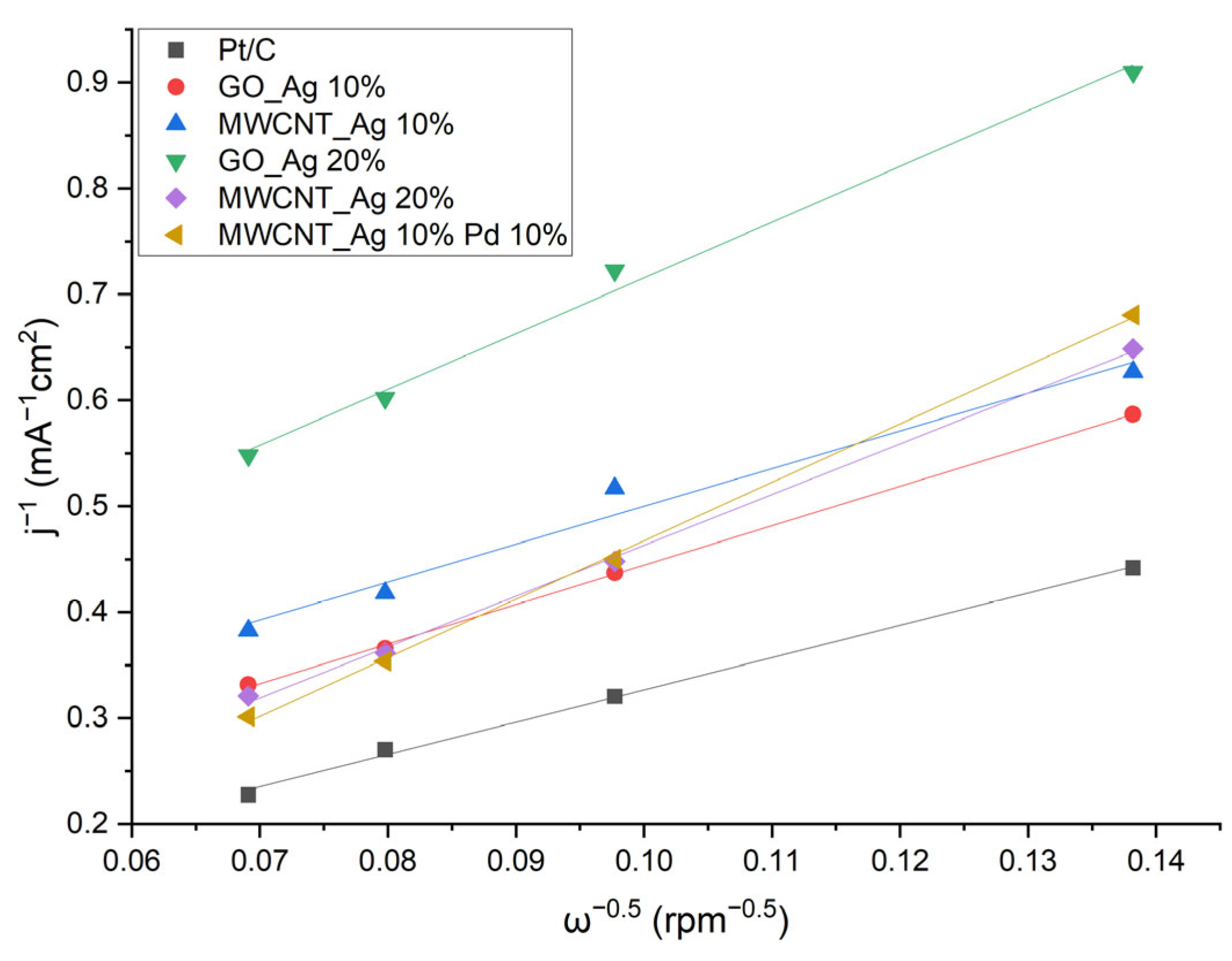
- j—measured current density;
- jk, jd—kinetic and limited diffusion current densities;
- k—electrochemical rate constant of oxygen reduction;
- —oxygen diffusion coefficient (1.9 × 10−5 cm2·s−1);
- —the concentration of oxygen in the volume;
- —kinematic viscosity of the solution;
- ω—angular velocity of rotation of the electrode;
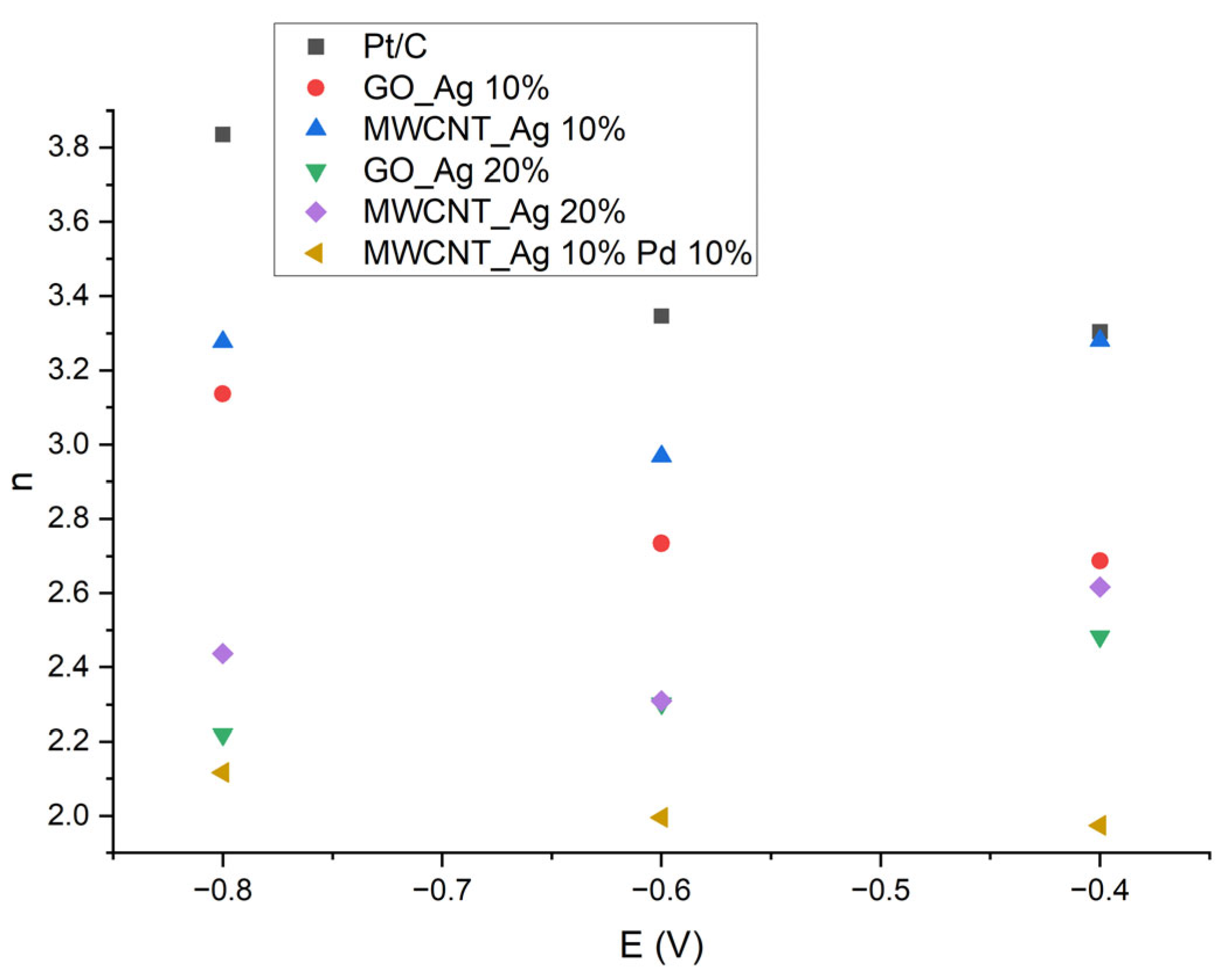
- n—the number of electrons transferred per oxygen molecule.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huya-Kouadio, J. Doe Hydrogen and Fuel Cells Program Record; US Departament of Energy: Washinton, DC, USA, 2017. [Google Scholar]
- Truong, V.M.; Yang, M.K.; Yang, H. Functionalized carbon black supported silver (Ag/C) catalysts in cathode electrode for alkaline anion exchange membrane fuel cells. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 711–721. [Google Scholar] [CrossRef]
- Kim, M.; Firestein, K.L.; Fernando, J.F.; Xu, X.; Lim, H.; Golberg, D.V.; Na, J.; Kim, J.; Nara, H.; Tang, J.; et al. Strategic design of Fe and N co-doped hierarchically porous carbon as superior ORR catalyst: From the perspective of nanoarchitectonics. Chem. Sci. 2022, 13, 10836–10845. [Google Scholar] [CrossRef] [PubMed]
- Nasim, F.; Ali, H.; Nadeem, M.A.; Nadeem, M.A. High-performance FeO x@ CoO x/NC electrocatalysts for the oxygen reduction reaction in alkaline media. Sustain. Energy Fuels 2023, 7, 190–200. [Google Scholar] [CrossRef]
- Sirirak, R.; Jarulertwathana, B.; Laokawee, V.; Susingrat, W.; Sarakonsri, T. FeNi alloy supported on nitrogen-doped graphene catalysts by polyol process for oxygen reduction reaction (ORR) in proton exchange membrane fuel cell (PEMFC) cathode. Res. Chem. Intermed. 2017, 43, 2905–2919. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. A highly active Pd coated Ag electrocatalyst for oxygen reduction reactions in alkaline media. Electrochim. Acta 2010, 55, 4506–4511. [Google Scholar] [CrossRef]
- Bharti, A.; Cheruvally, G. Influence of various carbon nano-forms as supports for Pt catalyst on proton exchange membrane fuel cell performance. J. Power Sources 2017, 360, 196–205. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Palafox-Segoviano, J.A.; Pérez-Díaz, P.J.; Medina-Ramírez, A. Synthesis of supported Pt nanoparticles by sonication for ORR: Effect of the graphene oxide-carbon composite. Int. J. Hydrogen Energy 2021, 46, 26027–26039. [Google Scholar] [CrossRef]
- Wang, T.; Chutia, A.; Brett, D.J.; Shearing, P.R.; He, G.; Chai, G.; Parkin, I.P. Palladium alloys used as electrocatalysts for the oxygen reduction reaction. Energy Environ. Sci. 2021, 14, 2639–2669. [Google Scholar] [CrossRef]
- Sravani, B.; Raghavendra, P.; Chandrasekhar, Y.; Reddy, Y.V.M.; Sivasubramanian, R.; Venkateswarlu, K.; Madhavi, G.; Sarma, L.S. Immobilization of platinum-cobalt and platinum-nickel bimetallic nanoparticles on pomegranate peel extract-treated reduced graphene oxide as electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 7680–7690. [Google Scholar] [CrossRef]
- He, X.; Li, D.; Bai, Z.; Chang, F.; Qiao, J.; Yang, L. Multi-wall carbon nanotube-supported palladium–cobalt oxide nanoparticle as efficient catalyst for oxygen reduction reaction. Ionics 2019, 25, 5929–5937. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, W.; Shao, Q.; Shi, H.; Liao, F.; Shao, C.; Shao, M. Palladium–copper bimetallic nanoparticles loaded on carbon black for oxygen reduction and zinc–air batteries. ACS Appl. Nano Mater. 2021, 4, 1478–1484. [Google Scholar] [CrossRef]
- Zhao, K.; Shu, Y.; Li, F.; Peng, G. Bimetallic catalysts as electrocatalytic cathode materials for the oxygen reduction reaction in microbial fuel cell: A review. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Medina-Ramírez, A.; Fuentes-Ramírez, R.; Navarro, R.; Goméz, C.M.; Pérez-Larios, A. Pt and Pt–Ag nanoparticles supported on carbon nanotubes (CNT) for oxygen reduction reaction in alkaline medium. Int. J. Hydrogen Energy 2022, 47, 30147–30159. [Google Scholar] [CrossRef]
- Linge, J.; Erikson, H.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on silver catalysts electrodeposited on various nanocarbon supports. SN Appl. Sci. 2021, 3, 263. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Li, F.; Cao, B.; Zhu, W.; Song, H.; Wang, K.; Li, C. Hydrogenation of phenol over Pt/CNTs: The effects of Pt loading and reaction solvents. Catalysts 2017, 7, 145. [Google Scholar] [CrossRef]
- Damdinov, B.B.; Ershov, A.A.; Krylov, A.S.; Maksimova, O.M.; Vtyurin, A.N. Investigation of silver nanoparticles by Raman scattering. In Proceedings of the XXXIV Session of the Russian Acoustic Society, Moscow, Russia, 14–18 February 2022; pp. 1192–1197. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Shafigulin, R.V.; Tokranova, E.O.; Bulanova, A.V.; Kazakevich, P.V.; Vostrikov, S.V.; Martynenko, E.A.; Zhu, H. Carbon black modified with silver and low concentration of palladium as effective catalysts for electroreduction of oxygen in alkaline solutions. React. Kinet. Mech. Catal. 2021, 133, 455–465. [Google Scholar] [CrossRef]
- Raghavendra, P.; Reddy, G.V.; Sivasubramanian, R.; Chandana, P.S.; Sarma, L.S. Reduced graphene oxide-supported Pd@ Au bimetallic nano electrocatalyst for enhanced oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2018, 43, 4125–4135. [Google Scholar] [CrossRef]
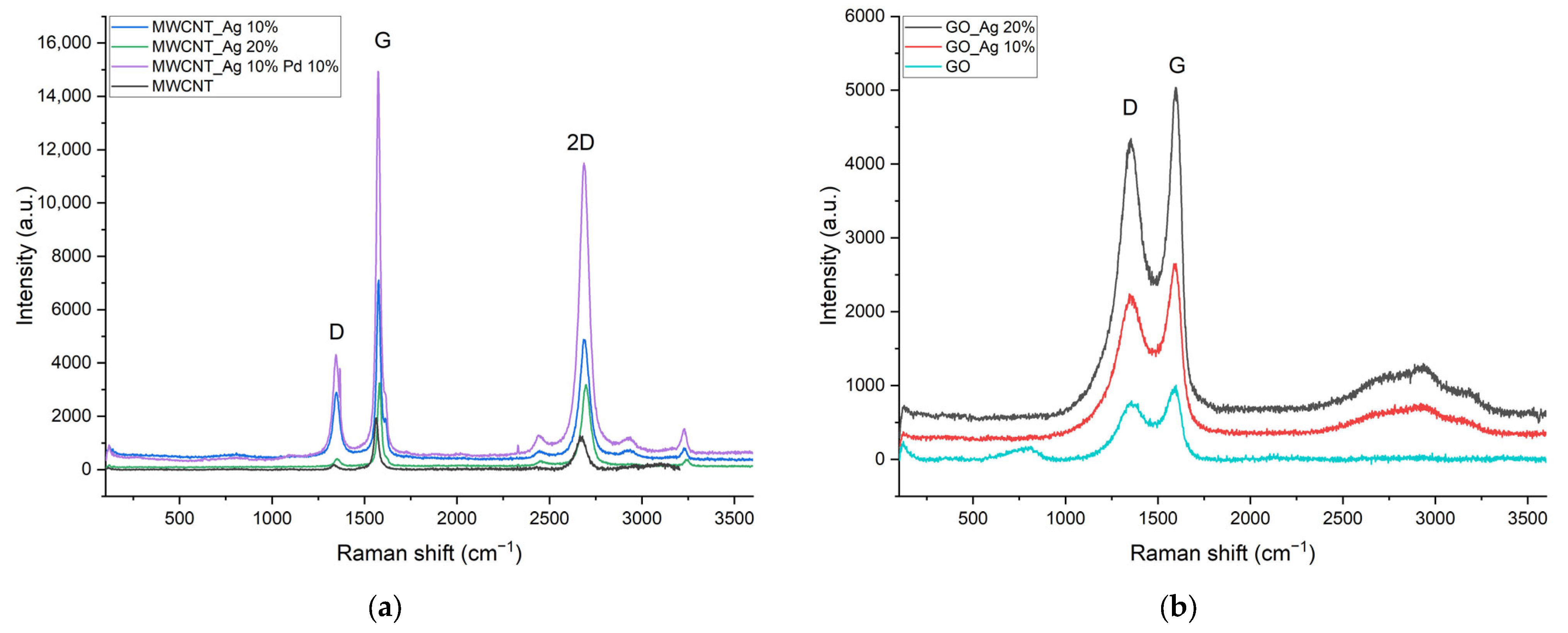

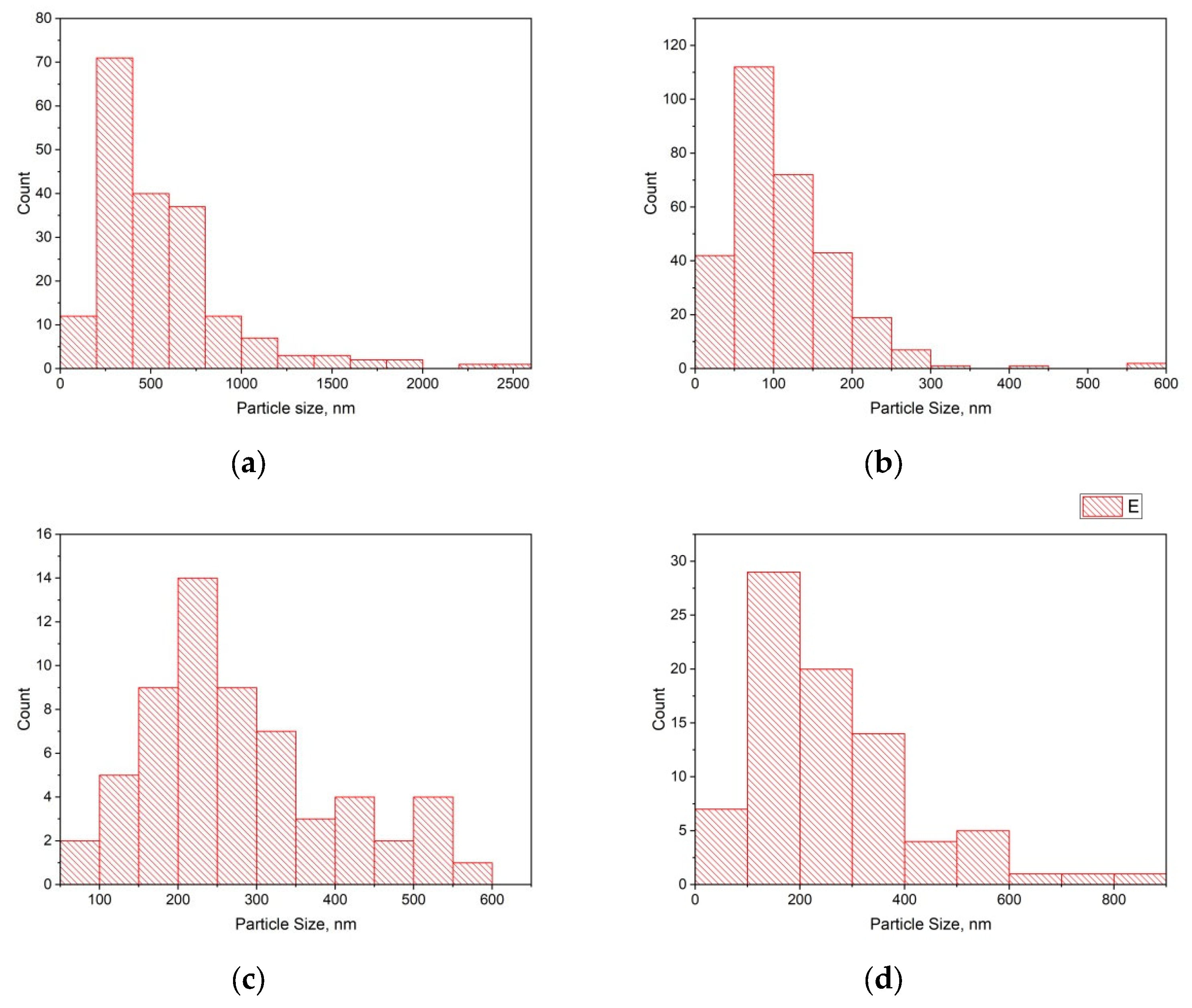

| Sample | ν(D), cm−1 | ν(G), cm−1 | ν(2D), cm−1 | ID | IG | I2D | IG/ID | I2D/IG |
|---|---|---|---|---|---|---|---|---|
| GO | 1357 | 1598 | - | 791 | 1004 | - | 1.27 | - |
| GO_Ag 10% | 1345 | 1598 | - | 2241 | 2650 | - | 1.18 | - |
| GO_Ag 20% | 1355 | 1594 | - | 4341 | 5036 | - | 1.16 | - |
| MWCNT | 1336 | 1565 | 2666 | 187 | 1942 | 1273 | 10.39 | 0.66 |
| MWCNT_Ag 10% | 1350 | 1577 | 2684 | 2899 | 7108 | 4874 | 2.45 | 0.69 |
| MWCNT_Ag 20% | 1353 | 1580 | 2700 | 415 | 3248 | 3184 | 7.83 | 0.98 |
| MWCNT_Ag 10% Pd 10% | 1345 | 1573 | 2686 | 4310 | 14,933 | 11,393 | 3.46 | 0.76 |
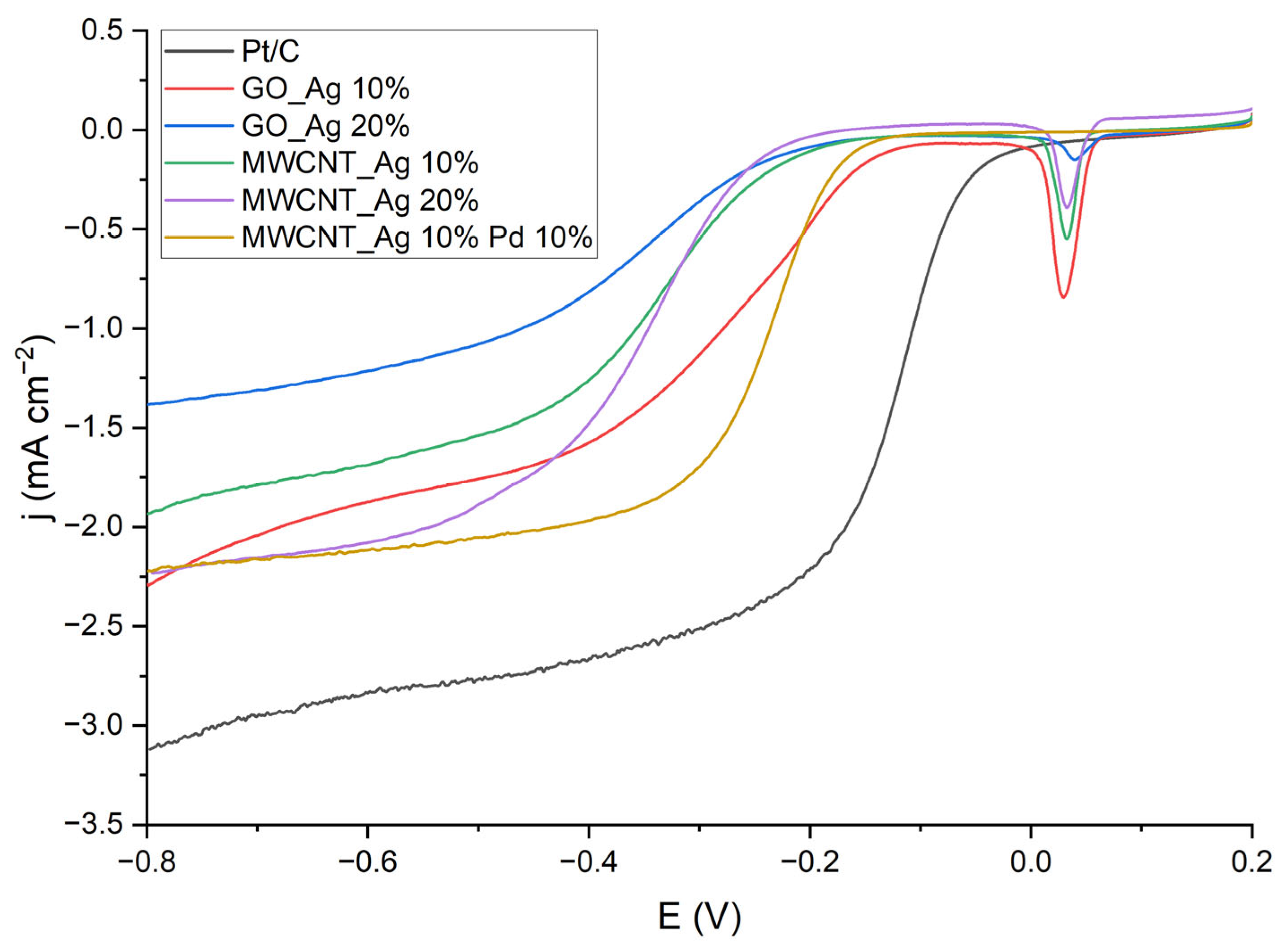
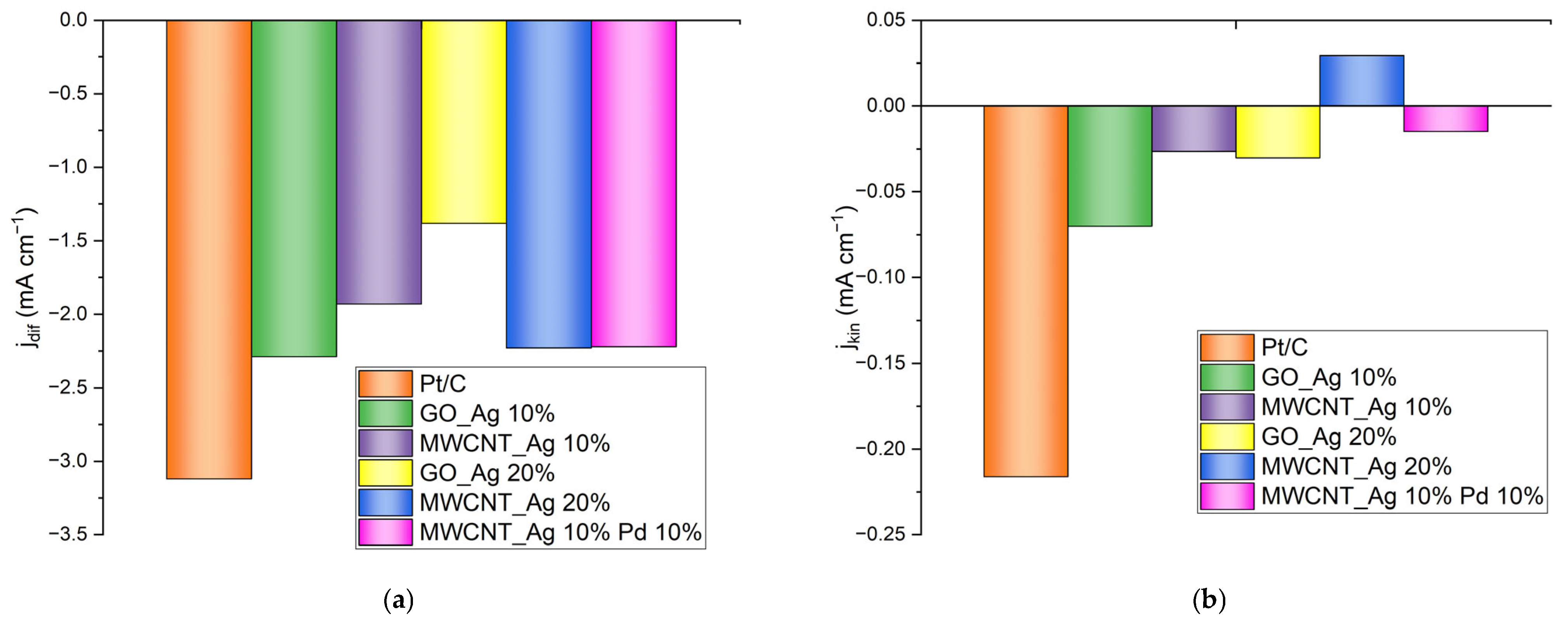
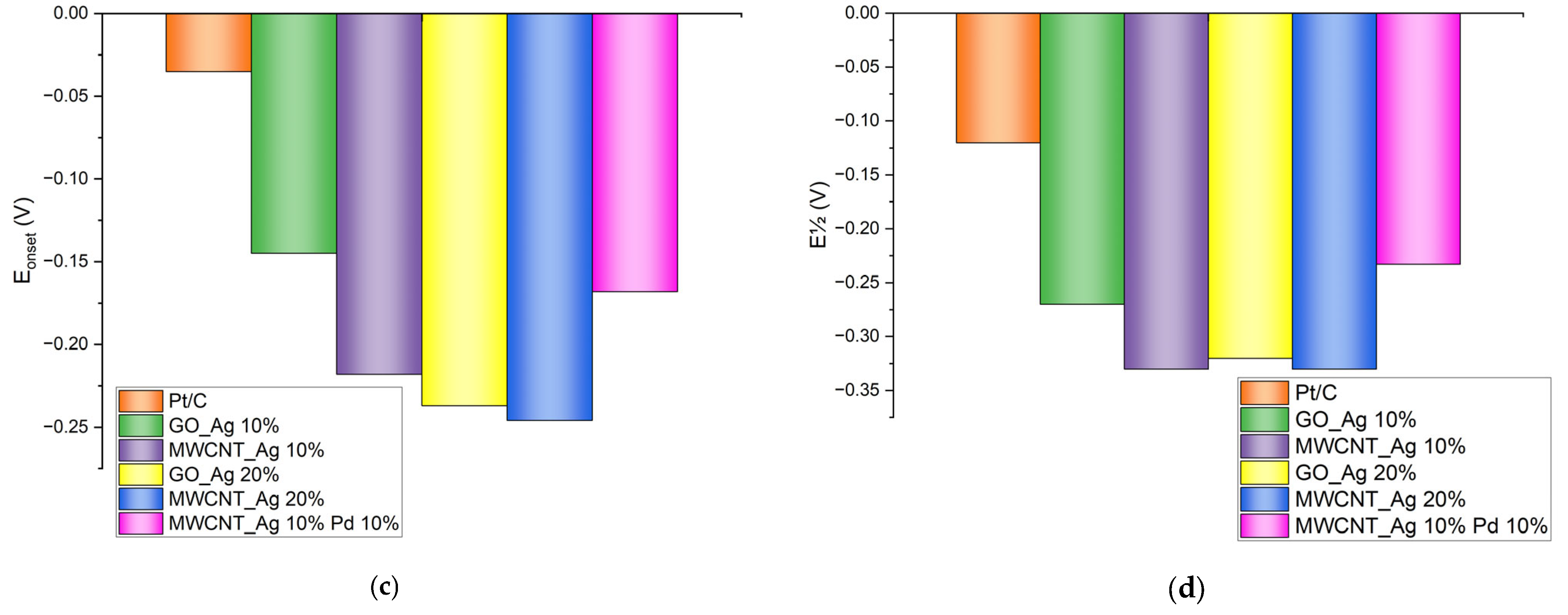
| Catalyst | Eonset,V | E½,V | Jkin, mA/cm2 | Jdif, mA/cm2 |
|---|---|---|---|---|
| Pt/C | −0.035 | −0.12 | −0.2161 | −3.12 |
| GO_Ag 10% | −0.145 | −0.27 | −0.0700 | −2.29 |
| MWCNT_Ag 10% | −0.218 | −0.33 | −0.0264 | −1.93 |
| GO_Ag 20% | −0.237 | −0.32 | −0.0302 | −1.38 |
| MWCNT_Ag 20% | −0.246 | −0.33 | 0.0294 | −2.23 |
| MWCNT_Ag 10% Pd 10% | −0.168 | −0.23 | −0.0149 | −2.22 |
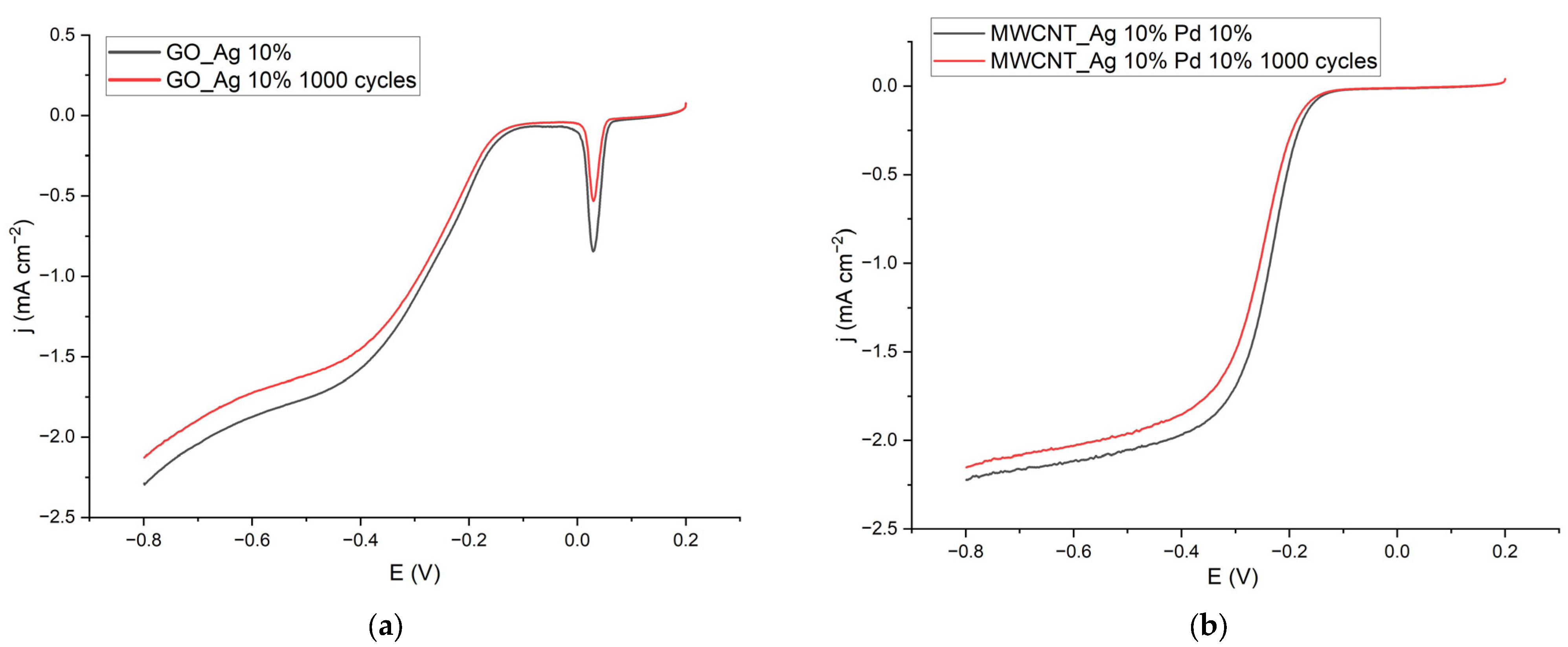
| Catalyst | E½, V (vs. Ag/AgCl) | j, mA cm−2 (E = −0.8 V) | E, V (j = 0.1 mA cm−2) |
|---|---|---|---|
| GO Ag 10% | −0.269 | −2.287 | −0.124 |
| GO Ag 10% 1000 cycles | −0.266 | −2.126 | −0.140 |
| MWCNT_Ag 10% Pd 10% | −0.233 | −2.222 | −0.157 |
| MWCNT_Ag 10% Pd 10% 1000 cycles | −0.239 | −2.152 | −0.166 |
| Catalyst | E½,V (vs. Ag/AgCl) | E½,V (vs. SCE) | Source |
|---|---|---|---|
| GO_Ag 10% | −0.27 | −0.32 | |
| MWCNT_Ag 10% | −0.33 | −0.38 | |
| MWCNT_Ag 10% Pd 10% | −0.23 | −0.28 | |
| Ag/NGO1 | −0.22 | −0.26 | [15] |
| Ag/NGO2 | −0.22 | −0.26 | [15] |
| Ag/MWCNT1 | −0.26 | −0.31 | [15] |
| Ag/MWCNT2 | −0.27 | −0.32 | [15] |
| Pd@Ag/RGO | −0.17 | −0.22 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradov, K.Y.; Shafigulin, R.V.; Tokranova, E.O.; Vostrikov, S.V.; Martynenko, E.A.; Podlipnov, V.V.; Kazakevich, P.V.; Sheldaisov-Meshcheryakov, A.A.; Vinogradov, N.A.; Bulanova, A.V. Catalysts for ORR Based on Silver-Modified Graphene Oxide and Carbon Nanotubes. Energies 2023, 16, 1526. https://doi.org/10.3390/en16031526
Vinogradov KY, Shafigulin RV, Tokranova EO, Vostrikov SV, Martynenko EA, Podlipnov VV, Kazakevich PV, Sheldaisov-Meshcheryakov AA, Vinogradov NA, Bulanova AV. Catalysts for ORR Based on Silver-Modified Graphene Oxide and Carbon Nanotubes. Energies. 2023; 16(3):1526. https://doi.org/10.3390/en16031526
Chicago/Turabian StyleVinogradov, Kirill Yurievich, Roman Vladimirovich Shafigulin, Elena Olegovna Tokranova, Sergey Vladimirovich Vostrikov, Evgeniya Andreevna Martynenko, Vladimir Vladimirovich Podlipnov, Pavel Vladimirovich Kazakevich, Artem Anatolevich Sheldaisov-Meshcheryakov, Nikolai Aleksandrovich Vinogradov, and Andzhela Vladimirovna Bulanova. 2023. "Catalysts for ORR Based on Silver-Modified Graphene Oxide and Carbon Nanotubes" Energies 16, no. 3: 1526. https://doi.org/10.3390/en16031526





