Phase-Homogeneous LiFePO4 Powders with Crystallites Protected by Ferric-Graphite-Graphene Composite
Abstract
1. Introduction
2. Synthesis of LiFePO4 Powders and Surface Coatings, and Characterization Techniques
2.1. Synthesis of LiFePO4 Powder and Coatings
- One-pot LiFePO4 liquid-phase synthesis using chemically pure lithium and iron acetates as starting materials.
- Using raw materials of organic nature.
- Using the tableting operation after the pyrolysis of organic matter, at 400 °C. This operation facilitates the course of the final synthesis of the topochemical reaction.
- Drying, before heat treatment, was carried out in a stream of hot air, which prevented the agglomeration of the starting materials.
- The synthesis intermediate is tableted, heat-treated at 400 °C, then re-milled and re-tableted before the final heat treatment at 670 °C. This made it possible to obtain a material of high phase purity.
- Before repeated tableting, adipic acid was introduced, the pyrolysis of which in an inert medium (high-purity nitrogen) led to encapsulation of LiFePO4 in a carbon shell.
- At all stages of the preparation, measures were taken to exclude the transition of Fe2+ to Fe3+, in particular, by isolation from atmospheric moisture.
2.2. Characterization Techniques
3. LiFePO4 Powders Phase Homogeneity Studies Using SXRD and Its Effect on the Test Cathodes Cyclability
3.1. Phase Homogeneity Using SXRD
3.2. Cycling Test Cathode Cells
- No impurity crystallites were found in the samples N1 and N4. However, the cyclability of the latter was significantly worse than that of the developed sample N1, despite the trend towards an increase in the role of the [100] surface of larger crystallites in their cyclability. Consequently, the absence of impurity crystallites is a necessary but obviously not a sufficient condition.
- Sample N1 had a high cyclability, but lower than that in the sample N3, which contained several impurity phases. In addition to lowering the resistance, these phases probably catch the degradation products, which slows down the formation of harmful impurity phases. It can be concluded that it is promising to search for such compositions or isovalent doping, including various mixed solid solutions, starting from our pure technology.
4. Crystallite Coating Composition and Properties Studied with TEM and RS
4.1. TEM Study
- -
- Large 40–150 nm particles of LiFePO4 are observed in all TEM images; an example is shown in Figure 3a for the developed sample N1.
- -
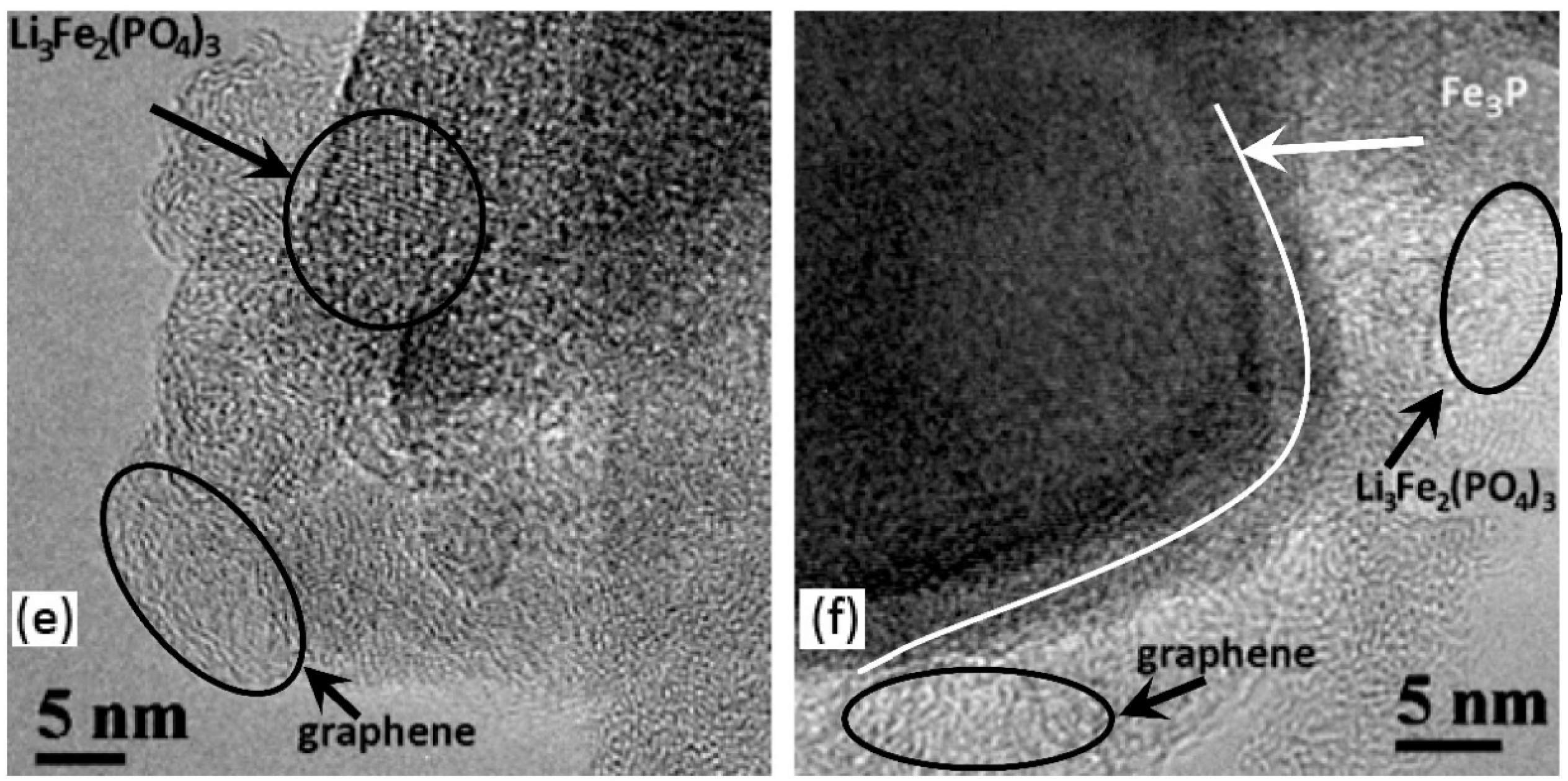
- In almost all samples, nanocrystalline 5–10 nm Li3Fe2(PO4)3 particles are observed (Figure 3b,e,f) with lattice spacing of 0.428 nm, which corresponds to (200) or (−121) planes. It should be noted, however, that the (011) planes of LiFePO4 have a similar interplanar distance. The ferric compound Li3Fe2(PO4)3 on the surface of LiFePO4 crystallites appears as a result of insufficient oxygen content to complete the oxidation reaction.
- -
- Figure 3f shows crystallites with interplanar spacing of 0.220 nm that corresponds presumably to the (321) planes of Fe3P in the N3 sample, even though a similar distance can be found in the structures of other phases. According to [91,101,102], at T > 850 °C and in the presence of carbon, LiFePO4 is reduced to form Fe3P. As seen from Table 2, a significant amount of that phase is reliably detected in the sample N3 using SXRD; the plate-like shape of the particles was described in detail in [103].
- -
- Various structures of the carbon layers encapsulating the LiFePO4 particles can be seen in the samples. More ordered carbon shells are up to 5 nm thick and the amorphous shells are up to 20 nm thick. In some cases, particles without a carbon shell are observed. The properties of the carbon coatings will be discussed below in the RS section.

4.2. Raman Spectroscopy Studies of Carbon Phases
5. The Role of Ferric Compounds Studied with MS; Degradation Mechanisms of LiFePO4 Test Cathodes with XRD
5.1. Mössbauer Spectroscopy Studies
5.2. Ageing Mechanisms of LiFePO4 and Test Cathode Study Using XRD and EDX
5.3. Test Cathode Aging Study Using XRD and EDX
6. The Galvanostatic Measurements of LiFePO4 Test Cathodes
6.1. Test Cathode Aging Study Using XRD and EDX
- -
- using weight (0.015 g) of the initial amount in the LiFePO4 powder sample and its pycnometric density (3.6 g/cm3), the total volume of all crystallites in the cathode is calculated (V = 4.2 × 1018 nm3),
- -
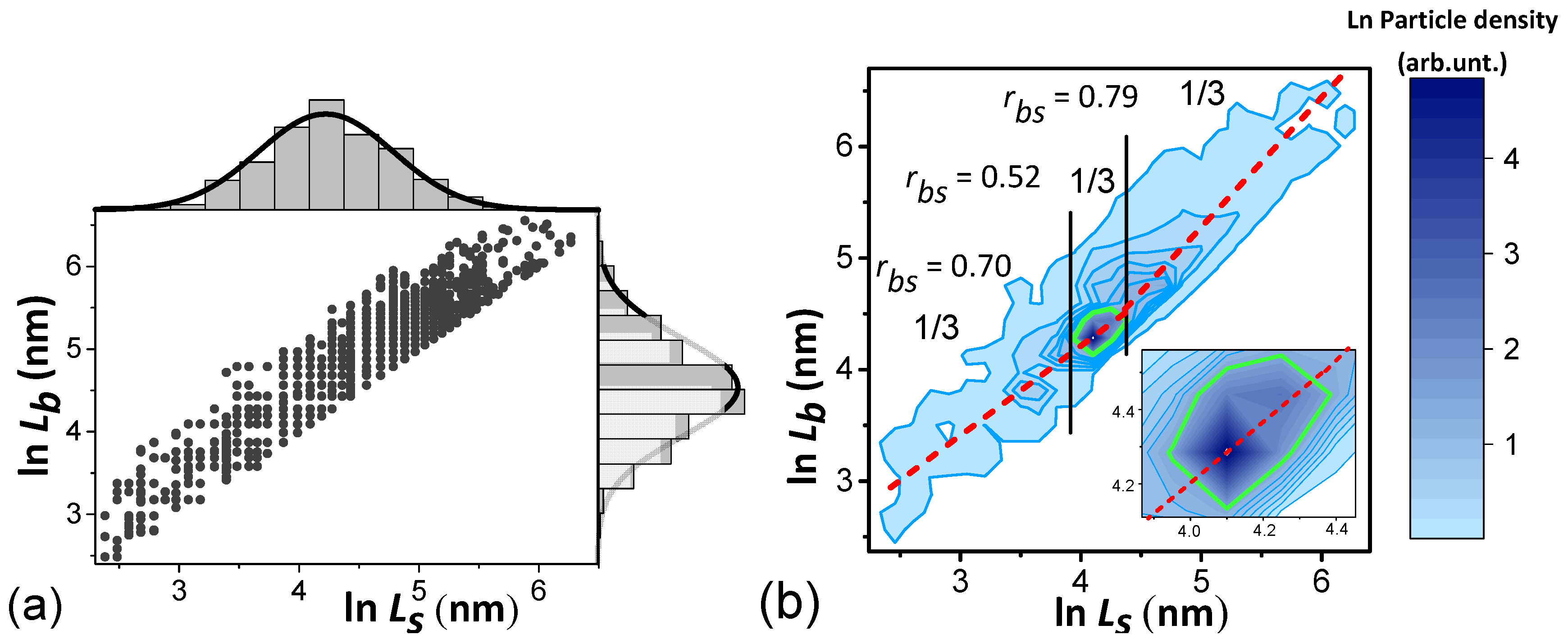
- the average crystallite volume is calculated (Mathematica 12 notation):where —3-dimensional N-bit matrix of particle volumes, each element of which for ellipsoid particles has a volume . Index n runs over values from 1 to N, while are the sizes of crystallites along the [010], [100] and [001] axes, respectively; is the discretization of function (1) normalized to 1 in the form of a 3-dimensional N-bit matrix, each element of which means the probability of occurrence of the crystallites with the corresponding sizes. The Total operator means the matrix elements product and all products summation:
- -
- dividing V by we obtain the number of particles Nct in the cathode and the S value by calculating an equation similar to (2). Instead of it uses the matrix —particle area projections onto the (010) plane, and the program line is as follows:
- -
- as a result, for the developed powder, we obtain the number of crystallites in the cathode Nct = 4.1 × 1012; the total areas S = 8.3 × 1016 nm2 and the specific interfacial area 2 × 107 m−1 can be calculated. The results for 2 samples are shown in Table 6.
6.2. Q(t) Dependence on Crystallite Parameters, Lithium Diffusion Coefficient D along [010] and the Quality of Their Coating (Electrical Relaxation Time τel)
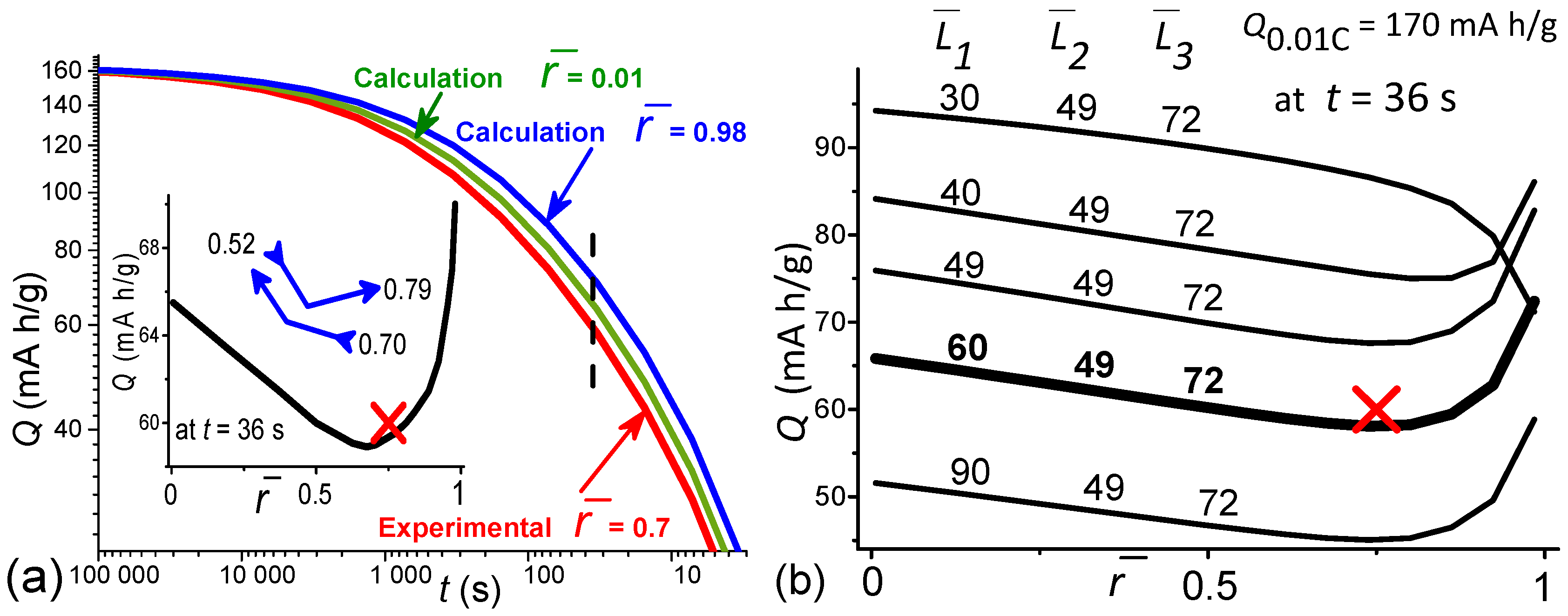
- In [152,158], for some current source, for which Q(t) asymptotically approaches the limit value at t→∞, and at t→0 it approaches the dependence , the following empirical equation was proposed:where is the time constant, and the exponent values n are defined in [152] for batteries and supercapacitors, as 0.5 and 1.0, respectively. That is, the exponent n is equal to the slope tangent of the dependence Q(t) in double logarithmic coordinates at small t. Equation (5) can be interpreted as follows: over time, Q(t) reaches its limit value with probability [1 − P], where P is equal to the subtract in the square bracket of Equation (5) and has the meaning of the process probability not being implemented due to the limited rate [159].
- According to [99], the crystallite is divided into columns with a cross-sectional area along the Li diffusion direction—axis [010], along which the coordinate axis is directed. The crystallite rate capability is determined by integrating over the plane (010) the rate capability of length M column:where are the crystallite dimensions along the [010], [100] and [001] axes, respectively.
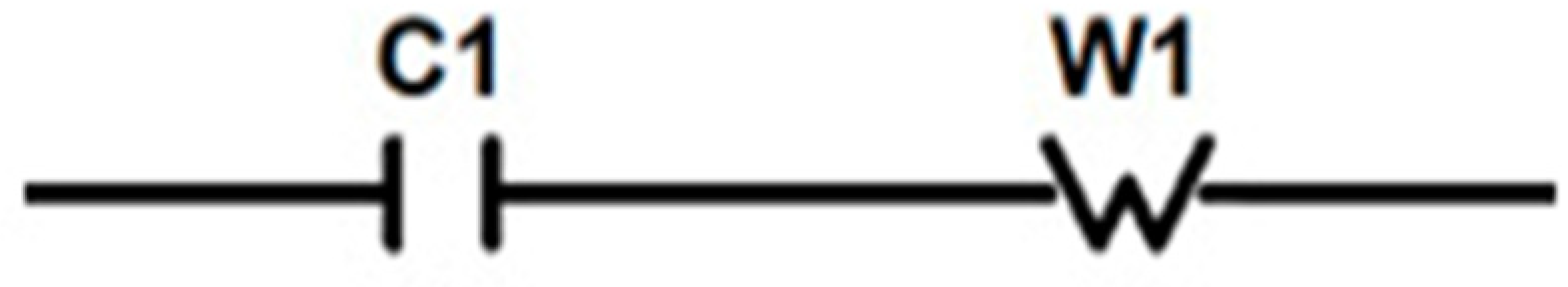
- Sequential charge carriers flow in a crystallite column through a capacitor (the model of a dense electric double layer) and an element with distributed parameters (the model of Warburg element diffusion of the stage limiting the rate of the Faradaic process). The model can be considered similar to the electrical circuit in which the capacitor and the Warburg element are series-connected [42,160].
- The chain Figure 7 corresponds to the probabilistic equation of the sequence of events [154]:in which the rate capability is not realized with probability in time t. The dependence is used, where D = Const, which describes the process of diffusion (desorption) from a finite size M with associated boundary conditions [161].
- Next, similarly to Equations (2) and (3), the desired dependences are calculated:
- Figure 8 shows the fitting of the dependence of the calculated sum results (7) on the discharge time with reference to the experimental normalization value of the rate capability at = 80 s (10 C rate), which is intermediate between the dominant contributions.

6.3. Q(t) Calculation in the Ranges of D, u , Close to the Values of the Developed LiFePO4 Powder to Assess the Possibility of Improving Technologies
7. Conclusions
- One-pot synthesis has been developed for LiFePO4 powders with impurity phase content of less than 0.1%, with 2–3 times smaller crystallites along the [010] axis, with 2–3 times greater cycling compared to the industrial samples, and with the particles covered by a mechanically strong, low-resistance ferric-graphite-graphene composite protective layer with inclusions of ferric (Fe3+) compound particles 5–10 nm in size. The ordered carbon shell thickness reaches 5 nm, and the amorphous shell is up to 20 nm.
- To detect impurity crystallite phases, SXRD was used, since conventional XRD is less sensitive due to the lower intensity of laboratory sources compared to the synchrotron.
- Control of adipic acid and polyvinyl alcohol concentrations and use of multistage annealing modes makes it possible to control the coating quality. The composite layer improves cyclability compared to industrial cathodes.
- The role of ferric Fe3+ compounds:
- -
- the content of ferric Fe3+ compounds is much higher (at least 6–8%) than the value expected from the electrochemical decrease in the Li content. The amount of Fe3+ reported in the literature varies from 2% to 30% (Table 7).
- -
- in a controlled way, Fe3+ compounds can be formed on the surface when the volatile components are not completely removed during LiFePO4 synthesis from an intermediate low-temperature amorphous phase.
- -
- To obtain highly cyclable and low resistant samples, it is necessary to have some optimal amount of the Fe3+ ferric compounds, which appear as LiFePO4 synthesis by-products. EDX studies of the tested cathode show that the total number of Fe atoms is reduced compared to the original samples. We have not detected Fe2O3, but it was observed in other technologies.
- -
- According to the corrosion degradation model, an increase in the cycle number leads to a decrease in the ferric Fe3+ compounds content on the surface of crystallites. These compounds play a certain sacrificial role [162,163], disappearing as the cathode resource is exhausted, and impurity phases can play the role of a catalyst for this breakdown. However, some of them, such as iron phosphide, weaken the catalytic activity. The degradation occurs in two stages: at the first stage, the layer on the crystallite surface is destroyed, and at the second stage, Fe escapes into the electrolyte and onto the anode with a decrease in the crystallite size due to increasing amorphization of the near-surface layer of crystallites.
- Galvanostatic studies of the N1 sample test cathodes were carried out in 3 stages with an assessment of the possibility to further improve the technology.
- 5.1
- To develop a theoretical dependence Q(t) that takes into account the 3D lognormal crystallite size distribution , the response time of the electrode/electrolyte interface is estimated using the specific interfacial area in the electrodes = S/V, where S is the total the projected area of the test cathode crystallites on the (010) plane, and V is their total volume. The value tc = 0.5 s is obtained.
- 5.2
- Comparing the value with those obtained in [155], it appears to be a minimal one in the hierarchy of relaxation times of electrochemical charge exchange. This makes it possible to simplify the theoretical equation for the Q(t) dependence on the parameters. Fitting the theoretical dependence to the experimental data gives the value of the Li diffusion coefficient, D = 0.12 nm2/s. The value of satisfies the inequality > = 0.5 s
- 5.3
- Q(t) calculations in the ranges of diffusion coefficients D, electrical relaxation times , and correlation coefficients close to the values characteristic of the developed LiFePO4 powder show that a decrease in D by 25% and a decrease in by a factor of 4 has practically no effect on the Q(t) value in the practically important range of discharge rates up to 50 C. Improving the powder technology should be aimed at increasing D three times and reducing to extremely small values closer to = 0.5 s. For an additional increase in Q(t) (and power) in the short recharge time region, it is necessary to optimize the values of the correlation coefficients between the anisotropic crystallite sizes.
- Table 7 summarizes the obtained parameters and compares them with the known ones, taking into account comments on them.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, A.; Chung, S.C.; Hinokuma, K.J. Optimized LiFePO4 for Lithium Battery Cathodes. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Ong, S.P.; Wang, L.; Kang, B.; Ceder, G. Li–Fe–P–O2 Phase Diagram from First Priciples Calculations. Chem. Mater. 2008, 20, 1798–1807. [Google Scholar] [CrossRef]
- Bezerra, C.A.G.; Davoglio, R.A.; Biaggio, S.R.; Bocchi, N.; Rocha-Filho, R.C. High-purity LiFePO4 prepared by a rapid one-step microwave-assisted hydrothermal synthesis. J. Mater. Sci. 2021, 56, 10018–10029. [Google Scholar] [CrossRef]
- Henriksen, C.; Mathiesen, J.K.; Ravnsbæk, D.B. Improving capacity and rate capability of Li-ion cathode materials through ball milling and carbon coating—Best practice for research purposes. Solid State Ion. 2020, 344, 115–152. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.Y.; Gu, Y.J.; Wu, F.Z.; Mai, Y.; Dai, X.Y. Study on the Preparation of LiFPO4 by Hydrothermal Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 761, 012004. [Google Scholar] [CrossRef]
- Nan, C.; Lu, J.; Li, L.; Li, L.; Peng, Q.; Li, Y. Size and shape control of LiFePO4 nanocrystals for better lithium ion battery cathode materials. Nano Res. 2013, 6, 469–477. [Google Scholar] [CrossRef]
- Kudryavtsev, E.N.; Sibiryakov, R.V.; Agafonov, D.V.; Naraev, V.N.; Bobyl, A.V. Modification of liquid-phase synthesis of lithium-iron phosphate, a cathode material for lithium-ion battery. Russ. J. Appl. Chem. 2012, 85, 879–882. [Google Scholar] [CrossRef]
- Janssen, Y.; Santhanagopalan, D.; Qian, D.; Chi, M.; Wang, X.; Hoffmann, C.S.; Meng, Y.; Khalifah, P.G. Reciprocal Salt Flux Growth of LiFePO4 Single Crystals with Controlled Defect Concentrations. Chem. Mater. 2013, 25, 4574–4584. [Google Scholar] [CrossRef]
- Abakumov, A.M.; Fedotov, S.S.; Antipov, E.V.; Tarascon, J.-M. Solid state chemistry for developing better metal-ion batteries. Nat. Commun. 2020, 11, 4976. [Google Scholar] [CrossRef]
- Ming, X.-L.; Wang, R.; Li, T.; Wu, X.; Yuan, L.-J.; Zhao, Y. Preparation of Micro-Nano-Structured FePO4·2H2O for LiFePO4 Cathode Materials by the Turbulent Flow Cycle Method. ACS Omega 2021, 6, 18957–18963. [Google Scholar] [CrossRef]
- Jittmonkong, K.; Roddecha, S.; Sriariyanun, M. One-pot Synthesis of LiFePO4 Nano-particles Entrapped in Mesoporous Melamine-Formaldehyde Matrix as the Promising Cathode Materials for the Next Generation Lithium Ion Batteries. Mater. Today Proc. 2019, 17, 1284–1292. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Liu, L.; Li, Y.; Yang, J. One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries. Materials 2022, 15, 4738. [Google Scholar] [CrossRef]
- Kaurz, G.; Gates, B.D. Review–Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Ren, W.; Wang, K.; Yang, J.; Tan, R.; Hu, J.; Guo, H.; Duan, Y.; Zheng, J.; Lin, Y.; Pan, F. Soft-contact conductive carbon enabling depolarization of LiFePO4 cathodes to enhance both capacity and rate performances of lithium ion batteries. J. Power Sources 2016, 331, 232–239. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Huang, J.; Deng, W.; Li, X.; Zhang, H.; Wen, Z. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Li, Y.; Qi, F.; Guo, H.; Guo, Z.; Li, M.; Wu, W. Characteristic investigation of an electrochemical-thermal coupled model for a LiFePO4/Graphene hybrid cathode lithium-ion battery. Case Stud. Therm. Eng. 2019, 13, 100387. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Wang, J.; Ma, X.; Hou, W.; Xue, X. Electrochemical performance of LiFePO4/graphene composites at low temperature affected by preparation technology. Electrochim. Acta 2021, 368, 137575. [Google Scholar] [CrossRef]
- Xi, Y.; Lu, Y. Toward Uniform In Situ Carbon Coating on Nano-LiFePO4 via a Solid-State Reaction. Ind. Eng. Chem. Res. 2020, 59, 13549–13555. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Chen, Y.; Wen, X. Regenerated LiFePO4/C for scrapped lithium iron phosphate powder batteries by pre-oxidation and reduction method. Ionics 2022, 28, 2125–2133. [Google Scholar] [CrossRef]
- Ravet, N.; Chouinard, Y.; Magnan, J.F.; Besner, S.; Gauthier, M.; Armand, M. Electroactivity of natural and synthetic triphylite. J. Power Sources 2001, 97–98, 503–507. [Google Scholar] [CrossRef]
- Huynh, L.T.N.; Nguyen, H.H.A.; Tran, T.T.D.; Nguyen, T.T.T.; Nguyen, T.M.A.; La, T.H.; Tran, V.M.; Le, M.L.P. Electrode composite LiFePO4@ Carbon: Structure and electrochemical performances. J. Nanomater. 2019, 2019, 2464920. [Google Scholar] [CrossRef]
- Altin, E.; Altundag, S.; Gultek, E.; Altin, S. Li1+xFePO4 (x = 0–0.5) production from Fe3+ sources by glass-ceramic technique with different carbon sources and investigation of structural, thermal and electrochemical performance. J. Non-Cryst. Solids 2022, 586, 121546. [Google Scholar] [CrossRef]
- Lim, H.H.; Jang, I.C.; Lee, S.B.; Karthikeyan, K.; Aravindan, V.; Lee, Y.S. The important role of adipic acid on the synthesis of nanocrystalline lithium iron phosphate with high rate performance. J. Alloys Compd. 2010, 495, 181–184. [Google Scholar] [CrossRef]
- Xu, H.; Jing, M.; Huang, Z.; Li, J.; Wang, T.; Yuan, W.; Ju, B.; Shen, X. Cross-Linked Polypropylene Oxide Solid Electrolyte Film with Enhanced Mechanical, Thermal, and Electrochemical Properties via Additive Modification. ACS Appl. Polym. Mater. 2021, 3, 6539–6547. [Google Scholar] [CrossRef]
- Ramkumar, V.; Gardas, R.L. Structural Arrangements of Guanidinium-Based Dicarboxylic Acid Ionic Liquids and Insights into Carbon Dioxide Uptake through Structural Voids. Cryst. Growth Des. 2022, 22, 3646–3655. [Google Scholar] [CrossRef]
- Guan, L.; Liu, M.; Yu, F.; Qiu, T.; Zhou, T.; Lin, X. A LiFePO4 regeneration method based on PVAc alcoholysis reaction. Renew. Energy 2021, 175, 559–567. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Zhou, S. An effective method for preparing uniform carbon coated nano-sized LiFePO4 particles. Electrochim. Acta 2011, 58, 359–363. [Google Scholar] [CrossRef]
- Ravet, N.; Gauthier, M.; Zaghib, K.; Goodenough, J.B.; Mauger, A.; Gendron, F.; Julien, C.M. Mechanism of the Fe3+ Reduction at Low Temperature for LiFePO4 Synthesis from a Polymeric Additive. Chem. Mater. 2007, 19, 2595–2602. [Google Scholar] [CrossRef]
- Liao, Y.; Li, G.; Xu, N.; Chen, T.; Wang, X.; Li, W. Synergistic effect of electrolyte additives on the improvement in interfacial stability between ionic liquid based gel electrolyte and LiFePO4 cathode. Solid State Ion. 2019, 329, 31–39. [Google Scholar] [CrossRef]
- Doeff, M.M.; Wilcox, J.D.; Kostecki, R.; Lau, G. Optimization of carbon coatings on LiFePO4. J. Power Sources 2006, 163, 180–184. [Google Scholar] [CrossRef]
- Kim, S.; Mathew, V.; Kang, J.; Gim, J.; Song, J.; Jo, J.; Kim, J. High rate capability of LiFePO cathodes doped with an excess amount of Ti. Ceram. Int. 2016, 42, 7230–7236. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, C.-C.; Wu, H.-C.; Guo, Z.-Z.; Yang, M.-H.; Chiang, Y.-P.; Sheu, H.-S.; Wu, N.-L. Kinetic study on low-temperature synthesis of LiFePO4 via solid-state reaction. J. Power Sources 2006, 158, 550–556. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Yu, X.; Cheng, L.; Singer, A.; Shpyrko, O.G.; Xin, H.L.; Tamura, N.; Tian, C.; Weng, T.-C.; et al. Synchrotron X-ray Analytical Techniques for Studying Materials Electrochemistry in Rechargeable Batteries. Chem. Rev. 2017, 117, 13123–13186. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Y. Fe excess in hydrothermally synthesized LiFePO4. Mater. Lett. 2012, 84, 139–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Alarco, J.A.; Nerkar, J.Y.; Best, A.S.; Snook, G.A.; Talbot, P.C.; Cowie, B.C.C. Observation of Preferential Cation Doping on the Surface of LiFePO4 Particles and Its Effect on Properties. ACS Appl. Energy Mater. 2020, 3, 9158–9167. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Novikova, S.A.; Kulova, T.L.; Skundin, A.M.; Yaroslavtsev, A.B. Synthesis of LiFePO4 nanoplatelets as cathode materials for Li-ion batteries. Nanotechnol. Russ. 2016, 11, 757–760. [Google Scholar] [CrossRef]
- Kim, J.-K.; Jeong, S.M. Physico-electrochemical properties of carbon coated LiFePO4 nanoparticles prepared by different preparation method. Appl. Surf. Sci. 2020, 505, 144630. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2020; pp. 1–288. [Google Scholar]
- Grissa, R.; Abramova, A.; Tambio, S.-J.; Lecuyer, M.; Deschamps, M.; Fernandez, V.; Greneche, J.-M.; Guyomard, D.; Lestriez, B.; Moreau, P. Thermomechanical Polymer Binder Reactivity with Positive Active Materials for Li Metal Polymer and Li-Ion Batteries: An XPS and XPS Imaging Study. ACS Appl. Mater. Interfaces 2019, 11, 18368–18376. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Effect of Time on a Hierarchical Corn Skeleton-Like Composite of CoO@ZnO as Capacitive Electrode Material for High Specific Performance Supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kumar, Y.A.; Sambasivam, S.; Hira, S.A.; Krishna, T.N.V.; Zeb, K.; Uddin, W.; Kumar, K.D.; Obaidat, I.M.; Kim, S.; et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. J. Energy Storage 2020, 32, 101988. [Google Scholar] [CrossRef]
- Eds Long, G.J.; Grandjean, F. Mossbauer Spectroscopy Applied to Magnetism and Materials Science; Springer Science and Business Media: New York, NY, USA, 1996; Volume 1, pp. 1–479. [Google Scholar]
- Wu, Y.; Holze, R. Surface Science in Batteries. In Surface and Interface Science: Applications of Surface Science I, 1st ed.; Wandelt, K., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2020; pp. 381–427. [Google Scholar] [CrossRef]
- Andersson, A.S.; Kalska, B.; Häggström, L.; Thomas, J.O. Lithium extraction/insertion in LiFePO4: An X-ray diffraction and Mössbauer spectroscopy study. Solid State Ion. 2000, 130, 41–52. [Google Scholar] [CrossRef]
- Jalkanen, K.; Lindén, J.; Karppinen, M. Local structures in mixed LixFe1–yMyPO4 (M=Co, Ni) electrode materials. J. Solid State Chem. 2015, 230, 404–410. [Google Scholar] [CrossRef]
- Khalifi, M.E.; Lippens, P.-E. First-Principles Investigation of the 57Fe Mössbauer Parameters of LiFePO4 and FePO. J. Phys. Chem. C 2016, 120, 28375–28389. [Google Scholar] [CrossRef]
- Yaroslavtsev, S.; Novikova, S.; Rusakov, V.; Vostrov, N.; Kulova, T.; Skundin, A.; Yaroslavtsev, A. LiFe1-XMgXPO4/C as cathode materials for lithium-ion batteries. Solid State Ion. 2018, 317, 149–155. [Google Scholar] [CrossRef]
- Hosono, E.; Wang, Y.; Kida, N.; Enomoto, M.; Kojima, N.; Okubo, M.; Matsuda, H.; Saito, Y.; Kudo, T.; Honma, I.; et al. Synthesis of Triaxial LiFePO4 Nanowire with a VGCF Core Column and a Carbon Shell through the Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Ferrari, S.; Capsoni, D.; Mustarelli, P.; Spina, G.; Giallo, F.D.; Lantieri, M.; Leonelli, C.; Rizzutie, A.; Massarotti, V. Pair distribution function analysis and Mo¨ssbauer study of defects in microwave-hydrothermal LiFePO4. RSC Adv. 2012, 2, 250–258. [Google Scholar] [CrossRef]
- Bazzi, K.; Mandal, B.P.; Nazri, M.; Naik, V.M.; Garg, V.K.; Oliveira, A.C.; Vaishnava, P.P.; Nazri, G.A.; Naik, R. Effect of surfactants on the electrochemical behavior of LiFePO4 cathode material for lithium ion batteries. J. Power Sources 2014, 265, 67–74. [Google Scholar] [CrossRef]
- Yaroslavtsev, S.A.; Vostrov, N.I.; Novikova, S.A.; Kulova, T.L.; Yaroslavtsev, A.B.; Rusakov, V.S. Study of Delithiation Process Features in LixFe0.8M0.2PO4 (M = Mg, Mn, Co, Ni) by Mössbauer Spectroscopy. J. Phys. Chem. C 2020, 124, 13026–13035. [Google Scholar] [CrossRef]
- Reklaitis, J.; Davidonis, R.; Dindune, A.; Valdniece, D.; Jasulaitiene, V.; Baltrunas, D. Characterization of LiFePO4/C composite and its thermal stability by Mossbauer and XPS spectroscopy. Phys. Status Solidi B 2016, 253, 2283–2288. [Google Scholar] [CrossRef]
- Jensen, K.M.Ø.; Christensen, M.; Gunnlaugsson, H.P.; Lock, N.; Bøjesen, E.D.; Proffen, T.; Iversen, B.B. Defects in Hydrothermally Synthesized LiFePO4 and LiFe1-xMnxPO4 Cathode Materials. Chem. Mater. 2013, 25, 2282–2290. [Google Scholar] [CrossRef]
- Lazarević, Z.Ž.; Križan, G.; Križan, J.; Milutinović, A.; Ivanovski, V.N.; Mitrić, M.; Gilić, M.; Umićević, A.; Kuryliszyn-Kudelska, I.; Romčević, N.Ž. Characterization of LiFePO4 samples obtained by pulse combustion under various conditions of synthesis. J. Appl. Phys. 2019, 126, 085109. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Tian, Z.; Zhao, X.; Shen, X. Low-Temperature Synthesis of LiFePO4 Nanoplates/C Composite for Lithium Ion Batteries. Energy Fuels 2020, 34, 11597–11605. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Hu, F.; Li, Y.; Ye, L.; Zhao, D.; Jin, W.; Chang, C.; Lai, Y.; Chen, Y. Gas evolution characterization and phase transformation during thermal treatment of cathode plates from spent LiFePO4 batteries. Thermochim. Acta 2020, 684, 178483. [Google Scholar] [CrossRef]
- Wilcox, J.D.; Doeff, M.M.; Marcinek, M.; Kostecki, R. Factors influencing the quality of carbon coatings on LiFePO4. J. Electrochem. Soc. 2007, 154, A389–A395. [Google Scholar] [CrossRef]
- Rouzaud, J.N.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and microtexture (optical and electron microscopy, Raman spectroscopy). Thin Solid Film. 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Bonhomme, F.; Lassegues, J.C.; Servant, L. Raman spectroelectrochemistry of a carbon supercapacitor. J. Electrochem. Soc. 2001, 148, E450–E458. [Google Scholar] [CrossRef]
- Duan, X.; Tian, W.; Zhang, H.; Sun, H.; Ao, Z.; Shao, Z.; Wang, S. sp2/sp3 Framework from Diamond Nanocrystals: A Key Bridge of Carbonaceous Structure to Carbocatalysis. ACS Catal. 2019, 9, 7494–7519. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Vejpravová, J. Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials 2021, 11, 2469. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Tan, P.-H. Raman Spectroscopy of Monolayer and Multilayer Graphenes Chapter 1. In Raman Spectroscopy of Two-Dimensional Materials; Tan, P.-H., Ed.; Springer Series in Materials Science 276; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. Carbon 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Casiraghi, C. Doping dependence of the Raman peaks intensity of graphene close to the Dirac point. Phys. Rev. B 2009, 80, 233407. [Google Scholar] [CrossRef]
- Sun, L.; Deng, Q.; Fang, B.; Li, Y.; Deng, L.; Yang, B.; Ren, X.; Zhang, P. Carbon-coated LiFePO4 synthesized by a simple solvothermal method. CrystEngComm 2016, 18, 7537–7543. [Google Scholar] [CrossRef]
- Public Spectra. Raman Spectrum of Ketjen Black. CAS Registry Number 1333-86-4.
- Vivo-Vilches, J.F.; Celzard, A.; Fierro, V.; Devin-Ziegler, I.; Brosse, N.; Dufour, A.; Etienne, M. Lignin-based carbon nanofibers as electrodes for vanadium redox couple electrochemistry. Nanomaterials 2019, 9, 106. [Google Scholar] [CrossRef]
- Vadahanambia, S.; Chunb, H.-H.; Jung, K.H.; Park, H. Nitrogen doped holey carbon nano-sheets as anodes in sodium ion battery. RSC Adv. 2016, 6, 38112–38116. [Google Scholar] [CrossRef]
- Tan, H.; Xu, L.; Geng, H.; Rui, X.; Li, C.; Huang, S. Nanostructured Li3V2(PO4)3 Cathodes. Small 2018, 14, 1800567. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Y.; Liu, D.; Zhang, P. Conductivity and electrochemical performance of LiFePO4 slurry in the lithium slurry battery. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012076. [Google Scholar] [CrossRef]
- Lion Specialty Chemicals Co. KETJENBLACK Highly Electro-Conductive Carbon Black; Lion: Tokyo, Japan, 2020. [Google Scholar]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Activated Carbon Blended with Reduced Graphene Oxide Nanoflakes for Capacitive Deionization. Nanomaterials 2021, 11, 1090. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Cong, C.; Wang, Y.; Yu, T. Raman Spectroscopy Study of Two-Dimensional Materials Under Strain. Chapter 6. In Raman Spectroscopy of Two-Dimensional Materials; Tan, P.-H., Ed.; Springer Series in Materials Science: Berlin/Heidelberg, Germany, 2019; Volume 276, pp. 111–129. [Google Scholar] [CrossRef]
- Yoon, D.; Son, Y.-W.; Cheong, H. Strain-dependent splitting of the double-resonance Raman scattering band in graphene. Phys. Rev. Lett. 2011, 106, 155502. [Google Scholar] [CrossRef] [PubMed]
- Davydov, V.V.; Grebenikova, N.M.; Smirnov, K.Y. Method of Monitoring the State of Flowing Media with Low Transparency That Contain Large Inclusions. Meas. Tech. 2019, 62, 519–526. [Google Scholar] [CrossRef]
- Kamzin, A.S.; Bobyl, A.V.; Ershenko, E.M.; Terukov, E.I.; Agafonov, D.V.; Kudryavtsev, E.N. Structure and Electrochemical Characteristics of LiFePO4 Cathode Materials for Rechargeable LiIon Batteries. Phys. Solid State 2013, 55, 1385–1394. [Google Scholar] [CrossRef]
- Doeff, M.; Hu, M.Y.; McLarnon, F.; Kostecki, R. Effect of surface carbon structure on the electrochemical performance of LiFePO4. Electrochem. Solid State Lett. 2003, 6, A207–A209. [Google Scholar] [CrossRef]
- Higuchi, M.; Katayama, K.; Azuma, Y.; Yukawa, M.; Suhara, M. Synthesis of LiFePO4 cathode material by microwave processing. J. Power Sources 2003, 119–121, 258–261. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.J. Nonaqueous sol-gel synthesis of high-performance LiFePO4. Electrochem. Solid State Lett. 2004, 7, A515–A518. [Google Scholar] [CrossRef]
- Deb, A.; Bergmann, U.; Cramer, S.P.; Cairns, E.J. Structural investigations of LiFePO4 electrodes and in situ studies by Fe X-ray absorption spectroscopy. Electrochim. Acta 2005, 50, 5200–5207. [Google Scholar] [CrossRef]
- Fu, Q.; Sarapulova, A.; Trouillet, V.; Zhu, L.; Fauth, F.; Mangold, S.; Welter, E.; Indris, S.; Knapp, M.; Dsoke, S.; et al. In Operando Synchrotron Diffraction and in Operando X-ray Absorption Spectroscopy Investigations of Orthorhombic V2O5 Nanowires as Cathode Materials for Mg-Ion Batteries. J. Am. Chem. Soc. 2019, 141, 2305–2315. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, J. Synthesis of lithium manganese phosphate nanoparticle and its properties. J. Phys. Chem. Solids 2007, 68, 734–737. [Google Scholar] [CrossRef]
- Lee, S.B.; Jang, I.C.; Lim, H.H.; Aravindan, V.; Kim, H.S.; Lee, Y.S. Preparation and electrochemical characterization of LiFePO4 nanoparticles with high rate capability by a sol–gel method. J. Alloys Compd. 2010, 491, 668–672. [Google Scholar] [CrossRef]
- Son, C.G.; Chang, D.R.; Kim, H.S.; Lee, Y.S. Synthesis and Electrochemical Properties of Nanocrystalline LiFePO4 Obtained by Different Methods. J. Electrochem. Sci. Technol. 2011, 2, 103–109. [Google Scholar] [CrossRef]
- Son, C.G.; Yang, H.M.; Lee, G.W.; Cho, A.R.; Aravindan, V.; Kim, H.S.; Kim, W.S.; Lee, Y.S. Manipulation of adipic acid application on the electrochemical properties of LiFePO4 at high rate performance. J. Alloys Compd. 2011, 509, 1279–1284. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Jugović, D.; Mitrić, M.; Cvjetićanin, N.; Jančar, B.; Mentus, S.; Uskoković, D. Synthesis and characterization of LiFePO4/C composite obtained by sonochemical method. Solid State Ion. 2008, 179, 415–419. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, Y.; Zhang, H.; Tao, H.; Zhong, M.; Jiao, Z. Morphology and electrical properties of carbon coated LiFePO4 cathode materials. J. Power Sources 2009, 189, 462–466. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Gong, W.; Xiang, K.; Sun, B.; Liu, J. Preparation and electrochemical performance of LiFePO4/C composite with network connections of nano-carbon wires. Mater. Lett. 2011, 65, 559–561. [Google Scholar] [CrossRef]
- Gauthier, M. Phosphate materials for lithium batteries and energy storage. Procedia Eng. 2012, 46, 234–254. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural materials science end-station at the Kurchatov synchrotron radiation source: Recent instrumentation upgrades and experimental results. Nucl. Instr. Meth. Phys. Res. A 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Ectors, D.; Goetz-Neunhoeffer, F.; Neubauer, J. A generalized geometric approach to anisotropic peak broadening due to domain morphology. J. Appl. Cryst. 2015, 48, 189–194. [Google Scholar] [CrossRef]
- Bobyl, A.; Kasatkin, I. Anisotropic crystallite size distributions in LiFePO4 powders. RSC Adv. 2021, 11, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Bobyl, A.; Nam, S.-C.; Song, J.-H.; Ivanishchev, A.; Ushakov, A. Rate Capability of LiFePO4 Cathodes and the Shape Engineering of Their Anisotropic Crystallites. J. Electrochem. Sci. Technol. 2022, 13, 438–452. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K. Surface effects on electrochemical properties of nano-sized LiFePO4. J. Mater. Chem. 2011, 21, 9955–9968. [Google Scholar] [CrossRef]
- Herle, P.S.; Ellis, B.; Coombs, N.; Nazar, L.F. Nano-network electronic conduction in iron and nickel olivine phosphates. Nat. Mater. 2004, 3, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kosova, N.V.; Devyatkina, E.T. Lithium Iron Phosphate Synthesis Using Mechanical Activation. Chem. Sustain. Dev. 2012, 20, 61–68. [Google Scholar]
- Ershenko, E.; Bobyl, A.; Boiko, M.; Zubavichus, Y.; Runov, V.; Trenikhin, M.; Sharkov, M. Fe3P impurity phase in high-quality LiFePO4: X-ray diffraction and neutron-graphical studies. Ionics 2017, 23, 2293–2300. [Google Scholar] [CrossRef]
- Babaev, A.A.; Zobov, M.E.; Kornilov, D.Y.; Tkachev, S.V.; Terukov, E.I.; Levitskii, V.S. Optical and electrical properties of graphene oxide. Opt. Spectrosc. 2018, 125, 1014–1018. [Google Scholar] [CrossRef]
- Mallet-Ladeira, P.; Puech, P.; Toulouse, C.; Cazayous, M.; Ratel-Ramond, N.; Weisbecker, P.; Vignoles, G.L.; Monthioux, M.A. Raman study to obtain crystallite size of carbon materials: A better alternative to the Tuinstra–Koenig law. Carbon 2014, 80, 629–639. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.-M.; Pellenq, R.; Monthioux, M. Analyzing the Raman spectra of graphenic carbon materials from kerogens to nanotubes: What type of information can be extracted from defect bands? C 2019, 5, 69. [Google Scholar] [CrossRef]
- Aldon, L.; Perea, A.; Womes, M.; Ionica-Bousquet, C.M.; Jumas, J.C. Determination of the Lamb-Mössbauer factors of LiFePO4 and FePO4 for electrochemical in situ and operando measurements in Li-ion batteries. J. Solid State Chem. 2010, 183, 218–222. [Google Scholar] [CrossRef]
- Li, Z.; Shinno, I. Next nearest neighbor effects in triphylite and related phosphate minerals. Mineral. J. 1997, 19, 99–107. [Google Scholar] [CrossRef]
- Lippens, P.-E.; Khalifi, M.E.; Chamas, M.; Perea, A.; Sougrati, M.-T.; Ionica-Bousquet, C.; Aldon, L.; Olivier-Fourcade, J.; Jumas, J.-C. How Mössbauer spectroscopy can improve Li-ion batteries. Hyperfine Interact 2012, 206, 35–46. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Z.; Chen, L. Regeneration and characterization of air-oxidized LiFePO4. Electrochem. Commun. 2008, 10, 1442–1444. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Donoue, K.; Kadohata, T.; Murata, T.; Matsuta, S.; Fujitani, S. Impurities in LiFePO4 and their influence on material characteristics. J. Electrochem. Soc. 2008, 155, A526–A530. [Google Scholar] [CrossRef]
- Dhindsa, K.S.; Mandal, B.P.; Bazzi, K.; Lin, M.W.; Nazri, M.; Nazri, G.A.; Naik, V.M.; Garg, V.K.; Oliveira, A.C.; Vaishnava, P.; et al. Enhanced electrochemical performance of graphene modified LiFePO4 cathode material for lithium ion batteries. Solid State Ion. 2013, 253, 94–100. [Google Scholar] [CrossRef]
- Shiratsuchi, T.; Okada, S.; Yamaki, J.; Yamashit, S.; Nishid, T. Cathode performance of olivine-type LiFePO4 synthesized by chemical lithiation. J. Power Sources 2007, 173, 979–984. [Google Scholar] [CrossRef]
- Maccario, M.; Croguennec, L.; Wattiaux, A.; Suard, E.; Le Cras, F.; Delmas, C. C-containing LiFePO4 materials-Part I: Mechano-chemical synthesis and structural characterization. Solid State Ion. 2008, 179, 2020–2026. [Google Scholar] [CrossRef]
- Salah, A.; Zaghib, K.; Mauger, A.; Gendron, F.; Julien, C. Magnetic studies of the carbothermal effect on LiFePO4. Phys. Status Solidi A Appl. Mater. Sci. 2006, 203, R1–R3. [Google Scholar] [CrossRef]
- Salah, A.A.; Mauger, A.; Zaghib, K.; Goodenough, J.B.; Ravet, N.; Gauthier, M.; Gendron, F.; Julien, C.M. Reduction Fe3+ of impurities in LiFePO4 from pyrolysis of organic precursor used for carbon deposition. J. Electrochem. Soc. 2006, 153, A1692–A1701. [Google Scholar] [CrossRef]
- Hirose, K.; Honma, T.; Doi, Y.; Hinatsu, Y.; Komatsu, T. Mössbauer analysis of Fe ion state in lithium iron phosphate glasses and their glass-ceramics with olivine-type LiFePO4 crystals. Solid State Commun. 2008, 146, 273–277. [Google Scholar] [CrossRef]
- Wang, Z.; Su, S.; Yu, C.; Chen, Y.; Xia, D. Synthesises, characterizations and electrochemical properties of spherical-like LiFePO4 by hydrothermal method. J. Power Sources 2008, 184, 633–636. [Google Scholar] [CrossRef]
- Bazzi, K.; Nazri, M.; Naik, V.; Garg, V.; Oliveira, A.; Vaishnava, P.; Nazri, G.; Naik, R. Enhancement of electrochemical behavior of nanostructured LiFePO4/Carbon cathode material with excess Li. J. Power Sources 2016, 306, 17–23. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Loble, M.W.; et al. Post-mortem analysis of aged lithium-ion batteries: Disassembly methodology and physico-chemical analysis techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode degradation in lithium-ion batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef]
- Sui, X.; Swierczynski, M.; Teodorescu, R.; Stroe, D.-I. The Degradation Behavior of LiFePO4/C Batteries during Long-Term Calendar Aging. Energies 2021, 14, 1732. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Bobyl, A.V.; Zabrodskii, A.G.; Malyshkin, V.G.; Novikova, O.V.; Terukov, E.I.; Agafonov, D.V. Degradation of Li-ion batteries. Application of Generalized Radon--Nikodym Approach to Direct Estimation of Degradation Rate Distribution. Izvestiya RAN Energetika 2018, 2, 46–58. Available online: https://www.researchgate.net/publication/309175377 (accessed on 23 November 2016).
- Scipioni, R.; Jørgensen, P.S.; Stroe, D.I.; Younesi, R.; Simonsen, S.B.; Norby, P.; Hjelm, J.; Jensen, S.H. Complementary analyses of aging in a commercial LiFePO4/graphite 26650 cell. Electrochim. Acta 2018, 284, 454–468. [Google Scholar] [CrossRef]
- Scipioni, R.; Jørgensen, P.S.; Ngo, D.-T.; Simonsen, S.B.; Liu, Z.; Yakal-Kremski, K.J.; Wang, H.; Hjelm, J.; Norby, P.; Barnett, S.A.; et al. Electron microscopy investigations of changes in morphology and conductivity of LiFePO4/C electrodes. J. Power Sources 2016, 307, 259–269. [Google Scholar] [CrossRef]
- Lia, X.; Jiang, F.; Qu, K.; Wang, Y.; Pan, Y.; Wang, M.; Liu, Y.; Xu, H.; Chen, J.; Huang, Y.; et al. First Atomic-Scale Insight on Degradation in Lithium Iron Phosphate Cathodes by Transmission Electron Microscopy. J. Phys. Chem. Lett. 2020, 11, 4608–4617. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Zhao, X.; Zhi, X.; Liang, G. Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material. J. Alloys Compd. 2016, 683, 123–132. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Tang, Y.; Li, R.; Liang, G.; Sham, T.-K.; Sun, X. Surface aging at olivine LiFePO4: A direct visual observation of iron dissolution and the protection role of nano-carbon coating. J. Mater. Chem. A 2013, 1, 1579–1586. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wang, J. Enhanced Electrochemical Performance of LiFePO4 Originating from the Synergistic Effect of ZnO and C Co-Modification. Nanomaterials 2021, 11, 12. [Google Scholar] [CrossRef]
- Tan, S.; Tieu, J.H.; Bélanger, D. Chemical Polymerization of Aniline on a Poly(styrene sulfonic acid) Membrane: Controlling the Polymerization Site Using Different Oxidants. J. Phys. Chem. B 2005, 109, 14085–14092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Hosono, E.; Wang, K.; Zhou, H. The Design of a LiFePO4/Carbon Nanocomposite with a Core–Shell Structure and Its Synthesis by an In Situ Polymerization Restriction Method. Angew. Chem. Int. Ed. 2008, 47, 7461–7465. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; 990p. [Google Scholar]
- Blyr, A.; Sigala, C.; Amatucci, G.; Guyomard, D.; Chabre, Y.; Tarascon, J.-M. Self-Discharge of LiMn2O4/C Li-Ion Cells in Their Discharged State: Understanding by Means of Three-Electrode Measurements. J. Electrochem. Soc. 1998, 145, 194–209. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Yang, J.; Li, R.; Liang, G.; Sun, X. Nature at LiFePO4 aging process: Roles of impurity phases. J. Power Sources 2013, 238, 454–463. [Google Scholar] [CrossRef]
- Cuisinier, M.; Dupré, N.; Moreau, P.; Guyomard, D. NMR Monitoring of Electrode/Electrolyte Interphase in the Case of Air-exposed and Carbon Coated LiFePO4. J. Power Sources 2013, 243, 682–690. [Google Scholar] [CrossRef]
- Chen, J.; Bai, J.; Chen, H.; Graetz, J. In Situ Hydrothermal Synthesis of LiFePO4 Studied by Synchrotron X-ray Diffraction. J. Phys. Chem. Lett. 2011, 2, 1874–1878. [Google Scholar] [CrossRef]
- Zhu, J.; Fiore, J.; Li, D.; Kinsinger, N.M.; Wang, Q.; DiMasi, E.; Guo, J.; Kisailus, D. Solvothermal Synthesis, Development, and Performance of LiFePO4 Nanostructures. Cryst. Growth Des. 2013, 13, 4659–4666. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Kim, Y.-M.; Kim, J.-G.; Kim, Y.-J. Multiphase transformation and Ostwald’s rule of stages during crystallization of a metal phosphate. Nat. Phys. 2009, 5, 68–73. [Google Scholar] [CrossRef]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle Size Dependence of the Ionic Diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef]
- Orikasa, Y.; Maeda, T.; Koyama, Y.; Murayama, H.; Fukuda, K.; Tanida, H.; Arai, H.; Matsubara, E.; Uchimoto, Y.; Ogumi, Z. Transient Phase Change in Two Phase Reaction between LiFePO4 and FePO4 under Battery Operation. Chem. Mater. 2013, 25, 1032–1039. [Google Scholar] [CrossRef]
- Raj, H.; Rani, S.; Sil, A. Two-Phase Composition (LiFePO4/FePO4) and Phase Transformation Dependence on Charging Current: In Situ and Ex Situ Studies. Energy Fuels 2020, 34, 14874–14881. [Google Scholar] [CrossRef]
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S. Recent Progress in Capacity Enhancement of LiFePO4 Cathode for Li-Ion Batteries. J. Electrochem. Energy Convers. Storage 2021, 18, 010801. [Google Scholar] [CrossRef]
- Tolganbek, N.; Yerkinbekova, Y.; Kalybekkyzy, S.; Bakenov, Z.; Mentbayeva, A. Current state of high voltage olivine structured LiMPO4 cathode materials for energy storage applications: A review. J. Alloys Compd. 2021, 882, 160774. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kumar, Y.A.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Enhanced electrochemical performance of nanoplate nickel cobaltite (NiCo2O4) supercapacitor applications. RSC Adv. 2019, 9, 1115–1122. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Wang, S.; Yub, J. How to make lithium iron phosphate better: A review exploring classical modification approaches in-depth and proposing future optimization methods. J. Mater. Chem. A 2016, 4, 18210–18222. [Google Scholar] [CrossRef]
- Wen, L.; Guan, Z.; Wang, L.; Hu, S.; Lv, D.; Liu, X.; Duan, T.; Liang, G. Effect of Carbon-Coating on Internal Resistance and Performance of Lithium Iron Phosphate Batteries. J. Electrochem. Soc. 2022, 169, 050536. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Tian, R.; Park, S.; King, P.; Cunningham, G.; Coelho, J.; Nicolosi, V.; Coleman, J. Quantifying the factors limiting rate performance in battery electrodes. Nat. Commun. 2019, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.; Balsara, N.P. Electrochemical Systems, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; 608p. [Google Scholar]
- Rice, J. Mathematical Statistics and Data Analysis; Brooks/Cole Cengage Learning: Belmont, CA, USA, 2007; p. 138. [Google Scholar]
- Jiang, F.; Peng, P. Elucidating the performance limitations of lithium-ion batteries due to species and charge transport through five characteristic parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef]
- Fang, W.; Ramadass, P.; Zhang, Z.J. Study of internal short in a Li-ion cell-II. Numerical investigation using a 3D electrochemical-thermal model. J. Power Sources 2014, 248, 1090–1098. [Google Scholar] [CrossRef]
- Davydov, V.V.; Myazin, N.S.; Velichko, E.N. Characteristics of spectrum registration of condensed medium by the method of nuclear-magnetic resonance in a weak field. Tech. Phys. Lett. 2017, 43, 607–610. [Google Scholar] [CrossRef]
- Higgins, T.M.; Coleman, J.N. Avoiding resistance limitations in high-performance transparent supercapacitor electrodes based on large-area, high-conductivity PEDOT: PSS films. ACS Appl. Mater. Interfaces 2015, 7, 16495–16506. [Google Scholar] [CrossRef]
- Triola, M.F. Elementary Statistics Technology Update, 11th ed.; Pearson Education: London, UK, 2012; 888p. [Google Scholar]
- Lvovich, V.F. Distributed Impedance Models. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; 368p. [Google Scholar]
- Beckman, I. Higher Mathematics: The Mathematical Apparatus of Diffusion; Yurayt Publishing House: Moscow, Russia, 2020; 459p. [Google Scholar]
- Zhang, Y.; Zhang, W.; Shen, S.; Yan, X.; Wu, R.; Wu, A.; Lastoskie, C.; Zhang, J. Sacrificial Template Strategy toward a Hollow LiNi1/3Co1/3Mn1/3O2 Nanosphere Cathode for Advanced Lithium-Ion Batteries. ACS Omega 2017, 2, 7593–7599. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Hong, B.; Lai, Y. Self-sacrificial organic lithium salt enhanced initial columbic efficiency for safer and greener lithium-ion batteries. Chem. Commun. 2019, 55, 10737–10739. [Google Scholar] [CrossRef]
- Yoo, J.W.; Zhang, K.; Patil, V.; Lee, J.T.; Jung, D.-W.; Pu, L.S.; Oh, W.; Yoon, W.-S.; Park, J.H.; Yi, G.-R. Porous supraparticles of LiFePO4 nanorods with carbon for high rate Li-ion batteries. Mater. Express 2018, 8, 316–324. [Google Scholar] [CrossRef]
- Churikov, A.V.; Ivanishchev, A.V.; Ushakov, A.V.; Romanova, V.O. Diffusion aspects of lithium intercalation as applied to the development of electrode materials for lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 1425–1441. [Google Scholar] [CrossRef]
- Zhang, W.-J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]




| № | Steps | Reagents |
|---|---|---|
| 1 | Preparation of acetates | Fe + LiCO3 +CH3COOH → Fe(CH3COO)2, LiCH3COO, H2O |
| 2 | Organic Additives | AA, PA |
| 3 | Phosphoric acid | H3PO4 |
| 4 | Pre-drying at 100 °C | Evaporation of CH3COOH, H2O |
| 5 | Annealing 1.5 h at 400 °C in an Ar atmosphere | Evaporation of CH3COOH, H2O, CO2, ((CH2))4CO |
| 6 | Annealing 1.5 h at 670 °C in an Ar atmosphere | Crystallization of LiFePO4 |
| Impurity Phase, % | Q, mAh/g | Forecast Cycling | Mössbauer | |||||
|---|---|---|---|---|---|---|---|---|
| 20 C Rate | Fe2+, % | Fe3+, % | ||||||
| N1 | not detected | 63 | 66 (5) | 82 (5) | 89 (7) | 3500 | 96 | 4 |
| N2 | Li3PO4, 1.01 (2) | 58 | 145 (26) | 131 (13) | 185 (17) | 1000 | 95 | 5 |
| N3 | Li3PO4, 2.39 (3) Fe2P, 2.34 (3) Fe3P, 2.02 (3) | 57 | 141 (5) | 146 (15) | 165 (7) | 5000 | 92 | 8 |
| N4 | not detected | 40 | 230 (20) | 261 (8) | 242 (30) | 800 | 98 | 2 |
| E1, I1, W1 | E2, I2, W2 | E3, I3, W3 | E4, I4, W4 | E5, I5, W5 | E6, I6, W6 | E7, I7, W7 | ID/IG (I2/I4) | Isp2/Isp3 ((I2 + I4)/(I1 + I3)) | (I1 + …AI)/ (I5 + …I7) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Excitation 532 nm | ||||||||||
| N1 | 1203 64,354 130 | 1346 464,462 191 | 1513 109,518 128 | 1596 183,271 64 | 2681 84,869 391 | 2915 114,677 376 | 3176 7613 112 | 2.53 | 3.73 | 3.96 |
| N4 | 1187 45,591 94 | 1341 733,854 190 | 1527 130,196 119 | 1599 244,016 58 | 2668 146,229 337 | 2919 231,601 344 | 3180 17,221 113 | 3.01 | 5.56 | 2.92 |
| N2 | 1190 22,138 110 | 1342 288,779 188 | 1522 53,844 120 | 1600 98,169 59 | 2651 37,209 307 | 2909 88,666 380 | 3185 5240 117 | 2.94 | 5.09 | 3.53 |
| Excitation 633 nm | ||||||||||
| N1 | 1201 63,280 131 | 1346 475,058 195 | 1514 106,974 126 | 1596 182,032 64 | 2716 65,290 328 | 2949 38,599 234 | 3178 9359 128 | 2.61 | 3.86 | 7.3 |
| N4 | 1193 11,389 112 | 1332 133,742 180 | 1528 21,170 123 | 1602 37,709 56 | 2630 16,408 293 | 2883 20,492 318 | 3178 692 78 | 3.55 | 5.26 | 5.42 |
| N2 | 1198 7329 117 | 1331 69,319 179 | 1516 11,644 127 | 1601 19,955 57 | 2594 9304 340 | 2870 9744 334 | 3178 350 130 | 3.47 | 4.70 | 5.58 |
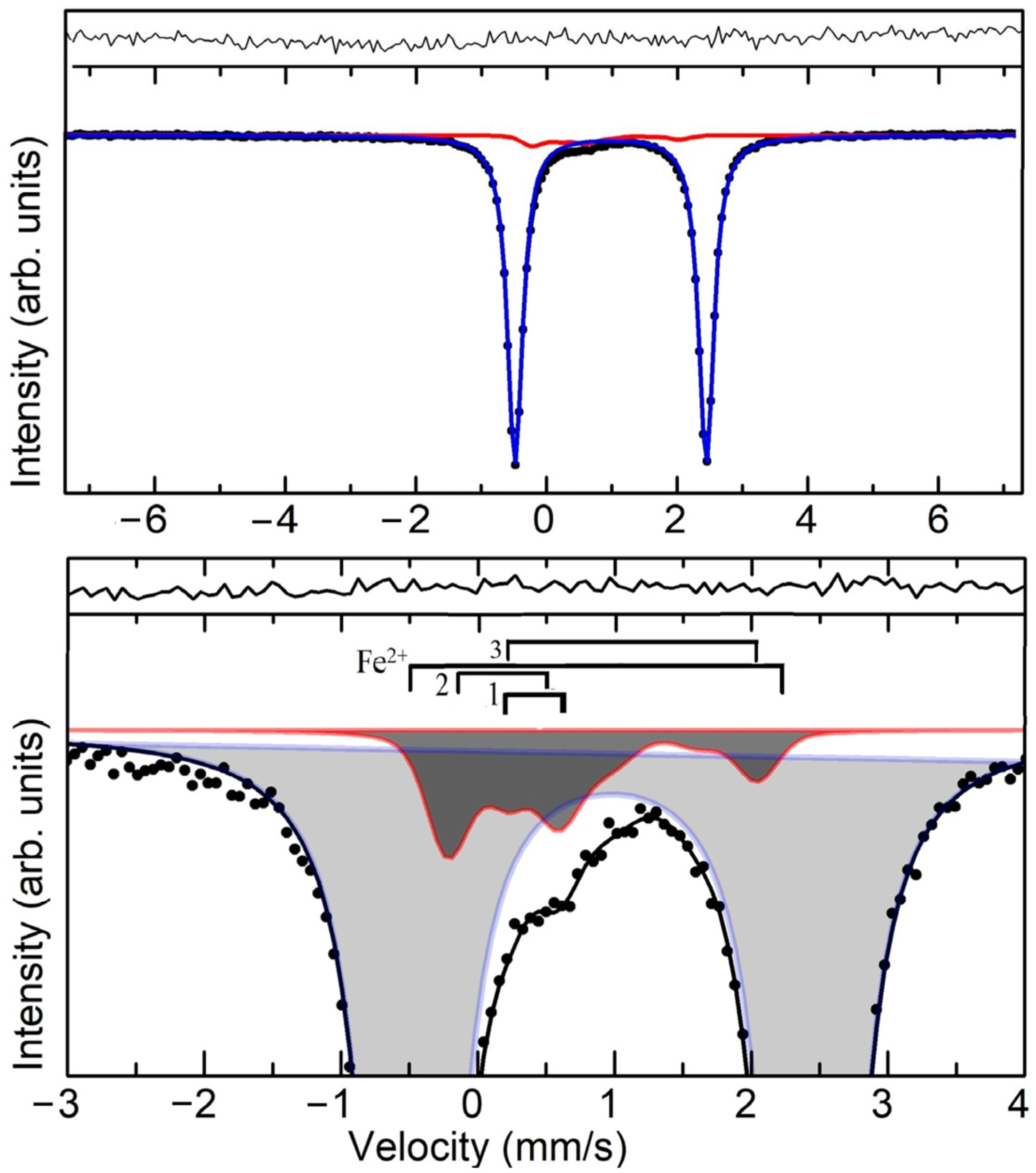
| Line Marking | IS, mm/s | QS, mm/s | G, mm/s | Int, (%) | Charge State Fe |
|---|---|---|---|---|---|
| Fe2+ | 0.981 ± 0.001 | 2.928 ± 0.001 | 0.281 ± 0.001 | 94.8 ± 0.2 | Fe2+ |
| 1 | 1.156 ± 0.040 | 1.818 ± 0.028 | 0.345 ± 0.025 | 1.4. ± 0.3 | Fe2+ |
| 2 | 0.172 ± 0.080 | 0.830 ± 0.080 | 0.345 ± 0.025 | 2.6 ± 0.4 | Fe3+ |
| 3 | 0.404 ± 0.400 | 0.452 ± 0.060 | 0.345 ± 0.025 | 1.2. ± 0.3 | Fe3+ |
| ΔQ, mAh/g | EDX, Fe, % | V = a × b × c, nm3 | Vb-Va, nm3 | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before–After | ||
| N1 | 0.7 | 13.7 | 5.47 | 190 (20) | 160 (50) | 291.183 | 291.37 | −0.09 |
| N2 | 4.0 | 6.28 | 2.98 | 220 (50) | 210 (50) | 291.376 | 290.069 | 1.303 |
| N3 | 0.4 | 7.45 | 2.92 | 119 (11) | 164 (17) | 290.723 | 290.633 | 0.09 |
| N4 | 6.2 | 11.17 | 12.5 | 800 (200) | 150 (20) | 291.184 | 290.88 | 0.304 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| r12 | r13 | r23 | as, m2 | D, nm2/s | τel, s | |||||
| N1 | 60, 0.41 | 49, 0.40 | 72, 0.38 | 0.87 | 0.64 | 0.73 | 0.75 | 2.0 × 107 | 0.16 (0.4) | 8 |
| N2 | 92, 0.43 | 108, 0.41 | 160, 0.35 | 0.72 | 0.56 | 0.53 | 0.60 | 3.1 × 107 | 0.3–2.1 (0.4) | 20 |
| Developed | Industrial | From References | |
|---|---|---|---|
| Growth T, °C | Two steps 400 °C, 670 °C | unknown | [1] 400–800; [3] 700; [5,18] 650; [6] 550; [8] 810; [19,22] 650–700; [24] 670; [33] 550–800; [37] 550; [49,55] 700; [51] 600. |
| Technology | one-pot liquid-phase | unknown | [143] More than 10 types, main: Solvothermal, Hydrothermal, Stripping synthesis, Sol-gel. |
| Protected layer | ferric-graphite-graphene | mostly ferric-graphite | [143] More than 30 types, main: [14] different carbon; [15] nanocarbons; [16,17,18] graphene; [19,20,21] sucrose; [22,23] glucose; [24,25,26] adipic acid; [27,28] polyvinyl alcohol; [29,30] polymeric additive; [31] ferrocene. |
| Q, mAh/g | 0.1 C 15110 C 82 | 128–163 72–80 | [151] commercial 121–160, best MWCNT, essentially mixed; [143] capacity growth of commercial powder from 160 to 208 mAh/g, more than theoretical 170 mAh/g (*) |
| Particle sizes, nm | 66 82 89 | 141–230 131–261 165–242 | [1] 300–7000; [22] 240–3000; [90] 180–300; [118] 500–30,200; [136] 120; [138] 95–280; [141] 60–1000; [144] 40–500; [149] 20–140; [151] 90–300; [164] 30–158. (**) |
| Cell volumes, 0A | [22] 290.63–290.94; [36] 291.08–292.07; [88] 289.7–291.2; [90] 291.3–292.3; [114] 291.33–291.63; [118] 289.8–291.9. | ||
| Cycling | 3500 | 800–1000, 5000 with ferric impurity | [121] 50–600; [143] 50–1000 with different capacity retentions 92–100% (**) |
| BET, m2/g | 12.5 | 9.4–13.4 | [1] 1–20; [6] 32–66; [16] 49.3–59.4; [19] 49.6; [121] 15.5; [132] 50; [136] 19–25; [164] 35. |
| D, nm2/s | 0.12 | 0.25–0.45 | [3] 2; [6] 2–3; [11,16] 0.01–1; [12] 4.9–7.2; [16] 3.4–8.8, 1.8–1000; [22] 109; [36] 1.2–8.2; [51] 900–2400; [119] 7400–42,000; [128] 0.83–27.3; [149] 1; [151] 0.67–11.7; [165] 1–100; [166] 0.01–10. (***) |
| τel, s | 8 | 3–30 | [152] 3–200 |
| tc, s | 0.5 | 0.5 | [155] 0.1 to >100 |
| Fe3+, % | 4 | 2–8 | [36] 0.28–0.39; [51] 9–17; [52] 5.16; [54] 2–12; [56] 5–26; [111] 7; [117] 7–25; [119] 5.4–17; [140] 1.13–17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agafonov, D.; Bobyl, A.; Kamzin, A.; Nashchekin, A.; Ershenko, E.; Ushakov, A.; Kasatkin, I.; Levitskii, V.; Trenikhin, M.; Terukov, E. Phase-Homogeneous LiFePO4 Powders with Crystallites Protected by Ferric-Graphite-Graphene Composite. Energies 2023, 16, 1551. https://doi.org/10.3390/en16031551
Agafonov D, Bobyl A, Kamzin A, Nashchekin A, Ershenko E, Ushakov A, Kasatkin I, Levitskii V, Trenikhin M, Terukov E. Phase-Homogeneous LiFePO4 Powders with Crystallites Protected by Ferric-Graphite-Graphene Composite. Energies. 2023; 16(3):1551. https://doi.org/10.3390/en16031551
Chicago/Turabian StyleAgafonov, Dmitry, Aleksandr Bobyl, Aleksandr Kamzin, Alexey Nashchekin, Evgeniy Ershenko, Arseniy Ushakov, Igor Kasatkin, Vladimir Levitskii, Mikhail Trenikhin, and Evgeniy Terukov. 2023. "Phase-Homogeneous LiFePO4 Powders with Crystallites Protected by Ferric-Graphite-Graphene Composite" Energies 16, no. 3: 1551. https://doi.org/10.3390/en16031551
APA StyleAgafonov, D., Bobyl, A., Kamzin, A., Nashchekin, A., Ershenko, E., Ushakov, A., Kasatkin, I., Levitskii, V., Trenikhin, M., & Terukov, E. (2023). Phase-Homogeneous LiFePO4 Powders with Crystallites Protected by Ferric-Graphite-Graphene Composite. Energies, 16(3), 1551. https://doi.org/10.3390/en16031551







