1. Introduction
In modern society, special attention should be paid to successfully implementing the main goals of sustainable development for humanity and creating a waste-free or ‘circular’ digital economy [
1]. Disposing of large quantities of single-use polymer wastes is one of the foremost concerns in the current era [
2]. Synthetic petroleum-based plastics are non-biodegradable even after disposal. Therefore, the accumulation of petroleum-based plastic waste has been recognized as a critical environmental issue [
3].
Nonetheless, producing biodegradable polymers is an effective strategy to resolve environmental and marine pollution caused by plastic waste [
4]. There has been significant interest in minimising the quantities of synthetic or petroleum-based waste. The use of biodegradable plastics that could quickly decompose after their disposal in the environment has received much interest and emphasis [
3]. The production of biodegradable polymers as alternatives to petroleum-based plastics has gained significant attention in recent years [
5].
Polylactic acid (PLA) is one of the most promising biocompostable and biodegradable thermoplastics made from renewable sources, and is one of the most commonly used plastics for packaging applications. PLA-layer-based composite materials are gaining huge interest due to their multiple applications (food, medical, etc.) as eco-friendly materials [
2]. Approximately 293,000 tonnes of PLA were produced in 2019, which will continue to increase [
3].
Lactic acid has been used to produce some essential compounds, specifically PLA [
6]. Lactic acid is one of the most commonly used organic acids in various industries due to its versatile applications [
6]. The application of lactic acid for producing PLA-based biopolymers used in the textile, automotive, and biomedical sectors is rapidly rising [
4]. According to the recent report of Global View Research, the global market size for lactic acid has been estimated at USD 2.64 billion in 2018 and is expected to grow further at a compound annual growth rate of 18.7% from 2019 to 2025 [
7]. Approximately 90% of lactic acid is produced microbiologically. However, the fermentative route is essential for the industrial production of lactic acid [
5]. The lactic acid manufacturing process by microbial fermentation is environmentally friendly due to using renewable resources as substrates. The synthesis reaction of butyl lactate from ammonium lactate is one of the polylactic acid production stages currently under development: this is an integrated technology stage where fermentation is applied. Microbes used for lactic acid fermentation, such as bacteria, fungi, yeast, and algae, could use lignocellulosic biomass, organic agro-waste, kitchen waste, dairy, and petroleum industry waste as feedstock. PLA production and exploitation facilitate resolving the global problem of polymer waste accumulation due to the biodegradable properties of PLA and raw plant material sources. Therefore, recycling various organic wastes to produce lactic acid not only resolves waste management issues, but may also help reduce the production cost of lactic acid [
6].
The obtained lactic acid is purified further by various mechanisms. Lactic acid purification from fermentation broth has several ecological and economic implications as it may constitute 50–80% of the production costs [
8]. Currently, the industrial process for separating lactic acid from the fermentation broth involves precipitation with calcium hydroxide or calcium carbonate. Then, lactic acid is recovered using excess sulfuric acid [
9]. The main drawback of this process is the generation of large gypsum quantity, which is considered a waste stream [
9]. For every 1t of lactic acid produced, 1t of gypsum is generated as solid waste. Research has focused on finding more environmentally friendly alternatives [
5]. One of the acceptable methods of lactic acid isolation from the standpoint of environmental and operating costs is to produce butyl lactate from ammonium lactate and
n-butanol. Butyl lactate could be used as an intermediate product for further synthesis [
10,
11].
A kinetic model of the process is essential for the mathematical modelling and scale-up of the lactic acid isolation technology based on the reaction between ammonium lactate and
n-butanol. Lactic acid ester formation is the target reaction, and the reaction proceeds through esterification, also known as Fischer–Speier esterification. This interaction reaction between carboxylic acids and alcohols was discovered quite a long time ago, and was first described by Emil Fischer and Arthur Speier in 1895 [
12]. Thus, the kinetics of lactic acid esterification have been actively studied for many years and described in many scientific papers. For example, Athana et al. [
13] developed a kinetic model for esterifying lactic acid and respective oligomers with ethanol on Amberlyst 15 cation exchange resin based on batch reaction studies.; their model predictions correlated with experimental results for 20%, 50%, and 88% lactic acid aqueous solution. Delgado et al. [
14] synthesized ethyl lactate from ethanol and lactic acid on an Amberlyst 15 and PERVAP
® 2201 hydrophilic membrane using a batch reactor combined with a pervaporation unit. Their kinetic model predictions correlated with experimental results for 20%, 50%, and 79-81% lactic acid aqueous solution. Additionally, Rattanaphanee [
15] derived a correlation between the lactic acid esterification with ethanol and n-butanol at different operating conditions.
To the authors’ knowledge, several researchers have focused on the kinetic studies of lactic acid esterification with n-butanol. For example, Qu et al. [
16] carried out a kinetic analysis of the lactic acid esterification with n-butanol and isobutyl alcohol on Weblyst D009 and Weblyst D80 ion exchange resins. The obtained experimental dependences were described using the pseudo-homogeneous model, the Langmuir–Hinshelwood model, and the Eley-Rideal model. The researchers derived the reaction rate and the Arrhenius equation for the rate constant of the direct reaction. The researchers investigated the influence of the alcohol and lactic acid molar ratio and temperature on the esterification process. Qu et al. [
16] observed that the pseudo-homogeneous model of kinetic description is preferred because it was simple with an accuracy comparable to the Langmuir–Hinshelwood and Eley–Rideal models. The paper presents the calculated values of the pre-exponential factor in the Arrhenius equation, the activation energy for each model. Schastlivaya et al. [
17] also studied the kinetic regularities of the lactic acid esterification process with butyl alcohol. In this case, cation exchange resins were used as a catalyst. Kinetic equations were obtained that describe the process under conditions of batch and flow reactors. The Langmuir–Hinshelwood model, similar to the research described earlier [
16], was used, considering the water adsorption on the catalyst’s sites. A mathematical model of a semi-batch reactor was derived. Thus, the kinetics of lactic acid esterification with various alcohols, including butanol, have been studied in sufficient detail in the literature provided here, among other existing literature. However, most of the researchers have focused on the catalytic process. As a rule, the study was carried out under atmospheric pressure and not-so-high temperatures (60–80 °C). These researchers have not focused on the esterification process at very high temperatures.
The kinetic reaction of alcohols with the lactic acid’s ammonium salt is of great interest since it is the form in which lactic acid is enzymatically produced in the industry. However, there is not sufficient data in the literature on the esterification kinetics of ammonium lactate directly. For example, Wang et al. [
18] studied the kinetics of ammonium lactate esterification with ethyl alcohol catalyzed by the enzyme-immobilized lipase Novozym 435 (N435). They proposed the Ping-Pong Bi-Bi enzymatic mechanism with competitive inhibition by both substrates. The corresponding kinetic parameters were calculated by non-linear regression. The direction of this work has a biotechnological specificity of using enzymatic catalysis. The kinetic data obtained in this regard do not apply to non-catalytic process models. Kasinathan et al. [
19] studied ethyl lactate synthesis from an ammonium lactate solution using joint extraction and esterification methods. The essence of the technique was that lactic acid was extracted from ammonium lactate solution with tributyl phosphate (TBP) or tridecylamine (TDA) with further esterification in the selected solutions. Etherification was carried out in the presence of a homogeneous catalyst, sulfuric acid, and on Amberlyst 15, 36, and Amberlite-IRP64 sulfonic cation exchangers. Kasinathan et al. derived a kinetic model of lactic acid esterification, and its parameters for each catalyst were obtained. The rate constants are calculated for different types of catalysts. Additionally, for the Amberlyst-36 catalyst, the activation energy for the esterification of the direct and reverse reactions was determined. Although this work studied the process of obtaining ethyl lactate from ammonium lactate, the researchers mainly focused on the kinetics of the lactic acid and ethanol interaction. In this case, the catalytic reaction alone was considered. As described above, most kinetic studies focused on the interaction of lactic acid with alcohol rather than ammonium lactate. In this case, the process was investigated at atmospheric pressure and temperatures not exceeding 80 °C. It is also worth noting that the processes were studied in the presence of various types of acid catalysts. However, in the case of using ammonium lactate as a raw material, this is impractical because of catalyst deactivation when the catalyst interacts with ammonium lactate. Furthermore, researchers have not conducted the processes in a closed system at temperatures greater than 80 °C.
In this work, we carried out a kinetic study of the non-catalytic interaction of ammonium lactate and n-butanol in the temperature range of 130–170 °C in a closed system for the first time. Carrying out the process in this temperature range significantly intensifies the respective process. In this regard, the industrial implementation of such a technology for the synthesis of butyl lactate is likely to be carried out at a similar temperature [
11].
The purpose of this work was to study the kinetics of the interaction between ammonium lactate and n-butanol. This study also aimed to obtain a kinetic model of the process and its parameters that adequately describe the experimental data in the studied ranges of conditions.
The study proposes the first kinetic model that accounts for lactamide formation through two routes. We also focused on the subsequent development of the kinetic model applicable to the mathematical modelling of the reactor unit for butyl lactate synthesis.
2. Materials and Methods
The initial reaction mass for the kinetic study was ammonium lactate aqueous solution and n-butanol. Aqueous ammonia solution (minimum 25 wt%), distilled water and lactic acid (80 wt%) from PURAC were used to prepare the ammonium lactate solution. Additionally, n-Butanol (minimum 99.5 wt%) was used without additional purification.
We conducted several experimental series to study the kinetic patterns of the reaction between ammonium lactate and n-butanol using varied conditions within the ranges close to those suggested for practical use. We studied the effects of changes in several parameters. The parameter ranges were as follows: initial ammonium lactate concentration was 0.464–1.584 mol/kg; initial water concentration was 0.3–5 wt%; n-butanol to ammonium lactate molar ratio was 6–25; temperature range was 130–170 °C.
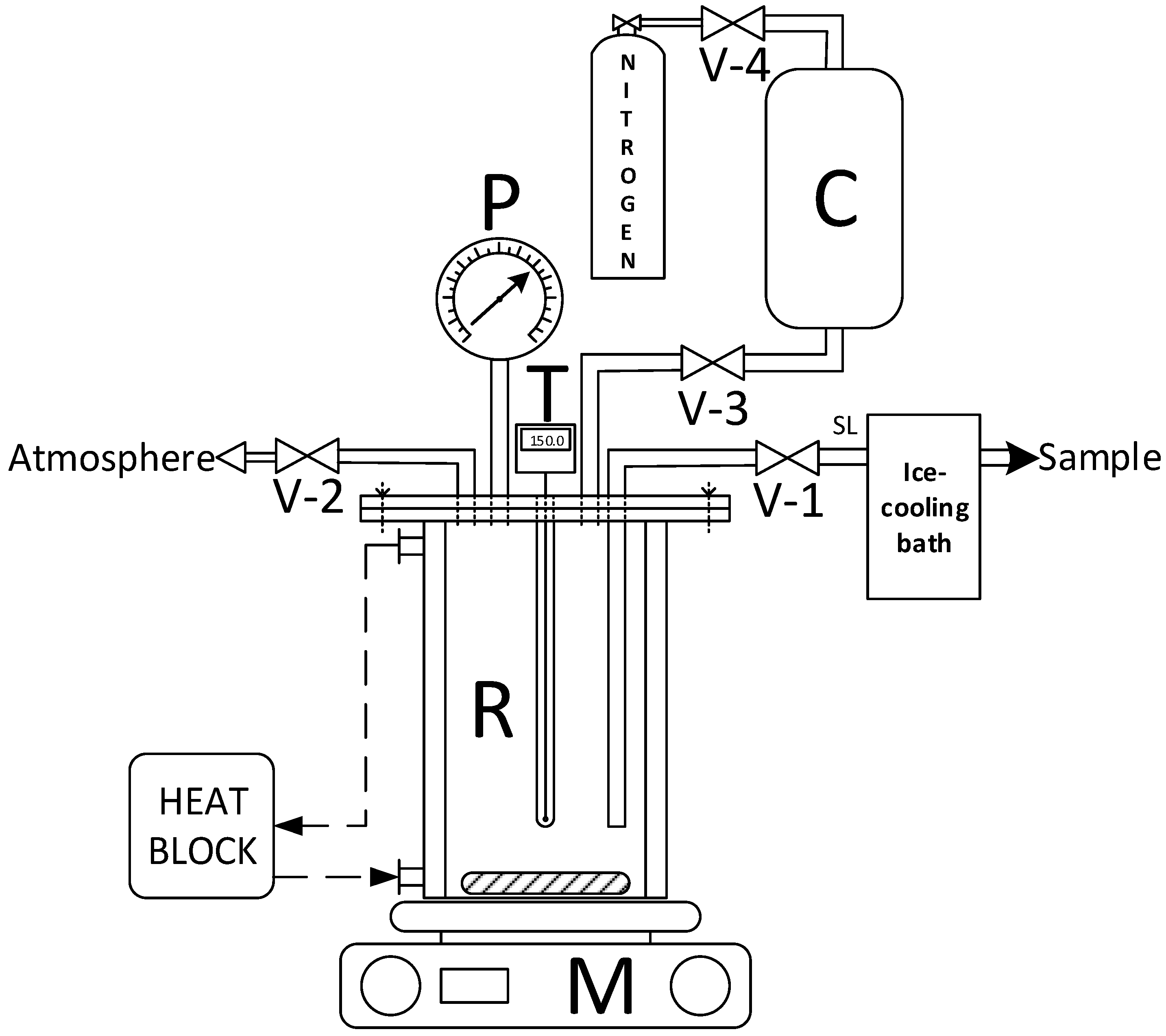
We conducted the kinetic study in a closed manner, and the system’s diagram is presented in
Figure 1. The reactor was a 285 mL steel autoclave with a jacket to maintain the required temperature, fitted with a pressure gauge and thermocouple. The reactor had a pressurization line, a reagent supply line, and a sampling line. The reaction mass was uniformly mixed with a magnetic stirrer.
Before initializing the experiment, we purged the rig with nitrogen through the pressurization line valves V-3 and V-4 and the sampling line valve V-1. The initial mixture was loaded into the feed cylinder tank C. Then, we switched on the thermostat and the heat-exchange fluid circulation to the jacket. After reaching the required temperature of 130–170 °C, we filled the reactor with the reaction mixture from C via valve V-3 via the nitrogen displacement method; the displacement nitrogen was continuously fed through V-4 valve until the system reached the working pressure of 13 atm. The kinetic experiment’s onset temperature was attained the moment the required reaction temperature was achieved. Samples were taken through valve V-1, and key components were analysed during the experiment. The concentrations of ammonium lactate, lactic acid and dissolved ammonia were determined using potentiometric titration (Metrohm 794 Basic Titrino autotitrator); we used 0.2 mol/L potassium hydroxide aqueous solution as the titrant. Butyl lactate, lactamide and n-butanol contents were determined using gas-chromatography (Crystallux-2000M instrument, Meta-chrom) with an internal standard (1-decanol).
3. Results and Discussion
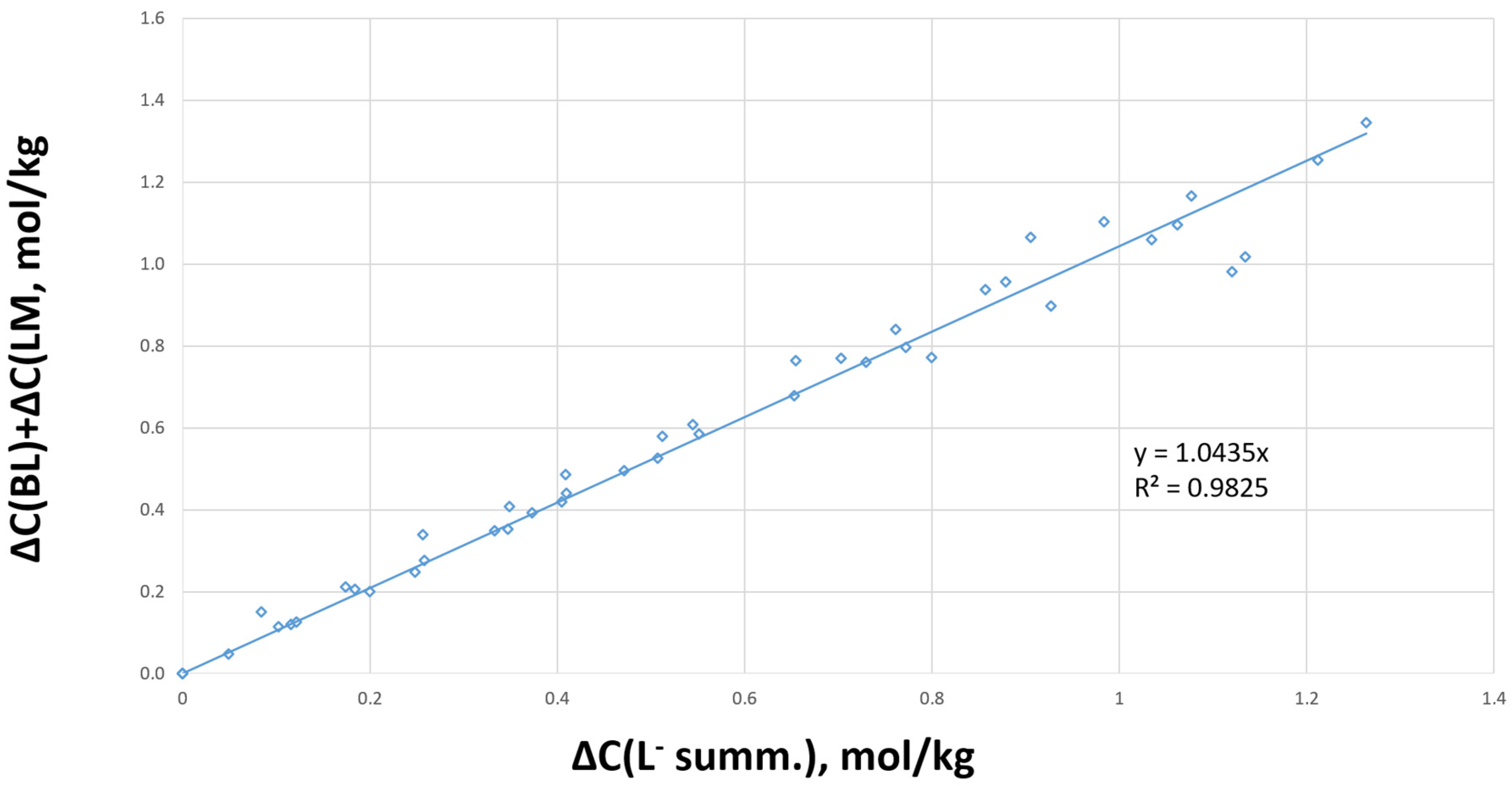
Preliminary experiments showed that the weight difference between the substances loaded into and unloaded from the reactor did not exceed 3%. We compared the increment in products (butyl lactate and lactamide) with the reagent consumption (ammonium lactate and lactic acid) to determine the chemical transformation routes (
Figure 2).
The linear characteristic of the dependence graph (
Figure 2) showed that the molar balance was maintained with its R
2 coefficient close to one; there were no unaccounted products. We suggested the following route for the chemical transformations that includes four reversible reactions based on these findings, as shown in Equations (1)–(4):
We derived the rate equations for the forward and backward reactions (Equations (6)–(11)) and the fast equilibrium equation (Equation (5)). Since lactic acid could catalyze the esterification process (Equation (2)), we included a catalytic factor [LH]
α in Equations (6) and (7), where α is the reaction order of the catalyst (lactic acid).
The kinetics of the lactic acid esterification with
n-butanol (Equation (2)) was separately studied in a series of experiments at 150 °C to determine variable α in Equations (6) and (7); the initial
n-butanol-to-lactic-acid molar ratio varied from 6 to 22, and the initial water concentration varied within 1.8–8.9 mol/kg. Molar balance analysis for this experiment series (
Figure 3) shows that 1 mol of consumed lactic acid generates 1 mol of butyl lactate. The reaction results agree with the reaction stoichiometry and indicate that there are no side reactions.
The obtained kinetic data for the esterification of lactic acid with
n-butanol were processed by numerical integration of Equations (6) and (7) using the least squares method. The minimum sum of squared deviations between the calculated and experimental concentrations of lactic acid, ammonium lactate, lactamide, and butyl lactate was α ≈ 1. The results were consistent with the general acid catalysis model of the esterification reaction (2) and the experimental data (
Figure 4).
Given α = 1, the kinetic model was converted to (5), (8)–(19):
We used the numerical integration using the least squares method to process the experimental data array for the lactic acid reaction with
n-butanol and calculated the kinetic model’s parameters (Equations (5) and (8)–(19)). As a result, the equilibrium and reaction constants and their respective activation parameters were determined at various temperatures; these data are provided in
Table 1 and
Table 2.
Table 1 shows the effect of temperature on the conversion of ammonium lactate and the selectivity to butyl lactate and lactamide.
The proposed kinetic models (Equations (5) and (8)–(19)) with the calculated parameters (
Table 1) enable the adequate description of the experimental kinetic dependences within the studied condition range. We confirmed the proposed kinetic model through the linear correlation between the experimental and calculated concentrations of the key components for the entire experimental array (
Figure 5).