Experimental Investigation of Graphene Nanoplatelets Enhanced Low Temperature Ternary Eutectic Salt Hydrate Phase Change Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Thermal Properties of Ternary Eutectic PCMs
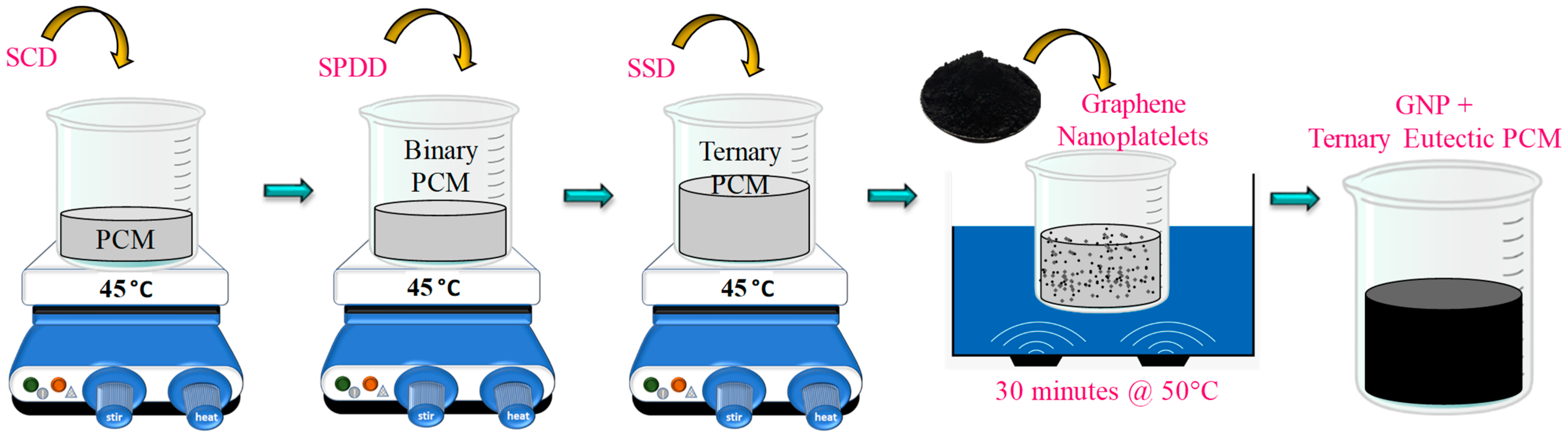
2.3. Preparation of Ternary Eutectic PCMs
2.4. Instruments and Characterization Techniques
3. Results and Discussion
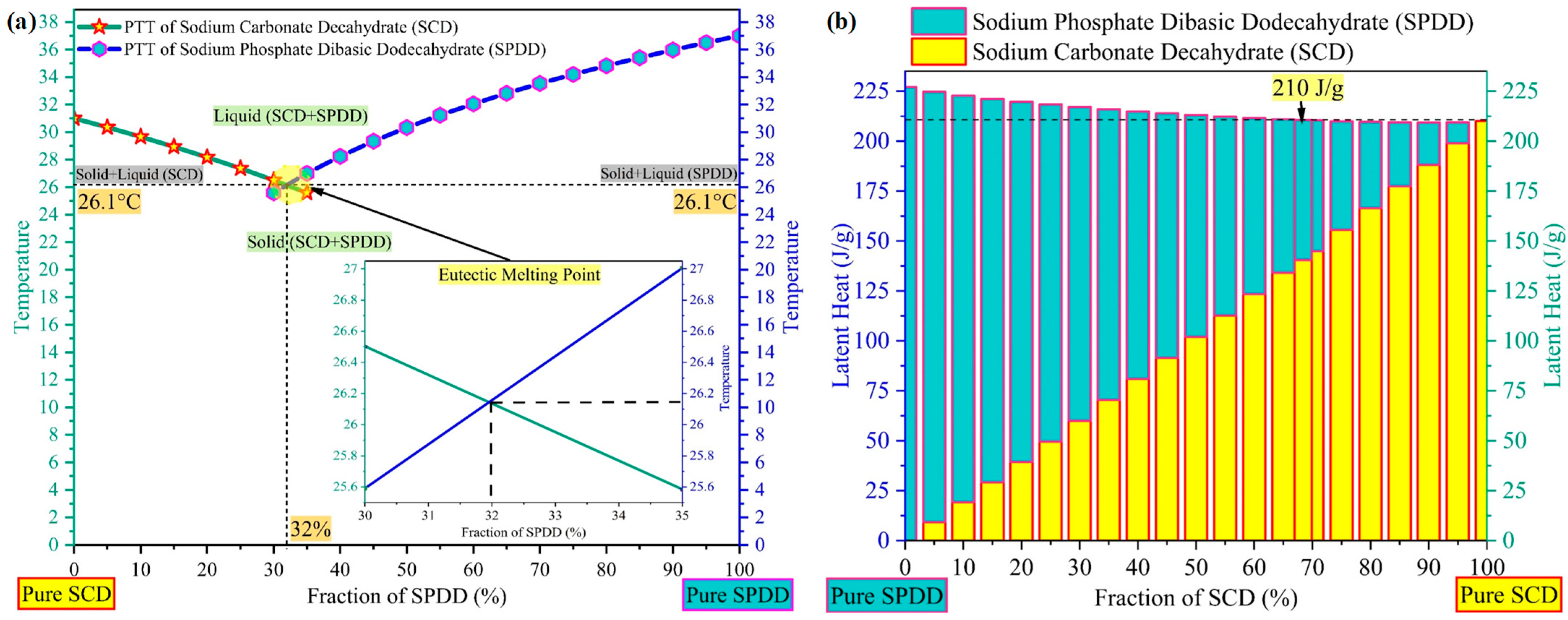
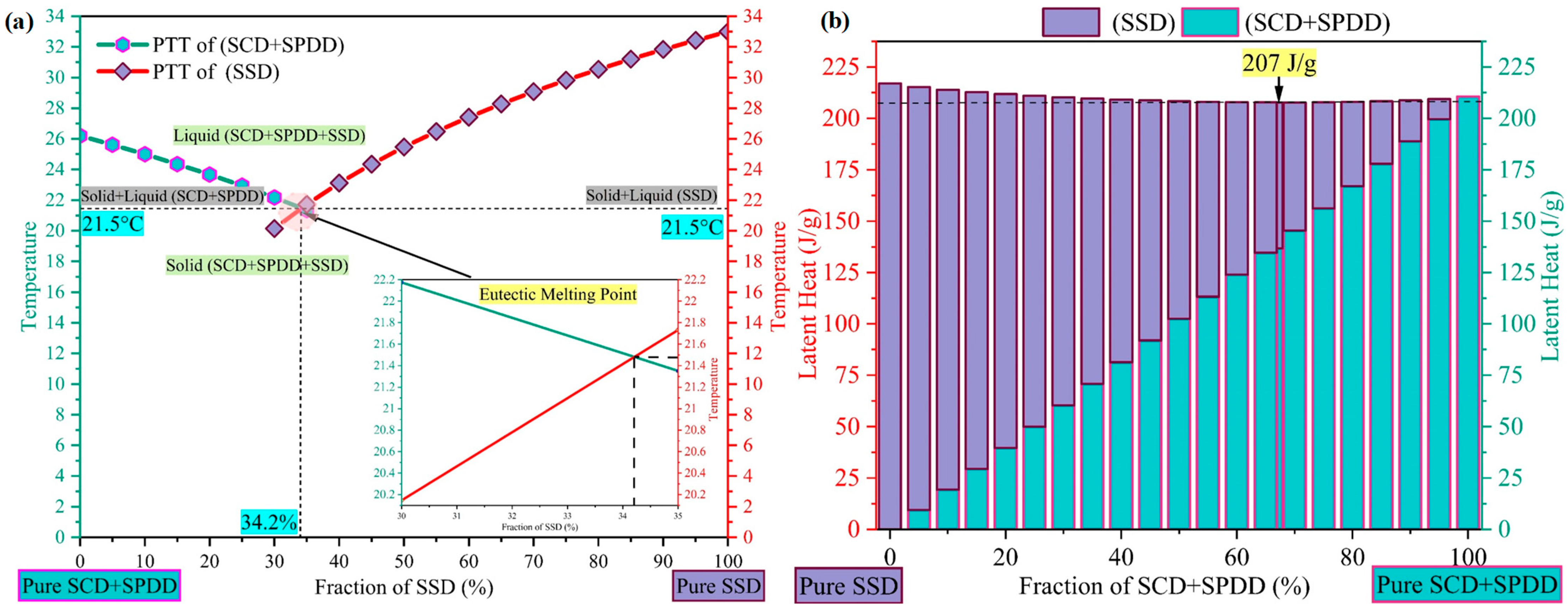
3.1. Design and Development of Ternary Eutectic Salt Hydrate PCM
3.2. Morphological Behavior
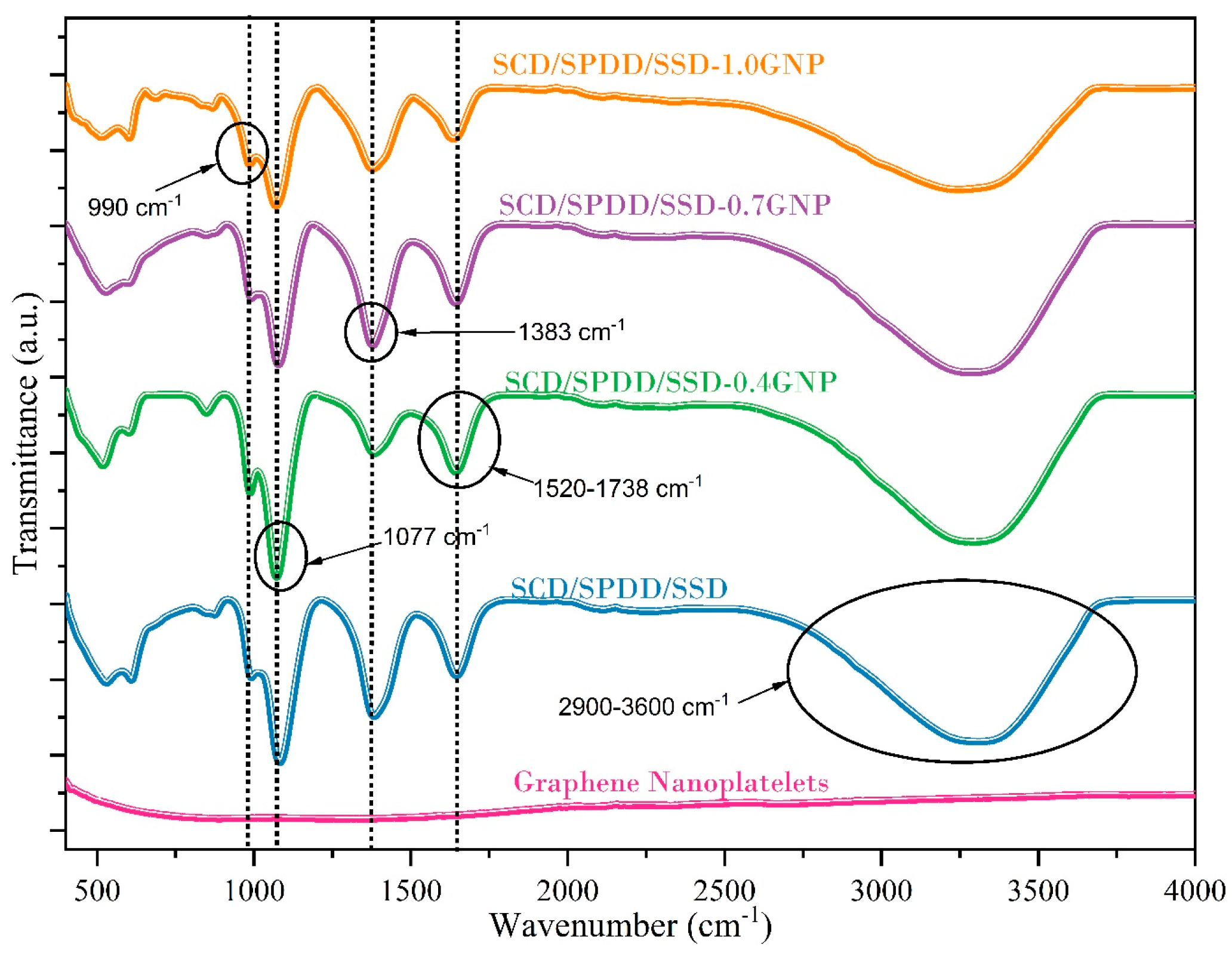
3.3. Chemical Stability
3.4. Optical Property Analysis
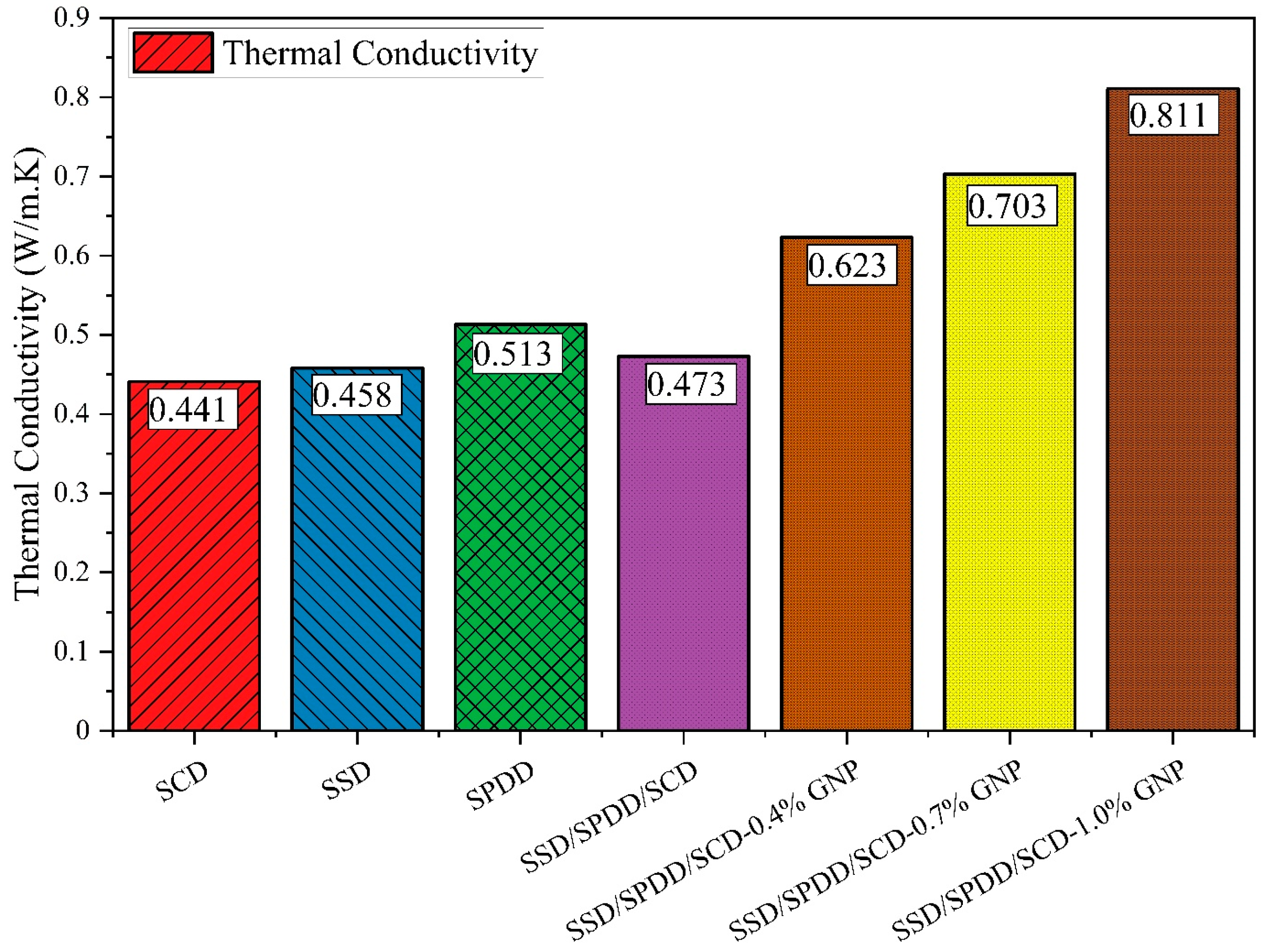
3.5. Thermal Conductivity
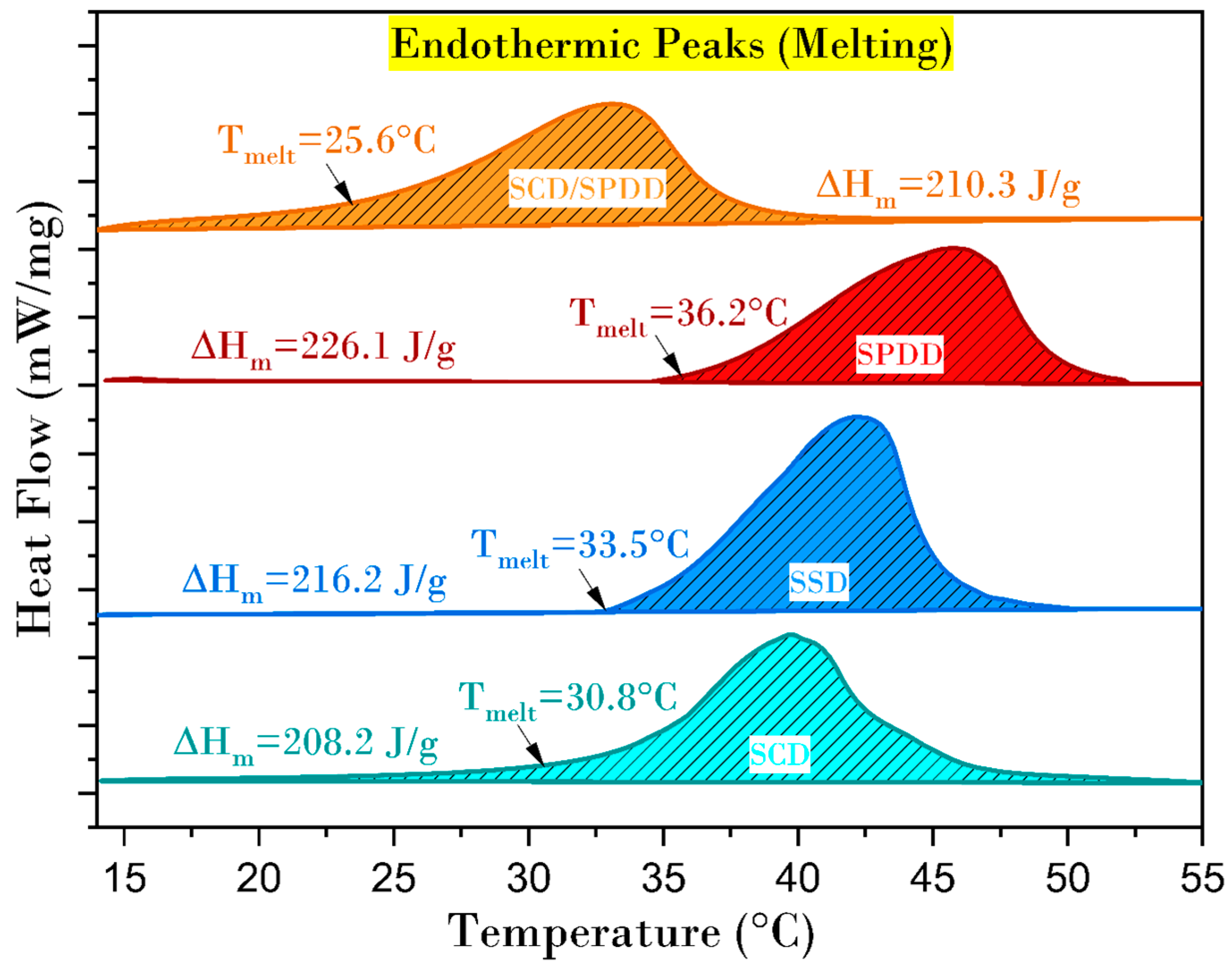
3.6. Melting Temperature, Latent Heat Storage and Degree of Supercooling
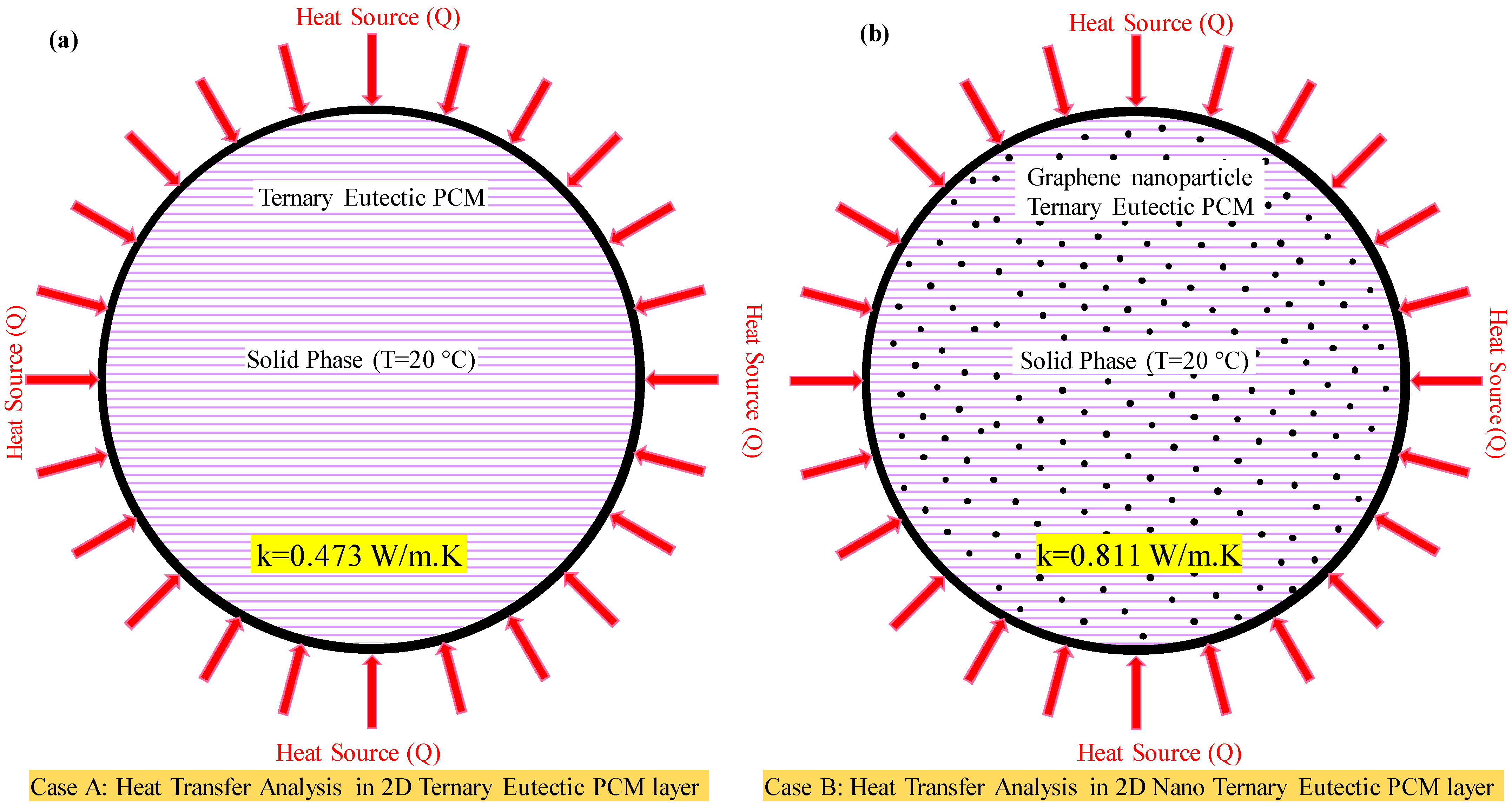
3.7. Numerical Analysis of Ternary Eutectic PCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, K.; Liu, Y.; Yang, Y. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.; Shahabuddin, S.; Samykano, M.; Thirugnanasambandam, M.; Saidur, R. Phase change materials integrated solar thermal energy systems: Global trends and current practices in experimental approaches. J. Energy Storage 2020, 27, 101118. [Google Scholar]
- Kalidasan, B.; Pandey, A.; Saidur, R.; Samykano, M.; Tyagi, V. Nano additive enhanced salt hydrate phase change materials for thermal energy storage. Int. Mater. Rev. 2022, 1–44. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Wang, S.; Ding, Y.; Gao, X.; Zhang, Z.; Fang, Y. SiO2 hydrophilic modification of expanded graphite to fabricate form-stable ternary nitrate composite room temperature phase change material for thermal energy storage. Chem. Eng. J. 2021, 413, 127549. [Google Scholar] [CrossRef]
- Tian, H.; Du, L.; Wei, X.; Deng, S.; Wang, W.; Ding, J. Enhanced thermal conductivity of ternary carbonate salt phase change material with Mg particles for solar thermal energy storage. Appl. Energy 2017, 204, 525–530. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Novel high thermal stability LiF–Na2CO3–K2CO3 eutectic ternary system for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2015, 140, 366–375. [Google Scholar] [CrossRef]
- Liang, L.; Chen, X. Preparation and thermal properties of eutectic hydrate salt phase change thermal energy storage material. Int. J. Photoenergy 2018, 2018, 6432047. [Google Scholar] [CrossRef]
- Sun, W.; Huang, R.; Ling, Z.; Fang, X.; Zhang, Z. Two types of composite phase change panels containing a ternary hydrated salt mixture for use in building envelope and ventilation system. Energy Convers. Manag. 2018, 177, 306–314. [Google Scholar] [CrossRef]
- Efimova, A.; Pinnau, S.; Mischke, M.; Breitkopf, C.; Ruck, M.; Schmidt, P. Development of salt hydrate eutectics as latent heat storage for air conditioning and cooling. Thermochim. Acta 2014, 575, 276–278. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and characterization of lauric–myristic–palmitic acid ternary eutectic mixtures/expanded graphite composite phase change material for thermal energy storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
- Jebasingh, B.E. Preparation of organic based ternary eutectic fatty acid mixture as phase change material (PCM), optimizing their thermal properties by enriched solar treated exfoliated graphite for energy storage. Mater. Today Proc. 2016, 3, 1592–1598. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Huang, Z.; Yin, Z.; Wen, R.; Huang, Y.; Wu, X.; Min, X. Preparation and characterization of capric-palmitic-stearic acid ternary eutectic mixture/expanded vermiculite composites as form-stabilized thermal energy storage materials. J. Mater. Sci. Technol. 2018, 34, 379–386. [Google Scholar] [CrossRef]
- Ke, H. Investigation of the effects of nano-graphite on morphological structure and thermal performances of fatty acid ternary eutectics/polyacrylonitrile/nano-graphite form-stable phase change composite fibrous membranes for thermal energy storage. Sol. Energy 2018, 173, 1197–1206. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, H.; Gao, X.; Xu, T.; Fang, Y.; Zhang, Z. Fabrication and characterization of form-stable capric-palmitic-stearic acid ternary eutectic mixture/nano-SiO2 composite phase change material. Energy Build. 2017, 147, 41–46. [Google Scholar] [CrossRef]
- He, Q.; Fei, H.; Zhou, J.; Du, W.; Pan, Y.; Liang, X. Preparation and characteristics of lauric acid-myristic acid-based ternary phase change materials for thermal storage. Mater. Today Commun. 2022, 32, 104058. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Ali, M.; Kannan, A.M. Fatty acids based eutectic phase change system for thermal energy storage applications. Appl. Therm. Eng. 2018, 142, 466–475. [Google Scholar] [CrossRef]
- Ke, H. Phase diagrams, eutectic mass ratios and thermal energy storage properties of multiple fatty acid eutectics as novel solid-liquid phase change materials for storage and retrieval of thermal energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Du, W.; Fei, H.; Pan, Y.; He, Q.; Zhou, J.; Liang, X. Development of capric acid-stearic acid-palmitic acid low-eutectic phase change material with expanded graphite for thermal energy storage. Constr. Build. Mater. 2022, 320, 126309. [Google Scholar] [CrossRef]
- Nordlie, B. Eutectic Melting. Petrology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 155–157. [Google Scholar]
- Nichols, L. Organic Chemistry Laboratory Techniques; Independent: Chicago, IL, USA, 2016. [Google Scholar]
- Lin, N.; Li, C.; Zhang, D.; Li, Y.; Chen, J. Emerging phase change cold storage materials derived from sodium sulfate decahydrate. Energy 2022, 245, 123294. [Google Scholar] [CrossRef]
- Shen, Z.; Oh, K.; Kwon, S.; Toivakka, M.; Lee, H.L. Use of cellulose nanofibril (CNF)/silver nanoparticles (AgNPs) composite in salt hydrate phase change material for efficient thermal energy storage. Int. J. Biol. Macromol. 2021, 174, 402–412. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, Y.; Xu, X.; Xu, X.; Fang, G.; Gu, M. Synthesis and characterization of microencapsulated sodium sulfate decahydrate as phase change energy storage materials. Appl. Energy 2019, 255, 113830. [Google Scholar] [CrossRef]
- Nickolov, Z.S.; Ozcan, O.; Miller, J. FTIR analysis of water structure and its significance in the flotation of sodium carbonate and sodium bicarbonate salts. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 231–239. [Google Scholar] [CrossRef]
- Kumar, R.; Samykano, M.; Ngui, W.; Pandey, A.; Kalidasan, B.; Kadirgama, K.; Tyagi, V. Investigation of thermal performance and chemical stability of graphene enhanced phase change material for thermal energy storage. Phys. Chem. Earth Parts A/B/C 2022, 128, 103250. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.; Rahman, S.; Yadav, A.; Samykano, M.; Tyagi, V. Graphene–Silver Hybrid Nanoparticle based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability 2022, 14, 13240. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, W.; Gou, Y.; Jia, Y.; Li, H. Thermal behaviors of energy storage process of eutectic hydrated salt phase change materials modified by Nano-TiO2. J. Energy Storage 2022, 53, 105077. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalidasan, B.; Pandey, A.K.; Rahman, S.; Sharma, K.; Tyagi, V.V. Experimental Investigation of Graphene Nanoplatelets Enhanced Low Temperature Ternary Eutectic Salt Hydrate Phase Change Material. Energies 2023, 16, 1574. https://doi.org/10.3390/en16041574
Kalidasan B, Pandey AK, Rahman S, Sharma K, Tyagi VV. Experimental Investigation of Graphene Nanoplatelets Enhanced Low Temperature Ternary Eutectic Salt Hydrate Phase Change Material. Energies. 2023; 16(4):1574. https://doi.org/10.3390/en16041574
Chicago/Turabian StyleKalidasan, B., A. K. Pandey, Saidur Rahman, Kamal Sharma, and V. V. Tyagi. 2023. "Experimental Investigation of Graphene Nanoplatelets Enhanced Low Temperature Ternary Eutectic Salt Hydrate Phase Change Material" Energies 16, no. 4: 1574. https://doi.org/10.3390/en16041574
APA StyleKalidasan, B., Pandey, A. K., Rahman, S., Sharma, K., & Tyagi, V. V. (2023). Experimental Investigation of Graphene Nanoplatelets Enhanced Low Temperature Ternary Eutectic Salt Hydrate Phase Change Material. Energies, 16(4), 1574. https://doi.org/10.3390/en16041574





