Control of the Energy Impact of Electric Discharges in a Liquid Phase
Abstract
1. Introduction
2. Materials and Methods
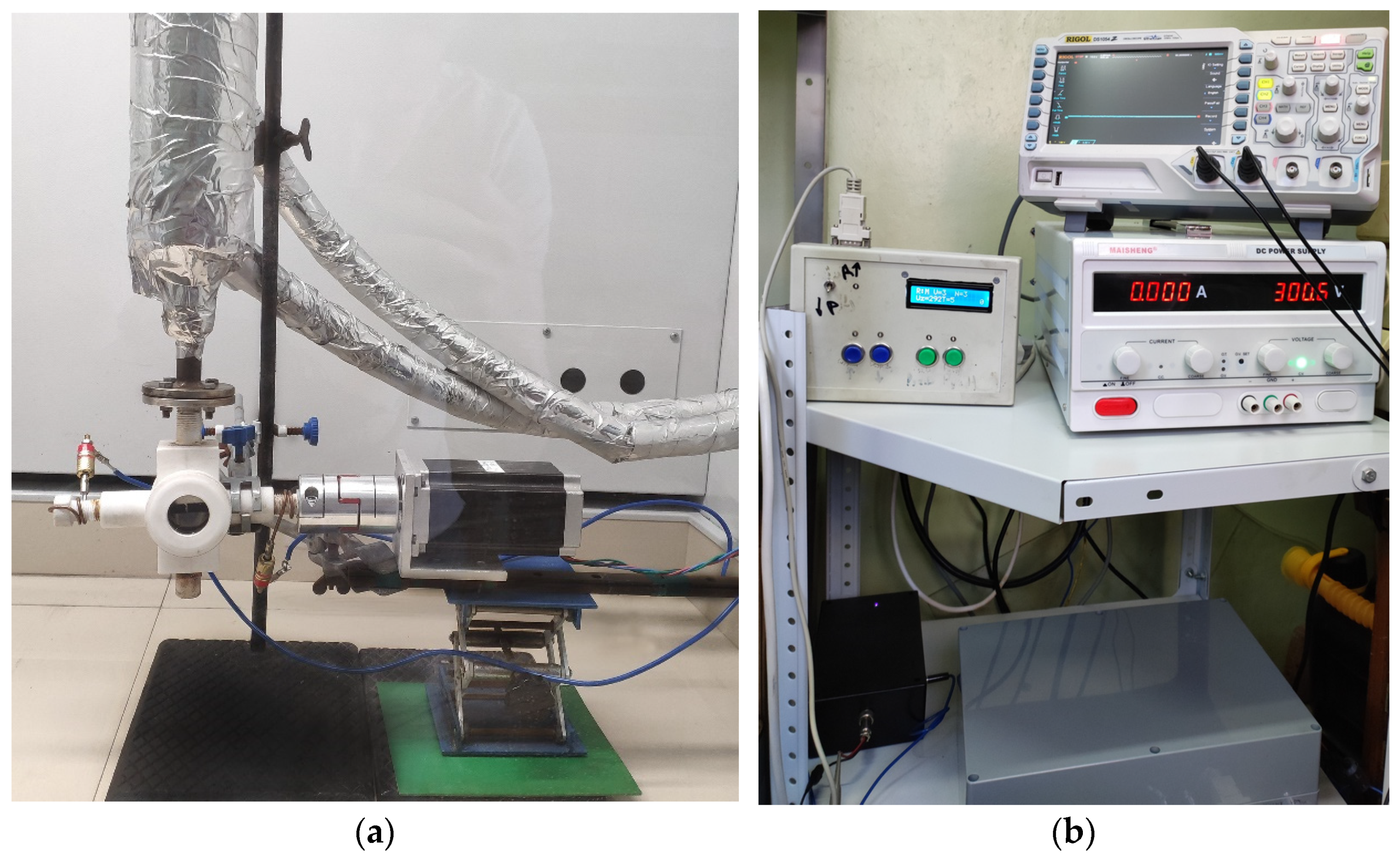
2.1. Description of the Laboratory Setup
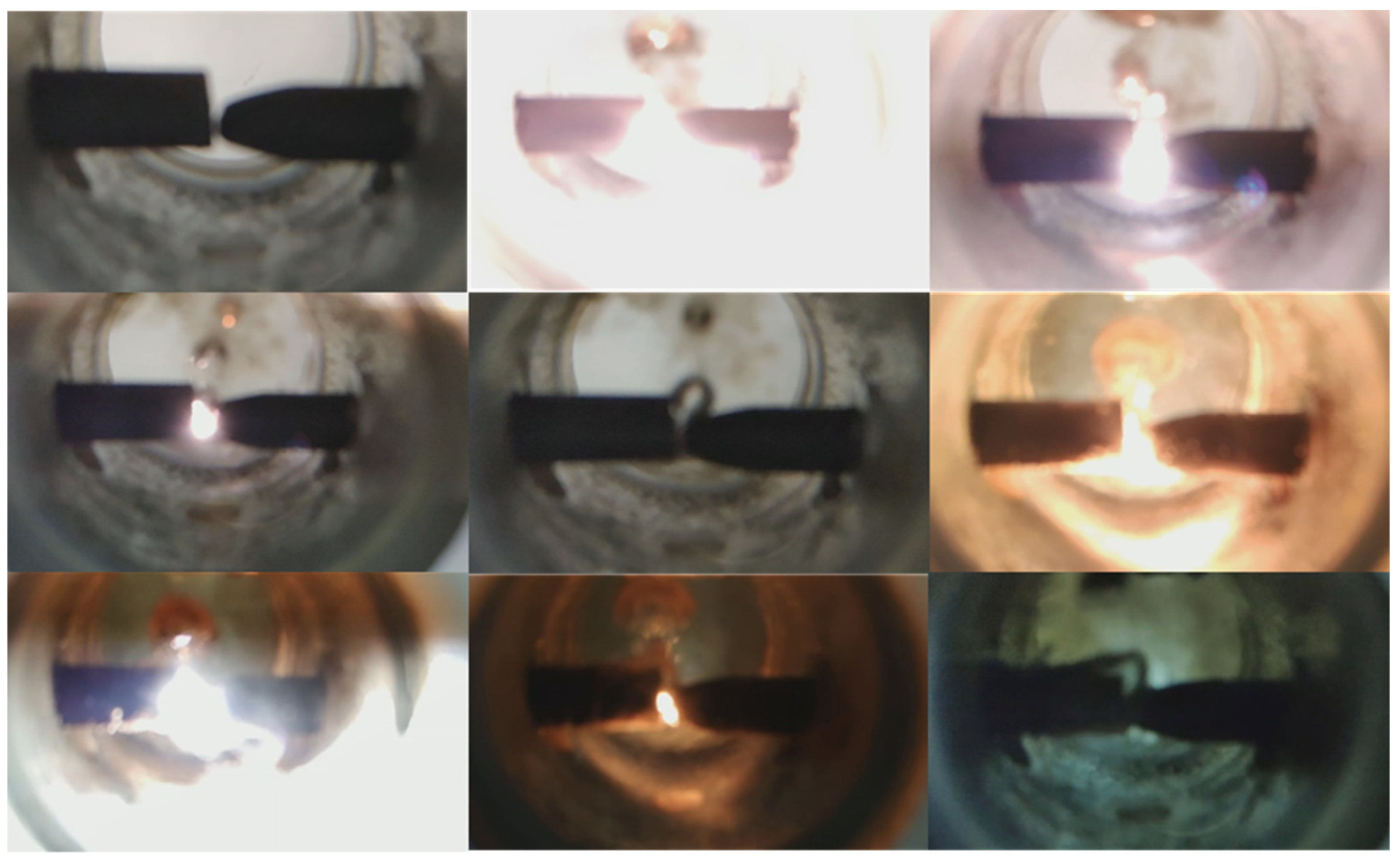
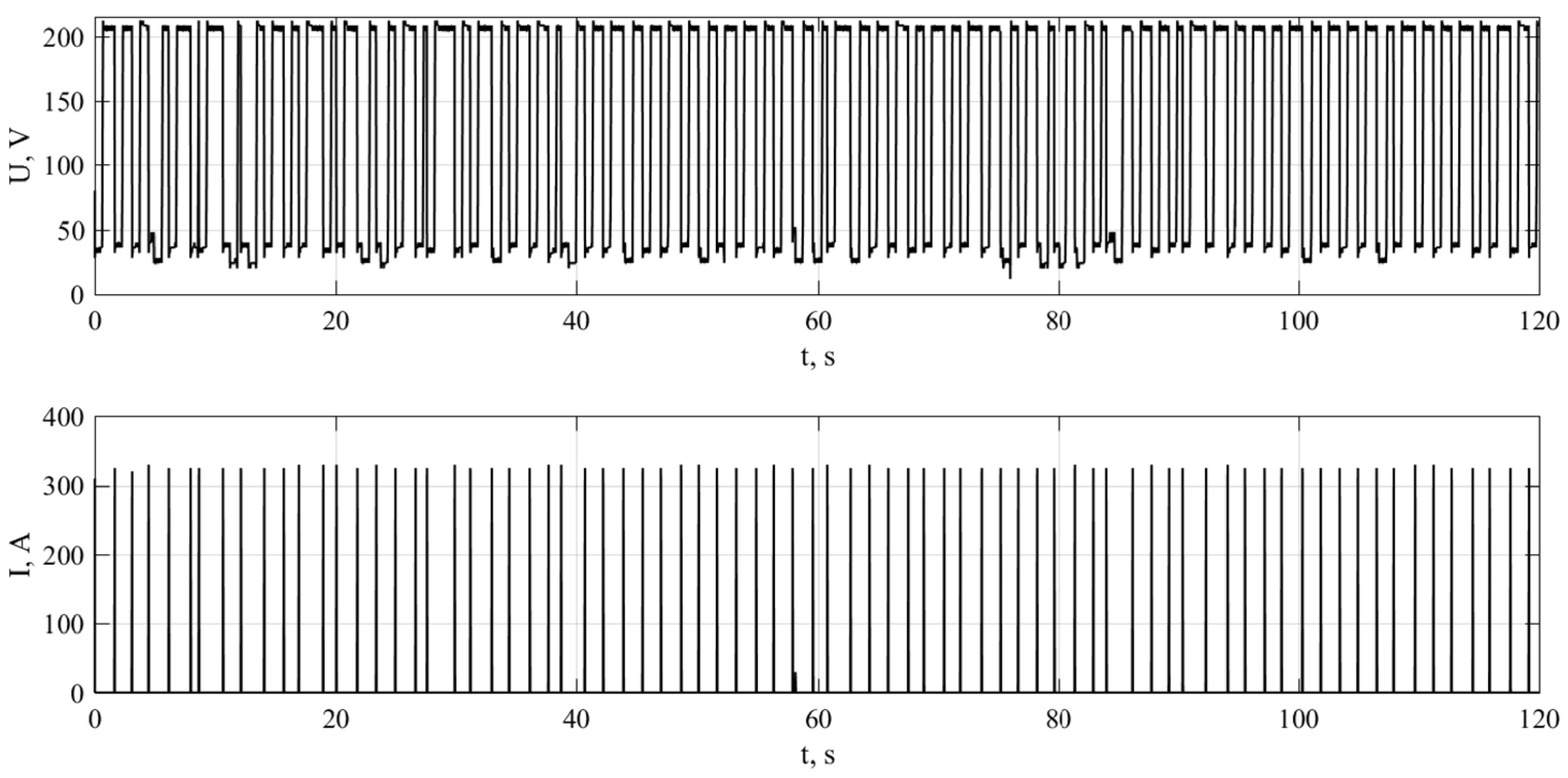
2.2. Methodology of Experiment
3. Results and Discussion
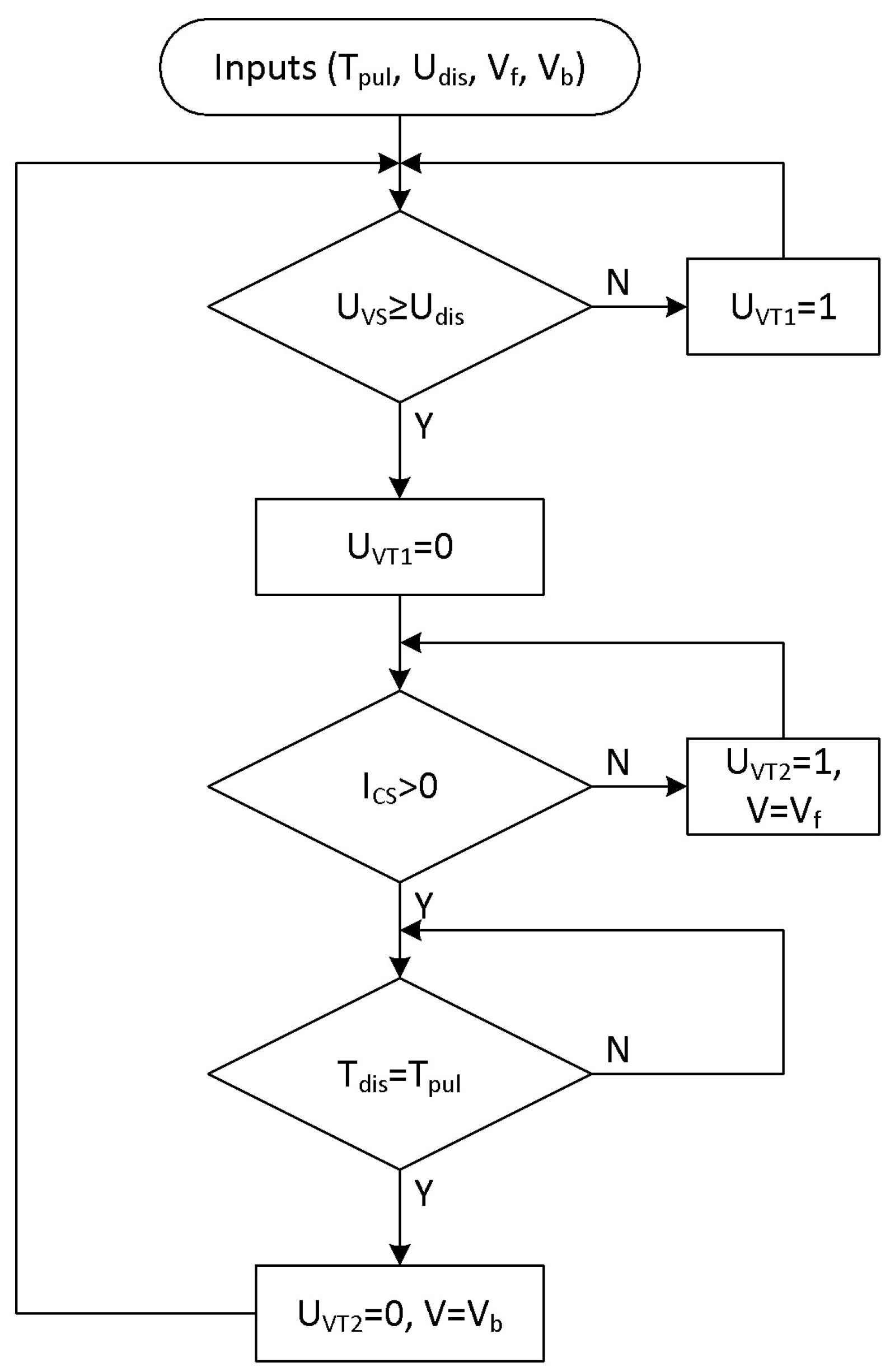
3.1. Method for Determining the Energy Impact of Electric Discharges
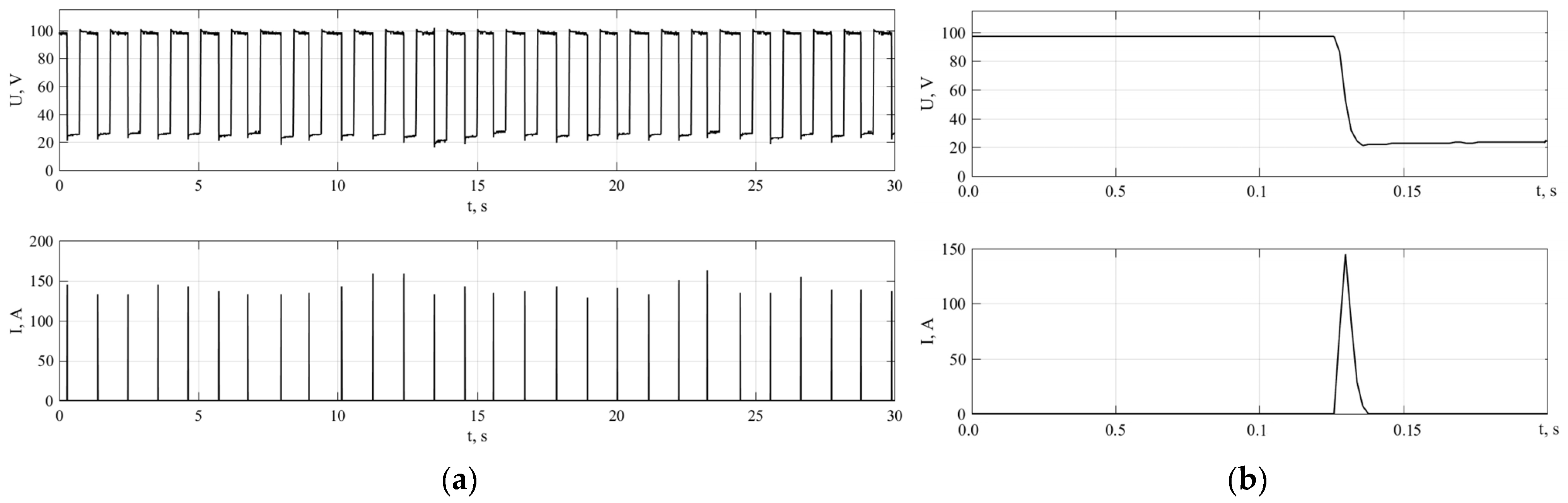
3.2. Results of Experiment
4. Conclusions
- (a)
- Search for optimal modes of processing substances to reduce energy costs, increase the productivity of the installation, and increase the yield of the main products;
- (b)
- Modification of the semiconductor key control circuit to limit the discharge time to nanosecond ranges, which will reduce the thermal effect of the plasma;
- (c)
- Expanding the scope of the application of plasma-chemical pyrolysis for the processing of renewable bio-raw materials, industrial waste, and carbon dioxide.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bataille, C.; Åhman, M.; Neuhoff, K.; Nilsson, L.J.; Fischedick, M.; Lechtenböhmer, S.; Solano-Rodriquez, B.; Denis-Ryan, A.; Stiebert, S.; Waisman, H.; et al. A review of technology and policy deep decarbonization pathway options for making energy-intensive industry production consistent with the Paris Agreement. J. Clean. Prod. 2018, 187, 960–973. [Google Scholar] [CrossRef]
- Rathore, K.; Bhuiyan, S.I.; Slavens, S.M.; Staack, D. Microplasma ball reactor for JP-8 liquid hydrocarbon conversion to lighter fuels. Fuel 2021, 285, 118943. [Google Scholar] [CrossRef]
- Riyanto, T.; Istadi, I.; Buchori, L.; Anggoro, D.D.; Dani Nandiyanto, A.B. Plasma-Assisted Catalytic Cracking as an Advanced Process for Vegetable Oils Conversion to Biofuels: A Mini Review. Ind. Eng. Chem. Res. 2020, 59, 17632–17652. [Google Scholar] [CrossRef]
- De Pee, A.; Pinner, D.; Roelofsen, O.; Somers, K.; Speelman, E.; Witteveen, M. Decarbonization of Industrial Sectors: The Next Frontier; McKinsey & Company: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Eryazici, I.; Ramesh, N.; Villa, C. Electrification of the chemical industry—Materials innovations for a lower carbon future. MRS Bull. 2021, 46, 1197–1204. [Google Scholar] [CrossRef]
- Wery, F.; Geerts, M.; Vandewalle, L.A.; Reyniers, P.A.; Heynderickx, G.J.; Marin, G.B.; Van Geem, K.M. Assessing the CO2 emission reduction potential of steam cracking furnace innovations via computational fluid dynamics: From high-emissivity coatings, over coil modifications to firing control. Chem. Eng. Res. Des. 2023, 190, 129–142. [Google Scholar] [CrossRef]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Dolganov, I.; Bunaev, A.; Dolganova, I. Unsteady-State Mathematical Modeling of Hydrocarbon Feedstock Pyrolysis. Processes 2020, 8, 1394. [Google Scholar] [CrossRef]
- Okubo, M. Recent Development of Technology in Scale-up of Plasma Reactors for Environmental and Energy Applications. Plasma Chem. Plasma Process. 2022, 42, 3–33. [Google Scholar] [CrossRef]
- Rabinovich, A.; Nirenberg, G.; Kocagoz, S.; Surace, M.; Sales, C.; Fridman, A. Scaling Up of Non-Thermal Gliding Arc Plasma Systems for Industrial Applications. Plasma Chem. Plasma Process. 2022, 42, 35–50. [Google Scholar] [CrossRef]
- Kuwahara, T.; Yoshida, K.; Kuroki, T.; Hanamoto, K.; Sato, K.; Okubo, M. High Reduction Efficiencies of Adsorbed NOx in Pilot-Scale Aftertreatment Using Nonthermal Plasma in Marine Diesel-Engine Exhaust Gas. Energies 2019, 12, 3800. [Google Scholar] [CrossRef]
- Khosravi, A.; Khani, M.R.; Goy, E.D.; Shokri, B. The Processing of Pyrolysis Fuel Oil by Dielectric Barrier Discharge Plasma Torch. Plasma Chem. Plasma Process 2018, 38, 365–378. [Google Scholar] [CrossRef]
- Titov, E.Y.; Bodrikov, I.V.; Serov, A.I.; Kurskii, Y.A.; Titov, D.Y.; Bodrikova, E.R. Liquid-Phase Non-Thermal Plasma Discharge for Fuel Oil Processing. Energies 2022, 15, 3400. [Google Scholar] [CrossRef]
- Wang, K.; Bhuiyan, S.I.; Baky, M.A.H.; Kraus, J.; Campbell, C.; Jemison, H.; Staack, D. Electric fuel conversion with hydrogen production by multiphase plasma at ambient pressure. Chem. Eng. J. 2022, 433, 133660. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Fabry, F.; Rehmet, C.; Rohani, V.; Fulcheri, L. Waste gasification by thermal plasma: A review. Waste Biomass Valorization 2013, 4, 421–439. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, T.; Yan, B.; Cheng, Y. Thermodynamic analysis of asphaltene pyrolysis in thermal plasma reactor. Shiyou Huagong/Petrochem. Technol. 2015, 44, 1168–1176. [Google Scholar]
- Shao, T.; Wang, R.; Zhang, C.; Yan, P. Atmospheric-pressure pulsed discharges and plasmas: Mechanism, characteristics and applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Lebedev, Y.A. Microwave discharges in liquid dielectrics. Plasma Phys. Rep. 2017, 43, 685–695. [Google Scholar] [CrossRef]
- Diono, W.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis. Plasma 2021, 4, 21. [Google Scholar] [CrossRef]
- Zhang, H.; Du, C.; Wu, A.; Bo, Z.; Yan, J.; Li, X. Rotating gliding arc assisted methane decomposition in nitrogen for hydrogen production. Int. J. Hydrog. Energy 2014, 39, 12620–12635. [Google Scholar] [CrossRef]
- Delikonstantis, E.; Scapinello, M.; Stefanidis, G.D. Process Modeling and Evaluation of Plasma-Assisted Ethylene Production from Methane. Processes 2019, 7, 68. [Google Scholar] [CrossRef]
- Zin, R.M.; Soon, C.F.; Yusof, N.M.; Rizon, E.R.; Morsin, M.; Tee, K.S.; Ahmad, M.K.; Nayan, N. Dimmer and neon transformer as a power controllable generator for atmospheric pressure plasma jet. Int. J. Power Elec. Drive Syst. 2019, 10, 594–601. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Titov, E.Y.; Titov, D.Y.; Bodrikov, I.V.; Kut’in, A.M.; Kurskii, Y.A.; Gazizzulin, R.R. A Device for Generation of Low-Voltage Discharges in Liquid Dielectric Media. High Energy Chem. 2018, 52, 512–513. [Google Scholar] [CrossRef]
- Bodrikov, I.V.; Kut’in, A.M.; Titov, E.Y.; Titov, D.Y.; Kurskii, Y.A.; Gazizullin, R.R. Fragmentation of thiophene and 3-methyl-2-thiophenecarboxaldehyde by direct liquid phase low-voltage discharges. Plasma Proc. Pol. 2018, 15, 1800094. [Google Scholar] [CrossRef]
- Watson, P.K.; Schneider, J.M.; Till, H.R. Electrohydrodynamic Stability of Space-Charge-Limited Currents in Dielectric Liquids. II. Experimental Study. Phys. Fluids 1970, 13, 1955–1961. [Google Scholar] [CrossRef]
- Sunka, P. Pulse electrical discharges in water and their applications. Phys. Plasm. 2001, 8, 2587–2594. [Google Scholar] [CrossRef]
- An, W.; Baumung, K.; Bluhm, H. Underwater streamer propagation analyzed from detailed measurements of pressure release. Appl. Phys. 2007, 101, 053302. [Google Scholar] [CrossRef]
- Xu, S.; Khalaf, P.I.; Martin, P.A.; Whitehead, J.C. CO2 dissociation in a packed-bed plasma reactor: Effects of operating conditions. Plasma Sources Sci. Technol. 2018, 27, 075009. [Google Scholar] [CrossRef]
- Bodrikov, I.V.; Ivanova, A.G.; Vasiliev, A.L.; Titov, E.Y.; Titov, D.Y.; Serov, A.I. Influence of low-voltage discharge energy on the morphology of carbon nanostructures in induced benzene transformation. RSC Adv. 2021, 11, 39428–39437. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Y.; Sun, H.; Fan, Z.; Shao, T. Dry reforming of methane by microsecond pulsed dielectric barrier discharge plasma: Optimizing the reactor structures. High Volt. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.T. Repetitively pulsed gas discharges: Memory effect and discharge mode transition. High Volt. 2020, 5, 569–582. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.; Guo, Y.; Zhou, Z.; Song, Y. Quantitative behavior of vibrational excitation in AC plasma assisted dry reforming of methane. J. Energy Chem. 2020, 46, 133–143. [Google Scholar] [CrossRef]
- Altgilbers, L.L.; Baird, J.; Freeman, B.L.; Lynch, C.S.; Shkuratov, S.I. Explosive Pulsed Power; Imperial College Press: London, UK, 2011. [Google Scholar]
- Sneyd, J.; Fewster, R.M.; McGillivray, D. Mathematics and Statistics for Science; Springer Nature Switzerland AG: Cham, Switzerland, 2022. [Google Scholar]
- Hu, S.; Zhang, C.; Wu, M.; Ye, R.; Shi, D.; Li, M.; Zhao, P.; Zhang, R.; Feng, G. Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts. Catalysts 2022, 12, 1072. [Google Scholar] [CrossRef]



| Characteristics of Electrical Discharges | Voltage, V | ||
|---|---|---|---|
| 100 | 200 | 300 | |
| Average pulse duration, ms | 0.84 | 1.139 | 1.146 |
| Average pulse frequency, Hz | 0.65 | 0.68 | 0.72 |
| Average pulse amplitude, A | 133.42 | 308.07 | 323.94 |
| Average pulse energy, J | 0.037 | 0.221 | 0.227 |
| Characteristics of Electrical Discharges | Voltage, V | ||
|---|---|---|---|
| 100 | 200 | 300 | |
| Average pulse duration, ms | 0.84 | 1.01 | 0.99 |
| Average pulse frequency, Hz | 0.65 | 0.67 | 0.69 |
| Average pulse amplitude, A | 131.14 | 312.97 | 326.75 |
| Average pulse energy, J | 0.036 | 0.199 | 0.201 |
| Voltage, V | 100 | 200 | 300 | |
| Energy consumption, kWh | 0.04 | 0.06 | 0.07 | |
| Energy consumption, kWh/kg of gas | 235.3 | 120.0 | 83.3 | |
| Gas flow, ml/h | 5.2 | 13.4 | 21.5 | |
| Gas yield, wt% | 0.8 | 2.2 | 3.7 | |
| Gas composition, mol% | ||||
| H2 | 56.0 | 53.3 | 51.8 | |
| CH4 | 4.6 | 5.0 | 5.6 | |
| C2H4 | 6.9 | 7.8 | 8.4 | |
| C2H6 | 0.3 | 0.4 | 0.4 | |
| C2H2 | 29.0 | 29.4 | 28.6 | |
| C3H8 | 1.1 | 1.6 | 2.0 | |
| C3H4 | 1.4 | 0.8 | 0.9 | |
| 1,3-C4H6 | 0.1 | 0.4 | 0.6 | |
| C4H10 | 0.1 | 0.2 | 0.2 | |
| C5H12 | 0.5 | 1.1 | 1.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titov, E.; Bodrikov, I.; Titov, D. Control of the Energy Impact of Electric Discharges in a Liquid Phase. Energies 2023, 16, 1683. https://doi.org/10.3390/en16041683
Titov E, Bodrikov I, Titov D. Control of the Energy Impact of Electric Discharges in a Liquid Phase. Energies. 2023; 16(4):1683. https://doi.org/10.3390/en16041683
Chicago/Turabian StyleTitov, Evgeniy, Ivan Bodrikov, and Dmitry Titov. 2023. "Control of the Energy Impact of Electric Discharges in a Liquid Phase" Energies 16, no. 4: 1683. https://doi.org/10.3390/en16041683
APA StyleTitov, E., Bodrikov, I., & Titov, D. (2023). Control of the Energy Impact of Electric Discharges in a Liquid Phase. Energies, 16(4), 1683. https://doi.org/10.3390/en16041683







