The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry—A Case Study from Poland
Abstract
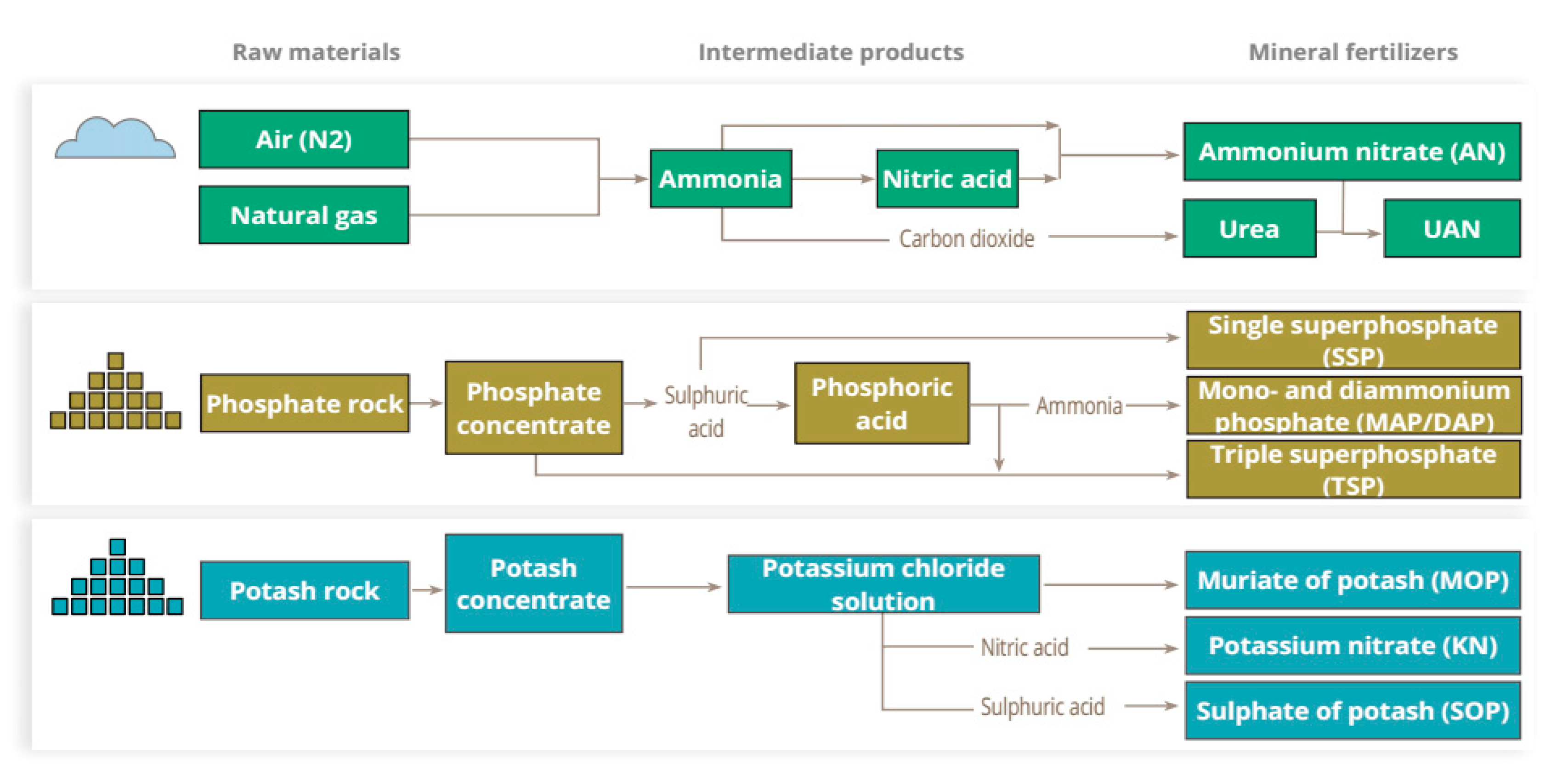
:1. Introduction
2. Energy Consumption of the Fertilizer Industry
3. Legal Regulations on the Use of Fertilizers in Poland
4. Liquid Fertilizers Developed and Used in Poland
5. Characteristics of the Most Used Waste Materials in the Production of Liquid Fertilizers in Terms of Their Fertilizer Properties
5.1. Algae
5.2. Digestate
5.3. Mine Minerals
6. Conclusions
- There is an opportunity to use waste products to produce liquid fertilizers due to their plentiful composition in plant and soil nutrients. In addition, managing waste products and demonstrating their reuse fits perfectly with the principles of a circular economy;
- In Poland the main substrates for liquid fertilizer production are mine minerals, algae, and digestate. The analysis shows that the latter substrate is most often used to produce organic liquid fertilizers (in 50% of cases), whereas in the production of organic–mineral fertilizers, the dominant base is algae. The largest group of fertilizer products are soil conditioners, produced most often from digestate, but also from mine minerals, algae, humic substances, or with the addition of micronutrients;
- The production of liquid fertilizers from waste materials seems to be an alternative to mineral solid fertilizers, as less energy is required to produce them and other mineral resources are not required;
- A sizable portion of this type of fertilizer is produced through the operation of biogas plants and can be used locally for fertilizer purposes, which is important in reducing carbon emissions and energy consumption for transportation;
- A major limitation during the creation of this work was the lack of research on the energy intensity of the liquid fertilizer industry, which may be a direction for future research, as this field is expected to grow significantly;
- Production of liquid fertilizer from waste is an economical solution for liquid fertilizer production due to the rather high cost determined by electricity consumption;
- It seems that a good direction for research now is to learn about the properties of different groups of wastes and the possibilities for their transformation and reuse;
- Management of the liquid form of sewage sludge free of animal products for fertilizer purposes may also be a correct direction of research in Poland.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AN | Ammonium Nitrate |
| AS | Ammonium Sulfate |
| ATP | Adenosine Triphosphate |
| BAT | Best Available Techniques |
| BTU | British Thermal Unit |
| CAN | Calcium Ammonium Nitrate |
| CE | Conformité Européenne |
| CJ | CheilJedang Corporation |
| D | Digestate |
| DM | Dry Matter |
| EC | Electrical Conductivity |
| F | Feedstock |
| HHV | Higher Heating Value |
| KN | Pottasium Nitrate |
| LHV | Lower Heating Value |
| MAP/DAP | Mono-and Diammonium Phosphate |
| MOP | Muriate of Potash |
| OM | Organic Matter |
| OS | Organic Suspension |
| RNA | Ribonucleic Acid |
| SOP | Sulphate of Potash |
| SSP | Single Superphosphate |
| TAM® | True Algae Max |
| TN | Total Nitrogen |
| TOC | Total Organic Carbon |
| TSP | Triple Superphosphate |
| TSS | Total Suspended Solids |
| UAN | Urea Ammonium Nitrate |
References
- Monforti-Ferrario, F.; Pascua, I.P.; Motola, V.; Banja, M.; Scarlat, N.; Medarac, H.; Castellazzi, L.; Labanca, N.; Bertoldi, P.; Pennington, D. Energy Use in the EU Food Sector: State of Play and Opportunities for Improvement; Publications Office Luxembourg: Luxembourg, 2015. [Google Scholar]
- Dimitrijevic, A.; Gavrilovic, M.; Ivanovic, S.M.; Mileusnic, Z.; Miodragovic, R.; Todorovic, S. Energy Use and Economic Analysis of Fertilizer Use in Wheat and Sugar Beet Production in Serbia. Energies 2020, 13, 2361. [Google Scholar] [CrossRef]
- Lopez-Vazquez, A.; Cadena-Zapata, M.; Campos-Magana, S.; Zermeno-Gonzalez, A.; Mendez-Dorado, M. Comparison of Energy Used and Effects on Bulk Density and Yield by Tillage Systems in a Semiarid Condition of Mexico. Agronomy 2019, 9, 189. [Google Scholar] [CrossRef]
- Domagala, J. Economic and Environmental Aspects of Agriculture in the EU Countries. Energies 2021, 14, 7826. [Google Scholar] [CrossRef]
- Smol, M. Transition to Circular Economy in the Fertilizer Sector-Analysis of Recommended Directions and End-Users’ Perception of Waste-Based Products in Poland. Energies 2021, 14, 4312. [Google Scholar] [CrossRef]
- McArthur, J.W.; McCord, G.C. Fertilizing growth: Agricultural inputs and their effects in economic development. J. Dev. Econ. 2017, 127, 133–152. [Google Scholar] [CrossRef]
- Favoino, E.; Hogg, D. The potential role of compost in reducing greenhouse gases. Waste Manag. Res. 2008, 26, 61–69. [Google Scholar] [CrossRef]
- Martinez-Alcantara, B.; Martinez-Cuenca, M.R.; Bermejo, A.; Legaz, F.; Quinones, A. Liquid Organic Fertilizers for Sustainable Agriculture: Nutrient Uptake of Organic versus Mineral Fertilizers in Citrus Trees. PLoS ONE 2016, 11, e0161619. [Google Scholar] [CrossRef]
- Walling, E.; Vaneeckhaute, C. Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manag. 2020, 276, 111211. [Google Scholar] [CrossRef]
- Fernandez-Delgado, M.; del Amo-Mateos, E.; Lucas, S.; Garcia-Cubero, M.T.; Coca, M. Liquid fertilizer production from organic waste by conventional and microwave-assisted extraction technologies: Techno-economic and environmental assessment. Sci. Total Environ. 2022, 806, 150904. [Google Scholar] [CrossRef]
- Worldbank.org. Available online: https://data.worldbank.org (accessed on 26 October 2022).
- IFA-International Fertilizer Association. Available online: https://www.fertilizer.org/ (accessed on 28 October 2022).
- Mahish, M.A.L. The impact of energy subsidy on nitrogen fertilizer producers in the GCC. Appl. Econom. Int. Dev. 2017, 17, 99–118. [Google Scholar]
- Huang, W.-y. Impact of Rising Natural Gas Prices on US Ammonia Supply; DIANE Publishing: Collingdale, PA, USA, 2007. [Google Scholar]
- Bloomberg.com. Available online: https://www.bloomberg.com (accessed on 27 October 2022).
- Eurativ.com. Available online: https://www.euractiv.com/ (accessed on 27 October 2022).
- Money.pl. Available online: https://www.money.pl/ (accessed on 27 October 2022).
- Szogi, A.A.; Vanotti, M.B.; Hunt, P.G. Phosphorus recovery from pig manure solids prior to land application. J. Environ. Manag. 2015, 157, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, R.; Gonzalez-Martinez, S. Influence of process parameters on the extraction of soluble substances from OFMSW and methane production. Waste Manag. 2017, 62, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, M.; Szara, E.; Was, A.; Sosulski, T.; van Pruissen, G.W.P.; Cornelissen, R.L. StruviteAn Innovative Fertilizer from Anaerobic Digestate Produced in a Bio-Refinery. Energies 2019, 12, 296. [Google Scholar] [CrossRef]
- Smol, M.; Adam, C.; Kugler, S.A. Thermochemical Treatment of Sewage Sludge Ash (SSA)-Potential and Perspective in Poland. Energies 2020, 13, 5461. [Google Scholar] [CrossRef]
- Fernandez-Delgado, M.; del Amo-Mateos, E.; Lucas, S.; Garcia-Cubero, M.T.; Coca, M. Recovery of organic carbon from municipal mixed waste compost for the production of fertilizers. J. Clean. Prod. 2020, 265, 121805. [Google Scholar] [CrossRef]
- Głodniok, M.; Deska, M.; Kaszycki, P. Impact of the Stabilized Sewage Sludge-Based Granulated Fertilizer on Sinapis alba Growth and Biomass Chemical Characteristics. Biol. Life Sci. Forum 2021, 35. [Google Scholar] [CrossRef]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 1047. [Google Scholar] [CrossRef]
- Piersa, P.; Szufa, S.; Czerwinska, J.; Uenyay, H.; Adrian, L.; Wielgosinski, G.; Obraniak, A.; Lewandowska, W.; Marczak-Grzesik, M.; Dzikuc, M.; et al. Pine Wood and Sewage Sludge Torrefaction Process for Production Renewable Solid Biofuels and Biochar as Carbon Carrier for Fertilizers. Energies 2021, 14, 8176. [Google Scholar] [CrossRef]
- Janiszewska, D.; Ossowska, L. The Role of Agricultural Biomass as a Renewable Energy Source in European Union Countries. Energies 2022, 15, 6756. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash-A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Zajac, G.; Szyszlak-Barglowicz, J.; Golebiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Jarosz-Krzeminska, E.; Poluszynska, J. Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies 2020, 13, 4805. [Google Scholar] [CrossRef]
- Czarnota, J.; Masłoń, A. Organic and organic-mineral fertilizers produced on the basis of municipal sewage sludge. Gas Water Sanit. Technol. 2020, 4, 14–18. (In Polish) [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Fawy, K.F.; Taher, M.A.; Hesham, A.; El-Shaboury, G.A.; Ahmed, M.T. Evaluation of the potential of sewage sludge as a valuable fertilizer for wheat (Triticum aestivum L.) crops. Environ. Sci. Pollut. Res. 2018, 26, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Styszko, K.; Durak, J.; Konczak, B.; Glodniok, M.; Borgulat, A. The impact of sewage sludge processing on the safety of its use. Sci. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef]
- Fachini, J.; de Figueiredo, C.C.; Frazao, J.J.; Rosa, S.D.; da Silva, J.; do Vale, A.T. Novel K-enriched organomineral fertilizer from sewage sludge-biochar: Chemical, physical and mineralogical characterization. Waste Manag. 2021, 135, 98–108. [Google Scholar] [CrossRef]
- Tang, Y.F.; Xie, H.; Sun, J.; Li, X.O.; Zhang, Y.; Dai, X.H. Alkaline thermal hydrolysis of sewage sludge to produce high-quality liquid fertilizer rich in nitrogen-containing plant-growth-promoting nutrients and biostimulants. Water Res. 2022, 211, 118036. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, L.P.; Babel, S. Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer. Sustainability 2022, 14, 387. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Potentiality of sewage sludge-based organo-mineral fertilizer production in Poland considering nutrient value, heavy metal content and phytotoxicity for rapeseed crops. J. Environ. Manag. 2019, 248, 109283. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (Text with EEA relevance). Regulation (EU) 2019/1009. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 26 October 2022).
- Cucina, M.; De Nisi, P.; Sordi, S.; Adani, F. Sewage Sludge as N-Fertilizers for Crop Production Enabling the Circular Bioeconomy in Agriculture: A Challenge for the New EU Regulation 1009/2019. Sustainability 2021, 13, 13165. [Google Scholar] [CrossRef]
- Rodias, E.; Aivazidou, E.; Achillas, C.; Aidonis, D.; Bochtis, D. Water-Energy-Nutrients Synergies in the Agrifood Sector: A Circular Economy Framework. Energies 2021, 14, 159. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Flammini, A.; Karl, K.; Obli-Laryea, G.; Qiu, S.Y.; Heiðarsdóttir, H.; Pan, X.; Conchedda, G. Methods for estimating greenhouse gas emissions from food systems—Part III: Energy use in fertilizer manufacturing, food processing, packaging, retail and household consumption. FAO Stat. Work. Pap. Ser. 2021, 29, 1–34. [Google Scholar] [CrossRef]
- Farm-Energy.extension.org. Available online: https://farm-energy.extension.org/ (accessed on 21 October 2022).
- Skowronska, M.; Filipek, T. Life cycle assessment of fertilizers: A review. Int. Agrophys. 2014, 28, 101–110. [Google Scholar] [CrossRef]
- Leigh, G.J. Haber-Bosch and Other Industrial Processes. In Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models and Commercial Processes; Smith, B.E., Richards, R.L., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 33–54. [Google Scholar]
- Jenssen, T.K.; Kongshaug, G. Energy Consumption and Greenhouse Gas Emissions in Fertiliser Production; Proceedings No. 509; The International Fertiliser Society: Colchester, UK, 2004. [Google Scholar]
- Nand, S.; Goswami, M. Recent efforts in energy conservation in ammonia and urea plants. Indian J. Fertil. 2021, 17, 628–634. [Google Scholar]
- IPTS/EC. Institute for Prospective Technology Studies (IPPC)-Reference Document on Best Available. Techniques for the Manufacture of Large Volume Inorganic Chemicals—Ammonia, Acids and Fertilizers. IPTS/EC. 2007. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/lvic_aaf.pdf (accessed on 29 October 2022).
- Farahani, S.S.; Rajabipour, A.; Keyhani, A.; Sharifi, M. Energy use and economic analysis of NPK-15: 8: 15 fertilizer granulation process in Iran. J. Saudi Soc. Agric. Sci. 2017, 16, 265–269. [Google Scholar] [CrossRef]
- Fadare, D.A.; Bamiro, O.A.; Oni, A.O. Energy and cost analysis of organic fertilizer production in Nigeria. Energy 2010, 35, 332–340. [Google Scholar] [CrossRef]
- Mazeika, R.; Staugaitis, G.; Baltrusaitis, J. Engineered Pelletized Organo-Mineral Fertilizers (OMF) from Poultry Manure, Diammonium Phosphate and Potassium Chloride. ACS Sustain. Chem. Eng. 2016, 4, 2279–2285. [Google Scholar] [CrossRef]
- Sarauskis, E.; Naujokiene, V.; Lekaviciene, K.; Kriauciuniene, Z.; Jotautiene, E.; Jasinskas, A.; Zinkeviciene, R. Application of Granular and Non-Granular Organic Fertilizers in Terms of Energy, Environmental and Economic Efficiency. Sustainability 2021, 13, 9740. [Google Scholar] [CrossRef]
- Latini, A.; Giagnacovo, G.; Campiotti, C.A.; Bibbiani, C.; Mariani, S. A Narrative Review of the Facts and Perspectives on Agricultural Fertilization in Europe, with a Focus on Italy. Horticulturae 2021, 7, 158. [Google Scholar] [CrossRef]
- Piwowar, A.; Dzikuc, M. Energy consumption and emissions of the fertilizer industry. Przem. Chem. 2020, 99, 564–568. [Google Scholar] [CrossRef]
- Fiamelda, L.; Suprihatin; Purwoko; IOP. Analysis of water and electricity consumption of urea fertilizer industry: Case study PT. X. In Proceedings of the International Conference on Innovation in Technology and Management for Sustainable Agroindustry (ITaMSA), Bogor, Indonesia, 9–10 October 2019. [Google Scholar]
- Salami, P.; Ahmadi, H.; Keyhani, A. Estimating the equivalent energy for single super phosphate production in Iran. J. Sci. Rev. 2010, 2, 1–10. [Google Scholar]
- Gellings, C.W.; Parmenter, K.E. Energy efficiency in fertilizer production and use. Efficient Use Conservation of Energy. Encycl. Life Support Syst. 2004, 2, 123–136. [Google Scholar]
- Kobayashi, H.; Sago, R. A study on life cycle assessment of energy consumption and CO2 emissions in the manufacturing and transportation processes of nitrogen and phosphate fertilizers. Jpn. J. Farm Work. Res. 2001, 36, 141–151. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling Agricultural Wastes and By-products in Organic Farming: Biofertilizer Production, Yield Performance and Carbon Footprint Analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Kytta, V.; Helenius, J.; Tuomisto, H.L. Carbon footprint and energy use of recycled fertilizers in arable farming. J. Clean. Prod. 2021, 287, 125063. [Google Scholar] [CrossRef]
- Gaidajis, G.; Kakanis, I. Life Cycle Assessment of Nitrate and Compound Fertilizers Production-A Case Study. Sustainability 2021, 13, 148. [Google Scholar] [CrossRef]
- Muscolo, A.; Romeo, F.; Marra, F.; Mallamaci, C. Recycling agricultural, municipal and industrial pollutant wastes into fertilizers for a sustainable healthy food production. J. Environ. Manag. 2021, 300, 113771. [Google Scholar] [CrossRef]
- Panuccio, M.; Marra, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Recycling of agricultural (orange and olive) bio-wastes into ecofriendly fertilizers for improving soil and garlic quality. Resour. Conserv. Recycl. Adv. 2022, 15, 200083. [Google Scholar] [CrossRef]
- Lisowska, A.; Filipek-Mazur, B.; Komorowska, M.; Niemiec, M.; Bar-Michalczyk, D.; Kubon, M.; Tabor, S.; Grodek-Szostak, Z.; Szelag-Sikora, A.; Sikora, J.; et al. Environmental and Production Aspects of Using Fertilizers Based on Waste Elemental Sulfur and Organic Materials. Materials 2022, 15, 3387. [Google Scholar] [CrossRef]
- Rehl, T.; Muller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid fertilizer products from anaerobic digestion of food waste: Mass, nutrient and energy balance of four digestate liquid treatment systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef]
- Xie, S.Y.; Zhang, T.; Mishra, A.; Tiwari, A.; Bolan, N.S. Assessment of catalytic thermal hydrolysis of swine manure slurry as liquid fertilizer: Insights into nutrients and metals. Front. Environ. Sci. 2022, 10, 12. [Google Scholar] [CrossRef]
- Law of July 10, 2007 on fertilizers and fertilization. J. Laws 2007, 147, 1033. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20071471033 (accessed on 31 October 2022). (In Polish).
- Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 on Fertilizers. Regulation (EC) No 2003/2003. 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32003R2003 (accessed on 26 October 2022).
- Regulation of the Minister of Agriculture and Rural Development of June 18, 2008 on the implementation of certain provisions of the Law on Fertilizers and Fertilization. J. Laws 2008, 119, 765. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20081190765 (accessed on 31 October 2022). (In Polish).
- Krystyna, M. New regulations on fertilizing products in EU countries from 2022. Biogas Mark. 2020, 11, 37–42. (In Polish) [Google Scholar]
- Benson, T.; Lavelle, F.; Bucher, T.; McCloat, A.; Mooney, E.; Egan, B.; Collins, C.E.; Dean, M. The Impact of Nutrition and Health Claims on Consumer Perceptions and Portion Size Selection: Results from a Nationally Representative Survey. Nutrients 2018, 10, 656. [Google Scholar] [CrossRef]
- Shambhavi, S.; Kumar, R.; Sharma, S.; Verma, G.; Sharma, R.; Sharma, S.K. Long-term effect of inorganic fertilizers and amendments on productivity and root dynamics under maize-wheat intensive cropping in an acid Alfisol. J. Appl. Nat. Sci. 2017, 9, 2004–2012. [Google Scholar] [CrossRef]
- Mazylyte, R.; Kaziuniene, J.; Orola, L.; Valkovska, V.; Lastauskiene, E.; Gegeckas, A. Phosphate Solubilizing Microorganism Bacillus sp. MVY-004 and Its Significance for Biomineral Fertilizers’ Development in Agrobiotechnology. Biology 2022, 11, 254. [Google Scholar] [CrossRef]
- IUNG.pl. Available online: http://www.ipm.iung.pulawy.pl/fert/fert.aspx?show=true (accessed on 16 March 2022).
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM(R)) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.X.; Hassanien, H.A.; Gaber, A.; Ammarr, G.A.G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Hrolfsdottir, A.P.; Arason, S.; Sveinsdottir, H.I.; Gudjonsdottir, M. Added Value of Ascophyllum nodosum Side Stream Utilization during Seaweed Meal Processing. Mar. Drugs 2022, 20, 340. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yan, M.Y.; Na, K.; Hwang, J.; Shin, S.; Yin, L.N.; Deng, X.P.; Wang, S.W. The New Soil Conditioner DewEco Could Improve Sandy Soil’s Properties for Efficient Maize Growth. Agronomy 2022, 12, 1124. [Google Scholar] [CrossRef]
- Szymanska, M.; Ahrends, H.E.; Srivastava, A.K.; Sosulski, T. Anaerobic Digestate from Biogas Plants-Nuisance Waste or Valuable Product? Appl. Sci. 2022, 12, 4052. [Google Scholar] [CrossRef]
- Abelenda, A.M.; Semple, K.T.T.; Aggidis, G.; Aiouache, F. Circularity of Bioenergy Residues: Acidification of Anaerobic Digestate Prior to Addition of Wood Ash. Sustainability 2022, 14, 3127. [Google Scholar] [CrossRef]
- Kovacevic, D.; Manojlovic, M.; Cabilovski, R.; Ilic, Z.S.; Petkovic, K.; Strbac, M.; Vijuk, M. Digestate and Manure Use in Kohlrabi Production: Impact on Plant-Available Nutrients and Heavy Metals in Soil, Yield, and Mineral Composition. Agronomy 2022, 12, 871. [Google Scholar] [CrossRef]
- Velechovsky, J.; Malik, M.; Kaplan, L.; Tlustos, P. Application of Individual Digestate Forms for the Improvement of Hemp Production. Agriculture 2021, 11, 1137. [Google Scholar] [CrossRef]
- Ran, Y.; Bai, X.L.; Long, Y.; Ai, P. Yield and Quality of Rice under the Effects of Digestate Application. Agriculture 2022, 12, 514. [Google Scholar] [CrossRef]
- Ntinas, G.K.; Bantis, F.; Koukounaras, A.; Kougias, P.G. Exploitation of Liquid Digestate as the Sole Nutrient Source for Floating Hydroponic Cultivation of Baby Lettuce (Lactuca sativa) in Greenhouses. Energies 2021, 14, 7199. [Google Scholar] [CrossRef]
- Rudawska, D.; Wiśniewska, J.; Drygaś, P.; Szyszkowska, A.; Drygaś, B. The importance of algae Brown algae (Phaeophyceae) and their impact on organisms plant and animal. Biol. Environ. Educ. 2018, 2, 3–9. (In Polish) [Google Scholar]
- Bień, J.; Zabochnicka-Świątek, M.; Sławik, L. Opportunities to use algae from the biomass of zeutrophized water bodies as a feedstock for biofuel production. Eng. Environ. Prot. 2010, 13, 197–209. (In Polish) [Google Scholar]
- Chojnacka, K.; Saeid, A.; Michalak, I. The possibilities of the application of algal biomass in the agriculture. Chemist 2012, 66, 1235–1248. (In Polish) [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Influence of Ecklonia maxima Extracts on Growth, Yield, and Postharvest Quality of Hydroponic Leaf Lettuce. Horticulturae 2021, 7, 440. [Google Scholar] [CrossRef]
- Knapik, M. Using biostimulants in modern agriculture. J. Stud. Sci. Mov. Jan Kochanowski Univ. Kielc. 2018, 27, 79–84. (In Polish) [Google Scholar]
- Collin, A. Analysis of the role of the HvABI5 gene in the drought stress response in barley (Hordeum vulgare L.); University of Silesia in Katowice: Katowice, Poland, 2021. (In Polish) [Google Scholar]
- Tuhy, L.; Chowañska, J.; Chojnacka, K. Seaweed extracts as biostimulants of plant growth: Review. Chemist 2013, 67, 636–641. (In Polish) [Google Scholar]
- Jastrząb, R.; Tylkowski, B. Biogenic amines in terms of their role in living organisms. Chem. News 2016, 70, 57–79. (In Polish) [Google Scholar]
- Kubiś, J. Polyamines and their involvement in plant response to environmental stress conditions. Cosmos 2006, 55, 209–215. (In Polish) [Google Scholar]
- Samaszko-Fiertek, J.; Kuźma, M.; Dmochowska, B.; Ślusarz, R.; Madaj, J. Bacterial exopolysaccharides: Structure and function. Chem. News 2016, 70, 473–496. (In Polish) [Google Scholar]
- Pielesz, A. Chemical composition of brown algae Fucus vesiculosus L. Post Fitoter 2011, 1, 9–17. (In Polish) [Google Scholar]
- Polifoska®. Available online: https://polifoska.pl/porady (accessed on 19 October 2022).
- Agrosimex.pl. Available online: https://doradca-rolniczy.pl/biostymulatory-fizjoaktywatory-aminokwasy-cz-2/ (accessed on 18 October 2022).
- Włodarek, A.; Badełek, E. Effect of different preparations applied during the growing season on the health of head cabbage heads after long-term storage. Prog. Plant Prot. 2020, 60, 105–110. (In Polish) [Google Scholar]
- Pipiak, P.; Skwarek, M. The use of amino acid fertilizers in agriculture. Technol. Prod. Qual. 2020, 65, 144–157. (In Polish) [Google Scholar]
- Sioubiz.pl. Available online: https://sioubiz.pl/pl/n/Jakich-witamin-potrzebuja-rosliny/15 (accessed on 11 October 2022).
- Jadczyszyn, T. Biogas plant digestate—Waste, by-product and fertilizer. Now Environ. 2019. (In Polish). Available online: https://www.teraz-srodowisko.pl/aktualnosci/poferment-biogazownia-odpad-produkt-uboczny-nawoz-7797.html (accessed on 26 October 2022).
- Łagocka, A.; Kamiński, M.; Cholewiński, M.; Pospolita, W. Environmental benefits of using digestate from agricultural biogas plants as organic fertilizer. Cosmos 2016, 65, 601–607. (In Polish) [Google Scholar]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Mostafazadeh-Fard, S.; Samani, Z.; Bandini, P. Production of Liquid Organic Fertilizer Through Anaerobic Digestion of Grass Clippings. Waste Biomass Valorization 2019, 10, 771–781. [Google Scholar] [CrossRef]
- Torres-Climent, A.; Martin-Mata, J.; Marhuenda-Egea, F.; Moral, R.; Barber, X.; Perez-Murcia, M.D.; Paredes, C. Composting of the Solid Phase of Digestate from Biogas Production: Optimization of the Moisture, C/N Ratio, and pH Conditions. Commun. Soil Sci. Plant Anal. 2015, 46, 197–207. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Restrepo, A.P.; Alburquerque, J.A.; Perez-Murcia, M.D.; Paredes, C.; Moral, R.; Bernal, M.P. Recycling of anaerobic digestates by composting: Effect of the bulking agent used. J. Clean. Prod. 2013, 47, 61–69. [Google Scholar] [CrossRef]
- Abdullahi, Y.A.; Akunna, J.C.; White, N.A.; Hallett, P.D.; Wheatley, R. Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresour. Technol. 2008, 99, 8631–8636. [Google Scholar] [CrossRef]
- Zeng, Y.; De Guardia, A.; Dabert, P. Improving composting as a post-treatment of anaerobic digestate. Bioresour. Technol. 2016, 201, 293–303. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Walker, L.; Charles, W.; Cord-Ruwisch, R. Comparison of static, in-vessel composting of MSW with thermophilic anaerobic digestion and combinations of the two processes. Bioresour. Technol. 2009, 100, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-Liquid Separation of Animal Slurry in Theory and Practice; Springer: Dordrecht, The Netherlands, 2011; pp. 953–986. [Google Scholar]
- Chiumenti, A.; da Borso, F.; Chiumenti, R.; Ted, F.; Segantin, P. Treatment of digestate from a co-digestion biogas plant by means of vacuum evaporation: Tests for process optimization and environmental sustainability. Waste Manag. 2013, 33, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Blecharczyk, A.; Paszkowski, A.; Dudzińska, A. Yield of winter oilseed rape grown in the second year of the following effects of legumes depending on the tillage and nitrogen fertilization. Fragm. Agron. 2020, 37, 38–46. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Jiang, G.; Liu, J.; Yin, H.; Xie, B.; Che, Z.; Jiang, F.; Zhang, T. Effect of Nitrogen Application on Root and Yield Traits of Chinese Spring Wheat (Triticum aestivum L.) under Drip Irrigation. Agronomy 2022, 12, 2618. [Google Scholar] [CrossRef]
- Makara, A.; Kowalski, Z.; Fela, K. Management of digestate in terms of environmental safety. Sci. Pap. Jan Długosz Acad. Częstochowa Technol. Inform. Secur. Eng. 2017, 5, 177–190. (In Polish) [Google Scholar]
- Majeed, A.; Farooq, M.; Naveed, M.; Hussain, M. Combined Application of Inorganic and Organic Phosphorous with Inoculation of Phosphorus Solubilizing Bacteria Improved Productivity, Grain Quality and Net Economic Returns of Pearl Millet (Pennisetum glaucum [L.] R. Br.). Agronomy 2022, 12, 2412. [Google Scholar] [CrossRef]
- Pitura, K.; Jarosz, Z. Chemical composition and biological value of medium-high kale in relation to differential mineral fertilization. Agron. Sci. 2020, 75, 97–107. (In Polish) [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Szymańska, M. Digestate a Fertilizer for Agriculture. Foundation for the Development of Polish Agriculture. 2017. Available online: https://ksow.pl/files/Bazy/Biblioteka/files/Poferment_nawozem_dla_rolnictwa_01.pdf (accessed on 26 November 2022).
- Ożyhar, T. Use of Calcium Carbonate-Based Mineral Additives in Wood-Based Panels and Wood Fiber-Reinforced Composites. 2022. (In Polish). Available online: https://indm.sggw.edu.pl/wp-content/uploads/sites/13/2022/06/Autoreferat_T.-Ozyhar_PL.pdf (accessed on 28 November 2022).
- Liu, X.; Hu, C.; Riaz, M.; Liu, X.; Sun, X.; Zhuang, M.; Tan, Q. Reducing Macronutrients and Increasing Micronutrient Fertilizers Are Key to Improving the Quality of Pomelo Citrus grandis (L.) Osbeck Cv. “Guanxi”. Agriculture 2022, 12, 1711. [Google Scholar] [CrossRef]
- Grzys, E. The role and importance of micronutrients in plant nutrition. Probl. Noteb. Prog. Agric. Sci. 2004, 502, 89–99. (In Polish) [Google Scholar]
- Potarzycki, J. The role of copper in winter wheat fertilization. Part I. Grain yield and quality. Probl. Noteb. Prog. Agric. Sci. 2004, 502, 953–959. (In Polish) [Google Scholar]
- Hallman, L.M.; Kadyampakeni, D.M.; Ferrarezi, R.S.; Wright, A.L.; Ritenour, M.A.; Johnson, E.G.; Rossi, L. Impact of Ground Applied Micronutrients on Root Growth and Fruit Yield of Severely Huanglongbing-Affected Grapefruit Trees. Horticulturae 2022, 8, 763. [Google Scholar] [CrossRef]
- Grzywnowicz-Gazda, Z. Effect of variation in zinc fertilization on the height and quality of grain yield of spring barley. Probl. Noteb. Prog. Agric. Sci. 1983, 242, 201–209. (In Polish) [Google Scholar]
- Kaningini, A.G.; Nelwamondo, A.M.; Azizi, S.; Maaza, M.; Mohale, K.C. Metal Nanoparticles in Agriculture: A Review of Possible Use. Coatings 2022, 12, 1586. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B.; Klimkowicz-Pawlas, A.; Maliszewska-Kordybach, B. The role of organic matter in the accumulation processes of persistent organic pollutants (POPs) in soils. Pol. J. Agron. 2015, 20, 15–23. (In Polish) [Google Scholar]
- Pontus, K. Digestion Sludge and Its Use. In Proceedings of the Baltic Biogas Forum IV, Gdansk, Poland, 11–12 September 2014; pp. 108–117. (In Polish). [Google Scholar]
- Tuszyńska, A. Analysis of the Possibility of Using Digestate as a Source of Bioavailable Phosphorus; Polish Academy of Sciences: Warsaw, Poland, 2019. (In Polish) [Google Scholar]
- Chaher, N.E.; Hemidat, S.; Chakchouk, M.; Nassour, A.; Hamdi, M.; Nelles, M. From anaerobic to aerobic treatment: Upcycling of digestate as a moisturizing agent for in-vessel composting process. Bioresour. Bioprocess. 2020, 7, 60. [Google Scholar] [CrossRef]
- Song, B.; Manu, M.K.; Li, D.Y.; Wang, C.; Varjani, S.; Ladumor, N.; Michael, L.; Xu, Y.J.; Wong, J.W.C. Food waste digestate composting: Feedstock optimization with sawdust and mature compost. Bioresour. Technol. 2021, 341, 125759. [Google Scholar] [CrossRef] [PubMed]
- Dobrzański, A.; Anyszka, Z.; Elkner, K. Response of carrots to extracts of natural origin from algae of the genus Sargassum-AlgaminoPlant and leonardite-HumiPlant. J. Res. Appl. Agric. Eng. 2008, 53, 53–58. (In Polish) [Google Scholar]
- Janas, R.; Szwejda, J.; Wojska, A. Cultivation of Onion (Allium cepa L.) for Seed in Organic Systems 1st Year of Cultivation-Production of Seedlings; Ministry of Agriculture and Rural Development: Skierniewice, Poland, 2020. (In Polish)
- Gawroński, D. Reducing the effects of drought on crops through the use of humic acids and biohumus. Agric. Advis. Issues 2021, 104, 48–59. (In Polish) [Google Scholar]
- Huculak-Mączka, M.; Nieweś, D.; Braun-Giwerska, M.; Porwoł, M.; Marecka, K.; Hoffmann, J. Use of fertilizer components to extract humic substances. Proc. ECOpole 2020, 14, 89–95. (In Polish) [Google Scholar]
- Dębska, B.; Banach-Szott, M.; Drąg, M. Influence of PAH contamination of hay soils on selected quality parameters of humic acids. Proc. ECOpole 2012, 6, 625–631. (In Polish) [Google Scholar]

| Process (Remarks) | Energy Use (GJ/Mg Urea)-LHV * | Energy Use (MMBtu/Mg urea)-HHV * |
|---|---|---|
| Conventional total recycle process | ||
| Conventional total recycle process (Toyo) (excl. electricity use for CO2 compression) | 2.7 | 2.6 |
| Existing installations (crystallization, natural draft prilling, compression with steam turbine) | 5.5 | 5.2 |
| NH3 stripping | ||
| Snamprogetti NH3 stripping (excl. electricity use for CO2 compression) | 1.7 | 1.6 |
| NH3 stripping (prilling, CO2 compression with steam turbine, prilling) | 2.9 | 2.7 |
| NH3 stripping (prilling, CO2 compression with steam turbine, granulation) | 3.1 | 2.9 |
| NH3 stripping (prilling, CO2 compression with electromotor, prilling) | 2.0 | 1.9 |
| NH3 stripping (prilling, CO2 compression with electromotor, granulation) | 1.9 | 1.8 |
| CO2 stripping | ||
| Stamicarbon CO2 stripping, (excl. electricity use for CO2 compression) | 1.9 | 1.8 |
| Stamicarbon CO2 stripping, (steam and electricity) | 2.7 | 2.6 |
| ACES stripping (spout fluid bed granulation, CO2/NH3/carbamate pumps driven by steam turbine) | 3.0 | 2.8 |
| ACES stripping (spout fluid bed granulation, only the CO2 pump driven by steam turbine) | 2.7 | 2.6 |
| Typ of Fertilizer | Unit Consumption | |||
|---|---|---|---|---|
| Total Energy | Incl. | |||
| Hydrocarbon Fuels | Heat | Electricity | ||
| MJ/Mg | MJ/Mg | MJ/Mg | kWh/Mg | |
| Urea (NH2)2CO | 3497.2–4985.7 | - | 4016.0–5256.1 | 109.0–134.8 |
| Calcium Ammonium Nitrate (CAN) | 545.5–1118.1 | 7.0–9.3 | 430.5–870.3 | 25.0–41.0 |
| Ammonium Nitrate (AN) NH4NO3 | 561.6–598.7 | 0.1 | 482.8–520.5 | 19.5–24.0 |
| Granular single superphosphate (SSP) | 688.4–962.0 | 610.5–850.8 | - | 16.4–30.9 |
| Triple superphosphate (TSP) | 1600.5–2011.9 | 1130.2–1352.9 | 203.8–482.7 | 50.5–83.8 |
| Typ of Fertilizer | Unit Consumption | |||
|---|---|---|---|---|
| Total Energy | Incl. | |||
| Hydrocarbon Fuels | Heat | Electricity | ||
| MJ/Mg | MJ/Mg | MJ/Mg | kWh/Mg | |
| NP Fertilizer | 895.8–1725.8 | 366.3–1024.1 | 341.0–499.8 | 30.6–56.1 |
| NPK Fertilizer | 990.5–1245.4 | 681.6–926.6 | 153.9–182.4 | 33.5–39.0 |
| Fertilizer Product | Primary Energy Consumption | Unit |
|---|---|---|
| Ammonium nitrate (AN) | 40 */29.8 | MJ/kg N |
| Urea | 51.6 */44.1 ** | |
| Calcium ammonium nitrate (CAN) | 42.6 */31.4 ** | |
| Ammonium sulphate (AS) | 42 | |
| Triple superphosphate (TSP) | 30.25 | MJ/kg P |
| Single superphosphate (SSP) | 13 | |
| Muriate of potash (MOP) | 10.06 | MJ/kg K |
| Limestone | 2.3 | MJ/kg Ca |
| Type | Characteristic | Required Permit |
|---|---|---|
| Mineral fertilizers | inorganic fertilizers, produced by chemical transformation, physical transformation or processing of mineral raw materials, including fertilizer lime, which includes fertilizer lime containing magnesium, as well as some fertilizers of organic origin | Yes—with the exception of fertilizers marked “EC FERTILIZER” and types of fertilizer lime in which impurities do not exceed the permissible values of impurities |
| Natural fertilizers | fertilizers intended for agricultural use without the addition of other substances, such as manure, guano, slurry | no |
| Organic fertilizers | fertilizers made from organic matter or from mixtures of organic matter, including composts, as well as composts produced using earthworms | yes |
| Organic-mineral fertilizers | mineral and organic fertilizer mixtures | yes |
| Indicator | Fertilizer in Liquid Form | ||
|---|---|---|---|
| Mineral | Organic | Organic-Mineral | |
| Nitrogen (N) | min. 1.0% (m/m) of total nitrogen | min. 0.08% (m/m) of total nitrogen | min. 0.5% (m/m) of total nitrogen |
| Phosphorus (P) | min. 1.0% (m/m) phosphorus per P2O5 | min. 0.05% (m/m) phosphorus per P2O5 | min. 0.2% (m/m) phosphorus per P2O5 |
| Potassium (K) | min. 1.0% (m/m) potassium per K2O | min. 0.12% (m/m) potassium per K2O | min. 0.5% (m/m) potassium per K2O |
| Commercial Name | Producer | Decision No. | Additional Information/Fertilizer Composition |
|---|---|---|---|
| AlfaMax | Agro Varichem Distribution LLC | 313/12 from year 2012 | Produced from algae. Contains: L-amino acids, hormones, auxins, gibberellins, cytokinins. Minimum parameters: N—1.0%, K2O—2.5%, dry matter content—26%, TOC—5.5%. |
| AlgaPlant | VARICHEM LLC | 284/11 from year 2011 | Algae extract 36%. Contains: auxins, gibberellins, cytokinins. Minimum parameters: N—0.15%, K2O—5.5%. |
| AlgaminoPlant | VARICHEM LLC | 316/13 from year 2013 | Brown algae extract, sargassum type and alphaaminoacids. Composition: N—1.56%, K2O—2.94%. |
| DARINA | TORTRANS LLC | 363/15 from year 2015 | Minimum parameters: N—4.0%, P2O5—2.5%, K2O—6.5%, organic matter content—30.0%. |
| FertiBio 48 | Moolenaar BV | NE/338/2017 year 2017 | Produced from corn grain. Composition: N—3.5%, P2O5—3.8, K2O—3.0%. |
| FERMROL | IMA Poland S.A. | 615/20 from year 2020 | Produced by methane fermentation of distillers’ stock. Composition: N—0.15%, K2O < 0.5%, TOC—26.9%, organic matter content—48.0%. |
| Gärrest | Biomethan Schöpstal GmbH & Co. KG | 350/14 from year 2014 | Produced from corn silage digestate, grass and GPS. Composition: N—0.47%, P2O5—0.18%, K2O—0.53%, MgO—0.07%, organic matter content—76.9%. |
| Green Plon NPK | Bio-Wat LLC | 371/15 from year 2015 | Produced from the remains of methane fermentation of silage from agricultural raw materials, i.e., corn, rye, haylage, beet pulp, waste from oil plants, vegetable waste. Composition: N—0.43%, N-NH4—0.15%, P2O5—0.18%, K2O—0.35%, organic matter content—74.4%. |
| HUMI BROWN GOLD | Generiks LLC | 549/19 from year 2019 | Composition: N—0.29%, K2O—1.13%, humic acids—79.0%, fulvic acids—21.0%, organic matter content—63.2%. |
| HUMIACID | “TOMATEX” | 372/15 from year 2015 | Produced from biogas digestate with a water content of 30%. Composition: N—0.15%, K2O—0.58%, organic matter content—63.2%. |
| INNBIO | Laseffre Polish JSC | 449/17 from year 2017 | Produced from baker’s yeast with the addition of bacteria Bacillus amyloloqiefaciens. Minimum parameters: N—2.4%, K2O—3.5%, organic matter content—55.0%. |
| Konzentrat | GENO Bioenergie Leasingfonds Erste GmbH&Co. KG | 257/11 from year 2011 | Produced from corn silage digestate 82.2%, rye—5.1%, barley—1.4% and water—11.3%. Composition: N—0.6%, P2O5—0.15%, K2O—0.45%, MgO—0.05%. |
| Nettle fertilizer | CDN Ireneusz Cal | 479/18 from year 2018 | Composition: N—0.2%, P2O5—0.1%, K2O—0.2%, organic matter content—30.0%. |
| PLANTEO | Green Energy LLC | 556a/19 from year 2019 | Corn silage digestate, haylage and beet pulp. Composition: N—0.45%, N-NH4—0.27%, P2O5—0.14%, K2O—0.32%. |
| Biogas digestate, liquid form | Pfeifer & Langen Poland S.A. | 491/19 from year 2019 | Digestate from beet root fragments and pulp. Composition: N—0.34%, N-NH4—0.17%, P2O5—0.07%, K2O—0.19%, organic matter content—72.6%. |
| Presswasser | Biomethan Schöpstal GmbH & Co. KG | 277/11 from year 2011 | Plant pulp digestate. Composition: N—0.44%, P2O5—0.15%, K2O—0.31%, organic matter content—73.2%. |
| GREEN ORGANIK | MAK Organic LLC | 521a/19 from year 2019 | Decoction of molasses with the addition of vegetable protein hydrolyzate. Composition: N—4.52%, P2O5—0.32%, K2O—6.94%, CaO—0.35%. |
| Commercial Name | Producer | Decision No. | Additional Information/Fertilizer Composition |
|---|---|---|---|
| Actifos | AGROPAK Ordinary Partnership; B. Pluta, G. Brzeziński, and Partners | 241/10 from year 2010 | Composition: N—10.2%, B—0.02%, Cu—0.08%, Fe—0.06%, Mn—0.04%, Mo—0.004%, Zn—0.02%. |
| ADIMIKS 7—solution 20% | Azoty-Adipol JSC | 272/12 from year 2012 | Composition: N—3.60%, N-NH4—0.80%, N-NH2—1.04%, N-NO3—1.76%, P2O5—1.7%, K2O—4.7%. |
| Aloes | BIOPON® Grzegorz Sobański | 73/04 from year 2004 | Minimum parameters: N—1.6%, N-NO3—1.0%, P2O5—4.6%, K2O—4.1%. |
| AMMIAK | TRANS-AMMIAK LLC | 169/06 from year 2006 | Ammonia water with a minimum N-NH4 content of 20%. |
| BARRIER Si-Ca | Osadkowski S.A. | 522/19 from year 2019 | Produced from calcium silicate. Composition: CaO—1.11%, SiO2—20.97%. |
| Bioflor popular | BIOPON® Grzegorz Sobański | 55/04 from year 2004 | Minimum parameters: N—3.0%, N-NH2—2.2%, P2O5—1.2%, K2O—2.4%. |
| FORTER | INTERMAG LLC | 608/20 from year 2020 | Composition: K2O—6.31%, I—15.8%, Se—1.13%. |
| Insol 4 | Fertilizer Research Institute | 340/13 from year 2013 | Composition: Mg—4.0%, B—0.5%, Cu—0.1%, Fe—0.35%, Mn—0.65%, Mo—0.005%, Zn—0.35%. |
| Mineral ammonium sulfate | Verbio Poland LLC | 613/20 from year 2020 | Distillers’ stock from grain with the addition of sulfuric acid, produced under anaerobic conditions. Composition: N—8.49%, S-SO3—24.4%. |
| NTS | Beiselen-ATR LLC | 173/06 from year 2006 | NS fertilizer, urea-ammonium nitrate solution with sulfur. Composition: N—27.3%, N-NH4—8.0%, N-NO3—5.9%, N-NH2—13.4%, S-SO3—3.4%. |
| OCTAN-PLUS | ALEKO Aleksandra Samuła | 648a/21 from year 2021 | Minimum parameters: CaO—6.65%, acetate content—18.9%. |
| PENTAKEEP-V | Agroniwa LLC | 179/07 from year 2007 | Composition: N—1.84%, P2O5—4.02%, K2O—0.16%, CaO—3.73%, MgO—0.46%, organic matter content—41.28%. |
| PLONURAN LIQUID | ARYSTA LIFESCIENCE Poland LLC | 118/05 from year 2005 | Composition: Cu—22.4%. |
| RSM + S 27/3 | Unibaltic Agro LLC | 164/06 from year 2006 | NS fertilizer, ammonium nitrate-urea solution with the addition of sulphate sulfur. Composition: N—27.6%, N-NH2—13.6%, N-NH4—8.2%, N-NO3—5.8%, S-SO3—3.1%. |
| Commercial Name | Producer | Decision No. | Additional Information/Fertilizer Composition |
|---|---|---|---|
| A.S.L | Verbio Poland LLC | 353/14 from year 2014 | Remains of methane fermentation of distillers’ stock, cereal straw. Composition: N—10.2%, N-NH4—8.28%. |
| ALGAREN BZn | GREEN HAS ITALIA JSC | 513/18 from year 2018 | Contains Ecklonia maxima sea algae extract. Composition: N—2.23%, B—2.02%, Zn—2.96%, TOC—6.58%. |
| BIOEKOR for geraniums and other balcony plants | EKOR WALKOWIAK Ordinary Partnership | 108/04 from year 2004 | Composition: N—3.6%, P2O5—6.0%, K2O—7.0%, microelements. |
| BM Start | Laboratoires Goëmar SAS | 481-18 from year 2018 | Fertilizer containing MgSBMo with the addition of brown algae filtrate—Ascophyllum nodosum (GA 142). Composition: MgO—3.25%, S-SO3—6.6%, B—2.09%, Mo—186 mg/kg, dry matter content—42.7%, organic matter content—73.8%. |
| CARBO’CAL | ARYSTA LIFESCIENCE Poland LLC | 542/19 from year 2019 | Contains Ascophyllum nodosum algae filtrate. Composition: CaO—15.4%, organic matter content—44.7%. |
| CARBO’FRUIT | ARYSTA LIFESCIENCE Poland LLC | 543/19 from year 2019 | Contains Ascophyllum nodosum algae filtrate. Composition: P2O5—27.02%, K2O—7.87%, organic matter content—18.7%. |
| COLORADO | ARYSTA LIFESCIENCE Poland LLC | 541/19 from year 2019 | Contains Ascophyllum nodosum algae filtrate. Composition: CaO—2.8%, MgO—2.21%, Mn—1.86%, Zn—1.88%, organic matter content—58.3%. |
| FoliQ Aminovigor | Kazgod LLC | 375/15 from year 2015 | Fertilizer with the addition of corn extract. Composition: N—2.56%, B—0.28%, Cu—0.6026%, Fe—1.4459%, Mn—0.6148%, Mo—0.2266%, Zn—0.5466%, organic matter content—73.4%. |
| FoliQ® Ascovigor | Kazgod LLC | 400/16 from year 2016 | NK fertilizer with microelements with the addition of algae extract as an adjuvant. Composition: N—3.04%, K2O—2.66%, B—4.15%, Mn—1.06%, Zn—0.59%, organic matter content—39.8%. |
| HALCZYNA | SENSO BARBARA KUKIEŁKA | 616/20 from year 2020 | Composition: N—1.55%, P2O5—0.5%, K2O—2.05%, Mn—0.004%, Fe—0.237%, Cu—0.0002%, Zn—0.0007%, humic acids—4.69%, fulvic acids—0.69%. |
| HUMUS-ONE PERFEKT | TTT LLC | 427/16 from year 2016 | Extract of humic acids from leonardites with the addition of plant extracts. Composition: N—1.4%, P2O5—0.5%, K2O—3.1%, TOC—19.0%. |
| Megafol | Amagro LLC | 194/07 from year 2007 | PK fertilizer, extract from fresh plant material—lucerne, seaweed, sugar beet molasses. Composition: N—3.2%, K2O—9.03%, organic matter content—61.39%. |
| Rooter | Laboratoires Goëmar SAS | 482/18 from year 2018 | Phosphorus-potassium fertilizer with the addition of Ascophyllum nodosum brown algae filtrate (GA 142). Composition: P2O5—12.6%, K2O—6.4%, dry matter content—23.7%, organic matter content—18.6%. |
| GREEN BUSH | HIMAL | 273/11 from year 2011 | A mixture of plant extracts with EC fertilizers. Composition: N—3.74%, P2O5—3.44%, K2O—3.25%, Cu—895 mg/kg. |
| Commercial Name | Producer | Decision No. | Additional Information/Fertilizer Composition |
|---|---|---|---|
| Acti Humus Pro | AGROSIMEX LLC | S-878/19 from year 2019 | Humic acids from leonardites. Composition: K2O—0.22%, Fe—0.013%, organic matter content—61.7%, humic acids—0.99%. |
| AGRO-plant | Producer Group Agro-Żabice LLC | G-1063/21 from year 2021 | Digestate from a biogas plant that uses distillers’ stock, haylage and molasses for the production of biogas. Composition: N—0.47%, K2O—0.59%, organic matter content—54.4%, pH—7.9. |
| AGROVIT II | “BIOGAS SERVICE” LLC | G-183/11 from year 2011 | Digestate obtained from stillage, waste plant mass. Composition: N—0.56%, K2O—1.16%, CaO—0.14%, organic matter content—51.2%. |
| ALGEEN VIT | Biohumuseco LLC | 696a/18 from year 2018 | Minumum parameters: B—1.0–3.50 mg/kg, Zn—0.1–2.0 mg/kg, Fe—6.0–20.0 mg/kg, organic matter content—2.5%, dry mass content—6.0%. |
| ALGIN-PLUS | ITADAM.NET Adam Samuła | G-812/19 from year 2019 | Algae extract. Composition: N—0.12%, P2O5—2.36%, K2O—1.30%, TOC—14.1%, organic matter content—83.2%. |
| APOL-HUMUS | Poli-Farm® LLC | S-326e/20 from year 2020 | Contains TOC in the form of dissolved humic substances—5.69 g/L. |
| ASX silicon plus | AGROSIMEX LLC | S-886a/20 from year 2020 | Mineral salts (copper chloride, orthosilicic acid, boric acid) dissolved in a mixture of choline chloride, hydrochloric acid, sorbitol and Yucca extract. Composition: SiO2—1.17%, B—0.47%, Cu—1.13%, organic matter content—85.6%. |
| Bactim soil | INTERMAG LLC | G-816/19 from year 2019 | Minumum prameters: Fe—0.007%, Zn—0.007%, number of bacteria of the genus Bacillus spp. 5 × 108 cfu/mL. |
| Bio-algeen S90 plus 2 | Service and Trade Enterprise Polger-Kido | S-3/08 from year 2008 | Composition: N—0.02%, P2O5—0.006%, K2O—0.096%, CaO—0.31%, MgO—0.021%, B—16 mg/kg, Fe—6.3 mg/kg, Cu—0.2 mg/kg, Mn—0.6 mg/kg, Zn—1.0 mg/kg. |
| Bioenergie flüssig | LINDHORST GRUPPE JLW HOLDING AG | G-810/19 from year 2019 | Biogas digestate. Composition: N—0.56%, N-NH4—0.26%, K2O—0.55%, organic matter content—76.0%. |
| Biomethan - Liquid | Biomethan Zittau GmbH | G-184/10 from year 2010 | Substrate after anaerobic fermentation of plant materials (corn, green rye, grass). Composition: N—0.47%, P2O5—0.11%, K2O—0.67%. |
| Florahumus Liquid | Brown coal mine Sieniawa LLC | S-1040/21 from year 2021 | Crushed brown coal from which humic acids are obtained in the form of salt. Composition: TOC—103.2 g/L, humic acids—88.8 g/L, dry mass content—21.3%, pH—9.0. |
| GAMAORGANIC | GAMAWIND LLC | G-1052/21 from year 2021 | Distillers’ stock from a biogas plant. Composition: N—0.43%, P2O5—0.12%, K2O—0.17%, organic matter content—75.6%, pH—7.9. |
| Germinator SL | NaturalCrop Poland LLC | S-526/15 from year 2015 | A concentrate of active humic and fulvic acids and bioactive chitosan (polymers of N-glucosamine and N-acetyl-glucosamine). Minimum parameters: TOC—7.5 g/L, macro- and microelements (N, P, K, Mg, S, Na, Cu, Zn, Mo, B). |
| GLEBOWIT II | ENEA Production LLC | G-323/13 from year 2013 | Distillers’ stock from an agricultural biogas plant. Composition: N—0.18%, K2O—1.26%, organic matter content—8.15%. |
| GREVITAX | AVIS NATURALL Poland LLC | S-290/12 from year 2012 | Organic grapefruit extract for watering or spraying. |
| HYDROHUMAT | AGROVITA LLC | G-557/16 from year 2016 | Humic acids extracted from peat treated with sodium hydroxide and then with hydrochloric acid. Composition: N—0.52%, N-NH4—0.04%, organic matter content—28.3%. |
| INGREEN SILVER | INWEX LLC | S-920/20 from year 2020 | Hydrogen peroxide stabilized with silver. Composition: Ag—0.04%. |
| KELPAK | PUH CHEMIROL LLC | S-220d/19 from year 2019 | Composition: TOC—0.36%, organic matter content—32.9%. |
| PERFEKT | ITADAM.NET Adam Samuła | G-813/19 from year 2019 | Produced from leonardite. Composition: N—1.09%, P2O5—0.12%, K2O—2.03%, TOC—18.0%, organic matter content—86.7%. |
| SEPTOVITAL 200 | AGROSIMEX LLC | S-297/12 from year 2012 | Crushed grapefruit, extracted with a solution of zucrroli. Composition: TOC—10.66%. |
| Synbio 600 | AGROL Krzysztof Świerzewski | S-1049/21 from year 2021 | Extract of humic acids from leonardites. Composition: dry matter content—5.1%, organic matter content—32.4%, pH—7.15. |
| TOTALSOIL | THE LLC | G-716/17 from year 2017 | Composition: K2O—2.62%, TOC—5.0%. |
| VANADOO | INTERMAG LLC | S-949/20 from year 2020 | A mixture of ascorbic acid, vanadyl sulfate and sodium hydroxide. Composition: V—2.40%, organic matter content—46.50%. |
| ZumSil® | EMC DENARIUS D. Lempkowski | S-717/18 from year 2018 | Contains silicon in the form of orthosilicic acid. Composition: SiO2—18.83%. |
| Dry Matter % | pHH2O | EC mS/cm | TN mg/kg | P mg/kg | K mg/kg | Ca mg/kg | Mg mg/kg | S mg/kg |
|---|---|---|---|---|---|---|---|---|
| 6.04 ± 0.127 | 8.35 ± 0.353 | >4000.0 ± 0.0 | 57,800.0 ± 1265.0 | 12,912.0 ± 562.0 | 42,988.0 ± 1214.0 | 39,996.0 ± 25,860 | 4268.0 ± 272.0 | 3228.0 ± 342.0 |
| Fe mg/kg | Zn mg/kg | Cu mg/kg | B mg/kg | Mn mg/kg | Pb mg/kg | Cd mg/kg | Cr mg/kg | As mg/kg |
| 267.0 ± 96.0 | 251.0 ± 68.0 | 6.1 ± 0.52 | 76.5 ± 12.5 | 189.0 ± 15.7 | 0.9 ± 0.02 | 0.09 ± 0.001 | 1.01 ± 0.02 | 3.79 ± 0.12 |
| N mg/dm3 | P mg/dm3 | K mg/dm3 | Ca mg/dm3 | Mg mg/dm3 | Na mg/dm3 | Fe mg/dm3 |
|---|---|---|---|---|---|---|
| 331.33 ± 47.65 | 153.62 ± 12.89 | 470.25 ± 2.05 | 12.9 ± 3.25 | 3.38 ± 0.31 | 106.2 ± 1.13 | 4.2 ± 1.56 |
| Cd mg/dm3 | Cu mg/dm3 | Cr mg/dm3 | Ni mg/dm3 | Mn mg/dm3 | Pb mg/dm3 | Zn mg/dm3 |
| 0.01 ± 0.0 | 0.4 ± 0.1 | 0.05 ± 0.01 | 0.28 ± 0.01 | 0.02 ± 0.0 | <0.042 | 2.42 ± 0.1 |
| Parameter | Example of Compounds | Role | References |
|---|---|---|---|
| Phytohormones | auxins, cytokinins, gibberellins, abscisic acid, ethylene | stimulation of stem elongation and leaf bud opening, regulation of RNA protein synthesis, enzyme activity, stimulation of flower production, increasing pollen viability and zygote viability, induction of seed germination, inhibition of lateral shoot growth | [92,93,94] |
| Polyamines | putrescine, spermidine | growth regulator, seed germination stimulation, pollen tube growth, anti-cellular aging, resistance to stressors | [95,96] |
| Polysaccharides | alginic acid | rheological properties that allow the fertilizer to adhere to the leaves | [97] |
| Elements (micro- and macroelements) | zinc, copper, bromine, iodine, iron, magnesium, manganese, calcium, phosphorus | properties that encourage bees to pollinate flowers, chlorophyll synthesis, electron transport to produce organic parts of carbon | [98,99] |
| Sugar alcohols | mannitol | improved uptake and transport of nutrients in the plant, stimulation of polyamine synthesis | [98,100] |
| Isoflavonoids | phytoalexins | inhibition of pathogen growth, protection against UV radiation and heavy metal ions and thermal shock | [100,101] |
| Amino-acids | glycoproteins, alanine, glycine, lysine, serine, leucine, methionine, tryptophan, valine | increasing the assimilation of fertilizer by plants, forming organic connections with nutrients, increasing the efficiency of photosynthesis | [98,102] |
| Vitamins | A, E, C, D, β-karoten | resistance to low temperatures, increased smog tolerance, intensification of photosynthesis, improved fruit quality, root system formation and germination | [98,103] |
| Parameter | Role | References |
|---|---|---|
| Nitrogen (including the ionic form of NH4+) * | extension of the vegetation period, stimulation of proper plant development and growth of aboveground and underground parts, maintenance of proper green color | [116,117,118] |
| Phosphorus (including the ionic form of PO43-) * | formation and growth of the root, increase the ability to form flowers and fruit, participation in ATP production | [118,119] |
| Potassium | increase disease and pest resistance, stimulation root system growth, resist cold, regulation plant water management, stimulation starch and sugar production | [120,121] |
| Calcium | strengthening of the root system, resistance to stress factors such as drought and frost, strengthening of mechanical tissues | [122] |
| Magnesium | participation in photosynthesis, mineral uptake, chlorophyll component, stimulation root system development | [99,123] |
| Iron | reducing the action of nitrates, enabling normal growth and development, participation in the formation of chlorophyll, transporting electrons to produce organic carbon compounds, participation in the process of photosynthesis | [124] |
| Copper | influence on the growth and development of the plant, influence on tissue structure, protein and vitamin C synthesis, develop more grain, strengthen resistance to permanent bending of stems | [125] |
| Manganese | influence on nitrogen management, support root growth deep into the soil profile, improve resistance to stress factors, toxic effect on soil pathogens, participation in photosynthesis and chlorophyll formation process | [99,126] |
| Zinc | increase plant resistance to diseases, influence on the yield, increase plant growth dynamics, enhance biological activity of roots | [127,128] |
| Organic carbon, organic matter | water retention in soil, source of humus in soil, renewable source of nutrients for plants | [129] |
| Parameter | Distillers’ Stock | Maize Silage | Maize Silage + Beet Pulp + Apple Pomace | Beet Pulp + Maize Silage + Distillers’ Stock | Maize Silage + Beet Pulp | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F * | D ** | F * | D ** | F * | D ** | F * | D ** | F * | D ** | |
| Unit | [mg/g s.m.] [%] ^ | [g/dm3] | [mg/g s.m.] | [g/dm3] | [mg/g s.m.] | [g/dm3] | [mg/g s.m.] | [g/dm3] | [mg/g s.m.] | [g/dm3] |
| Mg | 22.1 | 0.14 | 2.4 | 0.04 | 2.3 | 0.05 | 2.2 | 0.03 | 2.3 | 0.02 |
| Ca | 20.2 | 0.15 | 4.5 | 0.02 | 9.2 | 0.06 | 9.2 | 0.04 | 9.2 | 0.07 |
| K | 70.6 | 1.14 | 9.3 | 0.15 | 9.0 | 0.17 | 8.6 | 0.31 | 7.1 | 0.19 |
| Fe | 4.1 | 0.03 | 1.4 | 0.02 | 0.9 | 0.02 | 0.7 | 0.01 | 0.5 | 0.02 |
| TNNH4+ *** | 75.4 | 2.18 1.91 | 2.1 | 1.50 1.44 | 1.6 | 1.29 1.18 | 2.7 | 1.53 1.44 | 1.5 | 1.47 1.38 |
| DM ^ TSS ^^ | 4.1 | 16.2 | 27.3 | 10.7 | 26.4 | 10.5 | 19.3 | 13.8 | 23.2 | 20.5 |
| OM ^ OS ^^ | 82.1 | 15.6 | 88.4 | 10.5 | 84.8 | 9.60 | 88.8 | 12.8 | 89.1 | 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajura, R.; Masłoń, A.; Czarnota, J. The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry—A Case Study from Poland. Energies 2023, 16, 1747. https://doi.org/10.3390/en16041747
Pajura R, Masłoń A, Czarnota J. The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry—A Case Study from Poland. Energies. 2023; 16(4):1747. https://doi.org/10.3390/en16041747
Chicago/Turabian StylePajura, Rebeka, Adam Masłoń, and Joanna Czarnota. 2023. "The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry—A Case Study from Poland" Energies 16, no. 4: 1747. https://doi.org/10.3390/en16041747
APA StylePajura, R., Masłoń, A., & Czarnota, J. (2023). The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry—A Case Study from Poland. Energies, 16(4), 1747. https://doi.org/10.3390/en16041747







