Recent Approaches to Achieve High Temperature Operation of Nafion Membranes
Abstract
:1. Introduction
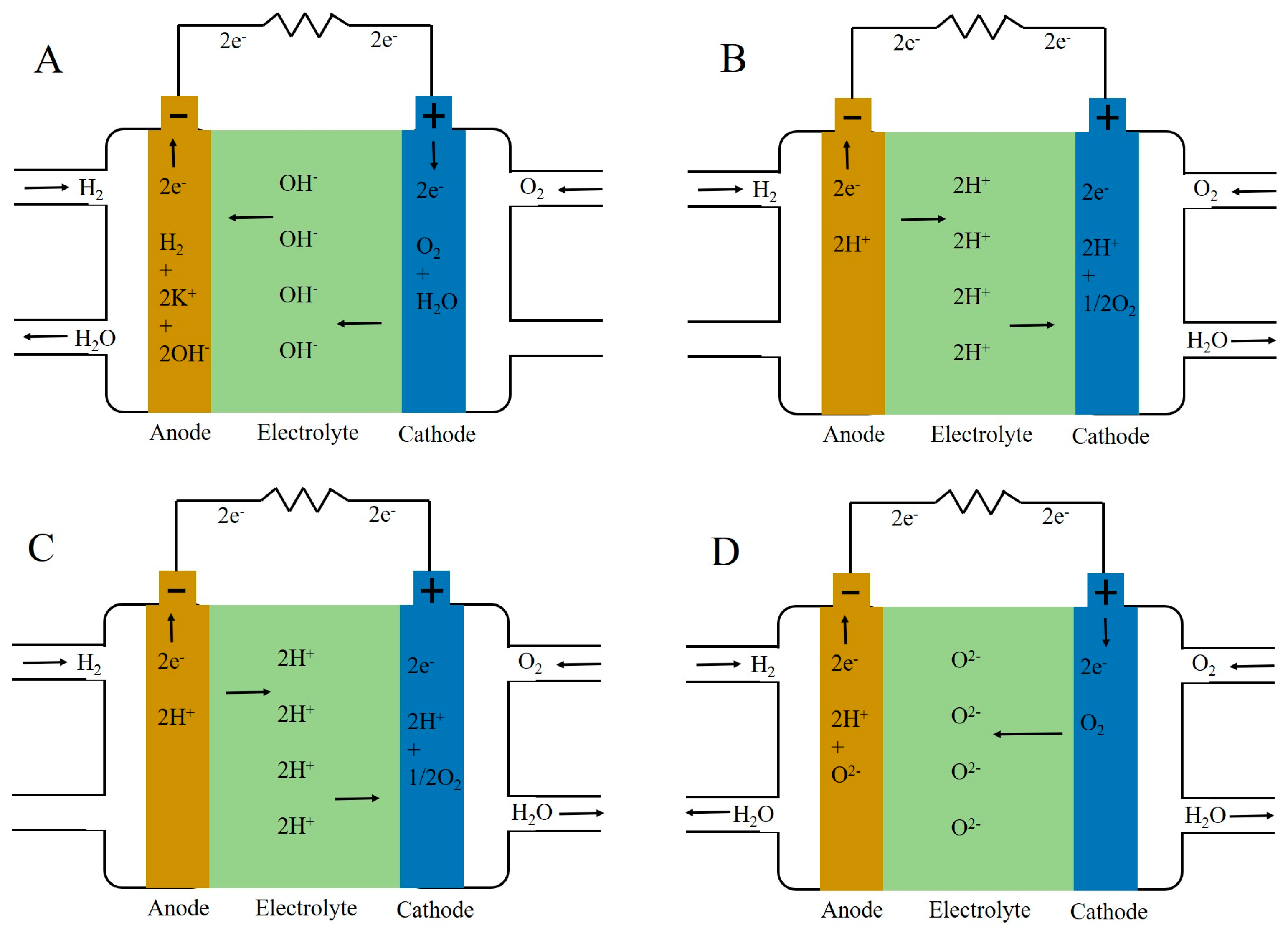
2. Proton Conduction Mechanism and Structural Characteristics of Nafion Membranes
3. Modifying Agent
3.1. Hygroscopic Materials
3.2. High-Temperature Proton Conductor Materials
3.3. Functional-Group-Modified Materials
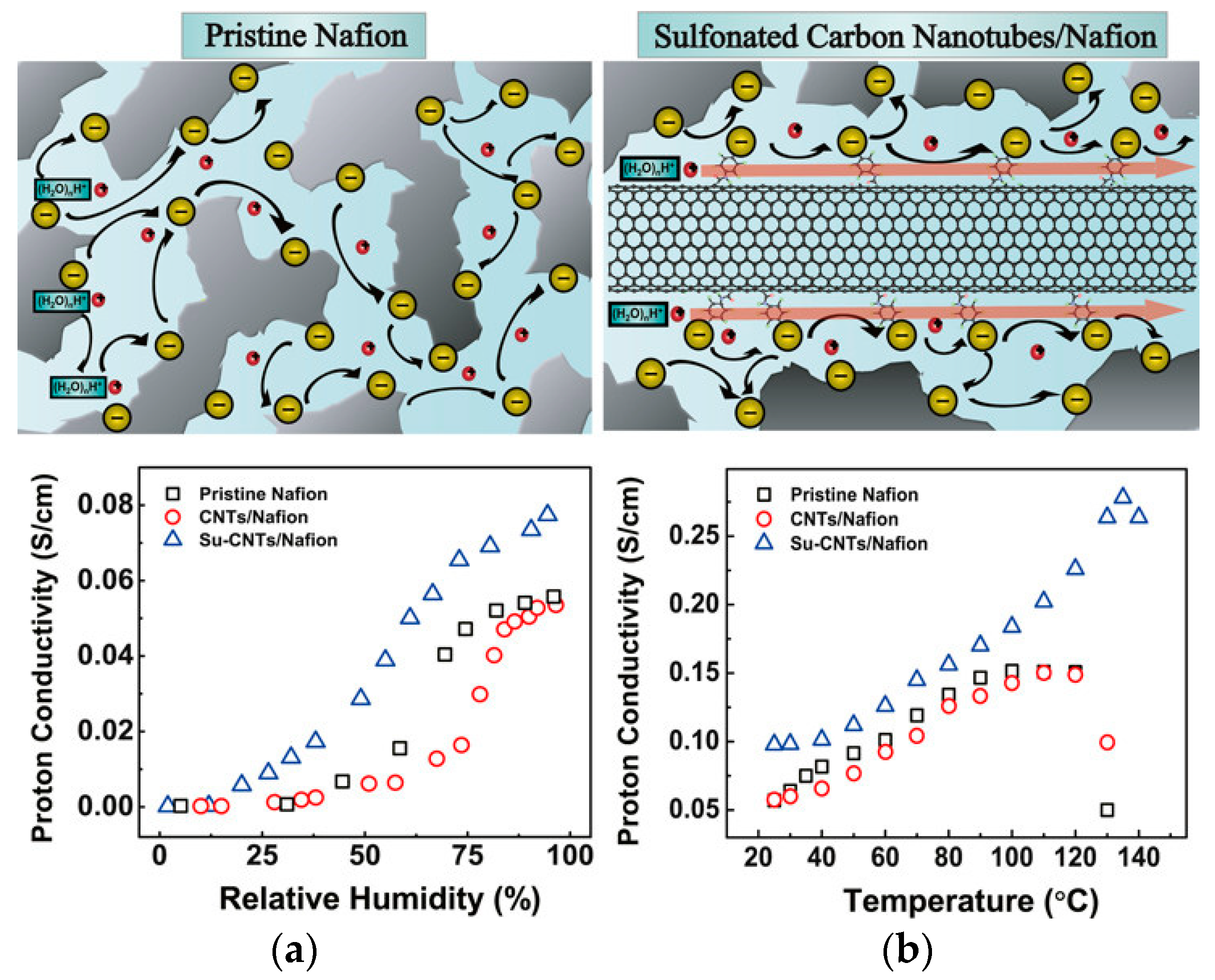
3.4. Promote Proton Conduction Materials
4. Modifying Methods Applied to Nafion Membranes
4.1. The Solution-Casting Method
4.2. The Swelling-Filling Method
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karanfil, G. Importance and applications of DOE/optimization methods in PEM fuel cells: A review. Int. J. Energy Res. 2019, 44, 4–25. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- Asim, A.Y.; Nabil, A.; Amira, S.Y.; Khalid, U. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar]
- Asim, A.Y.; Claudia, G.B.; Mohamad, N.M.I.; Khalid, U.A.; Suriaty, Y. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar]
- Xiao, X. The direct use of enzymatic biofuel cells as functional bioelectronics. eScience 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef]
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S. High-performance alkaline ionomer for alkaline exchange membrane fuel cells. Electrochem. Commun. 2013, 34, 278–281. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Adamiec, M. Research methodology of fuel cells. Prz. Elektrotechniczny 2010, 86, 334–337. [Google Scholar]
- Wang, S.; He, Z.; Wang, X.; Wang, C.; Li, X.; Zhao, Y. Ultrathin Semi-Interpenetrating Network Membranes Based on Perfluorinated Sulfonic Acid Resin and Polydivinylbenzene with Declined Hydrogen Crossover for Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2021, 168, 084508–084513. [Google Scholar] [CrossRef]
- He, H.; Zhu, Y.; Li, T.; Song, S.; Zhai, L.; Li, X.; Wu, L.; Li, H. Supramolecular Anchoring of Polyoxometalate Amphiphiles into Nafion Nanophases for Enhanced Proton Conduction. ACS Nano 2022, 16, 19240–19252. [Google Scholar] [CrossRef] [PubMed]
- Neelakandan, S.; Wang, L.; Zhang, B.; Ni, J.; Hu, M.; Gao, C.; Wong, W.-Y.; Wang, L. Branched Polymer Materials as Proton Exchange Membranes for Fuel Cell Applications. Polym. Rev. 2022, 62, 261–295. [Google Scholar] [CrossRef]
- Arslan, F.; Bohm, T.; Kerres, J.; Thiele, S. Spatially and temporally resolved monitoring of doping polybenzimidazole membranes with phosphoric acid. J. Membr. Sci. 2021, 625, 119145. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Lu, C.-L.; Chang, C.-P.; Guo, Y.-H.; Yeh, T.-K.; Su, Y.-C.; Wang, P.-C.; Hsueh, K.-L.; Tseng, F.-G. High-performance and low-leakage phosphoric acid fuel cell with synergic composite membrane stacking of micro glass microfiber and nano PTFE. Renew. Energy 2019, 134, 982–988. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L. Energy Efficiency Management of Coupling System for Molten Carbonate Fuel Cells. Int. J. Electrochem. Sci. 2022, 17, 220945. [Google Scholar] [CrossRef]
- Cassir, M.; McPhail, S.J.; Moreno, A. Strategies and new developments in the field of molten carbonates and high-temperature fuel cells in the carbon cycle. Int. J. Hydrogen Energy 2012, 37, 19345–19350. [Google Scholar] [CrossRef]
- Kulkarni, A.; Giddey, S. Materials issues and recent developments in molten carbonate fuel cells. J. Solid State Electrochem. 2012, 16, 3123–3146. [Google Scholar] [CrossRef]
- Kusnezoff, M.; Michaelis, A.; Schiel, J. Solid Oxide Fuel Cell Industry and Technology in Europe, Japan and the USA. Cfi-Ceram. Forum Int. 2013, 90, E27–E33. [Google Scholar]
- Wu, J.; Myung, J.-h.; Ding, D.; Zhu, T. Editorial: High Temperature Solid Oxide Cells. Front. Chem. 2021, 9, 719826. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, Y.S.; Lee, Y.D.; Kim, M.; Kim, D.K. Study on Internal Phenomena of Solid Oxide Fuel Cells Using Liquefied Natural Gas as Fuel. J. Electrochem. Soc. 2021, 168, 124513. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Jamil, A.; Rafiq, S.; Iqbal, T.; Khan, H.A.A.; Khan, H.M.; Azeem, B.; Mustafa, M.Z.; Hanbazazah, A.S. Current status and future perspectives of proton exchange membranes for hydrogen fuel cells. Chemosphere 2022, 303, 135204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Xing, L.; Tu, Z. Simulations and analysis of high-temperature proton exchange membrane fuel cell and its cooling system to power an automotive vehicle. Energy Convers. Manag. 2022, 253, 115182. [Google Scholar] [CrossRef]
- Chakraborty, U. Fuel crossover and internal current in proton exchange membrane fuel cell modeling. Appl. Energ. 2016, 163, 60–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Ma, L.; Cai, W.; Cheng, H. Recent Developments on Alternative Proton Exchange Membranes: Strategies for Systematic Performance Improvement. Energy Technol. 2015, 3, 675–691. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Huo, J.; Meng, X.; Cui, L.; Zhou, Q. Effects of Conducting Channels Microstructure in Proton Exchange Membrane on the Performance of Fuel Cells. Prog. Chem. 2015, 27, 395–403. [Google Scholar]
- Shimizu, G.K.H. Shrink-wrapping water to conduct protons. Nat. Energy 2017, 2, 842–843. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar] [CrossRef]
- Hasheminasab, M.; Kermani, M.J.; Nourazar, S.S.; Khodsiani, M.H. A novel experimental based statistical study for water management in proton exchange membrane fuel cells. Appl. Energy 2020, 264, 114713. [Google Scholar] [CrossRef]
- Janicka, E.; Mielniczek, M.; Gawel, L.; Darowicki, K.; Landowska, P. The impact of air humidity on the operation of proton exchange membrane fuel cells determined using dynamic electrochemical impedance spectroscopy. Electrochim. Acta 2020, 341, 136036. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications-A Review. Chemphyschem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, B.; Kim, J.-H.; Pak, C.; Moon, S.-H. High-temperature operation of PEMFC using pore-filling PTFE/Nafion composite membrane treated with electric field. Int. J. Energy Res. 2021, 45, 19136–19146. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Ni, J.; Xu, M.; Pan, C.; Wang, D.; Liu, D.; Wang, L. Preparation and investigation of block polybenzimidazole membranes with high battery performance and low phosphoric acid doping for use in high-temperature fuel cells. J. Membr. Sci. 2019, 572, 350–357. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Fang, M.; Chen, J.; Liu, X.; Yin, B.; Wang, L. Synthesis and preparation of branched block polybenzimidazole membranes with high proton conductivity and single-cell performance for use in high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2020, 602, 117981. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compan, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 81, 100859. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, H.; Kim, H.; Kim, D.; Kim, G.H.; Kwon, O.; Choi, H.; Cha, H.; Park, T. Non-destructive platinum catalyst-replenishment method for deteriorated polymer electrolyte membrane fuel cells. Energy Sci. Eng. 2022, 10, 414–422. [Google Scholar] [CrossRef]
- Friedman, A.; Mizrahi, M.; Levy, N.; Zion, N.; Zachman, M.; Elbaz, L. Application of Molecular Catalysts for the Oxygen Reduction Reaction in Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 58532–58538. [Google Scholar] [CrossRef]
- Zhang, Y.; Wilkinson, D.P.; Taghipour, F. Performance Analysis of an Air-Breathing Micro-Direct Methanol Fuel Cell with an Extended Anode Region. Fuel Cells 2020, 20, 634–642. [Google Scholar] [CrossRef]
- El-Hay, E.A.; El-Hameed, M.A.; El-Fergany, A.A. Performance enhancement of autonomous system comprising proton exchange membrane fuel cells and switched reluctance motor. Energy 2018, 163, 699–711. [Google Scholar] [CrossRef]
- Kuan, Y.-D.; Chang, J.-Y.; Ku, H.-T. Proton exchange membrane fuel cell purge and fan control using a microcontroller. Int. J. Green Energy 2017, 14, 86–91. [Google Scholar] [CrossRef]
- Sun, H.; Sun, Z.; Wu, Y. Proton transfer mechanism in perfluorinated sulfonic acid polytetrafluoroethylene. Int. J. Hydrogen Energy 2012, 37, 12821–12826. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Noto, V.D. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Oh, K.-H.; Kang, H.S.; Choo, M.-J.; Jang, D.-H.; Lee, D.; Lee, D.G.; Kim, T.-H.; Hong, Y.T.; Park, J.-K.; Kim, H.-T. Interlocking Membrane/Catalyst Layer Interface for High Mechanical Robustness of Hydrocarbon-Membrane-Based Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. 2015, 27, 2974–2980. [Google Scholar] [CrossRef]
- Raja, P.M.; Gayathri, R.; Cao, G.; Ramesh, P.M. Study of amine customized exfoliated BN sheets in SPEEK-PES based blend membrane for acid-base cation exchange membrane fuel cells. J. Environ. Chem. Eng. 2019, 10, 107025. [Google Scholar]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Liu, F.; Wang, D.; Li, J.; Mao, T.; Liu, G.; Wang, X.; Xu, J.; Wang, Z. Base-acid doped polybenzimidazole with high phosphoric acid retention for HT-PEMFC applications. J. Membr. Sci. 2019, 596, 117722. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.; Bjerrum, N. PBI-based polymer membranes for high temperature fuel cells–preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Qu, T.; Hu, J.; Tan, Q.; Liu, Y.; Chen, Y.; Sun, J.; Liu, Y. Effect of phosphoric acid-doped polybenzimidazole membranes on the performance of H+-ion concentration cell. Int. J. Hydrogen Energy 2021, 46, 4354–4364. [Google Scholar] [CrossRef]
- Fencl, V.; Leith, D.E. Stewart’s quantitative acid-base chemistry: Applications in biology and medicine. Respir. Physiol. 1993, 91, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, L.; Mu, D.; Yu, L.; Wang, L.; Xi, J. Acid-base membranes of imidazole-based sulfonated polyimides for vanadium flow batteries. J. Membr. Sci. 2018, 552, 167–176. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Abu-Saied, M.A.; Elzatahry, A.A.; El-Khatib, K.M.; Hassan, E.A.; El-Sabbah, M.M. Novel Acid-Base Poly vinyl chloride-Doped Ortho-Phosphoric Acid Membranes for Fuel Cell Applications. Int. J. Electrochem. Sci. 2011, 6, 5417–5429. [Google Scholar]
- Yan, H.; Peng, K.; Yan, J.; Jiang, C.; Wang, Y.; Feng, H.; Yang, Z.; Wu, L.; Xu, T. Bipolar membrane-assisted reverse electrodialysis for high power density energy conversion via acid-base neutralization. J. Membr. Sci. 2022, 647, 120288. [Google Scholar] [CrossRef]
- Silambarasan, P.; Ramu, A.G.; Govarthanan, M.; Kim, W.; Moon, I.S. Cerium-polysulfide redox flow battery with possible high energy density enabled by MFI-Zeolite membrane working with acid-base electrolytes. Chemosphere 2021, 291, 132680. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Dang, J.; Zhou, G.; Wang, Y.; Zhang, Y.; Qu, L.; Wu, W. Lamellar composite membrane with acid-base pair anchored layer-by-layer structure towards highly enhanced conductivity and stability. J. Membr. Sci. 2020, 602, 117978. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Che, Q. Multilayered Membrane Electrolytes Based on Aramid Nanofibers for High-Temperature Proton Exchange Membrane Fuel Cells. ACS Appl. Nano Mater. 2019, 2, 2160–2168. [Google Scholar] [CrossRef]
- Lee, H.-F.; Wang, P.-C.; Chen-Yang, Y.W. An electrospun hygroscopic and electron-conductive core-shell silica@carbon nanofiber for microporous layer in proton-exchange membrane fuel cell. J. Solid State Electrochem. 2019, 23, 971–984. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Wu, H.; Jiang, Z. Enhanced proton conductivity of the hybrid membranes by regulating the proton conducting groups anchored on the mesoporous silica. J. Power Sources 2014, 270, 292–303. [Google Scholar] [CrossRef]
- Matos, B.R.; Santiago, E.I.; Rey, J.F.Q.; Scuracchio, C.H.; Mantovani, G.L.; Hirano, L.A.; Fonseca, F.C. dcProton conductivity at low-frequency in Nafion conductivity spectrum probed by time-resolved SAXS measurements and impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 822–828. [Google Scholar] [CrossRef]
- Dresch, M.A.; Matos, B.R.; Fonseca, F.C.; Santiago, E.I.; Carmo, M.; Lanfredi, A.J.C.; Balog, S. Small-angle X-ray and neutron scattering study of Nafion-SiO2 hybrid membranes prepared in different solvent media. J. Power Sources 2015, 274, 560–567. [Google Scholar] [CrossRef]
- Haubold, H.G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 46, 1559–1563. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Deng, N.; Wang, X.; Xiang, H.; Cheng, B.; Kang, W. Electrospun multi-scale nanofiber network: Hierarchical proton-conducting channels in Nafion composite proton exchange membranes. Cellu 2021, 28, 6567–6585. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Non-humidified fuel cells using a deep eutectic solvent (DES) as the electrolyte within a polymer electrolyte membrane (PEM): The effect of water and counterions. Phys. Chem. Chem. Phys. 2020, 22, 2917–2929. [Google Scholar] [CrossRef]
- Han, A.; Fu, C.; Yan, X.; Chen, J.; Cheng, X.; Ke, C.; Hou, J.; Shen, S.; Zhang, J. Effect of cobalt ion contamination on proton conduction of ultrathin Nafion film. Int. J. Hydrogen Energy 2020, 45, 25276–25285. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sa, A.I.; Teixeira, A.P.S.; Rangel, C.M. Nafion phosphonic acid composite membranes for proton exchange membranes fuel cells. Appl. Surf. Sci. 2019, 487, 889–897. [Google Scholar] [CrossRef]
- Ressam, I.; Krins, N.; Laberty-Robert, C.; Selmane, M.; Lahcini, M.; Raihane, M.; El Kadib, A.; Perrot, H.; Sel, O. Sulfonic Acid Functionalized Chitosan as a Sustainable Component for Proton Conductivity Management in PEMs. Chemistryselect 2017, 2, 2503–2511. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Kompan, M.E.; Malyshkin, V.V.; Rosanov, V.V.; Stejskal, J. Properties of proton-conducting Nafion-type membranes with Nanometer-thick polyaniline surface layers. Russ. J. Electrochem. 2009, 45, 697–706. [Google Scholar]
- Zhou, D.; Ge, A.; Kogina, T.; Inoue, K.-i.; Chen, Y.-X.; Ye, S. Molecular Structures at Nafion/Graphene Interfaces Investigated by Sum-Frequency Generation Spectroscopy. J. Phys. Chem. C 2022, 126, 6523–6530. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Xu, W.; Long, J.; Huang, W.; He, Z.; Liu, S.; Zhang, Y. A Sulfonated Polyimide/Nafion Blend Membrane with High Proton Selectivity and Remarkable Stability for Vanadium Redox Flow Battery. Membranes 2021, 11, 946. [Google Scholar] [CrossRef]
- Asadchikov, V.E.; Bunkin, N.F.; Volkov, V.V.; Volkov, Y.O.; Nuzhdin, A.D.; Stepina, N.D.; Roshchin, B.S.; Tikhonov, A.M. On the Influence of the Alkaline Composition of Liquid Subphase on the Nafion Film Morphology. Phys. Wave Phenom. 2021, 29, 131–135. [Google Scholar] [CrossRef]
- Loccufier, E.; Geltmeyer, J.; Daelemans, L.; D’Hooge, D.R.; De Buysser, K.; De Clerck, K. Silica Nanofibrous Membranes for the Separation of Heterogeneous Azeotropes. Adv. Funct. Mater. 2018, 28, 1804138. [Google Scholar] [CrossRef]
- Feng, G.; Wang, J.; Boronat, M.; Li, Y.; Su, J.H.; Huang, J.; Ma, Y.; Yu, J. Radical-Facilitated Green Synthesis of Highly Ordered Mesoporous Silica Materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Hung, Y.; Mou, C.Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Kulkarni, S.; Mauritz, K.A. In situ grown titania composition for optimal performance and durability of Nafion® fuel cell membranes. J. Appl. Polym. Sci. 2011, 121, 2344–2353. [Google Scholar] [CrossRef]
- Santiago, E.I.; Isidoro, R.A.; Dresch, M.A.; Matos, B.R.; Linardi, M.; Fonseca, F.C. Nafion TiO2 hybrid electrolytes for stable operation of PEM fuel cells at high temperature. Electrochim. Acta 2009, 54, 4111–4117. [Google Scholar] [CrossRef]
- Divona, M.; Ahmed, Z.; Bellitto, S.; Lenci, A.; Traversa, E.; Licoccia, S. SPEEK-TiO2 nanocomposite hybrid proton conductive membranes via in situ mixed sol–gel process. J. Membr. Sci. 2007, 296, 156–161. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Vatanparast, M. Ultrasonic-assisted synthesis of ZrO2 nanoparticles and their application to improve the chemical stability of Nafion membrane in proton exchange membrane (PEM) fuel cells. J. Colloid Interface Sci. 2016, 483, 1–10. [Google Scholar] [CrossRef]
- Guzman, C.; Alvarez, A.; Godinez, L.A.; Ledesma-Garcia, J.; Arriaga, L.G. Evaluation of ZrO2 Composite Membrane Operating at High Temperature (100 degrees C) in Direct Methanol Fuel Cells. Int. J. Electrochem. Sci. 2012, 7, 6106–6117. [Google Scholar]
- Gandhi, K.; Dixit, B.K.; Dixit, D.K. Effect of addition of zirconium tungstate, lead tungstate and titanium dioxide on the proton conductivity of polystyrene porous membrane. Int. J. Hydrogen Energy 2012, 37, 3922–3930. [Google Scholar] [CrossRef]
- Ke, C.; Li, X.; Qu, S.; Shao, Z.; Yi, B. Preparation and properties of Nafion SiO2 composite membrane derived via in situ sol gel reaction size controlling and size effects of SiO2 nano particles. Polym. Adv. Technol. 2012, 23, 92–98. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Passalacqua, E.; D’Epifanio, A.; Licoccia, S.; Traversa, E.; Sala, E.; Traini, F.; Ornelas, R. Nafion–TiO2 hybrid membranes for medium temperature polymer electrolyte fuel cells (PEFCs). J. Power Sources 2005, 152, 16–21. [Google Scholar] [CrossRef]
- Baglio, V.; Di Blasi, A.; Aricò, A.S.; Antonucci, V.; Antonucci, P.L.; Trakanprapai, C.; Esposito, V.; Licoccia, S.; Traversa, E. Composite Mesoporous Titania Nafion Based Membranes for Direct Methanol Fuel Cell Operation at High Temperature. J. Electrochem. Soc. 2005, 152, A1373. [Google Scholar] [CrossRef]
- Yana, J.; Nimmanpipug, P.; Chirachanchai, S.; Gosalawit, R.; Dokmaisrijan, S.; Vannarat, S.; Vilaithong, T.; Lee, V.S. Molecular dynamics simulations of Krytox-Silica–Nafion composite for high temperature fuel cell electrolyte membranes. Polymer 2010, 51, 4632–4638. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhuang, X.; Cheng, B.; Wang, W.; Kang, W.; Shi, L.; Li, H. Modification of Nafion membrane with biofunctional SiO2 nanofiber for proton exchange membrane fuel cells. J. Power Sources 2017, 340, 201–209. [Google Scholar] [CrossRef]
- Xu, G.; Zou, J.; Guo, Z.; Li, J.; Ma, L.; Li, Y.; Cai, W. Bi-Functional Composting the Sulfonic Acid Based Proton Exchange Membrane for High Temperature Fuel Cell Application. Polymers 2020, 12, 1000. [Google Scholar] [CrossRef]
- Ko, E.H.; Yoon, Y.; Park, J.H.; Yang, S.H.; Hong, D.; Lee, K.B.; Shon, H.K.; Lee, T.G.; Choi, I.S. Bioinspired, cytocompatible mineralization of silica-titania composites: Thermoprotective nanoshell formation for individual chlorella cells. Angew. Chem. 2013, 52, 12279–12282. [Google Scholar] [CrossRef] [PubMed]
- López, G.P.; López, R.R.; Viveros, T. Dehydrocyclization of n-heptane over Pt catalysts supported on Al-and Si-promoted TiO2. Catal. Today 2014, 220, 61–65. [Google Scholar] [CrossRef]
- Byrne, H.E.; Mazyck, D.W. Removal of trace level aqueous mercury by adsorption and photocatalysis on silica titania composites. J. Hazard. Mater. 2009, 170, 915–919. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. Extension of the Stöber method to construct mesoporous SiO2 and TiO2 shells for uniform multifunctional core shell structures. Adv. Mater. 2013, 25, 142–149. [Google Scholar] [CrossRef]
- Sayeed, M.D.A.; Kim, H.J.; Park, Y.; Gopalan, A.I.; Kim, Y.H.; Lee, K.-P.; Choi, S.-J. Sulfated Titania Silica Reinforced Nafion Nanocomposite Membranes for Proton Exchange Membrane Fuel Cells. J. Nanosci. Nanotechnol. 2015, 15, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Wu, H.; Cao, L.; He, X.; Zhang, B.; Wang, C.; Jiang, Z. One pot synthesis of silica titania binary nanoparticles with acid base pairs via biomimetic mineralization to fabricate highly proton conductive membranes. J. Mater. Chem. A 2017, 5, 18585–18593. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.; Jeon, Y.; Park, J.-I.; Einaga, H.; Truong, Y.B.; Kyratzis, I.L.; Mochida, I.; Choi, J.; Shul, Y.-G. Phosphate-Modified TiO2/ZrO2 Nanofibrous Web Composite Membrane for Enhanced Performance and Durability of High-Temperature Proton Exchange Membrane Fuel Cells. Energy Fuels 2017, 31, 7645–7652. [Google Scholar] [CrossRef]
- Cavani, F. Heteropolycompound-based catalysts: A blend of acid and oxidizing properties. Catal. Today 1998, 41, 73–86. [Google Scholar] [CrossRef]
- Mioč, U.; Davidović, M.; Tjapkin, N.; Colomban, P.; Novak, A. Equilibrium of the protonic species in hydrates of some heteropolyacids at elevated temperatures. Solid State Ion. 1991, 46, 103–109. [Google Scholar] [CrossRef]
- Wu, H.; Shen, X.; Cao, Y.; Li, Z.; Jiang, Z. Composite proton conductive membranes composed of sulfonated poly (ether ether ketone) and phosphotungstic acid-loaded imidazole microcapsules as acid reservoirs. J. Membr. Sci. 2014, 451, 74–84. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Zhang, J.; Lan, F.; Lu, S. Phosphotungstic acid (HPW) molecules anchored in the bulk of Nafion as methanol-blocking membrane for direct methanol fuel cells. J. Membr. Sci. 2011, 368, 241–245. [Google Scholar] [CrossRef]
- Bose, A.B.; Gopu, S.; Li, W. Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes. J. Power Sources 2014, 263, 217–222. [Google Scholar] [CrossRef]
- Abouzari-lotf, E.; Nasef, M.M.; Ghassemi, H.; Zakeri, M.; Ahmad, A.; Abdollahi, Y. Improved Methanol Barrier Property of Nafion Hybrid Membrane by Incorporating Nanofibrous Interlayer Self-Immobilized with High Level of Phosphotungstic Acid. ACS Appl. Mater. Interfaces 2015, 7, 17008–17015. [Google Scholar] [CrossRef]
- Lu, S.; Wang, D.; Jiang, S.P.; Xiang, Y.; Lu, J.; Zeng, J. HPW/MCM-41 phosphotungstic acid/mesoporous silica composites as novel proton-exchange membranes for elevated-temperature fuel cells. Adv. Mater. 2010, 22, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Joghee, P.; Hsing, I.M. Preparation and characterization of hybrid Nafion silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Membr. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Xu, W.; Lu, T.; Liu, C.; Xing, W. Low methanol permeable composite Nafion/silica/PWA membranes for low temperature direct methanol fuel cells. Electrochim. Acta 2005, 50, 3280–3285. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; VanDersarl, J.J.; Bertsch, A.; Renaud, P.; Jacob, K.I. Ionic nanopeapods: Next-generation proton conducting membranes based on phosphotungstic acid filled carbon nanotube. Nano Energy 2016, 23, 114–121. [Google Scholar] [CrossRef]
- Yang, X.-B.; Meng, L.-H.; Sui, X.-L.; Wang, Z.-B. High proton conductivity polybenzimidazole proton exchange membrane based on phosphotungstic acid-anchored nano-Kevlar fibers. J. Mater. Sci. 2018, 54, 1640–1653. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, L.; Sui, X.L.; Meng, L.H.; Wang, Z.B. Phosphotungstic acid immobilized nanofibers-Nafion composite membrane with low vanadium permeability and high selectivity for vanadium redox flow battery. J Colloid Interface Sci 2019, 542, 177–186. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Jiang, S.; Li, Z.; He, G.; Wu, H. Enhanced proton conductivity of Nafion nanohybrid membrane incorporated with phosphonic acid functionalized graphene oxide at elevated temperature and low humidity. J. Membr. Sci. 2016, 518, 243–253. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hossain, O.; Chaggar, J.; Steinberger-Wilckens, R.; El-Kharouf, A. GO-nafion composite membrane development for enabling intermediate temperature operation of polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2020, 45, 5526–5534. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Liu, J.; Wang, J.; He, Y.; Davey, K.; Qiao, S.Z. Molecular-Level Hybridization of Nafion with Quantum Dots for Highly Enhanced Proton Conduction. Adv. Mater. 2018, 30, e1707516. [Google Scholar] [CrossRef]
- Firouz Tadavani, K.; Abdolmaleki, A.; Molavian, M.R.; Borandeh, S.; Sorvand, E.; Zhiani, M. Synergistic Behavior of Phosphonated and Sulfonated Groups on Proton Conductivity and Their Performance for High-Temperature Proton Exchange Membrane Fuel Cells (PEMFCs). Energy Fuels 2017, 31, 11460–11470. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Woo, S.P.; Yoon, Y.S. Nafion based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell. Compos. Sci. Technol. 2018, 155, 189–196. [Google Scholar] [CrossRef]
- Klose, C.; Breitwieser, M.; Vierrath, S.; Klingele, M.; Cho, H.; Büchler, A.; Kerres, J.; Thiele, S. Electrospun sulfonated poly(ether ketone) nanofibers as proton conductive reinforcement for durable Nafion composite membranes. J. Power Sources 2017, 361, 237–242. [Google Scholar] [CrossRef]
- Yan, L.M.; Zhu, S.H.; Ji, X.B.; Lu, W.C. Proton hopping in phosphoric acid solvated nafion membrane: A molecular simulation study. J. Phys. Chem. B 2007, 111, 6357–6363. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Pan, C.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Phosphoric acid doped membranes based on Nafion, PBI and their blends Membrane preparation, characterization and steam electrolysis testing. Int. J. Hydrogen Energy 2011, 36, 6985–6993. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Zhang, Y.; Xing, D. A novel H3PO4/Nafion PBI composite membrane for enhanced durability of high temperature PEM fuel cells. J. Power Sources 2007, 169, 259–264. [Google Scholar] [CrossRef]
- Kim, J.D.; Suzuki, A.; Jun, M.S. Nafion-Azole-H3PO4 Composite Membranes using Solution Processing for High Temperature PEMFCs. In Polymer Electrolyte Fuel Cells 2013, 58, 1185–1194. [Google Scholar]
- Yin, Y.; Li, Z.; Yang, X.; Cao, L.; Wang, C.; Zhang, B.; Wu, H.; Jiang, Z. Enhanced proton conductivity of Nafion composite membrane by incorporating phosphoric acid-loaded covalent organic framework. J. Power Sources 2016, 332, 265–273. [Google Scholar] [CrossRef]
- Yang, J.; Che, Q.; Zhou, L.; He, R.; Savinell, R.F. Studies of a high temperature proton exchange membrane based on incorporating an ionic liquid cation 1-butyl-3-methylimidazolium into a Nafion matrix. Electrochim. Acta 2011, 56, 5940–5946. [Google Scholar] [CrossRef]
- Lu, F.; Gao, X.; Yan, X.; Gao, H.; Shi, L.; Jia, H.; Zheng, L. Preparation and characterization of nonaqueous proton-conducting membranes with protic ionic liquids. ACS Appl. Mater. Interfaces 2013, 5, 7626–7632. [Google Scholar] [CrossRef]
- Sunda, A.P. Ammonium-based protic ionic liquid doped Nafion membranes as anhydrous fuel cell electrolytes. J. Mater. Chem. A 2015, 3, 12905–12912. [Google Scholar] [CrossRef]
- Lee, J.-W.; Yi, C.-W.; Kim, K. Phosphoric Acid-Functionalized Mesoporous Silica/Nafion Composite Membrane for High Temperature PEMFCs. Bull. Korean Chem. Soc. 2012, 33, 1397–1400. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Lu, S.; Zhu, H.; Aili, D.; De Marco, R.; Xiang, Y.; Forsyth, M.; Li, Q.; Jiang, S. Ion-Exchange-Induced Selective Etching for the Synthesis of Amino-Functionalized Hollow Mesoporous Silica for Elevated-High-Temperature Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 31922–31930. [Google Scholar] [CrossRef]
- Ketpang, K.; Son, B.; Lee, D.; Shanmugam, S. Porous zirconium oxide nanotube modified Nafion composite membrane for polymer electrolyte membrane fuel cells operated under dry conditions. J. Membr. Sci. 2015, 488, 154–165. [Google Scholar] [CrossRef]
- Li, J.; Fan, K.; Cai, W.; Ma, L.; Xu, G.; Xu, S.; Ma, L.; Cheng, H. An in-situ nano-scale swelling-filling strategy to improve overall performance of Nafion membrane for direct methanol fuel cell application. J. Power Sources 2016, 332, 37–41. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of nano-size of functionalized silica on overall performance of swelling-filling modified Nafion membrane for direct methanol fuel cell application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Xu, G.; Li, J.; Ma, L.; Xiong, J.; Mansoor, M.; Cai, W.; Cheng, H. Performance dependence of swelling-filling treated Nafion membrane on nano-structure of macromolecular filler. J. Membr. Sci. 2017, 534, 68–72. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Wei, Z.; Zhang, W.; Wu, J.; Li, Y.; Li, J.; Qu, K.; Cai, W. Non-destructive fabrication of Nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renew. Energy 2020, 153, 935–939. [Google Scholar] [CrossRef]
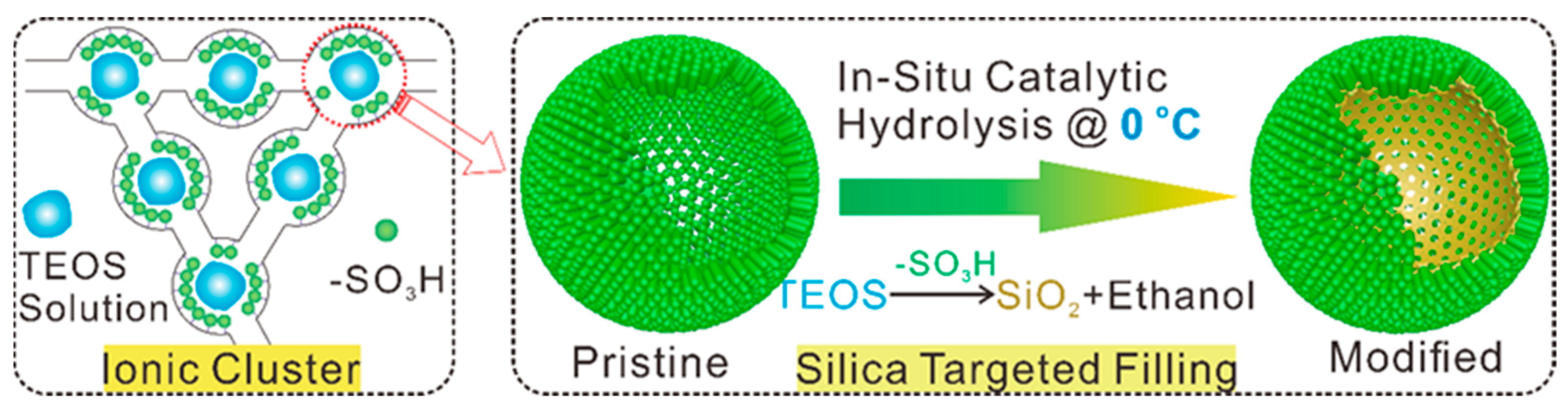
- Xu, G.; Li, S.; Li, J.; Liu, Z.; Li, Y.; Xiong, J.; Cai, W.; Qu, K.; Cheng, H. Targeted filling of silica in Nafion by a modified in situ sol-gel method for enhanced fuel cell performance at elevated temperatures and low humidity. Chem. Commun. 2019, 55, 5499–5502. [Google Scholar] [CrossRef] [PubMed]
- Stefanithis, I.D.; Mauritz, K.A. Microstructural Evolution of a Silicon Oxide Phase in a Perfluorosulfonic Acid Ionomer by an in Situ Sol-Gel Reaction. Macromolecules 1990, 23, 1380–1388. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Stefanithis, I.D.; Davis, V.; Scheetz, R.W.; Pope, R.K.; Wilkes, G.L.; Huanc, H. Microstructural Evolution of a Silicon Oxide Phase in a Perfluorosulfonic Acid lonomer by an In Situ Sol-Gel Reaction. J. Appl. Polym. Sci. 1995, 55, 181–190. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Xu, G.; Xue, S.; Wei, Z.; Li, J.; Qu, K.; Li, Y.; Cai, W. Stabilizing phosphotungstic acid in Nafion membrane via targeted silica fixation for high-temperature fuel cell application. Int. J. Hydrogen Energy 2021, 46, 4301–4308. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Xie, Z.; Wei, Z.; Li, J.; Qu, K.; Li, Y.; Cai, W. Graphene quantum dot reinforced hyperbranched polyamide proton exchange membrane for direct methanol fuel cell. Int. J. Hydrogen Energy 2021, 46, 9782–9789. [Google Scholar] [CrossRef]
- Xu, G.X.; Wei, Z.L.; Li, S.; Li, J.; Yang, Z.H.; Grigoriev, S.A. In-situ sulfonation of targeted silica-filled Nafion for high-temperature PEM fuel cell application. Int. J. Hydrogen Energy 2019, 44, 29711–29716. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Dong, X.; Xue, B.; Huang, J.; Wu, J.; Cai, W. Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies 2023, 16, 1565. https://doi.org/10.3390/en16041565
Xu G, Dong X, Xue B, Huang J, Wu J, Cai W. Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies. 2023; 16(4):1565. https://doi.org/10.3390/en16041565
Chicago/Turabian StyleXu, Guoxiao, Xinwei Dong, Bin Xue, Jianyou Huang, Junli Wu, and Weiwei Cai. 2023. "Recent Approaches to Achieve High Temperature Operation of Nafion Membranes" Energies 16, no. 4: 1565. https://doi.org/10.3390/en16041565
APA StyleXu, G., Dong, X., Xue, B., Huang, J., Wu, J., & Cai, W. (2023). Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies, 16(4), 1565. https://doi.org/10.3390/en16041565





